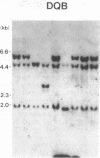
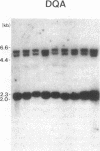
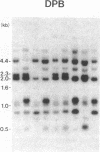
Abstract
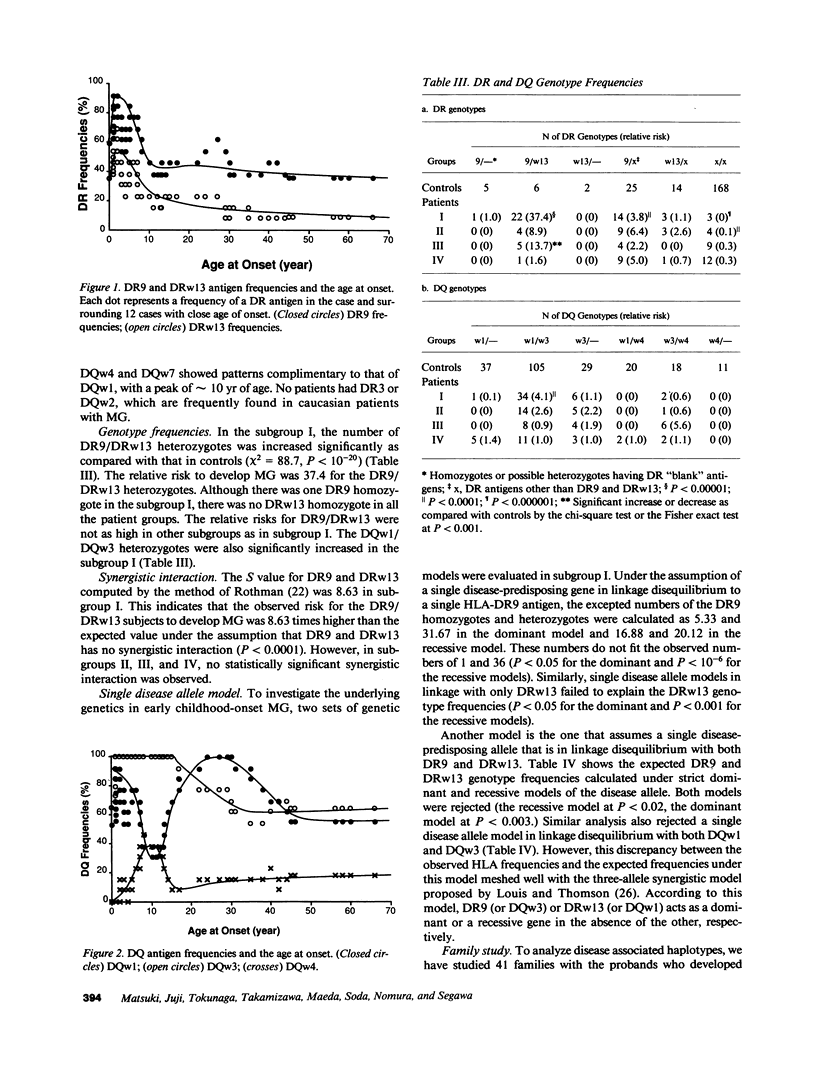
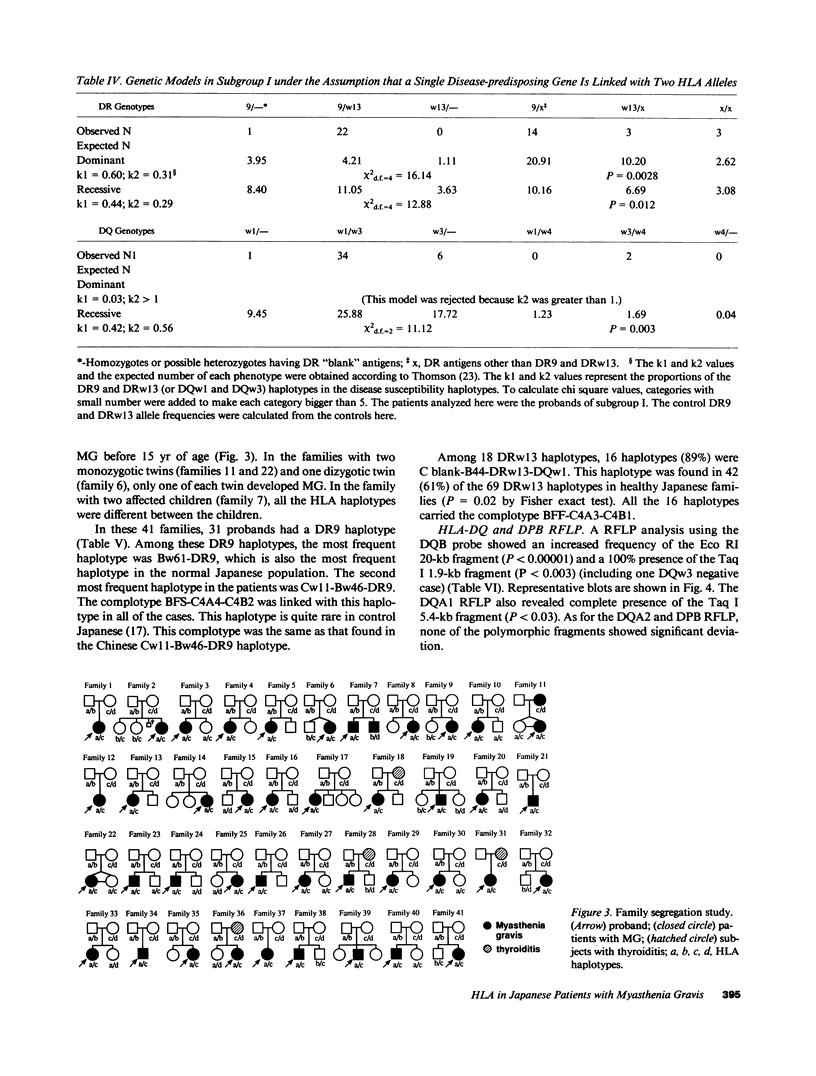
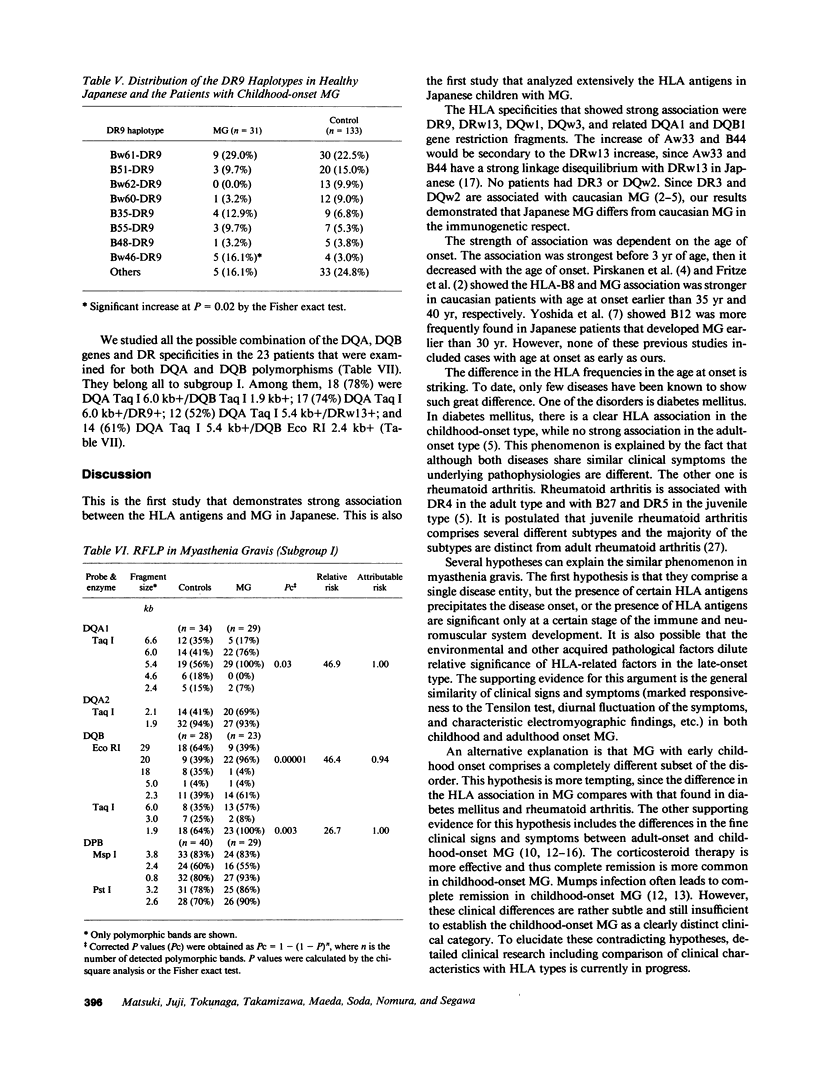
HLA antigens in 104 Japanese patients and 41 families with myasthenia gravis (MG) were investigated. The frequencies of DR9 and DRw13 were significantly increased in the patients who developed MG before 3 yr of age. The DQw3 antigen was positive for all the patients that developed MG before 15 yr with only one exception. All the examined cases that developed MG before 3 yr (including this DQw3 negative patient) had the same DQA and DQB DNA restriction fragments. These HLA frequencies decreased as the age of onset increased, and no significant association was observed in adult-onset MG. No patients had B8, DR3, and DQw2. The relative risk was higher for the DR9/DRw13 heterozygotes (37.4) than for DR9 (16.4) or DRw13 (7.1) in the childhood-onset MG. Statistical analysis suggested that DR9 and DRw13 (or DQw1 and DQw3) act synergistically in the disease development. Family study revealed diverse DR9 haplotypes. The most frequent DRw13 haplotype was Bw44-BFF-C4A3B1-DRw13-DQw1, which may be evolutionarily related to the caucasian B8-DR3-DQw2 haplotype. These results showed that MG in early childhood in Japanese individuals is genetically different from that in adulthood and that in caucasians.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper C. A., Boenisch T., Watson L. Genetic polymorphism in human glycine-rich beta-glycoprotein. J Exp Med. 1972 Jan;135(1):68–80. doi: 10.1084/jem.135.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer E. J., Giannini E. H., Person D. A. Juvenile rheumatoid arthritis. Second edition. Major Probl Clin Pediatr. 1982;6:1–351. [PubMed] [Google Scholar]
- Bugawan T. L., Angelini G., Larrick J., Auricchio S., Ferrara G. B., Erlich H. A. A combination of a particular HLA-DP beta allele and an HLA-DQ heterodimer confers susceptibility to coeliac disease. Nature. 1989 Jun 8;339(6224):470–473. doi: 10.1038/339470a0. [DOI] [PubMed] [Google Scholar]
- Dawkins R. L., Leaver A., Cameron P. U., Martin E., Kay P. H., Christiansen F. T. Some disease-associated ancestral haplotypes carry a polymorphism of TNF. Hum Immunol. 1989 Oct;26(2):91–97. doi: 10.1016/0198-8859(89)90094-3. [DOI] [PubMed] [Google Scholar]
- Fritze D., Herrmann C., Jr, Naeim F., Smith G. S., Zeller E., Walford R. L. The biologic significance of HL-A antigen markers in myasthenia gravis. Ann N Y Acad Sci. 1976;274:440–450. doi: 10.1111/j.1749-6632.1976.tb47705.x. [DOI] [PubMed] [Google Scholar]
- Hawkins B. R., Chan-Lui W. Y., Choi E. K., Ho A. Y. Strong association of HLA BW46 with juvenile onset myasthenia gravis in Hong Kong Chinese. J Neurol Neurosurg Psychiatry. 1984 May;47(5):555–557. doi: 10.1136/jnnp.47.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose S., Ueda G., Noguchi K., Okada T., Sekigawa I., Sato H., Shirai T. Requirement of H-2 heterozygosity for autoimmunity in (NZB X NZW)F1 hybrid mice. Eur J Immunol. 1986 Dec;16(12):1631–1633. doi: 10.1002/eji.1830161226. [DOI] [PubMed] [Google Scholar]
- Louis E. J., Thomson G. Three-allele synergistic mixed model for insulin-dependent diabetes mellitus. Diabetes. 1986 Sep;35(9):958–963. doi: 10.2337/diab.35.9.958. [DOI] [PubMed] [Google Scholar]
- Matsuki K., Juji T., Tokunaga K., Naohara T., Satake M., Honda Y. Human histocompatibility leukocyte antigen (HLA) haplotype frequencies estimated from the data on HLA class I, II, and III antigens in 111 Japanese narcoleptics. J Clin Invest. 1985 Dec;76(6):2078–2083. doi: 10.1172/JCI112211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuki K., Maeda H., Juji T., Inoko H., Ando A., Tsuji K., Honda Y. Taq I-generated HLA-DQ alpha polymorphism in Japanese patients with narcolepsy. Immunogenetics. 1988;27(2):87–90. doi: 10.1007/BF00351080. [DOI] [PubMed] [Google Scholar]
- Naeim F., Keesey J. C., Herrmann C., Jr, Lindstrom J., Zeller E., Walford R. L. Association of HLA-B8, DRw3, and anti-acetylcholine receptor antibodies in myasthenia gravis. Tissue Antigens. 1978 Nov;12(5):381–386. doi: 10.1111/j.1399-0039.1978.tb01347.x. [DOI] [PubMed] [Google Scholar]
- Nerup J. HLA studies in diabetes mellitus: a review. Adv Metab Disord. 1978;9:263–277. doi: 10.1016/b978-0-12-027309-6.50018-5. [DOI] [PubMed] [Google Scholar]
- Numaga J., Matsuki K., Mochizuki M., Minami M., Juji T. An HLA-D region restriction fragment associated with refractory Behçet's disease. Am J Ophthalmol. 1988 May 15;105(5):528–533. doi: 10.1016/0002-9394(88)90246-2. [DOI] [PubMed] [Google Scholar]
- Ono A., Kurita K., Tsuchiya M., Yamanaka I., Tsuji K. HLA antigens of intrathymic and peripheral lymphocytes in autoimmune disease. Keio J Med. 1975 Dec;24(4):367–376. doi: 10.2302/kjm.24.367. [DOI] [PubMed] [Google Scholar]
- Pirskanen R. Genetic associations between myasthenia gravis and the HL-A system. J Neurol Neurosurg Psychiatry. 1976 Jan;39(1):23–33. doi: 10.1136/jnnp.39.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollini P., Mach B., Gorski J. Linkage map of three HLA-DR beta-chain genes: evidence for a recent duplication event. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7197–7201. doi: 10.1073/pnas.82.21.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman K. J. The estimation of synergy or antagonism. Am J Epidemiol. 1976 May;103(5):506–511. doi: 10.1093/oxfordjournals.aje.a112252. [DOI] [PubMed] [Google Scholar]
- Sim E., Cross S. J. Phenotyping of human complement component C4, a class-III HLA antigen. Biochem J. 1986 Nov 1;239(3):763–767. doi: 10.1042/bj2390763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson G. A review of theoretical aspects of HLA and disease associations. Theor Popul Biol. 1981 Oct;20(2):168–208. doi: 10.1016/0040-5809(81)90009-5. [DOI] [PubMed] [Google Scholar]
- Thomson G. Investigation of the mode of inheritance of the HLA associated diseases by the method of antigen genotype frequencies among diseased individuals. Tissue Antigens. 1983 Feb;21(2):81–104. doi: 10.1111/j.1399-0039.1983.tb00377.x. [DOI] [PubMed] [Google Scholar]
- Tiwari J. L., Betuel H., Gebuhrer L., Morton N. E. Genetic epidemiology of coeliac disease. Genet Epidemiol. 1984;1(1):37–42. doi: 10.1002/gepi.1370010106. [DOI] [PubMed] [Google Scholar]
- Tokunaga K., Kay P. H., Christiansen F. T., Saueracker G., Dawkins R. L. Comparative mapping of the human major histocompatibility complex in different racial groups by pulsed field gel electrophoresis. Hum Immunol. 1989 Oct;26(2):99–106. doi: 10.1016/0198-8859(89)90095-5. [DOI] [PubMed] [Google Scholar]
- WOOLF B. On estimating the relation between blood group and disease. Ann Hum Genet. 1955 Jun;19(4):251–253. doi: 10.1111/j.1469-1809.1955.tb01348.x. [DOI] [PubMed] [Google Scholar]
- Wolf E., Spencer K. M., Cudworth A. G. The genetic susceptibility to type 1 (insulin-dependent) diabetes: analysis of the HLA-DR association. Diabetologia. 1983 Apr;24(4):224–230. doi: 10.1007/BF00282704. [DOI] [PubMed] [Google Scholar]
- Yanagi Y., Hirose S., Nagasawa R., Shirai T., Mak T. W., Tada T. Does the deletion within T cell receptor beta-chain gene of NZW mice contribute to autoimmunity in (NZB X NZW)F1 mice? Eur J Immunol. 1986 Sep;16(9):1179–1182. doi: 10.1002/eji.1830160925. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Tsuchiya M., Ono A., Yoshimatsu H., Satoyoshi E., Tsuji K. HLA antigens and myasthenia gravis in Japan. J Neurol Sci. 1977 Jun;32(2):195–201. doi: 10.1016/0022-510x(77)90234-9. [DOI] [PubMed] [Google Scholar]