Abstract
Chronic inflammation is an underlying etiological factor in carcinogenesis; nonsteroidal anti-inflammatory drugs (NSAIDs) and their chemically modified NO-releasing prodrugs (NO-NSAIDs) are promising chemopreventive agents. The aim of this study was to conduct a head-to-head comparison between two NO-ASAs possessing different NO donor groups, an organic nitrate [3-nitrooxyphenyl acetylsalicylate (NO-ASA; NCX-4016)] and an N-diazeniumdiolate [NONO-ASA, O2- (acetylsalicyloxymethyl)-1-(pyrrolidin-1-yl)diazen-1-ium-1,2-diolate (NONO-ASA; CVM-01)], as antiulcerogenic, analgesic, anti-inflammatory, and antipyretic agents. All drugs were administered orally at equimolar doses. For antiulcerogenic study, 6 h after administration, the number and size of hemorrhagic lesions in stomachs from euthanized animals were counted. Tissue samples were frozen for prostaglandin E2 (PGE2), superoxide dismutase (SOD), and malondialdehyde determination. For anti-inflammatory study, 1 h after drug administration, the volume of carrageenan-induced rat paw edemas was measured for 6 h. For antipyretic study, 1 h after dosing, fever was induced by intraperitoneal LPS, and body core temperatures measured for 5 h. For analgesic study, time-dependent analgesic effect of prodrugs was evaluated by carrageenan-induced hyperalgesia. Drugs were administered 30 min after carrageenan. NO-ASA and NONO-ASA were equipotent as analgesic and anti-inflammatory agents but were better than aspirin. Despite a drastic reduction of PGE2 in stomach tissue, both prodrugs were devoid of gastric side effects. Lipid peroxidation induced by aspirin was higher than that observed by prodrugs. SOD activity induced by both prodrugs was similar, but approximately 2-fold higher than that induced by aspirin. CVM-01 is as effective as NCX-4016 in anti-inflammatory, analgesic, and antipyretic assays in vivo, and it showed an equivalent safety profile in the stomach. These results underscore the use of N-diazeniumdiolate moieties in drug design.
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) are a diverse group of compounds used worldwide, predominantly to treat pain, fever, and inflammation. However, every day there are new studies supporting the fact that NSAIDs and, more importantly, their chemically modified prodrugs are expanding their repertoire of medical applications to include the prophylactic prevention of a wide variety of human diseases. These ailments include atherosclerosis (Yasuda et al., 2008; Zhao et al., 2008), thrombosis (FitzGerald, 2003; Yasuda et al., 2008), cancer (Chan et al., 2007; Flossmann et al. 2007; Orido et al., 2008; Spitz et al., 2009), Alzheimer's disease (Davies et al., 2001; Szekely et al., 2008; Vlad et al., 2008), and any other disease for which chronic inflammation is an etiological factor. The major anti-inflammatory and analgesic mechanism of action of NSAIDs is the inhibition of cyclooxygenase (COX) enzymes (COX-1 and COX-2). Because of differences in cellular localization and tissue expression, COX-1 and COX-2 produce a distinct set of prostaglandins that, depending on their type and tissue localization, operate as “normal” physiologic regulators or as proinflammatory molecules. Therefore, NSAIDs (including COX-2-selective inhibitors, “coxibs”) inhibit not only those prostaglandins involved in the inflammatory response but also those responsible for maintaining homeostasis (Cryer and Feldman, 1998). This is one of the main reasons the use of NSAIDs is often correlated with a relatively high incidence of adverse gastrointestinal (Aalykke and Lauritsen, 2001; Fiorucci and Del Soldato, 2003; Schaffer et al., 2006) and/or cardiovascular (Scheiman and Fendrick, 2007) side effects, and it is the main reason behind the withdrawal of highly selective COX-2 inhibitors such as rofecoxib (Vioxx) and valdecoxib (Bextra) (Jaksch et al., 2008).
To overcome this problem, comprehensive research studies have been carried out to modify the chemical structure of classic NSAIDs by forming hybrid (mixed) prodrugs, which upon metabolism release the parent NSAID and a second biologically active molecule that decreases or counteracts its mechanism-based toxicity (Wallace, 2008). In this regard, there are three main classes of hybrid NSAIDs: 1) the nitric oxide-releasing NSAIDs (NO-NSAIDs) (Davies et al., 2001; Fiorucci et al., 2007; Stefano and Distrutti, 2007), 2) the hydrogen sulfide-releasing NSAIDs (Fiorucci et al., 2007; Wallace et al., 2007), and 3) the phosphatidylcholine-conjugated NSAIDs (Kurinets and Lichtenberger, 1998; Anand et al., 1999; Lichtenberger et al., 2001). By far, the most studied type of hybrid NSAID is the NO-NSAID.
NO-NSAIDs were designed based on the assumption that NO (a potent vasodilator and inhibitor of leukocyte adherence to the gastric vascular endothelium) released from them would mimic most of the beneficial biological effects attributed to prostaglandins in the gastrointestinal tract (Martin and Wallace, 2006; Stanek et al., 2008). This approach effectively yielded safer anti-inflammatory, analgesic, antipyretic, and chemopreventive prodrugs in which the NO donor moiety is organic nitrate (-ONO2) (Wallace et al., 1994). Many other types of NO donors are described in the literature, but just a few have been employed to form new NO-NSAIDs. Velázquez et al. (2007, 2008) have described the use of N-diazen-1-ium-1,2-diolates, which represent improved NO donors, considering that unlike organic nitrates, they do not require metabolic activation to release NO. They release twice as much NO compared with organic nitrates, and they can be synthesized from a wide variety of secondary amines, which gives N-diazen-1-ium-1,2-diolates much more versatility over nitrates. Another advantage is that esterase-mediated hydrolysis of CVM-01 (NONO-ASA) generates both NO and ASA simultaneously in the same compartment, whereas the nitrates require two different activation steps to accomplish this: an ester hydrolysis and a separate nitrate reduction, which can occur in different places. This is a potential disadvantage for the nitrates if the NO-mimetic effect is most needed at the site where the freshly formed aspirin is irritating the tissue.
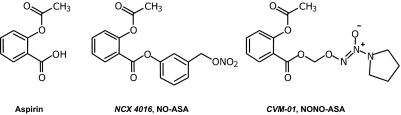
In the present study, we investigated and compared the anti-inflammatory, analgesic, antipyretic, and chemopreventive properties of one NO-ASA possessing an organic nitrate (NCX-4016) with those of a NONO-ASA possessing an N-diazen-1-ium-1,2-diolate (CVM-01) (Fig. 1). This head-to-head comparison was aimed at determining whether there is a statistically significant difference between hybrid NSAIDs possessing distinct NO donor groups and at finding possible mechanistic differences that might offer additional evidence to support the use of one over the other.
Fig. 1.
Chemical structures of aspirin and NO-releasing aspirins, NCX 4016 (NO-ASA) and CVM-01 (NONO-ASA).
Materials and Methods
Chemicals
3-Nitrooxyphenyl acetylsalicylate (Li-yuan et al., 2004) (NCX 4016; NO-ASA) and O2-(acetylsalicyloxymethyl)-1-(pyrrolidin-1-yl) diazen-1-ium-1,2-diolate (Velázquez et al., 2005) (CVM-01; NONO-ASA) were synthesized according to procedures described in the literature. Lipopolysaccharide (LPS) from Escherichia coli and carrageenan were purchased from Sigma-Aldrich (St. Louis, MO). Kits used for determination of prostaglandin E2 (PGE2), lipid peroxidation, superoxide dismutase (SOD), and total nitrate were from Cayman Chemical (Ann Arbor, MI).
Experimental Groups and Treatments
Animals.
Male Wistar rats (at least five per group) weighing 180 to 200 g were obtained from Charles River Laboratories, Inc. (Wilmington, MA). The rats were fed standard laboratory chow and water. All experimental procedures described below were approved by our institutional animal research committees and performed in accordance with nationally approved guidelines for the treatment of laboratory animals. Rats were fasted for 48 h with free access to drinking water. Prodrugs, administered orally by gavage, were suspended in 1% carboxymethylcellulose solution, at equimolar doses: ASA (180 mg/kg), NO-ASA (331 mg/kg), and NONO-ASA (323 mg/kg); the vehicle was 1% carboxymethylcellulose. Six hours after administration, animals were euthanized by suffocation in a CO2 chamber; stomachs were removed immediately after, cut along the greatest curvature, and rinsed with ice-cold distilled water. The ulcer index (UI) was determined as described by Best et al., (1984). Tissues from stomachs were excised and processed for measurement of PGE2, malondialdehyde (MDA), and SOD activity. Blood samples of the rat were taken by cardiac puncture into heparin-containing vials and used for determination of plasma TNF-α and total nitrite/nitrate level.
Determination of PGE2 Levels.
Tissue from each rat stomach was removed, weighed (approximately 1 g), and placed in a test tube containing 5 ml of 0.1 M phosphate buffer, pH 7.4, 1 mM EDTA, and 10 μM indomethacin. The tissue was homogenized and centrifuged for 10 min at 12,000 rpm at 4°C. PGE2 content in supernatant was determined in duplicate by an enzyme immunoassay kit following the protocol described by the manufacturer (Cayman Chemical). In brief, standard (50 μl) or homogenate (50 μl), enzymatic tracer (50 μl), and specific antiserum (50 μl) were mixed. After incubation for 17 h (overnight) at 4°C, the plates were washed with wash buffer, and Ellman's reagent (200 μl) was added into each well. The absorbance at 412 nm was measured after 1-h incubation at room temperature. Results are expressed as picograms of PGE2 per milligram of protein. Proteins were determined by assay (Bio-Rad Laboratories, Hercules, CA).
Index of Lipid Peroxidation.
Stomach tissue (25 mg) was snap-frozen and sonicated for 15 s at 40 V over ice with 250 μl of radioimmunoprecipitation assay buffer (25 mM Tris-HCl, pH 7.6, 150 mM NaCl, 1% Tergitol-type NP-40 (nonylphenoxypolyethoxyethanol), 1% sodium deoxycholate, and 0.1% SDS) with phenylmethylsulfonyl fluoride as protease inhibitor. Homogenates were centrifuged for 10 min at 1600 rpm at 4°C. Thiobarbituric acid reactant substances content was measured in the supernatant stored on ice by a colorimetric kit following the protocol described by the manufacturer (Cayman Chemical). In brief, reaction of MDA with thiobarbituric acid at high temperature (90–100°C) in acidic conditions produced an adduct with a chromophore that absorbed visible light at 530 to 540 nm. The results were expressed as picomoles of malondialdehyde per gram of protein. Proteins were determined by Bio-Rad assay.
Antioxidant Enzymes.
SOD activity in the gastric mucosa was assayed using a colorimetric kit according to the protocol described by the manufacturer (Cayman Chemical). Mucosal tissue (1 g) was homogenized with 5 ml of 20 mM HEPES buffer, pH 7.2, containing 1 mM EGTA and 300 mM sucrose solution. Homogenates were centrifuged at 1500 rpm for 10 min at 4°C. The supernatant was then removed and stored at −80°C until assayed. SOD activity was measured spectrophotometrically at 460 nm. As indicated in Cayman's SOD assay kit, “this procedure uses a tetrazolium salt for detection of superoxide radicals generated by xanthine oxidase and hypoxanthine.” SOD activity is expressed as the amount of the SOD standard showing activity equivalent to the determined activity. The results are expressed as units of SOD activity per milligram of protein. One unit of SOD is defined as the amount of enzyme needed to exhibit 50% dismutation of the superoxide radical.
Determination of Plasma TNF-α.
Fresh samples of blood from the animals were taken by cardiac puncture into heparin-containing vials. The determination of plasma TNF-α was made with an enzyme immunoassay kit from R&D Systems (Minneapolis, MN) according to the protocol described by the manufacturer. In brief, each sample (50 μl) was incubated with antibodies specific for rat TNF-α and washed three times with assay buffer. An enzyme-linked polyclonal antibody specific for rat TNF-α conjugated to horseradish peroxidase was then added to the wells. After washing of unbound antibody-enzyme reagent, a substrate solution [containing tetramethylbenzidine (TMB) plus hydrogen peroxide] was added to the wells. The enzyme reaction yielded a blue product (oxidized TMB) that turned yellow when the stop solution (dilute hydrochloride acid) was added. The intensity of the color was determined by measuring the OD of the yellow color in a standard enzyme-linked immunosorbent assay plate reader at 450 nm. Sensitivity of this TNF-α assay was determined by adding 2 standard deviations to the mean optical density value of 20× zero standard replicates and calculating the corresponding concentration. The kit contains all reagents and standards needed for the TNF-α sensitivity assay. We also employed the technical support services of R&D Systems to evaluate our raw data in their analysis tool. The results are expressed as picograms per milliliter. Sensitivity for TNF-α is estimated to be around 1.6 pg/ml.
Determination of Plasma NO Content.
Plasma concentration of NO was quantified indirectly as the concentration of nitrate (NO3−) and nitrite (NO2−) levels in plasma, by the Griess reaction using an assay kit, and according to the protocol described by the manufacturer. Rat plasma was filtered using a 10-kDa molecular mass cutoff filter from Millipore Corporation (Billerica, MA) before each analysis to reduce background absorbance because of the presence of hemoglobin. After centrifugation for 10 min at 3000 rpm, samples (40 μl/well) were mixed with 10 μl of nitrate reductase mixture and incubated for 3 h, after which Griess reagents 1 and 2 (50 μl each) were added. Absorbance was read after 10 min at 540 nm using a plate reader. The concentration of nitrate/nitrite was calculated graphically from a calibration curve prepared from NaNO2 standard solution, and it is expressed as micromolar nitrate.
AntiPyretic Activity.
Fever was induced in animals as described previously (Pinto et al., 1998). In brief, LPS (50 μg/kg i.p.; Sigma-Aldrich, St. Louis, MO) was administered to the animals 1 h before the administration of test drugs. Rectal temperature was measured by inserting a lubricated thermistor probe (external diameter, 3 mm) 2.8 cm into the rectum of the animal. The probe was linked to a digital reader, which displayed the temperature at the tip of the probe (± 0.1°C). The values displayed were manually recorded. Rectal temperatures were taken every hour for 5 h.
Inflammatory Edema Models.
Carrageenan (1%, 100 μl, suspended in sterile saline solution, type IV λ; Sigma-Aldrich) was subcutaneously injected into the plantar surface of the right hind paw in rat following the protocol described by Winter et al., (1962). Paw volume was measured using a water displacement plethysmometer (model 520; IITC/Life Sciences Instruments, Woodland Hills, CA) before carrageenan injection and thereafter at 1-h intervals for 6 h. The paw volume measured just before carrageenan injection was used as the control volume. Data are expressed as the change in paw volume (milliliters) at each time point.
Determination of PGE2 in Rat Paw Exudates.
Rats were euthanized by asphyxiation in a CO2 chamber. After cutting each hind paw at the level of the calcaneus bone, exudates (edema fluid) were collected and processed for measurement of PGE2 as described for the stomachs.
Induction and Assessment of Carrageenan-Evoked Hyperalgesia.
Rats were housed for 1 week before the experiment. They were weighed, marked for identification, and allowed to habituate to the thermostatically controlled test room (22°C) for at least 1 h before commencement of the experiment. Hind paw inflammation was produced by intraplantar injection of carrageenan (100 μl of 1% carrageenan in sterile saline solution) into either hind paw chosen at random. Suspensions of aspirin (180 mg/kg b.wt.), NO-ASA (331 mg/kg b.wt.), NONO-ASA (323 mg/kg b.wt.), or 0.5% (w/v) carboxymethylcellulose (vehicle, 10 ml/kg b.wt.) were administered orally 1 h after carrageenan injection, and the mechanical nociceptive threshold was determined 30 min after this and thereafter every 1 h for up to 5 h. The paw hyperalgesia was measured with an electronic pressure-meter. Each hind paw was positioned in turn under a conical probe surface (tip radius approximately 1 mm), and gradually increasing pressure was applied to the hind paw surface until the animal vocalized at which point the measurement was terminated. Mechanical nociceptive thresholds for both the injected and contralateral (i.e., noninjected) hind paw were determined. The animals were tested before and after treatments, and the results are expressed by the Δ reaction force (g).
Statistical Analysis
All data are presented as the mean ± S.E.M., with sample sizes of at least five rats/group (unless otherwise specified). Comparisons between groups were performed using a one-way analysis of variance followed by the Student t test.
Results
Gastric Mucosal Lesions.
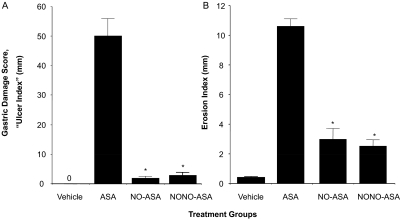
We conducted two different assays to determine the ulcerogenic properties of aspirin and its NO-releasing prodrugs. According to the gastric damage score (also described in the literature as “ulcer index”), animals receiving the vehicle (1% CMC solution) showed a normal glandular region on the surface of the stomach, and no ulcerative damage was observed (UI = 0). However, administration of aspirin (1 mmol/kg) resulted in extensive mucosal injury (UI = 50) to the glandular portion of the gastric fundus. Unlike aspirin, neither NO-releasing aspirin prodrug (NCX-4016 or CVM-01) produced significant ulcerative damage (UI = 2 and 3, respectively) compared with the parent NSAID at equimolar doses (1 mmol/kg), which represents a remarkable reduction (P < 0.01) in gastrointestinal toxicity (see Fig. 2A). The second screening assay compared the gastroprotective effects of NO released from aspirin prodrugs was the erosion index (EI). In this assay, we found a trend similar to that obtained in the ulcer index test; NO-ASA and NONO-ASA produced a significant reduction in gastric ulceration (EI approximately 3) compared with aspirin (EI = 11).
Fig. 2.
NO-ASA and NONO-ASA do not cause gastric damage. Drugs were administered orally at equimolar doses (1 mmol/kg), and effects on the stomach were evaluated as indicated under Materials and Methods. A, ASA caused severe gastric damage (UI = 50 ± 7 mm), whereas both NO-ASA and NONO-ASA were gastric damage-sparing (UI = 2 ± 0.2 and 3 ± 0.3 mm, respectively). B, all three drugs also caused erosions of the gastric mucosa, but the damage was significantly less in the NO-ASA and NONO-ASA groups compared with the ASA group. Results are mean ± S.E.M. for 9 to 12 rats in each group, ∗, P < 0.01 compared with the ASA group.
Gastric Mucosal and Paw Exudate Prostaglandin E2 Content.
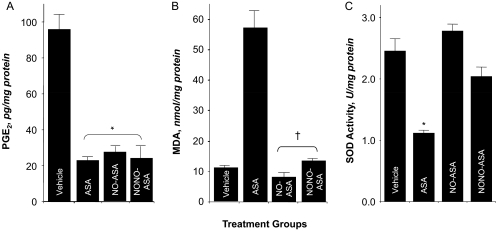
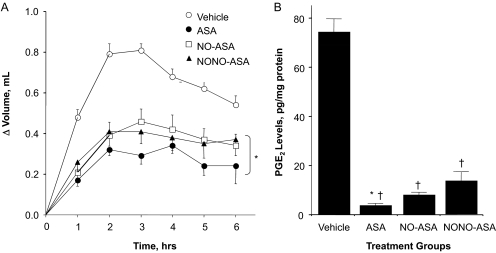
We investigated the effect of aspirin and aspirin prodrugs NCX-4016 and CVM-01 on PGE2 content in gastric mucosa (Fig. 3A) and paw exudates (Fig. 4B). Animals treated with aspirin (1 mmol/kg p.o.) produced approximately 78% less PGE2 than rats in the control group. Similar reductions in PGE2 levels were observed when NO-ASA and NONO-ASA were administered orally (1 mmol/kg, suspended in 1% CMC solution). Prostaglandins are the main product of cyclooxygenase-mediated arachidonic acid metabolism in gastric mucosa; therefore, comparison of PGE2 content in control and drug-treated groups showed a clear and significant COX inhibition by aspirin and its two NO-releasing prodrugs (Fig. 3A). Subsequently, we tested whether NO-releasing aspirins exerted a similar decrease in PGE2 levels in the carrageenan-induced paw edema model in rats. In this assay, aspirin (1 mmol/kg) induced a considerable decrease in PGE2 levels (5 pg/mg protein) compared with control group (75 pg/mg). Treatment with NO-ASA (1 mmol/kg) caused a similar decrease in PGE2 content (9 pg/mg), whereas NONO-ASA (1 mmol/kg) was approximately 3-fold less potent than aspirin, because it reduced PGE2 levels to only approximately 14 pg/mg protein (Fig. 4B).
Fig. 3.
Effects of ASA, NO-ASA, and NONO-ASA on gastric PGE2 level, lipid peroxidation (MDA), and SOD. Four groups of rats were treated with vehicle, ASA, NO-ASA, and NONO-ASA, and their stomachs were removed and processed as described under Materials and Methods. A, all three drugs caused a significant reduction in gastric mucosal PGE2 levels (A). Results are mean ± S.E.M. of 9 to 12 rats in each group, ∗, P < 0.05 versus vehicle group. B, ASA caused an almost 6-fold increase in MDA levels for NO-ASA- and NONO-ASA-treated rats, and MDA levels were comparable with those in the vehicle-treated rats. Results are mean ± S.E.M. for five to seven rats in each group, †, P < 0.01 versus ASA group. C, ASA caused a significant reduction in SOD activity, whereas NO-ASA and NONO-ASA did not have an effect. Results are mean ± S.E.M. of five to seven rats, ∗, P < 0.05 versus vehicle group.
Fig. 4.
Anti-inflammatory properties of ASA, NO-ASA, and NONO-ASA. Rat paw edema was induced by carrageenan injection, as described under Materials and Methods. A, all three drugs caused a significant reduction in paw volume at all time points. Results are mean ± S.E.M. of five rats in each group, ∗, P < 0.05 versus vehicle-treated rats at all time points. B, all three drugs also caused a significant reduction in PGE2 levels in the paw exudate. Results are mean ± S.E.M. for five rats in each group, †, P < 0.01 versus vehicle; ∗, P < 0.05 versus NO-ASA and NONO-ASA.
Lipid Peroxidation.
Oxidative stress in gastric tissue was assessed by measuring the concentration of MDA in intact mucosa 6 h after administration of drugs (1 mmol/kg p.o.). MDA levels were 10.1 ± 4.3, 58.3 ± 2.2, 10.3 ± 1.2, and 13.6 ± 1.9 nmol/mg protein for vehicle-, ASA-, NO-ASA-, and NONO-ASA-treated animals, respectively (Fig. 3B). ASA caused a 5.8-fold induction in MDA levels, whereas NO-ASA- and NONO-ASA-treated animals had MDA levels that were comparable with those of the vehicle-treated animals.
SOD Activity.
In intact gastric mucosal (control group), SOD activity was 2.4 ± 0.5 U/mg protein. After administration of ASA, a significant decrease in SOD activity (1.1 ± 0.2 U/mg protein) was observed (P < 0.05). Treatment with 1 mmol/kg NO-releasing aspirins had no effect on SOD activity, the respective values for NO-ASA and NONO-ASA being 2.7 ± 0.3 and 2.1 ± 0.4 U/mg protein (Fig. 3C).
Carrageenan-Induced Paw Swelling.
The most common use for NSAIDs (including aspirin) is the treatment of inflammatory conditions. We wanted to compare the COX-dependent anti-inflammatory activity of ASA to that obtained with NO-ASA and NONO-ASA. After inducing inflammation, animals receiving vehicle showed a fast time-dependent increase in paw volume (ΔV = 0.8 ml) after 2 h and decreased gradually every hour thereafter until the end of the experiment (6 h) (Fig. 4A). In contrast, animals receiving ASA showed a weak inflammatory response (ΔV = 0.3 ml) that was maintained 2 to 4 h after carrageenan injection, decreasing to approximately ΔV = 0.2 ml after 6 h. The anti-inflammatory effect registered in animals dosed with NO-releasing prodrugs was similar but a bit weaker (ΔV approximately 0.4 ml) compared with that observed with ASA (Fig. 4A).
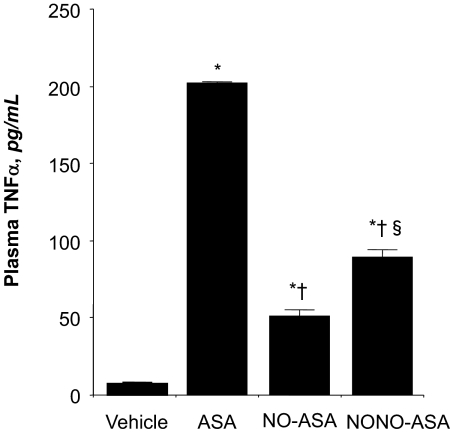
Plasma TNFα Levels.
We determined the inhibitory effect of ASA, NO-ASA, and NONO-ASA on proinflammatory cytokine tumor necrosis factor-α in plasma obtained from control and drug-treated animals. Administration of ASA (1 mmol/kg) increased TNFα concentration by approximately 20-fold (10 ± 0.4 control and 200 ± 2 pg/ml ASA); however, this rise was considerably lower in the NO-ASA (52 ± 2 pg/ml) and NONO-ASA (95 ± 2 pg/ml)-treated animals (Fig. 5).
Fig. 5.
Effect of ASA and its NO-releasing prodrugs on plasma TNF-α. Rats were treated with equimolar amounts (1 mmol/kg) of each drug, and plasma TNF-α was measured as described under Materials and Methods. ASA caused a significant rise in plasma TNF-α; however, this rise was significantly lower in the NO-ASA- and NONO-ASA-treated rats. Results are mean ± S.E.M. for three rats in each group: ∗, P < 0.01 versus vehicle; †, P < 0.01 versus ASA; §, P < 0.05 versus NO-ASA.
Antipyretic Activity.
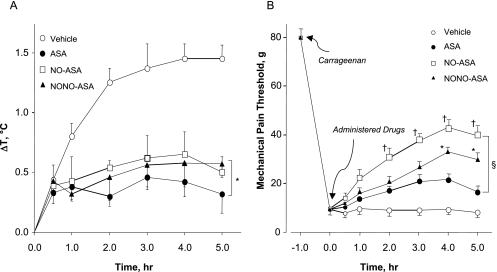
It is well known that aspirin exerts a moderate antipyretic effect when administered orally; therefore, we wanted to determine the decrease in body temperature induced by NO-ASA and NONO-ASA prodrugs compared with that obtained with the parent drug ASA. Experimental drugs (1 mmol/kg) were administered (by mouth) 30 min before injecting LPS (50 μg/kg i.p.) in experimental animals. In this regard, control animals showed a time-dependent increase in body temperature (ΔT = 1.5°C) up to 4 h and maintained it until the end of the screen (5 h). However, prodrug-treated animals showed only approximately a 0.5°C increase in body temperature 1 h after LPS injection and preserved it within this range throughout the experiment (Fig. 6A). Aspirin was more potent (ΔT = 0.3°C) than either prodrug, but all three drugs were effectively capable of reducing LPS-induced fever.
Fig. 6.
ASA, NO-ASA, and NONO-ASA reduce LPS-induced fever and raise the threshold for hyperalgesia. A, LPS (50 μg/kg i.p.) was administered to the animals 1 h before administration of the test drugs. Core body temperature was recorded at 30 min and hourly thereafter for 5 h. Results are mean ± S.E.M. for five rats in each group. ∗, P < 0.05 versus vehicle for all three drugs from 1 to 5 h. B, mechanical pain threshold was increased in a time-dependent manner by all three drugs; however, both NO-ASA and NONO-ASA were better than ASA, especially during the last 2 h of the experiment. Results are mean ± S.E.M. for five rats in each group. §, P < 0.05 versus vehicle for all three drugs from 1 to 5 h; †, P < 0.05 for NO-ASA versus ASA and NONO-ASA from 2 to 5 h; ∗, P < 0.05 for NONO-ASA versus ASA from 4 to 5 h.
Carrageenan-Induced Mechanical Hyperalgesia.
This assay measures the ability of the test drugs to reverse hyperalgesia (decreased threshold to a painful stimuli) produced by injection of carrageenan reagent. The mechanical pain threshold was increased upon time by administering aspirin or its two NO-releasing prodrugs at an equimolar dose. Pain threshold was markedly reduced from 80 to approximately 10 g in animals receiving vehicle (control group), indicating a higher sensitivity to mechanical stimuli (nonpainful at normal conditions). Hyperalgesia was decreased in animals receiving NO-ASA (1 mmol/kg p.o.), which produced a decrease in mechanical pain threshold to approximately 40 g (50% reduction compared with the initial response). The response obtained with NONO-ASA (1 mmol/kg p.o.) was a reduced hyperalgesia (mechanical pain threshold approximately 30 g), similar to that observed with NO-ASA. However, according to this assay, the analgesic effect exerted by aspirin was weaker (pain threshold in the 15–20-g range) than that observed with either NO-ASA or NONO-ASA (Fig. 6B).
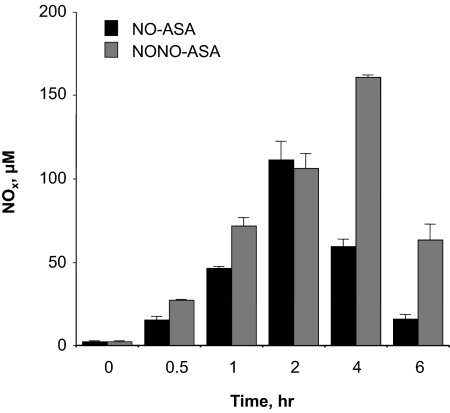
Nitric Oxide Release.
It was essential for us to evaluate the extent of nitric oxide release from aspirin prodrugs possessing different NO donor groups, namely an organic nitrate (present in NO-ASA) and an N-diazen-1-ium-1,2-diolate (present in NONO-ASA). The NO-release profile from these drugs was monitored every hour (up to 6 h) using the Griess reaction to measure NO as total NO3−/NO2− levels (Fig. 7). Both prodrugs showed a time-dependent release of NO. After administration of NO-ASA (1 mmol/kg p.o.), NO levels increased to 120 ± 3 μM after 2 h, but decreased thereafter to approximately 25 ± 2 μM at the end of the assay (6 h). In the case of NONO-ASA, plasma NO levels peaked to 160 ± 3 μM at 4 h after administration (1 mmol/kg p.o.), and at the end of the experiment (6 h), the NO level was approximately 4-fold higher than that observed in animals receiving NO-ASA (Fig. 7).
Fig. 7.
Time course of NOx levels in plasma after NO-ASA and NONO-ASA administration. Plasma concentration of NO was quantified indirectly as the concentration of NO3−/NO2− using the Griess method. Results are mean ± S.E.M. of three rats at each time point, P < 0.01 versus zero time at all time points.
Discussion
Hybrid anti-inflammatory prodrugs possessing organic nitrates are described in the literature as effective anti-inflammatory, analgesic, antipyretic, and chemopreventive drugs (Wallace and Vong, 2008). We conducted an extensive head-to-head comparison between two NO-releasing aspirins, one of them (NCX-4016) possessing an organic nitrate and the other (CVM-01) possessing an N-diazeniumdiolate. The replacement of organic nitrates by N-diazeniumdiolates, and its biological evaluation under identical experimental conditions, offered us the opportunity to assess physicochemical and pharmacological differences inherent to each drug, and to evaluate unique mechanistic features required for the release of the active components from the prodrug. Furthermore, the use of aspirin as parent NSAID was a starting point for comparison purposes.
We initiated our comparison by measuring the extent of gastric protection exerted by the two hybrid prodrugs. As described previously, nitric oxide release from hybrid prodrugs was based on the fact that NO is one of the gastric mucous membrane-protecting factors (Brown et al., 1992; Brown et al., 1993). Continuous release of NO (through nitric-oxide synthase expression) contributes to the physiologic gastrointestinal mobility, tonus, permeability, and blood flow to the vessels of the gastric wall (Kato et al., 2001), which protects the epithelial layer against the mechanism-based toxicity of aspirin. However, the mechanism of NO release from these prodrugs was expected to be different and therefore constitute a pharmacological difference between them. In this regard, organic nitrates require an in vivo three-electron reduction to release NO, whereas N-diazeniumdiolate ions spontaneously release NO in physiologic media. Furthermore, N-diazeniumdiolates release two equivalents of NO (hence their name NONOates) per molecule (Davies et al., 2001). Both prodrugs were significantly less toxic than aspirin, which is consistent with prior evidence describing the role of NO in reducing the gastric damage produced by NSAIDs (Fiorucci and Del Soldato, 2003; Fiorucci et al., 2003). Nevertheless, there was no statistically significant difference between groups of animals receiving the NO-ASA (NCX-4016) and rats dosed with NONO-ASA (CVM-01). This was confirmed by the ulcer and erosion indexes. The first assay measures clearly visible ulcers (elongated, hemorrhagic lesions varying in length), whereas the second accounts for less noticeable microhemorrhagic lesions, which are only visible with the use of a magnifying lens. It appears that gastroprotection provided by both hybrid prodrugs was not entirely dependent on the amount of NO released from them. Despite the observed higher levels of NO released from the N-diazeniumdiolate ion present in CVM-01 (confirmed by the measurement of plasma nitrite/nitrite levels), the extent of gastric protection was practically identical for both prodrugs. For this reason, it might be speculated that NO-dependent protection from hybrid prodrugs reaches a “saturation point,” where the excess of NO (approximately 2 equivalents) released from CVM-01 does not produce additional gastric protection. We also found an increase in the concentration of thiobarbituric acid reactant substances, an index of lipid peroxidation, and a decrease in TNF-α, whereas the antioxidative marker (SOD) increased in NO-ASA- and NONO-ASA-treated rats. All changes in the gastric mucosal tissue may be the result of the antioxidative effects of the prodrug.
In addition to reducing gastric damage, it was essential for us to evaluate and compare the anti-inflammatory profiles of aspirin, NO-ASA, and NONO-ASA. We conducted this evaluation by measuring the in vivo carrageenan-induced rat paw edema assay and direct measurement of cyclooxygenase-dependent production of PGE2 in gastric tissue as well as plasma. When administered at equimolar doses (1 mmol/kg po), all test drugs showed significant anti-inflammatory activity by decreasing the inflammatory response (paw volume) and reducing the amount of PGE2 in rats.
In the rat paw edema model, however, there were some differences noted among the three compounds. Both aspirin and NO-ASA produced a significant reduction (approximately 60%) in paw inflammation compared with the vehicle control group. NONO-ASA, on the other hand, produced a 40% reduction in rat paw swelling. One possible reason for this might be found in the PGE2 measurements in the paw exudates. ASA and NO-ASA both produced a 90% or greater reduction in PGE2 levels, whereas NONO-ASA produced approximately a 70% reduction in PGE2 levels. It may be necessary, therefore, to inhibit PGE2 levels almost completely to have a significant effect on inflammation (Wallace et al., 1999). In models of inflammatory pain (hyperalgesia), NCX 4016 and CVM-01 showed a significant analgesic activity for 6 h after treatment and had a longer duration of action compared with ASA-treated rats. Aspirin, NO-ASA, and NONO-ASA demonstrated the analgesic effect, NO-donating compounds being more potent than aspirin on a molar basis. These two compounds also provide antipyretic activity as the potential to decrease body temperature induced by LPS (50 μg/kg).
In conclusion, we have demonstrated that the hybrid aspirin possessing N-diazen-1-ium-1,2-diolate (CVM-01) has equipotent anti-inflammatory, analgesic, and antipyretic activity compared with the hybrid aspirin possessing an organic nitrate (NCX-4016). Furthermore, it was demonstrated that gastric protection exerted by the release of NO from hybrid NO-releasing aspirins appears to be independent of the extent and mechanism of NO release, because replacement of N-diazen-1-ium-1,2-diolates for organic nitrates did not produce a statistically significant difference in the ulcerogenic profile measured for both prodrugs.
This work was supported in part by the Intramural Research Program of the National Institutes of Health National Cancer Institute, Division of Basic Sciences, Center for Cancer Research [Project ZIA-BC005673].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.171017
- NSAID
- nonsteroidal anti-inflammatory drug
- COX
- cyclooxygenase
- CVM-01
- O2-(acetylsalicyloxymethyl)-1-(pyrrolidin- 1-yl)diazen-1-ium-1,2-diolate
- NONO-ASA
- O2-(acetylsalicyloxymethyl)-1-(pyrrolidin-1-yl)diazen-1-ium-1,2-diolate
- NCX 4016
- 3-nitrooxyphenyl acetylsalicylate
- NO-ASA
- 3-nitrooxyphenyl acetylsalicylate
- ASA
- acetylsalicylic acid
- LPS
- lipopolysaccharide
- PGE2
- prostaglandin E2
- SOD
- superoxide dismutase
- UI
- ulcer index
- MDA
- malondialdehyde
- TNFα
- tumor necrosis factor α
- CMC
- carboxymethyl cellulose
- EI
- erosion index
References
- Aalykke C, Lauritsen K. ( 2001) Epidemiology of NSAID-related gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol 15: 705– 722 [DOI] [PubMed] [Google Scholar]
- Anand BS, Romero JJ, Sanduja SK, Lichtenberger LM. ( 1999) Phospholipid association reduces the gastric mucosal toxicity of aspirin in human subjects. Am J Gastroenterol 94: 1818– 1822 [DOI] [PubMed] [Google Scholar]
- Best R, Lewis DA, Nasser N. ( 1984) The anti-ulcerogenic activity of the unripe plantain banana (Musa species). Br J Pharmacol 82: 107– 116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JF, Hanson PJ, Whittle BJ. ( 1992) Nitric oxide donors increase mucus gel thickness in rat stomach. Eur J Pharmacol 223: 103– 104 [DOI] [PubMed] [Google Scholar]
- Brown JF, Keates AC, Hanson PJ, Whittle BJ. ( 1993) Nitric oxide generators and cGMP stimulate mucus secretion by rat gastric mucosal cells. Am J Physiol 265: G418– G422 [DOI] [PubMed] [Google Scholar]
- Chan AT, Ogino S, Fuchs CS. ( 2007) Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med 356: 2131– 2142 [DOI] [PubMed] [Google Scholar]
- Cryer B, Feldman M. ( 1998) Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. American Journal of Medicine 104: 413– 421 [DOI] [PubMed] [Google Scholar]
- Davies KM, Wink DA, Saavedra JE, Keefer LK. ( 2001) Chemistry of the diazeniumdiolates. 2. Kinetics and mechanism of dissociation to nitric oxide in aqueous solution. J Am Chem Soc 123: 5473– 5481 [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Del Soldato P. ( 2003) NO-aspirin: mechanism of action and gastrointestinal safety. Dig Liver Dis 35 ( Suppl 2): S9– S19 [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Santucci L, Distrutti E. ( 2007) NSAIDs, coxibs, CINOD and H2S-releasing NSAIDs: what lies beyond the horizon. Dig Liver Dis 39: 1043– 1051 [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Santucci L, Gresele P, Faccino RM, Del Soldato P, Morelli A. ( 2003) Gastrointestinal safety of NO-aspirin (NCX-4016) in healthy human volunteers: a proof of concept endoscopic study. Gastroenterology 124: 600– 607 [DOI] [PubMed] [Google Scholar]
- FitzGerald GA. ( 2003) COX-2 and beyond: Approaches to prostaglandin inhibition in human disease. Nat Rev Drug Discov 2: 879– 890 [DOI] [PubMed] [Google Scholar]
- Flossmann E, Rothwell PM, British Doctors Aspirin Trial and the UK-TIA Aspirin Trial ( 2007) Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet 369: 1603– 1613 [DOI] [PubMed] [Google Scholar]
- Jaksch W, Dejaco C, Schirmer M. ( 2008) 4 years after withdrawal of rofecoxib: where do we stand today? Rheumatol Int 28: 1187– 1195 [DOI] [PubMed] [Google Scholar]
- Kato S, Suzuki K, Ukawa H, Komoike Y, Takeuchi K. ( 2001) Low gastric toxicity of nitric oxide-releasing aspirin, NCX-4016, in rats with cirrhosis and arthritis. Dig Dis Sci 46: 1690– 1699 [DOI] [PubMed] [Google Scholar]
- Kurinets A, Lichtenberger LM. ( 1998) Phosphatidylcholine-associated aspirin accelerates healing of gastric ulcers in rats. Dig Dis Sci 43: 786– 790 [DOI] [PubMed] [Google Scholar]
- Li-yuan J, Li C, Yi-hua Z. ( 2004) Improved synthesis of 2-(acetyloxy)benzoic acid 3-(nitrooxymethyl)phenyl ester. Chin J Med Chem 14: 178– 179 [Google Scholar]
- Lichtenberger LM, Romero JJ, de Ruijter WM, Behbod F, Darling R, Ashraf AQ, Sanduja SK. ( 2001) Phosphatidylcholine association increases the anti-inflammatory and analgesic activity of ibuprofen in acute and chronic rodent models of joint inflammation: relationship to alterations in bioavailability and cyclooxygenase-inhibitory potency. J Pharmacol Exp Ther 298: 279– 287 [PubMed] [Google Scholar]
- Martin GR, Wallace JL. ( 2006) Gastrointestinal inflammation: a central component of mucosal defense and repair. Exp Biol Med (Maywood) 231: 130– 137 [DOI] [PubMed] [Google Scholar]
- Orido T, Fujino H, Hasegawa Y, Toyomura K, Kawashima T, Murayama T. ( 2008) Indomethacin decreases arachidonic acid uptake in HCA-7 human colon cancer cells. J Pharmacol Sci 108: 389– 392 [DOI] [PubMed] [Google Scholar]
- Pinto L, Borreli F, Bomberdelli E, Cristonic A, Capasso F. ( 1998) Antiinflammatory, analgesis and antipyretic effects of glaucine in rats and mice. Pharm Pharmacol Commun 4: 502– 505 [Google Scholar]
- Schaffer D, Florin T, Eagle C, Marschner I, Singh G, Grobler M, Fenn C, Schou M, Curnow KM. ( 2006) Risk of serious NSAID-related gastrointestinal events during long-term exposure: a systematic review. Med J Aust 185: 501– 506 [DOI] [PubMed] [Google Scholar]
- Scheiman JM, Fendrick AM. ( 2007) Summing the risk of NSAID therapy. Lancet 369: 1580– 1581 [DOI] [PubMed] [Google Scholar]
- Spitz GA, Furtado CM, Sola-Penna M, Zancan P. ( 2009) Acetylsalicylic acid and salicylic acid decrease tumor cell viability and glucose metabolism modulating 6-phosphofructo-1-kinase structure and activity. Biochem Pharmacol 77: 46– 53 [DOI] [PubMed] [Google Scholar]
- Stanek A, Gadowska-Cicha A, Gawron K, Wielkoszyński T, Adamek B, Cieślar G, Wiczkowski A, Sieroń A. ( 2008) Role of nitric oxide in physiology and pathology of the gastrointestinal tract. Mini Rev Med Chem 8: 1549– 1560 [DOI] [PubMed] [Google Scholar]
- Stefano F, Distrutti E. ( 2007) Cyclo-oxygenase (COX) inhibiting nitric oxide donating (CINODs) drugs: a review of their current status. Curr Top Med Chem 7: 277– 282 [DOI] [PubMed] [Google Scholar]
- Szekely CA, Breitner JC, Fitzpatrick AL, Rea TD, Psaty BM, Kuller LH, Zandi PP. ( 2008) NSAID use and dementia risk in the Cardiovascular Health Study: role of APOE and NSAID type. Neurology 70: 17– 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velázquez C, Praveen Rao PN, Knaus EE. ( 2005) Novel nonsteroidal antiinflammatory drugs possessing a nitric oxide donor diazen-1-ium-1,2-diolate moiety: design, synthesis, biological evaluation, and nitric oxide release studies. J Med Chem 48: 4061– 4067 [DOI] [PubMed] [Google Scholar]
- Velázquez CA, Chen QH, Citro ML, Keefer LK, Knaus EE. ( 2008) Second-generation aspirin and indomethacin prodrugs possessing an O(2)-(acetoxymethyl)-1-(2-carboxypyrrolidin-1-yl)diazenium-1,2-diolate nitric oxide donor moiety: design, synthesis, biological evaluation, and nitric oxide release studies. J Med Chem 51: 1954– 1961 [DOI] [PubMed] [Google Scholar]
- Velázquez CA, Praveen Rao PN, Citro ML, Keefer LK, Knaus EE. ( 2007) O2-acetoxymethyl-protected diazeniumdiolate-based NSAIDs (NONO-NSAIDs): synthesis, nitric oxide release, and biological evaluation studies. Bioorg Med Chem 15: 4767– 4774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlad SC, Miller DR, Kowall NW, Felson DT. ( 2008) Protective effects of NSAIDs on the development of Alzheimer disease. Neurology 70: 1672– 1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JL. ( 2008) Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn't the stomach digest itself? Physiol Rev 88: 1547– 1565 [DOI] [PubMed] [Google Scholar]
- Wallace JL, Caliendo G, Santagada V, Cirino G, Fiorucci S. ( 2007) Gastrointestinal safety and anti-inflammatory effects of a hydrogen sulfide-releasing diclofenac derivative in the rat. Gastroenterology 132: 261– 271 [DOI] [PubMed] [Google Scholar]
- Wallace JL, Chapman K, McKnight W. ( 1999) Limited anti-inflammatory efficacy of cyclo-oxygenase-2 inhibition in carrageenan-airpouch inflammation. Br J Pharmacol 126: 1200– 1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JL, Reuter BK, Cirino G. ( 1994) Nitric oxide-releasing non-steroidal anti-inflammatory drugs: a novel approach for reducing gastrointestinal toxicity. J Gastroenterol Hepatol 9 (Suppl 1): S40– S44 [DOI] [PubMed] [Google Scholar]
- Wallace JL, Vong L. ( 2008) NSAID-induced gastrointestinal damage and the design of GI-sparing NSAIDs. Curr Opin Investig Drugs 9: 1151– 1156 [PubMed] [Google Scholar]
- Winter CA, Risley EA, Nuss GW. ( 1962) Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med 111: 544– 547 [DOI] [PubMed] [Google Scholar]
- Yasuda O, Takemura Y, Kawamoto H, Rakugi H. ( 2008) Aspirin: recent developments. Cell Mol Life Sci 65: 354– 358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Qi R, Li R, Wu W, Gao X, Bao L, Lu S. ( 2008) Protective effects of aspirin against oxidized LDL-induced inflammatory protein expression in human endothelial cells. J Cardiovasc Pharmacol 51: 32– 37 [DOI] [PubMed] [Google Scholar]