Abstract
The putative cardioprotective and chemopreventive properties of the red wine phenolic resveratrol (RES) have made it the subject of a growing body of clinical and basic research. We have begun investigations focusing on the effects of RES on the activity of the aryl hydrocarbon receptor (AHR) complex. Our evidence suggests that RES is a potent repressor of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-inducible gene transcription in estrogen receptor (ER)-positive human breast, lung, and colon cancer cell lines. RES activates the transcription of the ER target genes to the same degree as estradiol (E2) in human MCF-7 breast cancer cells. Unlike E2, which can only diminish TCDD-inducible CYP1A1 gene transcription by approximately 50%, RES can completely abrogate this response. Furthermore, 50% repression of TCDD-inducible transcription can be achieved with 100 nM RES, approximately 2.5 orders of magnitude lower than concentrations required for maximal inhibition, suggesting that multiple mechanisms are responsible for this effect. RES (100 nM) does not prevent ligand binding of a TCDD analog, nor does it prevent AHR from binding to its response element in the 5′-regulatory region of the CYP1A1 gene. Small inhibitory RNAs directed to ERα have demonstrated that RES-mediated repression of CYP1A1 depends on ERα. Whereas CYP1A1 protein levels in MCF-7 cells are refractory to the low-dose transcriptional effects of RES, a concomitant decrease in CYP1A1 protein levels is observed in Caco-2 cells. These results highlight a low-dose RES effect that could occur at nutritionally relevant exposures and are distinct from the high-dose effects often characterized.
Introduction
Resveratrol (3,4′,5-trihydroxystilbene, RES) is found in abundance in many foodstuffs, including grapes and berries, and is available as an over-the-counter dietary supplement. Its virtues as a cardioprotective agent are thought to account for the beneficial effects of red wine—the so-called “French Paradox” (Ulrich et al., 2005). RES inhibits proliferation in many tumor cell lines, including breast, colon, and cervical carcinomas. RES can repress the activity of several transcription factors, including NF-κB, AP-1, and the androgen receptor (Mitchell et al., 1999; Manna et al., 2000; Yu et al., 2001). As such, the putative cardioprotective and chemopreventive properties of RES have made it the focus of intense research over the past decade. However, most of the documented pharmacological effects of RES, including inhibition of MAP kinases, activation of sirtuins, inhibition of cell-cycle progression, and pharmacological antagonism of the aryl hydrocarbon receptor (AHR), occur at concentrations in the mid- to high-micromolar range (Casper et al., 1999; Ciolino and Yeh, 1999; Ulrich et al., 2005). In addition, the estrogenic activity of RES is well documented, acting as an agonist at micromolar concentrations for both estrogen receptors (ERs) α and β (Gehm et al., 1997; Bowers et al., 2000). Concentrations of RES in red wines range from 1.5 to 3 mg/l (Goldberg et al., 1995). Because of these factors and the low bioavailability of RES after oral ingestion (Marier et al., 2002), micromolar plasma concentrations cannot be achieved by normal food intake or moderate consumption of red wine (for a review, see Cottart et al., 2010). Thus, the mechanisms by which the chemoprotective effects of RES, associated with the so-called French Paradox, are manifested remain to be determined.
One potential explanation stems from the observation that nanomolar concentrations of RES can activate membrane-associated ERs, leading to MAPK activation (Klinge et al., 2005) and, ultimately, ER target gene expression. This intrigued us because we observed in MCF-7 cells that ERα was greatly enriched at the CYP1A1 enhancer in response to estrogen during 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-activated transcription (Beischlag and Perdew, 2005). Likewise, we were able to monitor AHR on the pS2 promoter under similar conditions, consistent with the observations of other investigators (Ohtake et al., 2003; Matthews et al., 2005).
AHR and ARNT form a heterodimeric transcription factor that binds a variety of environmental pollutants, including TCDD, and mediate an organism's response to these contaminants. The AHR activates the transcription of several genes, including those encoding several xenobiotic-metabolizing enzymes (e.g., CYP1A1/1A2/1B1). Unliganded AHR exists in the cytoplasm as part of a multimeric complex. Upon ligand binding, AHR translocates to the nucleus, where it associates with ARNT to form a functional transcription factor, the aryl hydrocarbon receptor complex (AHRC) (Beischlag et al., 2008). It does so by the recruitment of transcriptional coactivators and corepressors that serve to modify chromatin structure, stabilize core transcriptional machinery, and mediate RNA chain elongation. AHR is capable of recruiting the p160/ basic-helix-loop-helix/PER-ARNT-SIM coactivators SRC-1, NCoA2/GRIP1, and p/CIP (Kumar and Perdew, 1999; Beischlag et al., 2002), NcoA4 (Kollara and Brown, 2006), CoCoA (Kim and Stallcup, 2004), GAC63 (Chen et al., 2006), CREB-binding protein (Kobayashi et al., 1997), and TRIP230 (Beischlag et al., 2004). In addition to these classic transcriptional coactivators, AHR appears to recruit other transcription factors during transcription, including ERα (Ohtake et al., 2003; Beischlag and Perdew, 2005; Matthews et al., 2005) and NF-κB (Tian et al., 1999). The possibility that environmental contaminants and naturally occurring dietary compounds can differentially regulate the AHRC and its recruitment of other transcription factors is intriguing. An understanding of the mechanisms by which this regulation occurs could aid in our understanding of the AHRC and its role in the development, homeostasis, and complex pathologies responsible for a wide spectrum of human diseases, including chemical carcinogenesis, solid tumor growth, and atherosclerosis.
We wanted to test the hypothesis that RES mediates some of its effects on AHR-activated transcription through binding to the ER. To address this issue, we examined the effect of a range of concentrations of RES on AHR-mediated gene expression in several human cell lines. Both low concentrations similar to those readily achievable through regular dietary intake as well as high concentrations, commonly used to activate sirtuins, were tested. RES at nanomolar concentrations was able to repress AHR-mediated induction of CYP1A1 in an ERα-dependent manner without reducing the level of AHRC at the CYP1A1 promoter. In contrast, 10 μM RES markedly reduced AHRC on the CYP1A1 promoter and resulted in near-complete inhibition of CYP1A1 expression and metabolic activity.
Materials and Methods
Cell Lines and Reagents.
Polyclonal anti-ERα and CYP1A1 antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Rabbit anti-AHR polyclonal antibody (SA-210) was purchased from Enzo Life Sciences (Plymouth Meeting, PA). Anti-XAP2 (anti-ARA9) monoclonal antibody was purchased from Novus Biologicals, Inc. (Littleton, CO), and anti-HOP mouse monoclonal antibody F5 was kindly provided by David Toft (Mayo Clinic, Rochester, MN). Human lung cancer BEAS-2B, human colon cancer Caco-2, and human breast cancer MCF-7 cells were purchased from the American Type Culture Collection (Manassas, VA). BEAS-2B cells were maintained in serum-free LHC-9 media (Invitrogen, Carlsbad, CA). MCF-7 cells were maintained in serum-free Dulbecco's modified Eagle's medium (DMEM; Lonza, Walkersville, MD) lacking phenol red, with high glucose for at least 48 h before any treatment or experimental manipulation to ensure that any unnecessary activation/down-regulation of ER or other signal transduction pathways would not confound the experimental parameters examined. Likewise, Caco-2 cells were maintained in DMEM and 2% dextran-treated, charcoal-stripped fetal bovine serum for 48 h before any treatment. TCDD was kindly provided by Steve Safe (Texas A&M University, College Station, TX).
Reverse Transcription and Real-Time Polymerase Chain Reaction.
Reverse transcription and real-time PCR were performed as described previously (Beischlag and Perdew, 2005). In brief, before treatment with the ligand, MCF-7 cells were exposed to cycloheximide (10 μg/ml) for 1 h to halt protein translation. Cells were subsequently treated either with vehicle (Me2SO), TCDD, RES, or a combination of TCDD and increasing concentrations of RES for 6 h. Cells were harvested in TRIzol (Invitrogen), and total RNA was isolated and subjected to reverse transcription using a high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA). Complementary DNA was amplified by real-time PCR using a Power SYBR Green PCR kit (Applied Biosystems) according to the manufacturer's protocols. Oligonucleotide pairs used to amplify human cDNA sequences were described previously (Beischlag and Perdew, 2005). DNA was amplified for 40 cycles in a StepOne Plus Real-Time PCR system (Applied Biosystems). Oligonucleotides for amplification of the CYP1A1 enhancer chromatin isolated using the ChIP assay were identical to those reported by Hestermann and Brown (2003).
Western Blot Analysis.
To determine the effects of RES on TCDD-inducible CYP1A1 protein levels, MCF-7 cells were treated either with vehicle (Me2SO), TCDD, RES, or a combination of TCDD and increasing concentrations of RES for 24 h. Whole-cell extracts of MCF-7 cells were resolved by SDS-PAGE, and protein blot analysis was performed as described previously (Beischlag et al., 2002), with minor modifications. Western blots were probed with affinity-purified rabbit anti-CYP1A1 (Santa Cruz Biotechnology, Inc.) and anti-XAP2 monoclonal antibody, or anti-HOP F5 monoclonal antibody (as a loading control in MCF-7 and Caco-2 cells, respectively). After incubation with primary antibodies, blots were incubated with a biotin-labeled goat anti-rabbit IgG. Blots were washed and incubated with 125I-labeled streptavidin (Amersham, Little Chalfont, Buckinghamshire, UK) and exposed to film overnight. The level of radioactivity was quantified using a Cyclone filmless autoradiographic analysis system and OptiQuant software (PerkinElmer Life and Analytical Sciences, Waltham, MA).
Cytochrome P450 Assays.
We assessed cytochrome P450 1A1 (CYP1A1) enzymatic activity by using the P450-Glo (Promega, Madison, WI) indirect luminescence assay according to the manufacturer's protocols. This assay depends on the formulation of luciferin from a synthetic CYP1A1 substrate, luciferin-chloroethyl ether (L-CEE). In brief, cells were maintained as described above in 24-well plates. After 24 h of serum deprivation in phenol red-free media, cells were treated either with vehicle or with TCDD and various concentrations of RES for an additional 24 h. Media were replaced with fresh media containing 50 μM L-CEE and incubated overnight. Eighteen hours later, 100 μl of media was mixed with 100 μl of luciferase detection reagent, and luminescence was measured with a GloMax 20/20 luminometer (Promega).
Competition Ligand Binding Assays.
MCF-7 cytosol was prepared by manual cell disruption of cells in MENG supplemented with 20 mM Na2MoO4 and a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) using a steel Dounce homogenizer (Wheaton Instruments, Millville, NJ). The crude homogenate was centrifuged at 100,000g for 60 min at 4°C, and the supernatant was collected as cytosol. Protein content was determined by BCA assay (Thermo Fisher Scientific, Waltham, MA), and cytosol samples were stored at −80°C until use. Ligand binding assays were performed using the previously described AHR photoaffinity ligand 2-azido-3-[125I]iodo-7,8-dibromodibenzo-p-dioxin (Poland et al., 1986). Varying concentrations of benzo[a]pyrene or RES were added to 150-μl aliquots of cytosol and incubated at room temperature for 10 min, followed by the addition of a saturating amount (700,000 counts per min, 1.25 nM) of photoaffinity ligand. After a 30-min incubation at room temperature to achieve steady-state receptor binding levels, samples were placed on ice for 5 min to prevent further association or dissociation of receptor-ligand complexes. Next, 75 μl of a charcoal (1%)/dextran (0.1%) solution (made in MENG) was added to each tube and incubated for 10 min on ice, after which samples were centrifuged at 3000g for 10 min at 4°C. Ligand-receptor complexes were covalently cross-linked by exposure to ultraviolet light at a distance of 8 cm. Aliquots were resolved by 8% SDS-PAGE, and gels were transferred to a polyvinylidene difluoride membrane. Cross-linked bands were visualized by autoradiography and quantified using a Cyclone PhosphorImager. Data were plotted and statistical analysis was performed using GraphPad Prism 4.00 (GraphPad Software Inc., San Diego, CA).
Chromatin Immunoprecipitation Assays.
Chromatin immunopreciptation (ChIP) assays from fixed MCF-7 cell lysates were performed as described previously (Beischlag et al., 2004). In brief, cells were treated with 0.1% vehicle as a control, 10 nM E2, 2 nM TCDD, or a combination of E2 and TCDD for 1 h. Cells were fixed in 1% formaldehyde for 10 min at room temperature and subjected to ChIP analysis using agarose gel electrophoreses and real-time PCR.
Subcellular Localization Assay.
MCF-7 cells were transfected with 500 ng of human AHR-eGFP expression vector using SuperFect transfection reagent (QIAGEN, Valencia, CA). Five hours after transfection, media were replaced, and cells were cultured in phenol red-free DMEM without sera for 24 h before treatment with ligand. Cells were then treated with vehicle (DMSO), RES (50 μM), TCDD (2 nM), or a combination of TCDD and increasing concentrations of RES (1–10 μM). To identify nuclei, media were supplemented with Hoechst 33342 dye (bisbenzimide H) (Sigma-Aldrich) to a final concentration of 5 μg/ml at 50 min after treatment with ligand tissue culture, and cells were allowed to incubate for an additional 10 min at 37°C in a humidified CO2 incubator. One hour after treatment, cells were fixed in 4% paraformaldehyde at room temperature for 15 min. Fluorescence micrographs of cells at 24 h after transfection were obtained with an Olympus Fluoview FV10i laser scanning confocal microscope (Olympus, Tokyo, Japan) at 60× objective.
ERα Fluorescence Polarization Competition Assay.
ERα competition assay was performed using the Estrogen Receptor-α Competitor Assay kit (green, PanVera P2614, P2698; Invitrogen). In essence, compounds and vehicle were diluted to twice their final assay concentrations in PanVera-supplied ES2 screening buffer and added to 6 × 50-mm borosilicate glass cell culture tubes. A master mix was made of screening buffer with human recombinant ERα added for a final concentration of 6 pmol/μl, and ES2 fluoromone was added for a final concentration of 400 nM. The master mix was added to diluted test compounds in a 1:1 volume, mixed gently, and incubated in the dark at room temperature for 2 h. Samples then were measured for fluorescence polarization using the PanVera Beacon 2000 polarization reader with 485-nm excitation and 530-nm emission filters at 25°C.
RNA Interference.
“Knockdown” of ER was performed essentially as described previously (Hollingshead et al., 2008). In brief, MCF-7 and Caco-2 cells were maintained in DMEM supplemented with Na+-pyruvate and 7% FBS, and cells were grown to approximately 50% confluence, at which time cells were washed with phosphate-buffered saline and maintained in DMEM with 1% dextran/charcoal-stripped FBS, without antibiotics, 24 h before transfection. BEAS-2B cells were maintained in LHC-9 media. Cells were transfected with ERα, ERβ, or siGFP shortcut siRNA (New England Biolabs, Ipswich, MA) using Dharmafect I (Dharmacon RNA Technologies, Lafayette, CO) according to the manufacturers' protocols. Twenty-four hours after transfection, cells were supplemented with 5 mg/ml bovine serum albumin and treated with 10 μg/ml cycloheximide. One hour later, cells were treated as described above, and CYP1A1 mRNA levels were determined.
Statistical Analysis.
For multiple comparisons, statistical analyses were performed using a one-way ANOVA and Tukey's multiple comparison test. For multiple comparisons using more than one variable (i.e., siRNA experiments), statistical significance was determined using a two-way ANOVA with Tukey's multiple comparison test. Values are presented as means ± S.E.M. A p value of <0.05 was considered significant.
Results
Effects of Resveratrol on TCDD-Mediated Accumulation of CYP1A1 mRNA.
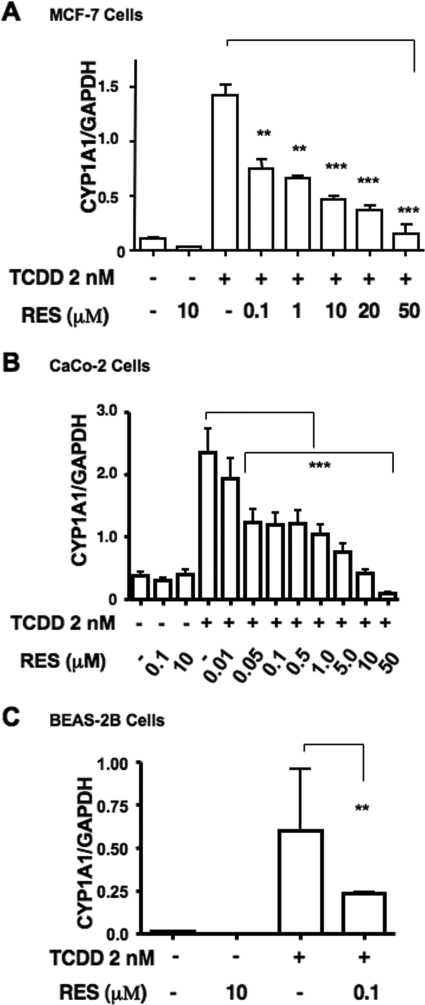
Various reports have identified RES as a pharmacological antagonist of the AHR (Ciolino et al., 1998; Casper et al., 1999) and a partial agonist or superagonist of the ER (Ciolino et al., 1998). We were interested in exploring the outcome of RES on the cross-talk that exists between the AHR and ER. Phenol red dye found in regular DMEM displays ER agonist activity (Berthois et al., 1986) and, similar to endogenous estrogens found in regular fetal bovine sera, can lead to extreme down-regulation of ER, ablating the pharmacological effects of any modulatory agent. Therefore, to ensure an appropriate and robust ER-dependent response, all cell lines were either deprived of fetal bovine sera or maintained in low levels (≤2%) of dextran-treated, charcoal-stripped sera in phenol red-free media for at least 24 h before any experimental manipulation. The TCDD-dependent accumulation of CYP1A1 mRNA in various human cancer cell lines was first examined after exposure to increasing amounts of RES. To eliminate secondary transcriptional effects of RES, cells were pretreated for 1 h with 10 μg/ml cyclohexamide. Initial studies with RES have shown that it is capable of repressing TCDD-responsive CYP1A1 transcription in MCF-7, Caco-2, and BEAS-2B human cell lines (Fig. 1 , A–C). TCDD-dependent CYP1A1 mRNA accumulation was reduced by approximately 50% by 100 nM RES in each of the three cell lines tested, which is well below the EC50 reported for RES to displace [1,6-3H]TCCD from the receptor (Casper et al., 1999). Approximately 90 to 95% repression of CYP1A1 expression was achieved with 50 μM RES in MCF-7 and Caco-2 cells, which were treated with a wide range of doses. Furthermore, the response at the mRNA level appeared to be biphasic, especially in Caco-2 cells. The lower range of RES repression (below 100 nM) was not explored in all cell lines. Superinduction of CYP1A1 transcription by cycloheximide is common in many cancer cell lines (Joiakim et al., 2004). Superinduction in response to cycloheximide was negligible in the MCF-7 cells (Beischlag and Perdew, 2005) and modest in Caco-2 and BEAS-2B cells. We did observe an increase in basal CYP1A1 expression that resulted in a proportional decrease in the fold expression by TCDD, which we have reported previously (Beischlag and Perdew, 2005). Treatment with cycloheximide had no effect on the profile of TCDD activation or RES repression in either of these cells as shown in Fig. 7, where cycloheximide was not used.
Fig. 1.
Dose-dependent repression of CYP1A1 transcription by resveratrol. Human MCF-7 cells (A) and human Caco-2 cells (B) were maintained in phenol red-free, FBS-free media for at least 24 h before treatment, whereas human BEAS-2B (C) cells were maintained in LHC-9 media. Cells were preincubated with 10 μg/ml cycloheximide 1 h before the addition of ligand. Cells were treated for 6 h with DMSO, RES alone (10 μM), TCDD alone (2 nM), or TCDD in combination with varying doses of RES (0.05–50 μM). Measurement of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used to standardize CYP1A1 mRNA accumulation. Data were analyzed using a one-way ANOVA and Bonferroni's multiple comparison test (∗∗, p < 0.01; ∗∗∗, p < 0.001).
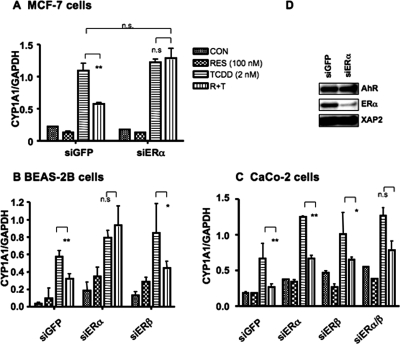
Fig. 7.
Role of estrogen receptors in RES-mediated repression of AHR-driven transcription. Small inhibitory RNAs directed against ERα, ERβ, and GFP were used to test their effects on AHR-mediated gene transcription in MCF-7 (A), BEAS-2B (B), and Caco-2 (C) cells. Cells were transfected with either siGFP as a control or ERα and/or ERβ RNAs 24 h before ligand treatment; otherwise, cells were subsequently treated as described in Fig. 1. CYP1A1 mRNA levels were determined as described under Materials and Methods. Data are presented as mean ± S.D. (n = 3; ∗, p < 0.05; ∗∗, p < 0.01). D, Western blot of ERα protein knockdown of target proteins in MCF-7 cells; AHR and XAP2 are shown to control for protein loading. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Effects of Resveratrol on TCDD-Inducible CYP1A1 Protein Levels.
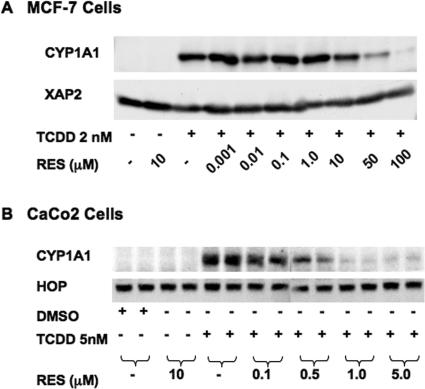
Next, we examined the functional consequences of decreased CYP1A1 mRNA accumulation after RES treatment on protein levels in MCF-7 and Caco-2 cells. It is surprising that no decrease in CYP1A1 protein levels in MCF-7 cells was observed in response to nanomolar concentrations of RES (Fig. 2 A). Only concentrations in excess of the reported EC50 for RES to displace [1,6-3H]TCCD from the receptor led to a concomitant decrease in CYP1A1 protein (Casper et al., 1999). To examine changes in protein levels, a longer-exposure time frame of 16 h was used, as opposed to 6-h treatment used in Fig. 1. This longer time frame of exposure may lead to significant RES metabolism in MCF-7 cells reducing its effect on CYP1A1 expression. However, Western blots of Caco-2 lysates reveal that 100 nM RES efficiently blocks CYP1A1 protein expression (approximately 50%; Fig. 2B), and exposure to increasing concentrations of RES led to a dose-dependent reduction in TCDD-dependent protein levels. These data suggest that multiple mechanisms are responsible for the repressive effect of RES on AHR activity, and the degree of their respective effects may be cell type-specific. Furthermore, the more physiologically relevant mode of action would be at concentrations below those that act as a pharmacological antagonist of AHR.
Fig. 2.
Effect of resveratrol on CYP1A1 protein expression in MCF-7 and Caco-2 cells. Western blots of MCF-7 cells (A) and Caco-2 cell lysates (B) are shown. Cells were treated with vehicle, RES, 2 nM TCDD alone, or TCDD with various concentrations of RES and were harvested 16 h later; lysates were fractionated by SDS-PAGE. B, an analysis of lysates from two separate experiments is shown.
Effects of Resveratrol on TCDD-Inducible CYP1A1 Activity.
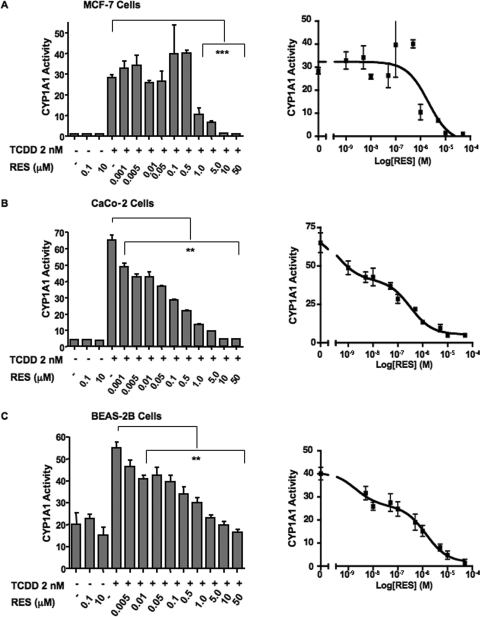
To confirm these findings, we performed correlative studies to determine CYP1A1 enzymatic activity using the P450-Glo assay. Because of the assay's reliance on CYP1A1 activity to convert a synthetic inert precursor of luciferin to a usable substrate, we expected the results to coincide with protein expression. The effect of RES in both the MCF-7 and Caco-2 cell lines (Fig. 3 , A and B) indeed mirrored the observed changes in CYP1A1 protein levels. For these studies, we used a larger range of doses for RES: from 1 nM to 50 μM. In MCF-7 cells, by use of doses ranging from 1 to 500 nM RES, the trend was toward a mild enhancement of TCDD-inducible CYP1A1 activity. A sharp decrease in this activity was observed at doses of RES above 500 nM. Likewise, Caco-2 CYP1A1 oxidative activity mirrored changes observed at the protein level. Doses of RES above 10 nM resulted in a significant decrease in TCDD-inducible activity. A distinct biphasic response was observed in both Caco-2 and BEAS-2B cells, with a dose-dependent decrease in CYP1A1 activity to approximately 50% of the maximal observed with 100 and 500 nM RES, respectively (Fig. 3, B and C). Nonlinear regression analysis using an F test to determine the best fit to a one- or two-site model confirmed this (p < 0.001). In addition, concentrations above 500 nM further decrease CYP1A1 activity, with complete abrogation of activity observed at 10 μM.
Fig. 3.
Alterations in CYP1A1 activity in response to RES in MCF-7, Caco-2, and BEAS-2B cells as assessed by P450-Glo assay. MCF-7 cells (A), Caco-2 cells (B), and BEAS-2B cells (C) were maintained as described under Materials and Methods before treatment with ligand. Cells were treated for 24 h with either DMSO, resveratrol alone (100 nM and 10 μM), TCDD alone (2 nM), or TCDD in combination with varying doses of resveratrol (0.005–50 μM). CYP1A1 activity was measured by using the P450-Glo assay (Promega) according to the manufacturer's protocol. After 24 h, L-CEE was added to each well, and cells were incubated overnight. After incubation, recombinant luciferase was added to each well, and luminescence was measured. Data are presented as mean ± S.E.M. (n = 3; ∗∗, p < 0.01; ***, p 0.001). Right, the data presented in a log-dose response relationship. Curves were fitted using GraphPad Prism 4.0 and nonlinear regression analysis (F test, for best-fit comparisons) to predict a one- or two-site model.
Pharmacological Effect of RES on AHR-Ligand Binding.
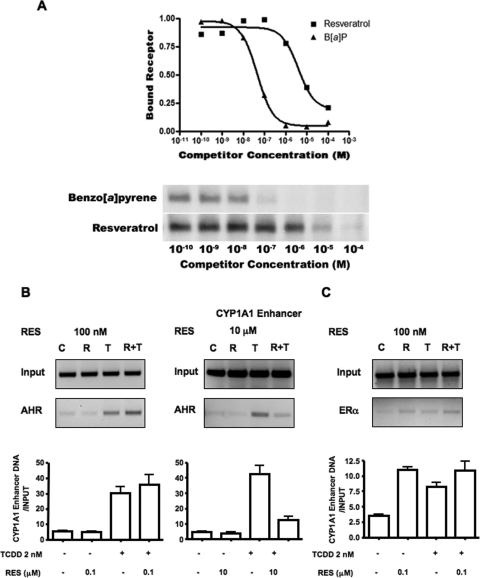
Our preliminary results indicate that RES has the ability to repress TCDD signaling at concentrations lower than what could be attributed to the pharmacologically relevant antagonist properties reported in other studies (Ciolino et al., 1998; Casper et al., 1999; Ciolino and Yeh, 1999). Furthermore, we observed a biphasic response to RES in all cell lines exposed to TCDD at the level of mRNA. Therefore, we wanted to confirm observations reported previously that RES is an AHR antagonist only in the micromolar range, to eliminate the possibility of experimental error on our part, and to resolve micromolar and submicromolar actions of RES. MCF-7 cells were chosen for these studies because they express primarily ERα and not ERβ in estrogen-deprived cells (Shaw et al., 2006), and thus the likelihood of observing effects attributable to a mixed population of receptors was reduced. A pilot displacement study using cytosolic lysates derived from MCF-7 cells and the TCDD analog, 2-azido-3-[125I]iodo-7,8-dibromodibenzo-p-dioxin, suggests that the IC50 for RES is approximately 5 μM (Fig. 4 A). This is in agreement with other investigators' previously determined values (Casper et al., 1999; Ciolono and Yeh, 1999). Furthermore, the apparent RES-dependent displacement of radioactive ligand displayed simple first-order kinetics with little or no displacement at 500 nM. This result also indicates that the effects observed in this report at high nanomolar concentrations of RES are not due to its pharmacological antagonist properties with the AHR, and thus, the concentrations used had been titrated appropriately.
Fig. 4.
Low-dose resveratrol (100 nM) does not displace TCDD from the receptor or prevent DNA binding. A, an AHR ligand displacement study using 2-azido-3-[125I]iodo-7,8-dibromodibenzo-p-dioxin was performed. The image below the displacement plot depicts an autoradiograph of an SDS-PAGE-fractionated receptor coupled to the photoaffinity ligand after preincubation with increasing concentrations of RES. B, representative micrographs of ChIP analysis of AHR at the CYP1A1 enhancer during repression by low (100 nM) and high (10 μM) doses of RES. Graphs show real-time PCR analysis of immunoprecipitated CYP1A1 enhancer chromatin. Data were obtained from at least two separate experiments. C, ChIP analysis of ERα at the CYP1A1 enhancer in response to combinations of 100 nM RES and 2 nM TCDD. B[a]P, benzo[a]pyrene.
Effects of RES on AHR DNA Binding and Nuclear Translocation.
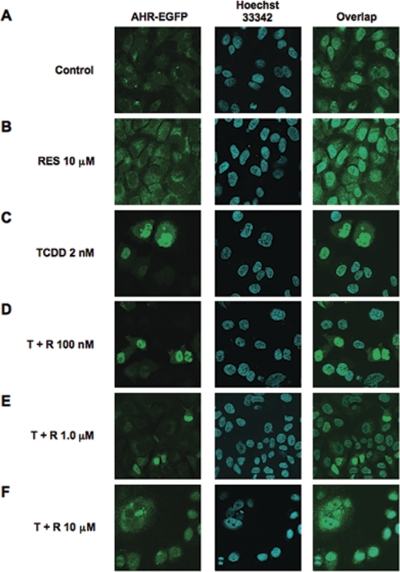
To better understand this phenomenon, we asked whether nanomolar RES had any effect on the translocation profile of the AHR or its ability to associate with dioxin response elements (DREs) in the upstream activating sequences of the CYP1A1 gene. Therefore, we performed ChIP experiments followed by real-time PCR in MCF-7 cells to determine whether nanomolar RES concentrations blocked the association of AHR with its cognate DNA-response element, the DRE. Enrichment of the CYP1A1 upstream activating sequences is seen in both panels in a TCDD-dependent fashion. Cotreatment of MCF-7 cells with TCDD and 100 nM RES did not diminish this enrichment (Fig. 4B, bottom left). However, cotreatment of cells with 10 μM RES is effective in preventing TCDD-dependent AHR association with DREs (Fig. 4B, bottom right), consistent with recent observations made in Oliver Hankinson's laboratory (Beedanagari et al., 2009). In addition, the amount of CYP1A1 enhancer precipitated with an antibody directed toward ERα was increased by 100 nM RES and 2 nM TCDD, demonstrating that ERα is present at the CYP1A1 regulatory regions under these conditions (Fig. 4C). Likewise, concentrations of RES that can partially displace 2-azido-3-[125I]iodo-7,8-dibromodibenzo-p-dioxin from the receptor (i.e., 1 and 5 μM) did not alter the pattern of fluorescence of an AHR-enhanced green fluorescence protein (eGFP) moiety on treatment with TCDD (Fig. 5). In the absence of dioxin, green fluorescence appears to be homogenous (Fig. 5A), and treatment with RES has no effect on this pattern (Fig. 5B). Upon treatment with 2 nM TCDD, a profound and rapid translocation of the AHR-eGFP moiety to the nucleus is observed within 45 min, which can be confirmed by the nuclear fluorescence of the Hoechst 33342 dye stain in Fig. 5C, middle. After cotreatment of MCF-7 cells with TCDD and either 100 nM or 1 μM RES, the majority of fluorescence in the cell appears to be nuclear (Fig. 5, D and E). Only cotreatment with 10 μM RES was sufficient to block the accumulation of nuclear fluorescence in MCF-7 cells (Fig. 5F). These data strongly suggest that nanomolar concentrations of RES are sufficient to repress the accumulation of TCDD-dependent CYP1A1 mRNA and protein expression, but they do not affect the ability of the AHR to translocate to the nucleus upon exposure to TCDD, nor do they interfere with the ability of the AHR to associate with its cognate DNA-response element.
Fig. 5.
RES does not prevent nuclear translocation of AHR in MCF-7 cells. MCF-7 cells were transfected with AHR-eGFP using SuperFect transfection reagent (QIAGEN). Cells were maintained in phenol red-free, FBS-free media for at least 24 h after transfection and before treatment with ligand. Cells were treated with either vehicle (DMSO), RES (50 μM), TCDD (2 nM), or TCDD in combination with various concentrations of RES (T + R, 0.1–10 μM) for 1 h. Cells were fixed, and receptor status was assessed by immunofluorescence. Nuclei were identified using Hoechst 33342 dye.
ERα Fluorescence Polarization Competition Assay.
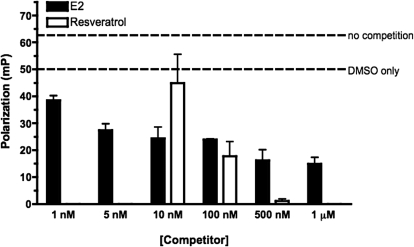
The data presented thus far suggest that RES is capable of repressing AHR activity at a 100 nM concentration. To test whether RES can significantly interact with the ERα in the nanomolar range, a competition assay that measures the ability of a compound to displace a fluorescent ERα ligand, estradiol (ES2), was used. ES2 fluoromone bound to the ERα tumbles slowly in the solution, giving a higher polarization reading (mP). Compounds that compete with the fluoromone away from the ERα result in free fluoromone that tumbles faster and provides a lower mP value. Nonspecific binding effects can be seen by the shift of mP from ERα and fluoromone alone to the addition of 2% DMSO in the reaction (Fig. 6). The addition of E2 at higher concentrations displaces the fluoromone. Resveratrol shows that minimal ERα competition at a dose of 10 nM is at parity with E2 at a dose of 100 nM and is stronger competition at higher doses. These data suggest that resveratrol at 100 nM would be capable of significantly binding to the ER. The ability of 500 nM RES to inhibit almost totally in this assay relative to E2 was surprising and suggests that the manner in which RES interacts with ERα in vitro differs.
Fig. 6.
Fluorescence polarization ERα competition assay. ERα competition assay was performed using the estrogen receptor-α competitor assay kit (green, PanVera P2614, P2698). Vehicle (DMSO), varying concentrations of RES (10–200 nM), or E2 (1 nM to 1 μM) were diluted in ES2 screening buffer and added to 60 × 150-mm borosilicate glass cell culture tubes. Recombinant ERα was added for a final concentration of 6 pmol/μl, and ES2 fluoromone was added for a final concentration of 400 nM. Samples were incubated in the dark at room temperature for 2 h and were measured for fluorescence polarization using a PanVera Beacon 2000 polarization reader with 485-nm excitation and 530-nm emission filters at 25°C. Results shown are the mean of samples done in triplicate.
Role of Estrogen Receptors in RES-Mediated Repression of AHR Signaling.
There have been few reports documenting significant perturbations in cellular physiology mediated by RES at submicromolar concentrations. One report described the activation of the MAP kinase signaling cascade by 100 nM RES, mimicking the effect of estradiol (Klinge et al., 2005). We have previously shown that estradiol can repress TCDD-dependent CYP1A1 expression (Beischlag and Perdew, 2005). Therefore, we were interested in determining whether estrogen receptors were essential for the repressive effects of RES on AHR activity. To this end, we used small inhibitory RNA directed against either ERα or ERβ to investigate the possible contribution of these proteins to this phenomenon. All cell lines transfected with a nonspecific, scrambled GFP siRNA displayed a repression profile for RES similar to that observed in Fig. 2 (Fig. 7, A–C). In MCF-7 cells, which express predominantly ERα and little ERβ, only siRNA directed to ERα was used. This completely ablated the repressive effect of RES on TCDD-inducible CYP1A1 mRNA accumulation (Fig. 7A). A representative Western blot analysis of ERα protein levels in MCF-7 cells after transfection with either GFP or siRNA directed toward ERα is displayed in Fig. 7D. A similar profile for the ERα siRNA was observed in BEAS-2B cells, whereas siRNA directed to ERβ was ineffective (Fig. 7B). Knockdown of either ERα or ERβ failed to prevent repression of TCDD signaling in Caco-2 cells, although knockdown did enhance the accumulation of TCDD-dependent CYP1A1 mRNA levels (Fig. 7C). This may reflect transfection efficiency and hence the ability of these siRNAs to block target protein expression in this cell line as transfection with a combination of both siRNAs blocked the ability of RES to repress TCDD-mediated accumulation of CYP1A1 mRNA. These results suggest that both ERα and ERβ are capable of repressing CYP1A1 induction in the presence of RES, depending on the cell line examined.
Discussion
The putative intrinsic, antiaging, cardioprotective, and chemoprotective effects of resveratrol, a red wine phenolic, have made RES an attractive focus for the development of new pharmacological strategies for the treatment of a myriad of disease states. At high (micromolar) concentrations, RES acts as a pharmacological antagonist of the aryl hydrocarbon receptor and a weak agonist for estrogen receptors, and it binds sirtuins. However, these levels are not attainable through regular diet and thus are not likely to account for the protective effects attributed to RES. We have observed that low, nanomolar doses of RES are capable of significantly repressing dioxin-inducible CYP1A1 transcription in human MCF-7 breast tumor, Caco-2 colonic tumor, and BEAS-2B lung epithelial cell lines. These effects are dose-dependent and lead to a concomitant decrease in CYP1A1 protein and activity levels. Whereas 100 nM RES repressed dioxin-inducible CYP1A1 transcription by approximately 50%, it did not prevent nuclear translocation of the activated receptor or binding of the receptor to the regulatory regions of the CYP1A1 gene, nor did it displace 2-azido-3-[125I]iodo-7,8-dibromodibenzo-p-dioxin from the aryl hydrocarbon receptor. These data strongly suggest a role for nanomolar concentrations of RES in the assembly and disassembly of transcription complexes at the CYP1A1 promoter and ultimately in the physiological manifestation of AHR activation by environmental pollutants. Therefore, this mechanism may account for some of the putative chemoprotective effects of dietary RES.
RES is marketed commercially as a nutritional supplement and as a preventive medicine (Jang et al., 1997; Kundu and Surh, 2004). However, the effects of RES most often studied, namely, those mediated by NF-κB, AP-1, and sirtuins and other targets, are realized at concentrations (30–100 μM) well in excess of those reasonably achievable even by the overconsumption of concentrated dietary supplements. Thus, the mechanisms responsible for the chemoprotective properties of resveratrol observed in dietary studies remain to be determined. Mean peak plasma concentrations in humans range from the picomolar to mid-nanomolar after oral ingestion of quantities associated with normal dietary consumption and reach the low micromolar range only after consumption of gram quantities (for a review, see Cottart et al., 2010). It is certain that local concentrations could be higher in gastrointestinal mucosa where there is significant first-pass metabolism.
We have found that concentrations of RES that are more likely achievable through dietary sources have a profound effect on CYP1A1 mRNA accumulation, protein levels, and activity. With the exception of MCF-7 cells, the effects of RES on CYP1A1 mRNA accumulation lead to a concomitant decrease in CYP1A1 protein levels and/or CYP1A1 enzymatic activity. The rapid and extensive biotransformation of RES has been noted and metabolism is likely to occur in cell culture. Despite this, the acute effects of RES on CYP1A1 mRNA accumulation can be observed as perturbations in CYP1A1 protein levels and associated enzymatic activity (in BEAS-2B and Caco-2 cells) for as much as 24 h after treatment. The unexpected increase in CYP1A1 protein levels observed in MCF-7 cells after exposure to nanomolar concentrations of RES is not consistent with observed decreases in TCDD-dependent CYP1A1 mRNA levels. However, the modest increases in protein levels are mirrored in the P450-Glo assay, an indirect indicator of enzymatic activity. These data suggest that potential carcinogen (e.g., benzo[a]pyrene) metabolism would be decreased by low-dose resveratrol exposure. Because concentrations lower than 5 nM RES were not tested in the P450-Glo assay in Caco-2 and BEAS-2B cells, it is difficult to ascertain whether the submicromolar effects demonstrate the kinetics of a one-site model. Therefore, RES may be eliciting its effects via more than one pathway. However, ablation of ERα expression seems to indicate that ERα is the primary target of RES at nanomolar concentrations in these studies. In addition, these findings indicate that RES concentrations as low as 5 nM may have significant chemoprotective properties.
Several findings presented in this article support a distinct mechanism(s) or target that is sensitive to low, nanomolar concentrations of RES that differ mechanistically from the effects of RES most widely reported in the literature. First, interrogation of the CYP1A1 TCDD-responsive upstream enhancer region by chromatin immunoprecipitation with an affinity-purified antibody directed against AHR revealed that 100 nM RES could not prevent TCDD-inducible association of AHR with its cognate response elements (Fig. 4B). However, 10 μM RES efficiently blocked TCDD-mediated binding of AHR to this region of chromatin. Second, these effects are elicited at concentrations that do not displace 2-azido-3-[125I]iodo-7,8-dibromodibenzo-p-dioxin from the receptor and are below the EC50 reported for RES to displace [1,6-3H]TCDD from the receptor (Casper et al., 1999). Finally, RES concentrations below 10 μM are not effective at blocking the TCDD-dependent translocation of AHR to the nucleus. These data strongly suggest that the intrinsic properties of RES lead to action via multiple mechanisms. Therefore, the low-dose effect of RES on TCDD-responsive gene transcription is distinct from the high-dose effects associated with its properties as a pharmacological antagonist of AHR. Furthermore, any nutritional benefits derived from RES ingestion are likely to involve mechanisms separate from those seen with high doses. For example, the physiological effects on sirtuins and other cellular factors are likely unattainable by RES administered orally.
It is well established that treatment of cells with TCDD or other activators of AHR alone is sufficient to recruit ER to the dioxin response element-containing regulatory regions of the CYP1A1 gene (Beischlag and Perdew, 2005; Matthews et al., 2005; Abdelrahim et al., 2006). In addition, we have demonstrated previously by sequential ChIP using affinity-purified antibodies to AHR and ER that activated ER associates with the CYP1A1 enhancer in an AHR-dependent fashion (Beischlag and Perdew, 2005). Therefore, it may not be necessary for RES to direct estrogen receptors to AHR target genes—RES simply binds to ERα while at the enhancer region. In addition, RES has been reported to activate ERα via an estrogen-sensitive site in the cell membrane that activates the MAPK signaling cascade (Klinge et al., 2005). This finding, along with the fact that we have previously implicated ER in estrogen-mediated repression of AHR transcriptional activity (Beischlag and Perdew, 2005), led us to investigate the role of ER in these phenomena. Immunoprecipitated chromatin fractions from MCF-7 cells using an antibody directed to ERα were enriched with CYP1A1 enhancer sequences in both a RES- (100 nM) and TCDD-dependent fashion. Reduction of estrogen receptor expression with siRNAs abrogated the repressive effect of resveratrol on dioxin-inducible gene transcription. In addition, direct measurement of RES binding to ERα by time-resolved fluorescence indicates that RES is capable of interacting with ERα at nanomolar concentrations. This may indicate its potential as a selective modulator of ER in addition to its type I ER ligand activity (Levenson et al., 2003). Indeed, the ability to selectively modulate ERα activity with a synthetic compound, WAY-169916 [4-[1-allyl-7-(trifluoromethyl)-1H-indazol-3-yl]benzene-1,3-diol], to yield ER-mediated repression of NF-κB-induced gene expression has been established (Chadwick et al., 2005). It is worth noting that this compound is both a selective ER and AHR ligand (Murray et al., 2009). Clearly, further studies are needed to ascertain whether RES is a selective ER ligand.
To facilitate the transcription of TCDD-responsive genes in the appropriate spatial and temporal manner, the AHR must assemble a large array of ancillary coactivators, corepressors, and chromatin-remodeling enzymes, such as Brahma/brg1 (Wang and Hankinson, 2002) and CREB-binding protein (Kobayashi et al., 1997). Indeed, AHR may recruit ERα/β for a similar purpose. Hence, the RES-mediated repression of TCDD-inducible gene induction seems to be mediated by the presence of ER in an AHR transcription complex at the CYP1A1 promoter and may not directly mediate signals received from a RES-activated MAPK signaling cascade. However, there are conflicting reports concerning activation of the MAPK signaling cascade and AHR activity (Andrieux et al., 2004). For example, U0126 [1,4-diamino-2,3-dicyano-1,4-bis(methylthio)butadiene], an inhibitor of the MAP kinase kinase MEK1, leads to increased activation of the receptor even in the absence of ligand (Andrieux et al., 2004). This suggests that MAPK plays a role in suppressing the basal function of AHR. We did not examine the MAPK signaling cascade pharmacologically in this study, but further investigation into the role of this signaling cascade seems warranted.
In conclusion, we have documented the repression of ligand-inducible AHR signaling by concentrations of RES well below those attributed to either the pharmacological antagonism of the receptor or the widely reported activation of other cellular constituents such as sirtuins. Furthermore, we have established that ERα is a target for low-dose exposure to RES. The data presented here provide a plausible explanation for some of the chemopreventive effects of dietary consumption of RES-containing foodstuffs.
Acknowledgments
We thank Marcia H. Perdew for critically reviewing this article.
This work was supported by National Institutes of Health National Institute of Environmental Health Sciences [Grant ES04869] (to G.H.P.) and the Natural Science and Engineering Research Council of Canada (to T.V.B.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- RES
- 3,4′,5-trihydroxystilbene, resveratrol
- NF-κB
- nuclear factor-κB
- AP-1
- activating protein
- MAP
- mitogen-activated protein
- AHR
- aryl hydrocarbon receptor
- ER
- estrogen receptor
- MAPK
- MAP kinase
- TCDD
- 2,3,7,8-tetrachlorodibenzo-p-dioxin
- ARNT
- aryl hydrocarbon receptor nuclear translocator
- AHRC
- aryl hydrocarbon receptor complex (AHR/ARNT)
- DMEM
- Dulbecco's modified Eagle's medium
- PCR
- polymerase chain reaction
- ChIP
- chromatin immunoprecipitation
- PAGE
- polyacrylamide gel electrophoresis
- L-CEE
- luciferin-chloroethyl ether
- MENG
- 25 mM 3-(N-morpholino)propanesulfonic acid, 2 mM EDTA, 0.02% NaH3, and 10% glycerol, pH 7.5
- eGFP
- enhanced green fluorescence protein
- DMSO
- dimethyl sulfoxide
- FBS
- fetal bovine serum
- siGFP
- small interfering RNA to green fluorescent protein
- siER
- small interfering RNA to estrogen receptor
- siRNA
- small interferring RNA
- ANOVA
- analysis of variance
- DRE
- dioxin response element
- E2
- 17-β-estradiol
- GFP
- green fluorescence protein
- WAY-169916
- 4-[1-allyl-7-(trifluoromethyl)-1H-indazol-3-yl]benzene-1,3-diol
- U0126
- 1,4-diamino-2,3-dicyano-1,4-bis(methylthio)butadiene.
References
- Abdelrahim M, Ariazi E, Kim K, Khan S, Barhoumi R, Burghardt R, Liu S, Hill D, Finnell R, Wlodarczyk B, et al. ( 2006) 3-Methylcholanthrene and other aryl hydrocarbon receptor agonists directly activate estrogen receptor alpha. Cancer Res 66: 2459– 2467 [DOI] [PubMed] [Google Scholar]
- Andrieux L, Langouët S, Fautrel A, Ezan F, Krauser JA, Savouret JF, Guengerich FP, Baffet G, Guillouzo A. ( 2004) Aryl hydrocarbon receptor activation and cytochrome P450 1A induction by the mitogen-activated protein kinase inhibitor U0126 in hepatocytes. Mol Pharmacol 65: 934– 943 [DOI] [PubMed] [Google Scholar]
- Beedanagari SR, Bebenek I, Bui P, Hankinson O. ( 2009) Resveratrol inhibits dioxin-induced expression of human CYP1A1 and CYP1B1 by inhibiting recruitment of the aryl hydrocarbon receptor complex and RNA polymerase II to the regulatory regions of the corresponding genes. Toxicol Sci 110: 61– 67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beischlag TV, Perdew GH. ( 2005) ER alpha-AHR-ARNT protein-protein interactions mediate estradiol-dependent transrepression of dioxin-inducible gene transcription. J Biol Chem 280: 21607– 21611 [DOI] [PubMed] [Google Scholar]
- Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH. ( 2008) The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr 18: 207– 250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beischlag TV, Taylor RT, Rose DW, Yoon D, Chen Y, Lee WH, Rosenfeld MG, Hankinson O. ( 2004) Recruitment of thyroid hormone receptor/retinoblastoma-interacting protein 230 by the aryl hydrocarbon receptor nuclear translocator is required for the transcriptional response to both dioxin and hypoxia. J Biol Chem 279: 54620– 54628 [DOI] [PubMed] [Google Scholar]
- Beischlag TV, Wang S, Rose DW, Torchia J, Reisz-Porszasz S, Muhammad K, Nelson WE, Probst MR, Rosenfeld MG, Hankinson O. ( 2002) Recruitment of the NCoA/SRC-1/p160 family of transcriptional coactivators by the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator complex. Mol Cell Biol 22: 4319– 4333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. ( 1986) Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci USA 83: 2496– 2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers JL, Tyulmenkov VV, Jernigan SC, Klinge CM. ( 2000) Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology 141: 3657– 3667 [DOI] [PubMed] [Google Scholar]
- Casper RF, Quesne M, Rogers IM, Shirota T, Jolivet A, Milgrom E, Savouret JF. ( 1999) Resveratrol has antagonist activity on the aryl hydrocarbon receptor: implications for prevention of dioxin toxicity. Mol Pharmacol 56: 784– 790 [PubMed] [Google Scholar]
- Chadwick CC, Chippari S, Matelan E, Borges-Marcucci L, Eckert AM, Keith JC, Jr, Albert LM, Leathurby Y, Harris HA, Bhat RA, et al. ( 2005) Identification of pathway-selective estrogen receptor ligands that inhibit NF-kappaB transcriptional activity. Proc Natl Acad Sci USA 102: 2543– 2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Beischlag TV, Kim JH, Perdew GH, Stallcup MR. ( 2006) Role of GAC63 in transcriptional activation mediated by the aryl hydrocarbon receptor. J Biol Chem 281: 12242– 12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciolino HP, Yeh GC. ( 1999) Inhibition of aryl hydrocarbon-induced cytochrome P-450 1A1 enzyme activity and CYP1A1 expression by resveratrol. Mol Pharmacol 56: 760– 767 [PubMed] [Google Scholar]
- Ciolino HP, Daschner PJ, Yeh GC. ( 1998) Resveratrol inhibits transcription of CYP1A1 in vitro by preventing activation of the aryl hydrocarbon receptor. Cancer Res 58: 5707– 5712 [PubMed] [Google Scholar]
- Cottart CH, Nivet-Antoine V, Laguillier-Morizot C, Beaudeux JL. ( 2010) Resveratrol bioavailability and toxicity in humans. Mol Nutr Food Res 54: 7– 16 [DOI] [PubMed] [Google Scholar]
- Gehm BD, McAndrews JM, Chien PY, Jameson JL. ( 1997) Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc Natl Acad Sci USA 94: 14138– 14143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg DM, Yan J, Ng E, Diamandis EP, Karumanchiri A, Soleas G, Waterhouse AL. ( 1995) A global survey of trans-resveratrol concentraions in commercial wines. Am J Enol Vitic 46: 159– 165 [Google Scholar]
- Hestermann EV, Brown M. ( 2003) Agonist and chemopreventative ligands induce differential transcriptional cofactor recruitment by aryl hydrocarbon receptor. Mol Cell Biol 23: 7920– 7925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead BD, Beischlag TV, Dinatale BC, Ramadoss P, Perdew GH. ( 2008) Inflammatory signaling and aryl hydrocarbon receptor mediate synergistic induction of interleukin 6 in MCF-7 cells. Cancer Res 68: 3609– 3617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, et al. ( 1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275: 218– 220 [DOI] [PubMed] [Google Scholar]
- Joiakim A, Mathieu PA, Elliott AA, Reiners JJ., Jr ( 2004) Superinduction of CYP1A1 in MCF10A cultures by cycloheximide, anisomycin, and puromycin: a process independent of effects on protein translation and unrelated to suppression of aryl hydrocarbon receptor proteolysis by the proteasome. Mol Pharmacol 66: 936– 947 [DOI] [PubMed] [Google Scholar]
- Kim JH, Stallcup MR. ( 2004) Role of the coiled-coil coactivator (CoCoA) in aryl hydrocarbon receptor-mediated transcription. J Biol Chem 279: 49842– 49848 [DOI] [PubMed] [Google Scholar]
- Klinge CM, Blankenship KA, Risinger KE, Bhatnagar S, Noisin EL, Sumanasekera WK, Zhao L, Brey DM, Keynton RS. ( 2005) Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors alpha and beta in endothelial cells. J Biol Chem 280: 7460– 7468 [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Numayama-Tsuruta K, Sogawa K, Fujii-Kuriyama Y. ( 1997) CBP/p300 functions as a possible transcriptional coactivator of Ah receptor nuclear translocator (Arnt). J Biochem 122: 703– 710 [DOI] [PubMed] [Google Scholar]
- Kollara A, Brown TJ. ( 2006) Functional interaction of nuclear receptor coactivator 4 with aryl hydrocarbon receptor. Biochem Biophys Res Commun 346: 526– 534 [DOI] [PubMed] [Google Scholar]
- Kumar MB, Perdew GH. ( 1999) Nuclear receptor coactivator SRC-1 interacts with the Q-rich subdomain of the AhR and modulates its transactivation potential. Gene Expr 8: 273– 286 [PMC free article] [PubMed] [Google Scholar]
- Kundu JK, Surh YJ. ( 2004) Molecular basis of chemoprevention by resveratrol: NF-kappaB and AP-1 as potential targets. Mutat Res 555: 65– 80 [DOI] [PubMed] [Google Scholar]
- Levenson AS, Gehm BD, Pearce ST, Horiguchi J, Simons LA, Ward JE, 3rd, Jameson JL, Jordan VC. ( 2003) Resveratrol acts as an estrogen receptor (ER) agonist in breast cancer cells stably transfected with ER alpha. Int J Cancer 104: 587– 596 [DOI] [PubMed] [Google Scholar]
- Manna SK, Mukhopadhyay A, Aggarwal BB. ( 2000) Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol 164: 6509– 6519 [DOI] [PubMed] [Google Scholar]
- Marier JF, Vachon P, Gritsas A, Zhang J, Moreau JP, Ducharme MP. ( 2002) Metabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J Pharmacol Exp Ther 302: 369– 373 [DOI] [PubMed] [Google Scholar]
- Matthews J, Wihlén B, Thomsen J, Gustafsson JA. ( 2005) Aryl hydrocarbon receptor-mediated transcription: ligand-dependent recruitment of estrogen receptor alpha to 2,3,7,8-tetrachlorodibenzo-p-dioxin-responsive promoters. Mol Cell Biol 25: 5317– 5328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SH, Zhu W, Young CY. ( 1999) Resveratrol inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells. Cancer Res 59: 5892– 5895 [PubMed] [Google Scholar]
- Murray IA, Morales JL, Flaveny CA, Dinatale BC, Chiaro C, Gowdahalli K, Amin S, Perdew GH. ( 2009) Evidence for ligand-mediated selective modulation of aryl hydrocarbon receptor activity. Mol Pharmacol 77: 247– 254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake F, Takeyama K, Matsumoto T, Kitagawa H, Yamamoto Y, Nohara K, Tohyama C, Krust A, Mimura J, Chambon P, et al. ( 2003) Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature 423: 545– 550 [DOI] [PubMed] [Google Scholar]
- Poland A, Glover E, Ebetino FH, Kende AS. ( 1986) Photoaffinity labeling of the Ah receptor. J Biol Chem 261: 6352– 6365 [PubMed] [Google Scholar]
- Shaw LE, Sadler AJ, Pugazhendhi D, Darbre PD. ( 2006) Changes in oestrogen receptor-alpha and -beta during progression to acquired resistance to tamoxifen and fulvestrant (Faslodex, ICI 182,780) in MCF7 human breast cancer cells. J Steroid Biochem Mol Biol 99: 19– 32 [DOI] [PubMed] [Google Scholar]
- Tian Y, Ke S, Denison MS, Rabson AB, Gallo MA. ( 1999) Ah receptor and NF-kappaB interactions, a potential mechanism for dioxin toxicity. J Biol Chem 274: 510– 515 [DOI] [PubMed] [Google Scholar]
- Ulrich S, Wolter F, Stein JM. ( 2005) Molecular mechanisms of the chemopreventive effects of resveratrol and its analogs in carcinogenesis. Mol Nutr Food Res 49: 452– 461 [DOI] [PubMed] [Google Scholar]
- Wang S, Hankinson O. ( 2002) Functional involvement of the Brahma/SWI2-related gene 1 protein in cytochrome P4501A1 transcription mediated by the aryl hydrocarbon receptor complex. J Biol Chem 277: 11821– 11827 [DOI] [PubMed] [Google Scholar]
- Yu R, Hebbar V, Kim DW, Mandlekar S, Pezzuto JM, Kong AN. ( 2001) Resveratrol inhibits phorbol ester and UV-induced activator protein 1 activation by interfering with mitogen-activated protein kinase pathways. Mol Pharmacol 60: 217– 224 [DOI] [PubMed] [Google Scholar]