Abstract
Adolescence is a well defined developmental period during which marijuana use is common. However, little is known about the response to marijuana in adolescents compared with adults. We have shown previously that adolescent rats are more impaired than adults by Δ9-tetrahydrocannabinol (THC), the main psychoactive compound in marijuana, in a spatial learning task, but the mechanism responsible for this differential impairment is not understood. We determined the role of THC tolerance and cannabinoid receptor type 1 (CB1) regulation in THC-induced spatial learning impairment in adolescent and adult rats. We measured the development of tolerance to THC-induced learning impairment in adolescent (postnatal days 30–35) and adult (postnatal days 70–75) rats. We pretreated them for 5 days with 10 mg/kg THC, and then evaluated the effects of vehicle or THC treatment on learning during training in the Morris water maze. We also determined CB1 number and functional coupling in the hippocampus of adolescents and adults. Finally, we measured the time course of hippocampal CB1 desensitization in adolescents and adults during treatment with 10 mg/kg THC or vehicle. Our results indicate that adults, but not adolescents, become tolerant to the effects of THC during water maze training after 5 days of pretreatment. CB1s in adolescent hippocampus are less functionally coupled to G proteins and desensitize more slowly in response to THC treatment than those of adults. THC may impair learning in adolescents more than in adults because of delayed activation of cellular homeostatic adaptive mechanisms underlying cannabinoid tolerance in the hippocampus.
Introduction
Marijuana is the most commonly used illicit drug in the United States (Substance Abuse and Mental Health Services Administration, 2007). Marijuana use is most prevalent in adolescents, with a trend of increasing use through late adolescence, which then falls in adulthood (Substance Abuse and Mental Health Services Administration, 2007). Reports of persevering effects of adolescent marijuana use include an elevated risk for both psychosis and long-term cognitive deficits (Pope et al., 2003; Moore et al., 2007). Animal studies of adolescent cannabinoid exposure have reported persevering anxiety, impairment of object memory, altered sensitivity to reward, and increased depressive behavior in females in adulthood (Schneider and Koch 2003; O'Shea et al., 2006; Quinn et al., 2008; Rubino et al., 2008b). In summary, both epidemiologic and animal research have raised significant concerns about the long-term consequences of adolescent marijuana use.
One of the most profound pharmacologic effects of marijuana is disruption of learning (Abel, 1971). Spatial learning impairment has been attributed to pharmacologic action of Δ9-tetrahydrocannabinol (THC), the main psychotropic ingredient of marijuana, on cannabinoid receptor type 1 (CB1) in the hippocampus (Lichtman et al., 1995; Wise et al., 2009). THC disrupts neuronal firing rhythms required for information integration and processing in the hippocampus, producing learning impairment comparable with hippocampal lesion in rats (Hampson and Deadwyler, 2000). Microinjections of THC into the hippocampus, but not other brain areas relevant to maze learning, impair learning in the radial arm maze (Egashira et al., 2002). Our studies have shown that adolescent rats treated with THC are more impaired on both spatial and nonspatial learning tasks than adults (Cha et al., 2006, 2007), suggesting that adolescents might be particularly vulnerable to cognitive impairment by marijuana.
Previously reported effects of chronic adolescent and adult treatment on memory and other behaviors in animal models have produced divergent results. Chronic WIN 55212-2 [[(3R)-2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-napthalenylmethanone mesylate] treatment caused persevering effects on object recognition memory, reward, and prepulse inhibition only after adolescent treatment (Schneider and Koch, 2003). Chronic treatment with THC (Quinn et al., 2008) or CP 55940 [2-[(1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl) cyclohexyl]-5-(2-methyloctan-2-yl)phenol] (O'Shea et al., 2006) caused comparable persevering effects on object recognition memory and social interaction after adolescent or adult treatment. One study reported persevering depressive-like behavior after adolescent exposure, but only in females (Rubino et al., 2008b). One potential cause for these disparate findings could be that the dose regimens used produced varying effects in adolescents and adults. Another consideration is the use of THC versus synthetic cannabinoid agonists. The most commonly used synthetic agonists (WIN 55212-2 and CP 55940) are full agonists, whereas THC is a partial agonist at CB1. The discordance of these results could reflect varying magnitude and duration of receptor adaptation, which depends on the efficacy of the chosen drug (Childers, 2006). In addition, persevering effects of prolonged intoxication can contribute to cognitive impairment (Pope et al., 2001).
The purpose of the present study was to determine whether tolerance development contributed to our previous findings of age differences in THC-induced learning impairment in the Morris water maze. Our previous studies were conducted over a 5-day period of training. In the present study, we explored the possibility that the apparent age difference in THC-induced learning impairment may have been caused by development of tolerance in adults, but not adolescents, over the 5 training days. We also investigated the contribution of hippocampal CB1 desensitization to this tolerance in adolescents and adults. We focused on the hippocampus because of its pivotal role in spatial learning required for the Morris water maze task. We report that adolescents do not become tolerant to THC-induced learning impairment in the Morris water maze after a 5-day THC pretreatment, whereas adults develop substantial tolerance. In addition, adolescent hippocampal CB1s are less functionally active, and THC desensitizes CB1 more slowly in adolescents than adults. Age differences in the mechanisms underlying tolerance development may contribute to the differential acute THC-induced learning impairment observed in adolescents and adults.
Materials and Methods
Materials.
[3H] SR141716A [rimonabant; 5-(4-chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide] was from GE Healthcare (Little Chalfont, Buckinghamshire, UK), and [35S] GTPγS was from PerkinElmer Life and Analytical Sciences (Waltham, MA). GDP, adenosine deaminase, WIN 55212-2, and GTPγS were from Sigma-Aldrich (St. Louis, MO). SR141716A was from the National Institute of Mental Health Chemical Synthesis and Drug Supply Program (Bethesda, MD), and Δ9THC was from the National Institute on Drug Abuse Drug Supply Program (RTI International, Triangle Park, NC). CB1 antibody was from Thermo Fisher Scientific (Waltham, MA), and Alexa Fluor-conjugated secondary antibody was from Invitrogen (Carlsbad, CA).
Subjects.
Male Sprague-Dawley CD rats were obtained from Charles River Laboratories (Wilmington, MA) and housed in an Association for Assessment and Accreditation of Laboratory Animal Care-approved animal care facility on a 12:12 light/dark cycle with ad libitum access to food and water. Rats were allowed to acclimate to the housing facilities for 5 days before the start of treatment. All animal handling procedures were approved by the Duke University or Durham Veterans Affairs Medical Center Institutional Animal Care and Use Committees. Adolescent rats used were 30 to 35 days old at the start of each experiment. Adult rats were 70 to 75 days old at the start of each experiment.
Drugs.
THC was dissolved in normal saline, ethanol, and Emulphor (18:1:1) on the morning of injection and administered intraperitoneally at a volume of 1 μl/g of rat weight, with vehicle control animals receiving an equal volume of vehicle. A single daily dose of 10 mg/kg THC was used to replicate the regimen that produced significant age differences in previous studies (Cha et al., 2006).
Hippocampal Spatial Learning.
Adolescent and adult rats were pretreated for 5 days with daily intraperitoneal injections of 10 mg/kg THC or vehicle solution. On days 6 to 10, the same rats were treated with daily injections of 10 mg/kg THC or vehicle, and then trained in the Morris water maze spatial learning task 30 min after injection as described previously (Cha et al., 2006, 2007). Each day, rats swam six trials in which they were released from a randomized sequence of release points along the wall of the water tank. Rats were released from each of three release points twice during the six trials. All subjects completed the water maze task regardless of THC treatment condition. The platform remained static throughout the experiment. Rat swim paths were tracked by using the ANY-Maze video tracking system version 4.5 (Stoelting Co, Wood Dale, IL).
Radioligand Binding.
CB1 number and drug binding affinity were quantified in hippocampal homogenates by using saturation binding as described previously (Breivogel et al., 1997). Drug-naive adolescent and adult rats were killed by decapitation. Brains were immediately removed and placed into ice-cold saline. Hippocampi were dissected and snap-frozen on dry ice. Hippocampal tissue was homogenized in a glass-Teflon homogenizer in homogenization buffer (50 mM Tris-HCl, 3 mM MgCl2, 1 mM EGTA, pH 7.4), then centrifuged 10 min at 48,000g at 4°C. Pellets were resuspended in homogenization buffer and centrifuged again. Resulting pellets were resuspended in assay buffer (50 mM Tris-HCl, 3 mM MgCl2, 0.2 mM EGTA, 100 mM NaCl, 0.1 mg/ml bovine serum albumin, pH 7.4) and centrifuged a final time. Final pellets were resuspended in 5 ml of assay buffer. Binding assays were performed in triplicate. Tissue containing 6 μg of protein was incubated with [3H] SR141716A at concentrations ranging from 0.04 to 2 nM and 40 μM GDP. Nonspecific binding was determined for each concentration of [3H] SR141716A by using 1 μM unlabeled SR141716A. Assay tubes were incubated in a shaking water bath at 30°C for 1 h. Reactions were stopped by using ice-cold assay buffer in a Brandel Inc. (Gaithersburg, MD) harvester with filtration through GF/B glass filter paper. Radioactivity was extracted overnight with shaking in 4 ml of scintillation fluid and counted the next morning.
CB1 Distribution: Immunofluorescence.
Distribution of CB1 in dorsal hippocampus was determined by using immunofluorescence. Drug-naive rats were terminally anesthetized with 75 mg/kg pentobarbital and transcardially perfused with 10% formalin. Brains were removed, stored overnight in 10% formalin at 4°C, cryoprotected in 30% sucrose phosphate buffer for 3 days at 4°C, and frozen in tissue-embedding medium. Twenty-micron-thick coronal sections of dorsal hippocampus were collected and placed free-floating into Tris-buffered saline (TBS). Sections were incubated in blocking buffer (5% normal goat serum, 0.3% Triton X-100 in TBS) for 1 h at room temperature, then incubated overnight on a shaker at 4°C in rabbit anti-CB1 polyclonal antibody (1:1000) in antibody buffer (0.5% normal goat serum, 0.3% Triton X-100 in TBS). Sections were washed the next morning in blocking buffer. They were incubated with Alexa Fluor anti-rabbit 594 secondary antibody (1:400) in antibody buffer for 2 h at room temperature after shaking. They were washed again in blocking buffer and mounted onto slides. Slides were imaged with a Zeiss Axio Imager wide-field fluorescence microscope (Carl Zeiss Inc., Thornwood, NY) with a 10× objective and MetaMorph version 7.5 software (Molecular Devices, Sunnyvale, CA). Images were analyzed with ImageQuant TL version 2003 (GE Healthcare).
CB1 Coupling: Hippocampal Section GTPγS Incorporation.
To determine whether tolerance was related to CB1 desensitization, adolescent and adult rats were treated with 10 mg/kg THC or vehicle as described for 3 or 7 days to bracket the time period that animals became tolerant to THC-induced impairment of learning. Receptor number was not measured, because major desensitization, but only modest receptor down-regulation, develops within this time frame during repeated THC treatment (Sim-Selley, 2003). We hypothesized that desensitization would correlate better with tolerance to THC-induced impairment of learning.
Agonist-stimulated GTPγS autoradiography was performed as described previously (Sim et al., 1995). Rats were killed by decapitation 24 h after the last injection. Brains were snap-frozen in tissue-embedding medium. Twenty-micron coronal sections of dorsal hippocampus were collected and thaw-mounted onto gelatin-subbed slides. Adjacent sections were used to compare basal and agonist-stimulated GTPγS incorporation in triplicate. Before use, sections were dried under a stream of room temperature air for 30 min. Sections were rinsed in TME buffer (50 mM Tris, 3 mM MgCl2, 0.2 mM EGTA, 100 mM NaCl, 0.1 mg/ml bovine serum albumin, pH 7.4) for 10 min at 25°C followed by incubation in TME buffer with 10 mU/ml adenosine deaminase and 2 mM GDP for 10 min at 25°C. Agonist stimulation was determined by 2-h incubation in the TME buffer + adenosine deaminase + GDP solution with 10 μM cannabinoid agonist WIN 55212-2 and 0.04 nM [35S]GTPγS for 2 h at 25°C. Full agonist was used in these assays to determine the maximal stimulation of hippocampal CB1 and the extent to which functional CB1 activity was lost in THC-treated animals. Use of a partial agonist such as THC in this assay results in low levels of GTPγS incorporation and a poor signal/noise ratio. After agonist stimulation, sections were dipped in room-temperature 50 mM Tris, pH 7.4, for 1 min, and then dipped in dH2O briefly. Sections were dried under a stream of air for 30 min and exposed to a storage phosphor screen overnight. Screens were scanned with a Typhoon phosphorimager (GE Healthcare).
Data Analysis.
All graphs were generated by using Prism version 5 (GraphPad Software, Inc., San Diego, CA). Morris water maze data were analyzed by using SPSS version 15.0 software (SPSS Inc., Chicago, IL). Results from the six trials from each day were averaged to produce a mean daily value for each parameter. Repeated-measures analysis of variance (ANOVA) (age × pretreatment × challenge, repeated measure of day) was used to analyze the mean daily values of swim speed and distance swum to reach the platform. When interactions of age × pretreatment × challenge were found, data from each age group were separately subjected to a repeated-measures ANOVA (pretreatment × challenge, repeated measure of day).
For binding assays, Bmax and Kd values were determined by using the best-fit nonlinear dose-response curves. These values were compared between adolescents and adults by using one-way ANOVA in JMP (SAS Institute, Cary, NC). Negative data were analyzed post hoc for statistical power by using Fisher's Z test.
ImageQuant TL version 2003 was used to quantify autoradiograms of brain sections. Image pixel intensity was measured in CA1, CA3, and dentate gyrus (DG) regions of the hippocampus. Stimulated binding was divided by basal [35S] GTPγS incorporation and multiplied by 100 to give the percentage stimulation by WIN 55212-2. Maximal stimulation and EC50 were determined for each subject by using values from the best-fit dose-response curves, and age groups were compared in naive animals by using one-way ANOVA in JMP. One-way ANOVA was used to determine effect of treatment in desensitization studies. Percentage stimulation data were analyzed with a three-way ANOVA (age × days × treatment) using JMP. In addition, percentage change from control of WIN 55212-2-induced CB1 stimulation was calculated for THC-treated rat brain sections. Percentage change data were analyzed with a two-way ANOVA (age × days), also using JMP.
Results
Adults but Not Adolescents Become Tolerant to THC-Induced Learning Impairment after 5 Days of THC Treatment.
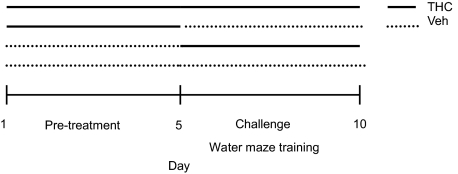
To elucidate age differences in THC tolerance in a spatial learning task, we examined learning performance in the Morris water maze after a 5-day pretreatment with THC or vehicle in adolescents and adults. The design of the treatment paradigm is shown in Fig. 1. Water maze performance demonstrated that adolescents did not become tolerant to THC-induced learning impairment in the water maze after THC pretreatment, whereas adults did.
Fig. 1.
Time course of treatment for Morris water maze training. During a pretreatment phase, rats were treated with either THC or vehicle (Veh) daily for 5 days. The challenge treatment was either THC or vehicle. Rats were trained in the water maze 30 min after injection during the challenge treatment phase.
Adolescent and adult results are depicted separately for visual clarity. Subjects of both ages were run in cohorts together, and data were analyzed together. Adolescents overall swam more quickly in the water maze than adults (main effect of age, F1,69 = 14.2, p < 0.001) (Table 1). However, swim speed decreased as a function of day in all animals (main effect of day, F1,69 = 39.3, p < 0.001). In addition, THC challenge increased swim speed in all rats (main effect of challenge, F1,69 = 20.2, p < 0.001). Adolescents pretreated with THC swam faster than adults (age × pretreatment interaction, F1,69 = 6.4, p = 0.014) and also swam faster after THC challenge (age × challenge, F1,69 = 4.6, p = 0.035). These two differences probably mediate the observed main effect of age. Adult rats became tolerant to the effects of THC on swim speed after a 5-day pretreatment. Adolescents treated chronically with THC, however, swam even more quickly than adolescents treated with acute THC challenge only, possibly indicating a sensitization effect in adolescents.
TABLE 1.
Daily swim speed (cm/s) in adolescent and adult rats during Morris water maze training
Data are presented as mean ± S.E.M., n = 10 rats per group.
| Day | Veh/Veh | Veh/THC | THC/Veh | THC/THC |
|---|---|---|---|---|
| Adolescent | ||||
| 1 | 20.2 ± 0.4 | 24.3 ± 2.6 | 21.6 ± 0.9 | 27.4 ± 1.0‡ |
| 2 | 16.8 ± 0.6 | 22.1 ± 2.0* | 19.1 ± 1.1 | 22.5 ± 1.2* |
| 3 | 16.2 ± 0.9 | 19.8 ± 1.8 | 18.6 ± 0.9 | 23.5 ± 1.6* |
| 4 | 15.0 ± 0.9 | 19.6 ± 1.3 | 17.2 ± 1.4 | 22.9 ± 2.1‡ |
| 5 | 14.9 ± 0.9 | 18.1 ± 1.7 | 16.7 ± 1.2 | 21.4 ± 1.5* |
| Adult | ||||
| 1 | 19.5 ± 0.5 | 20.9 ± 1.3 | 20.0 ± 0.7 | 21.2 ± 1.1 |
| 2 | 17.1 ± 0.8 | 19.9 ± 1.6 | 18.2 ± 0.5 | 17.9 ± 1.4 |
| 3 | 14.5 ± 0.8 | 20.0 ± 1.8* | 16.4 ± 1.0 | 15.6 ± 1.4 |
| 4 | 15.3 ± 1.0 | 18.4 ± 1.8† | 15.3 ± 0.7 | 13.4 ± 0.9 |
| 5 | 14.3 ± 0.6 | 18.8 ± 2.4 | 14.7 ± 0.7 | 15.3 ± 1.4 |
Veh/Veh, vehicle pretreated, vehicle challenged. Veh/THC, vehicle pretreated, THC challenged. THC/Veh, THC pretreated, vehicle challenged. THC/THC, THC pretreated, THC challenged.
Significantly different from Veh/Veh treatment on the same day.
Significantly different from THC/THC treatment.
Significantly different from THC/Veh and Veh/Veh treatments.
The results of the distance measure showed that adolescent rats did not become tolerant to the impairing effects of THC, whereas adults did (Fig. 2). All subjects showed continual improvement of performance in the water maze task across days (main effect of day, F4,276 = 103.9, p < 0.001). Challenge treatment with THC impaired all subjects compared with controls (main effect of challenge, F1,69 = 102.0, p < 0.001). Adults pretreated with THC, however, were less impaired by the THC challenge than treatment-matched adolescents (age × pretreatment × challenge interaction, F1,69 = 4.7, p = 0.033). No residual effects of THC pretreatment were observed upon vehicle challenge performance in the distance measure.
Fig. 2.
Mean swim distance per day for rats of each treatment group and age. Notations are the same as those described for Table 1. Circles represent animals challenged with vehicle, and squares represent animals challenged with THC. Open symbols represent animals pretreated with vehicle, and filled symbols represent animals pretreated with THC. Adolescent rats challenged with THC swam farther than vehicle-challenged rats regardless of pretreatment. Adults pretreated with THC swam less in response to acute THC challenge than adults pretreated with vehicle. No pretreatment effect was observed in vehicle-challenged rats. Data are presented as mean ± S.E.M., n = 10 rats per group.
Adolescent learning was impaired by THC challenge (main effect of challenge, F1,35 = 63.3, p < 0.001). This learning impairment in response to the THC challenge was not affected by pretreatment with THC. In adults, pretreatment with THC decreased distance traveled (main effect of pretreatment, F1,34 = 5.3, p = 0.028). THC challenge, however, increased distance traveled (main effect of challenge, F1,34 = 39.6, p < 0.001). THC challenge increased distance swum less in adult rats pretreated with THC than those pretreated with vehicle (pretreatment × challenge interaction, F1,34 = 8.8, p = 0.006). This result indicates that adults became tolerant to THC during a 5-day THC treatment, whereas adolescents did not.
In summary, THC-pretreated adults were less impaired in the water maze task after THC challenge compared with vehicle-pretreated controls, indicating tolerance to learning impairment had developed during the pretreatment phase. In contrast, this 5-day pretreatment did not cause tolerance in adolescents. In addition, 5 days of THC pretreatment did not cause residual learning impairment in vehicle-challenged animals.
Hippocampal CB1 Number Does Not Differ In Adolescents and Adults.
The age differences in tolerance to learning impairment during repeated THC treatment could reflect underlying differences in CB1 function or homeostatic regulation after chronic treatment. To elucidate the biochemical mechanisms underlying this differential tolerance by age, we characterized CB1 number, distribution, function, and desensitization in the hippocampus of adolescent and adult rats. To verify that CB1 number is similar in adolescent and adult hippocampus, we analyzed total hippocampal CB1 number from drug-naive rats by using saturation radioligand binding. Table 2 shows Bmax and Kd values for [3H] SR141716A binding in adolescent and adult hippocampal membranes. Bmax or Kd were not different across age groups, indicating that CB1 number and binding affinity are similar in adolescents and adults.
TABLE 2.
CB1 number in hippocampus of adolescent and adult rats
Saturation radioligand binding to CB1 in hippocampal tissue from naïve adolescent and adult rats is shown. Data are presented as mean ± S.E.M., n = 9/group. No differences are observed between ages.
| Bmax | Kd | |
|---|---|---|
| Adolescent | 1.7 ± 0.2 | 3.5 × 10−10 ± 0.5 × 10−10 |
| Adult | 1.7 ± 0.1 | 2.0 × 10−10 ± 0.3 × 10−10 |
Bmax, pmol [3H] SR141716A bound per mg protein. Kd, molar concentration.
Hippocampal CB1 Distribution Does Not Differ in Adolescents and Adults.
To determine whether subfield distribution of CB1 differed in adolescent and adult hippocampus, immunofluorescence staining for CB1 was performed in fixed hippocampal sections from drug-naive adolescent and adult rats. The CA1 and CA3 subfields were analyzed for fluorescence staining intensity. Presynaptic, perisomatic staining was found mostly in the pyramidal layer of CA1 and CA3 hippocampus (Fig. 3), concordant with previously published observations (Tsou et al., 1999). Although pyramidal layer staining intensity was higher in CA3 than CA1, the distribution of receptors did not differ by age (data not shown).
Fig. 3.
Representative 10× magnification photomicrograph of CB1 immunofluorescence staining in adolescent CA1 (top) and CA3 (bottom) hippocampus. CB1 staining is confined largely to perisomatic terminals in the pyramidal layer. n = 5/group.
Adolescent CB1 Is Less Functionally Coupled to Downstream G Proteins.
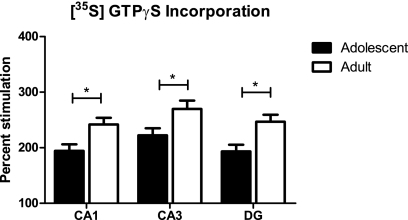
Receptor coupling to downstream signaling molecules is another possible source of differential cannabinoid effects by age. We investigated the normal functional activity of CB1 in hippocampal sections from drug-naive adolescents and adults. CB1 agonist-stimulated incorporation of [35S] GTPγS in all hippocampal subfields was greater in adults than adolescents (main effect of age, F1,114 = 22.7, p < 0.0001). CB1 in adolescents was less functionally coupled to Gαi/o proteins than in adults (Fig. 4 ).
Fig. 4.
WIN 55212-2-stimulated incorporation of the radiolabeled, nonhydrolyzable GTP analog [35S]GTPγS in coronal sections of drug-naive adolescent and adult rat dorsal hippocampus. Data are presented as mean ± S.E.M. of percentage of unstimulated [35S] GTPγS incorporation. n = 10.
THC Desensitizes Adolescent CB1 More Slowly Than Adult.
To determine the role of CB1 desensitization in tolerance to spatial learning impairment, CB1 agonist-stimulated [35S] GTPγS incorporation was quantified in brain sections from adolescents and adults after 3 or 7 days of treatment with 10 mg/kg THC or vehicle. Three days of THC treatment did not affect adolescent CB1 function relative to vehicle treatment. THC-treated adults, however, had reduced CB1 function after 3 days of THC treatment (Fig. 5 , top). After 7 days, adult CB1 did not desensitize further, and adolescent CB1 desensitized only slightly (Fig. 5, bottom). THC treatment reduced GTPγS incorporation in CA1 (effect of treatment, F1,29 = 9.5, p = 0.003), CA3 (effect of treatment F1,29 = 9.8, p = 0.003), and DG (effect of treatment F1,29 = 5.1, p = 0.027) areas of hippocampus. The effect of THC varied by duration of treatment in CA1 (effect of day, F1,29 = 8.0, p = 0.007), CA3 (effect of day, F1,29 = 5.2, p = 0.026), and DG (effect of day, F1,29 = 6.9, p = 0.011). In CA1 hippocampus, THC treatment reduced GTPγS incorporation (effect of treatment, F1,29 = 9.5, p = 0.003). This effect varied with duration of treatment and age (interaction of day × treatment, F1,29 = 4.8, p = 0.034). When normalized to controls and expressed as a percentage change from control, adolescent CB1 did not desensitize after 3 days of THC treatment, whereas adults had reduced CB1 function (Fig. 6 , top). After 7 days of THC treatment, adult receptors remained desensitized, whereas adolescent receptors showed only minor desensitization (Fig. 6, bottom). ANOVA of desensitization data reported an interaction of age × days in all subfields (CA1, F1,25 = 4.7, p = 0.040; CA3, F1,25 = 7.1, p = 0.013; DG, F1,25 = 6.2, p = 0.020.) These results demonstrate that adolescents undergo delayed desensitization of CB1 compared with adults over a time course of treatment with THC.
Fig. 5.
WIN 55212-2-stimulated incorporation of [35S]GTPγS in adolescent and adult coronal sections of dorsal hippocampus. Data are from adolescent and adult animals treated for 3 or 7 days with THC or vehicle. Data are presented as percentage of unstimulated [35S]GTPγS incorporation. Data represent mean ± S.E.M. Day 3, n = 10; day 7, n = 5.
Fig. 6.
THC treatment-induced desensitization expressed as normalized to vehicle-treated controls in coronal sections of dorsal hippocampus. Data are from animals treated for 3 or 7 days with THC or vehicle. Data are presented as mean ± S.E.M. Day 3, n = 10; day 7, n = 5.
Discussion
The purpose of this study was to determine whether tolerance contributed to the differential impairment of learning by THC in adolescent and adult rats. We found that THC-pretreated adults were significantly less impaired by a THC challenge than control adults, whereas adolescents did not develop tolerance to THC. We also found that hippocampal CB1s in drug-naive adolescent rats were less able to activate G proteins than those in drug-naive adults, and that THC desensitized these CB1s more slowly in adolescents than adults over 7 days of THC treatment. We conclude that delays in CB1 homeostatic adaptation to THC treatment contributed to differential learning impairment in adolescents in the Morris water maze.
The present results suggest that tolerance develops quickly to the effects of THC in the Morris water maze in adults but not in adolescents. This finding is consistent with studies using relatively high doses of THC (2.5–10 mg/kg), which typically report tolerance (Cha et al., 2006, 2007; Niyuhire et al., 2007), although exceptions exist (Nava et al., 2001). Rats also develop tolerance to the disruptive effects of THC or full cannabinoid agonists in a delayed nonmatch to sample task after 35 days of repeated administration (Hampson et al., 2003) and to elevated error rates in operant behaviors (Delatte et al., 2002).
Age differences in tolerance to THC effects on learning have not been previously reported. However, tolerance to the tetrad of THC effects on body temperature, locomotion, catalepsy, and antinociception have been reported: they are complex and vary by effect, initial sensitivity, and dose of THC. Age differences in tolerance are not uniform across variables measured. Adolescent males exhibit more tolerance to THC-induced inhibition of locomotion and temperature, but less tolerance to antinociception compared with adults (Wiley et al., 2007). Adolescent males also exhibit comparable acute catalepsy and antinociception relative to adults, with comparable tolerance to catalepsy across ages (Wiley et al., 2007). THC dose used may also contribute to initial differences in sensitivity. These findings with the tetrad indicate that multiple factors can contribute to apparent age differences in tolerance development. However, differences in tolerance in the present study were not related to initial drug effects: adolescents and adults both were significantly impaired by this dose at the start of treatment. In our previous studies, initial learning impairment was greater in adolescents, but the duration of adolescent impairment was greater throughout THC treatment, concordant with the present results (Cha et al., 2006, 2007). In summary, previously described age differences in initial action and tolerance to THC-induced changes in locomotion, the similarity of impairment in the water maze in adolescents and adults by the first dose, and the consistency of developmental differences in THC-induced learning impairment suggest that the present results do reflect differential tolerance development to learning in adolescents and adults.
All animals swam comparably greater distances after THC on the first day of water maze training, after which the age groups diverged. Increased swim distance on the first day can reflect a number of phenomena, including anxiety, stress, or sensorimotor impairment (Vorhees and Williams, 2006). THC causes anxiety at high doses (Onaivi et al., 1990; Rubino et al., 2008a) and tolerance to this phenomenon has not been described. Although initial swim distances were comparable across ages, THC causes less anxiety in adolescents than in adults (Schramm-Sapyta et al., 2007). An interaction of THC treatment with the novelty of the task might have contributed to the initial increased distance and swim speed observed. We are conducting further analyses to address these possibilities.
CB1 number in hippocampus did not change between early adolescence and adulthood. Several studies have investigated ontogeny of CB1 number (Rodríguez de Fonseca et al., 1993; McLaughlin et al., 1994; Morozov and Freund, 2003), but most report findings only at weaning and adulthood. The one study that reported data through adolescence reported a peak between days 30 and 40 with a slight decline to adulthood (Rodríguez de Fonseca et al., 1993). Differences in the strain of rats or radioligand used to identify receptors might contribute to differences between that study and the present findings. Our findings suggest that CB1 populations are mature by early adolescence, consistent with the behavioral evidence that THC profoundly impairs learning during adolescence.
Although CB1 number did not differ between adolescents and adults, the CB1s in naive adolescent hippocampus were less efficiently coupled to G proteins than those in adults. Although CB1–Gα coupling is well characterized in adults (Childers, 2006), developmental changes in CB1 function have not been well described. Consistent with our results, one previous study reported that CB1 functional coupling to downstream signaling pathways in developing human brain increased from the neonatal period to adulthood (Mato et al., 2003). Differential CB1–G protein coupling does not likely reflect ontogenetic changes in inhibitory G protein number, because adult expression levels are reached by postnatal day 25 in the hippocampus (Ihnatovych et al., 2002). However, it might reflect the gradual maturation of the complex of multiple components that must assemble for normal receptor–G protein signaling including receptor, G protein, and β-arrestin-dependent signaling (Defea, 2008).
The lesser G protein coupling observed in adolescent rats relative to adults may have contributed to the delayed CB1 desensitization observed in adolescents. Previous studies have extensively characterized the desensitization and down-regulation of CB1 in response to THC in rodent models (Sim-Selley, 2003; McKinney et al., 2008). CB1 is phosphorylated by G protein-coupled receptor kinase, desensitized by association with β-arrestin, and internalized. The delayed desensitization of CB1 observed in the present study might reflect immaturity at any step in this pathway.
The contribution of delayed receptor desensitization to more long-lasting effects of adolescent cannabinoid exposure is more difficult to assess. Few studies have evaluated receptor function in adulthood after adolescent exposure. Robust down-regulation and desensitization of CB1 were reported after 11 days of adolescent THC treatment, which had attenuated but remained significant in adulthood (Rubino et al., 2008b). Another study did not detect CB1 desensitization or down-regulation after adolescent THC exposure, although the dose regimen was modest (1.5 mg/kg every 3 days) (Ellgren et al., 2007). One caveat in interpreting our results is that we studied only males. Receptor down-regulation in hippocampus was reported to be greater in females than in males (Rubino et al., 2008b). The present study observed a significant parallel between behavioral tolerance and receptor desensitization. A more thorough study of receptor desensitization and behavioral tolerance at multiple time points of treatment would provide additional insight into the correlation between behavior and receptor function.
The observed developmental differences in receptor desensitization could have contributed to the age differences in tolerance development. The developmental difference (less desensitization in adolescents) and time frame over which changes occurred (the first 7 days of treatment) are consistent with this interpretation. Furthermore, CB1 in the hippocampus desensitizes and down-regulates more quickly than most other regions (Sim-Selley, 2003; McKinney et al., 2008), and microinjection studies strongly support the hippocampus as a major site of THC effects on learning (Egashira et al., 2002). However, the relationship currently is only correlative, and it is possible that other brain regions contribute to THC-induced learning impairment.
In summary, the present results identify a potential mechanism for increased adolescent sensitivity to acute THC compared with adults. These findings suggest that delayed tolerance development in adolescents may lead to longer-lasting learning impairment during acute THC administration. Future studies comparing CB1 function and homeostatic regulation in response to repeated THC exposure in adolescents and adults are needed. In addition, it will be important to evaluate differential effects of THC in adolescents and adults by using models of learning and memory that do not depend on repeated exposure to THC.
One implication of the present findings for adolescent marijuana users is that adolescents might be more vulnerable to persevering effects that result from chronic intoxication. Depending on the regional specificity of receptor adaptation in humans, this could be reflected in both effects on cognitive function and reinforcing effects of marijuana, which could enhance motivation for continued use.
Acknowledgments
We thank Dr. Steven R. Childers and members of his laboratory at Wake Forest University (Winston-Salem, NC) for helpful guidance in performing the [35S]GTPγS assay in brain sections; Drs. Theodore A. Slotkin and Nicole L. Schramm-Sapyta for valuable advice on statistical analyses; and Reynold Francis for expert technical assistance.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant R01-DA019346] (to H.S.S.).
Disclosure/conflicts of interest: C.M.K. has received compensation from Duke University and the National Institutes of Health for grant review consultations. H.S.S., W.A.W., and S.K.A. have received compensation from Veterans Affairs, Duke University, and the National Institutes of Health for grant review consultations. C.M.K., H.S.S., and W.A.W. have received compensation from Norton Publishing for book royalties. N.L.T.M. and A.L.R.G. have received compensation only from Duke University.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.169359.
- THC
- Δ9-tetrahydrocannabinol
- CB1
- cannabinoid receptor type 1
- DG
- dentate gyrus
- TBS
- Tris-buffered saline
- ANOVA
- analysis of variance
- WIN 55212-2
- [(3R)-2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-napthalenylmethanone mesylate
- CP 55940
- 2-[(1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohexyl]-5-(2-methyloctan-2-yl)phenol
- SR141716A
- 5-(4-chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide.
References
- Abel EL. ( 1971) Marihuana and memory: acquisition or retrieval? Science 173: 1038– 1040. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Sim LJ, Childers SR. ( 1997) Regional differences in cannabinoid receptor/G-protein coupling in rat brain. J Pharmacol Exp Ther 282: 1632– 1642 [PubMed] [Google Scholar]
- Cha YM, Jones KH, Kuhn CM, Wilson WA, Swartzwelder HS. ( 2007) Sex differences in the effects of Δ9-tetrahydrocannabinol on spatial learning in adolescent and adult rats. Behav Pharmacol 18: 563– 569 [DOI] [PubMed] [Google Scholar]
- Cha YM, White AM, Kuhn CM, Wilson WA, Swartzwelder HS. ( 2006) Differential effects of Δ9-THC on learning in adolescent and adult rats. Pharmacol Biochem Behav 83: 448– 455 [DOI] [PubMed] [Google Scholar]
- Childers SR. ( 2006) Activation of G-proteins in brain by endogenous and exogenous cannabinoids. AAPS J 8: E112– E117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defea K. ( 2008) β-Arrestins and heterotrimeric G-proteins: collaborators and competitors in signal transduction. Br J Pharmacol 153 (Suppl 1): S298– S309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatte MS, Winsauer PJ, Moerschbaecher JM. ( 2002) Tolerance to the disruptive effects of Δ9-THC on learning in rats. Pharmacol Biochem Behav 74: 129– 140 [DOI] [PubMed] [Google Scholar]
- Egashira N, Mishima K, Iwasaki K, Fujiwara M. ( 2002) Intracerebral microinjections of Δ9-tetrahydrocannabinol: search for the impairment of spatial memory in the eight-arm radial maze in rats. Brain Res 952: 239– 245 [DOI] [PubMed] [Google Scholar]
- Ellgren M, Spano SM, Hurd YL. ( 2007) Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology 32: 607– 615 [DOI] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. ( 2000) Cannabinoids reveal the necessity of hippocampal neural encoding for short-term memory in rats. J Neurosci 20: 8932– 8942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, Simeral JD, Kelly EJ, Deadwyler SA. ( 2003) Tolerance to the memory disruptive effects of cannabinoids involves adaptation by hippocampal neurons. Hippocampus 13: 543– 556 [DOI] [PubMed] [Google Scholar]
- Ihnatovych I, Novotny J, Haugvicova R, Bourova L, Mares P, Svoboda P. ( 2002) Opposing changes of trimeric G protein levels during ontogenetic development of rat brain. Brain Res Dev Brain Res 133: 57– 67 [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Dimen KR, Martin BR. ( 1995) Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology 119: 282– 290 [DOI] [PubMed] [Google Scholar]
- Mato S, Del Olmo E, Pazos A. ( 2003) Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. Eur J Neurosci 17: 1747– 1754 [DOI] [PubMed] [Google Scholar]
- McKinney DL, Cassidy MP, Collier LM, Martin BR, Wiley JL, Selley DE, Sim-Selley LJ. ( 2008) Dose-related differences in the regional pattern of cannabinoid receptor adaptation and in vivo tolerance development to Δ9-tetrahydrocannabinol. J Pharmacol Exp Ther 324: 664– 673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin CR, Martin BR, Compton DR, Abood ME. ( 1994) Cannabinoid receptors in developing rats: detection of mRNA and receptor binding. Drug Alcohol Depend 36: 27– 31 [DOI] [PubMed] [Google Scholar]
- Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. ( 2007) Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet 370: 319– 328 [DOI] [PubMed] [Google Scholar]
- Morozov YM, Freund TF. ( 2003) Post-natal development of type 1 cannabinoid receptor immunoreactivity in the rat hippocampus. Eur J Neurosci 18: 1213– 1222 [DOI] [PubMed] [Google Scholar]
- Nava F, Carta G, Colombo G, Gessa GL. ( 2001) Effects of chronic Δ9-tetrahydrocannabinol treatment on hippocampal extracellular acetylcholine concentration and alternation performance in the T-maze. Neuropharmacology 41: 392– 399 [DOI] [PubMed] [Google Scholar]
- Niyuhire F, Varvel SA, Martin BR, Lichtman AH. ( 2007) Exposure to marijuana smoke impairs memory retrieval in mice. J Pharmacol Exp Ther 322: 1067– 1075 [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Green MR, Martin BR. ( 1990) Pharmacological characterization of cannabinoids in the elevated plus maze. J Pharmacol Exp Ther 253: 1002– 1009 [PubMed] [Google Scholar]
- O'Shea M, McGregor IS, Mallet PE. ( 2006) Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar long-lasting deficits in object recognition and reduced social interaction in rats. J Psychopharmacol 20: 611– 621 [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. ( 2003) Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend 69: 303– 310 [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Yurgelun-Todd D. ( 2001) Residual neuropsychologic effects of cannabis. Curr Psychiatry Rep 3: 507– 512 [DOI] [PubMed] [Google Scholar]
- Quinn HR, Matsumoto I, Callaghan PD, Long LE, Arnold JC, Gunasekaran N, Thompson MR, Dawson B, Mallet PE, Kashem MA, et al. ( 2008) Adolescent rats find repeated Δ9-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology 33: 1113– 1126 [DOI] [PubMed] [Google Scholar]
- Rodríguez de Fonseca F, Ramos JA, Bonnin A, Fernández-Ruiz JJ. ( 1993) Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport 4: 135– 138 [DOI] [PubMed] [Google Scholar]
- Rubino T, Guidali C, Vigano D, Realini N, Valenti M, Massi P, Parolaro D. ( 2008a) CB1 receptor stimulation in specific brain areas differently modulate anxiety-related behaviour. Neuropharmacology 54: 151– 160 [DOI] [PubMed] [Google Scholar]
- Rubino T, Vigano' D, Realini N, Guidali C, Braida D, Capurro V, Castiglioni C, Cherubino F, Romualdi P, Candeletti S, et al. ( 2008b) Chronic Δ9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology 33: 2760– 2771 [DOI] [PubMed] [Google Scholar]
- Schneider M, Koch M. ( 2003) Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology 28: 1760– 1769 [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Cha YM, Chaudhry S, Wilson WA, Swartzwelder HS, Kuhn CM. ( 2007) Differential anxiogenic, aversive, and locomotor effects of THC in adolescent and adult rats. Psychopharmacology (Berl )191: 867– 877 [DOI] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Childers SR. ( 1995) In vitro autoradiography of receptor-activated G proteins in rat brain by agonist-stimulated guanylyl 5′-[γ-[35S]thio]-triphosphate binding. Proc Natl Acad Sci USA 92: 7242– 7246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ. ( 2003) Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev Neurobiol 15: 91– 119 [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration ( 2007) National Survey on Drug Use and Health, Substance Abuse and Mental Health Services Administration, Rockville, MD [Google Scholar]
- Tsou K, Mackie K, Sañudo-Peña MC, Walker JM. ( 1999) Cannabinoid CB1 receptors are localized primarily on cholecystokinin-containing GABAergic interneurons in the rat hippocampal formation. Neuroscience 93: 969– 975 [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. ( 2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1: 848– 858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, O'Connell MM, Tokarz ME, Wright MJ., Jr ( 2007) Pharmacological effects of acute and repeated administration of Δ9-tetrahydrocannabinol in adolescent and adult rats. J Pharmacol Exp Ther 320: 1097– 1105 [DOI] [PubMed] [Google Scholar]
- Wise LE, Thorpe AJ, Lichtman AH. ( 2009) Hippocampal CB(1) receptors mediate the memory impairing effects of Δ9-tetrahydrocannabinol. Neuropsychopharmacology 34: 2072– 2080 [DOI] [PMC free article] [PubMed] [Google Scholar]