Abstract
Smith-Magenis syndrome (SMS; OMIM 182290) is a neurodevelopmental disorder characterized by a well-defined pattern of anomalies. The majority of cases are due to a common deletion in chromosome 17p11.2 that includes the RAI1 gene. In children with SMS, autistic-like behaviors and symptoms start to emerge around 18 months of age. This study included 26 individuals (15 females and 11 males), with a confirmed deletion (del 17p11.2). Parents/caregivers were asked to complete the Social Responsiveness Scale (SRS) and the Social Communication Questionnaire (SCQ) both current and lifetime versions. The results suggest that 90% of the sample had SRS scores consistent with autism spectrum disorders. Moreover, females showed more impairment in total T-scores (p=0.02) and in the social cognition (p=0.01) and autistic mannerisms (p=0.002) subscales. The SCQ scores are consistent to show that a majority of individuals may meet criteria for autism spectrum disorders at some point in their lifetime. These results suggest that SMS needs to be considered in the differential diagnosis of autism spectrum disorders but also that therapeutic interventions for autism are likely to benefit individuals with SMS. The mechanisms by which the deletion of RAI1 and contiguous genes cause psychopathology remain unknown but they provide a solid starting point for further studies of gene-brain-behavior interactions in SMS and autism spectrum disorders.
Keywords: del 17p11.2, RAI1, microdeletion syndrome, behavioral phenotype, social communication
Introduction
Microdeletion syndromes associated with defined neurobehavioral phenotypes offer unique genetic models of haploinsufficiency to identify/discover critical gene(s) and/or gene-networks involved in cognitive and neurospsychiatric disorders. Smith-Magenis syndrome (SMS; OMIM 182290) is a neurodevelopmental disorder characterized by a well-defined pattern of anomalies including a distinct craniofacial dysmorphic phenotype, abnormalities of sleep-wake circadian rhythm, and cognitive impairment with behavioral and psychiatric symptoms [Smith et al., 2010]. The majority of cases (∼90%) are due to a common 3.7Mb interstitial deletion of chromosome 17p11.2 that includes the RAI1 gene. Heterozygous mutations in RAI1 gene account for fewer than 10% of cases [Elsea and Girirajan, 2008]. A number of genes have been mapped and isolated to the critical region, but except for RAI1, their participation in the pathogenesis of the syndrome remains unclear. First described in early 1980's [Smith et al., 1986; Smith et al., 1982], the syndrome prevalence is now estimated to be 1/15,000. Virtually all cases occur de novo, suggesting a low recurrence risk. SMS is probably under diagnosed due to mild facial abnormalities and the behavioral problems that are not prominent until the affected child is older [Gropman et al., 2006; Smith et al., 1998a].
Variable levels of cognitive impairment, most frequently in the moderate range of intellectual disability, are universal in individuals with Smith-Magenis syndrome. Speech and language delays are present in most cases, with receptive skills generally better than expressive language skills. Distractibility is characteristic of the syndrome. Learning abilities are characterized by strength in visual reasoning tasks and weakness in sequential processing (counting, mathematical, and multi-step tasks). Short-term memory is poor, but long-term memory is considered a relative strength [Dykens et al., 1997].
The striking neurobehavioral phenotype that characterizes the syndrome emerges over time, beginning between 18-36 months of age, when head-banging and autistic-like behaviors are seen [Gropman et al., 2006; Wolters et al., 2009]. Mild gross and fine motor delays with age-appropriate social skills and minimal maladaptive behaviors can be observed in infants <18 months; however, at ages 2-3 years, global psychomotor, expressive language delays and mild to moderate autistic behaviors begin to emerge [Wolters et al., 2009]. Initially, infants are sociable and are frequently described as a “perfect baby” who “never cries” [Gropman et al., 2006]. Parents generally do not report issues with sleep until after 18 months of age; however, sleep actigraphy data suggests sleep dysfunction as early as 9 months of age [Duncan et al., 2003; Gropman et al., 2006]. With increasing age, psychomotor delays and the emerging neurobehavioral and sleep difficulties often lead to referral and pursuit of diagnostic workup.
Previous studies suggest that age, degree of cognitive delay and levels of sleep disturbance are associated with maladaptive behavior [Dykens and Smith, 1998; Finucane et al., 2001]. Maladaptive behaviors include hyperactivity, impulsivity, temper tantrums (mainly in response to changes in routine), and aggression. Self-injurious behavior (SIB), reported in over 90% [Dykens and Smith, 1998], includes wrist-biting, head-banging, hitting self or objects, skin picking, and two behaviors unique to SMS, onychotillomania (i.e., pulling out nails/nail yanking) and polyembolokoilamania (the insertion of foreign bodies in their body orifices). The latter, could be so severe that in some cases, parents have been reported to social services for suspicion of child abuse [Smith et al., 1998a]. An additional salient feature, thought to be unique to the syndrome, is the involuntary spasmodic upper body squeeze or “self-hug” first described by Finucane et al., [1994]. Two types of self-hugging are described: (1) self-hugging (i.e, arms tightly wrapped around upper arms) and spasmodically tensing the upper body and (2) hand clasping at chest level or under the chin while squeezing their arms tightly against their chests and sides. These movements appear as an expression of happiness or excitement and are involuntary, with a tic-like quality. Mild to moderate generalized hypotonia impacts early motor development and a fine motor tremor may be observed [Gropman et al., 2006; Wolters et al., 2009]. Individuals with SMS also have been described to have stereotypies, sensory integration difficulties and social communication problems consistent with select features of autism spectrum disorders (ASD). Despite being described as “friendly,” their social awareness is extremely limited. This study expands our previous work to describe co-morbid autism spectrum disorders (ASD) in individuals diagnosed with Smith-Magenis Syndrome.
Materials and Methods
Participants
The study includes 26 individuals (15 females and 11 males), with a confirmed deletion (del 17p11.2) diagnosis of Smith-Magenis syndrome (SMS) ranging in age from 4.2 years to 49.9 years. All participants were recruited among those currently participating in the ongoing natural history study of SMS (01-HG-0109) at National Institutes of Health and provided consent/assent for the study. Parents/caregivers were asked to complete a battery of tools to assess autism spectrum and other co-morbid disorders. Full-scale intelligence quotients (IQ) for 21of the 26 subjects enrolled in the natural history study of SMS were available for inclusion in the data analysis. The IQ scores in this population ranged from 40 to 80, with a mean and standard deviation of 56, and 10 respectively.
Instruments
Social Responsiveness Scale
(SRS), developed by Constantino and Bruber [2005], is a well validated 65-item questionnaire used to evaluate the current behavioral and developmental history for children and adolescents between 4-18 years of age. The SRS provides gender specific T-scores for five “treatment” subscales, i.e., receptive, cognitive, expressive, motivational aspects of social behavior, and autistic preoccupations.
Social Communication Questionnaire
(SCQ), developed by Rutter et al.[2003] and previously known as the Autism Screening Questionnaire (ASQ), is a 40-item questionnaire for children ages 4 years and older. The SCQ-Current is based on the individual's behavior during the most recent 3-month period and the SCQ-Lifetime focuses on the individual's entire developmental history. Both versions of the SCQ yield a single total score. The SCQ was developed as a companion to the more detailed Autism Diagnostic Interview – Revised (ADI-R). The SCQ uses an easy yes/no response format that can generally be completed by parent/primary caregiver in ≤10 minutes and scored in 5 minutes to yield a total score for interpretation. For the SCQ-Lifetime, a cutoff score of 15 or greater is used as indicator of possible ASD and reason for referral for more comprehensive assessment. The SCQ assists in the differentiation of children at risk for ASD compared to other developmental disabilities (DD) [Allen et al., 2007]. Agreement between SCQ and ADI-R scores is high, (r=0.71) and unaffected by age, gender, language level, and performance intelligence quotient (IQ).
Statistical Analysis
Data was compiled for statistical analysis using Statview® software version.5.0. Descriptive statistics were derived and the total scores and subscores obtained from the SRS and the SCQ were analyzed as continuous dependent variables using t-tests. Categorical variables were analyzed using chi square tests. Alpha was set at <0.05.
Results
Demographics
Demographics for the study group (n=26) are summarized in Table I. Mean age was 14.4 years (SD 10.0) for the group. No differences in age by gender were observed. Mean IQ was 53.8 (SD 10.9) for females (n=13) and 58.2 (SD 9.4) for males (n=9). All subjects were Caucasian, including 25 non-Hispanics and 1 Hispanic.
Table I. Demographics of the study population.
| Total | Females | Males | |
|---|---|---|---|
| N | 26 | 15 | 11 |
| Mean age (years) | 14.4 SD 10.0 | 14.7 SD 7.8 | 14.1 SD 12.9 |
| Range | 4.2-49.9 years | 5.7-30.5 years | 4.2-49.9 years |
| IQ* | 55.6 SD 10.3 | 53.8 SD 19.9 | 58.2 SD 9.3 |
| SRS (age 4-18 yr) | 20 | 11 | 9 |
| SCQ (age 4 and over) | 26 | 15 | 11 |
IQ scores available on 13 females and 9 males.
Social Responsiveness Scale (SRS)
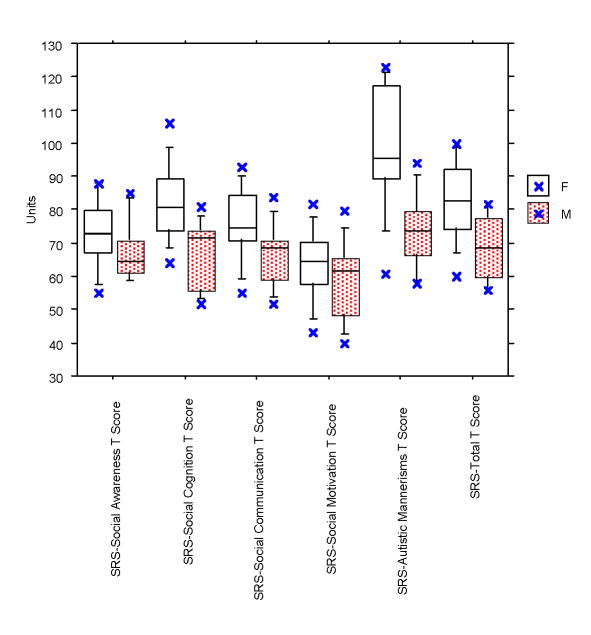
The SRS was analyzed for subjects between ages 4-18 years only (n=20). A comparison of total T-score and five subscale scores obtained for the SRS are summarized in Table 2. In this sample, 90% of participants scored within the autism range. T-scores fell in the mild/moderate range (60-75) for 35% of SMS subjects and in the severe range (> 76 or higher) for the remaining 55%, reflecting over half of the group. While females demonstrated consistently higher T-scores than males for all five subscales and total SRS score, significant gender differences were documented for only two of the five mean subscales (Social Cognition (p =.01) and Autistic Mannerisms (p=.002)) and the mean Total T-score (p=.02). Male mean T-scores were in the mild/moderate range for four of five subscales; only the mean Social Motivation T-score (58.6) was within the normal range. In contrast, the mean T-scores in females were in the “severe range” for three of the five subscales (Table 2): Social awareness, Social cognition, and Autistic mannerisms. The observed distribution frequency of SRS T-Score ranges (normal, mild/moderate, severe ranges) by gender (Fig. 1), however, was not statistically significant (Chi-square=4.258; p=ns).
Table II. Social Responsiveness Scale (SRS) Mean Subscale and Total T-scores1.
| SRS T-Scores | Group n=20 | Females n=11 | Males n=9 | t-test2 | DF | Significance p value |
|---|---|---|---|---|---|---|
| Social Awareness | ||||||
| Mean (SD) | 70.6 (10.1) | 72.9 (10.7) | 67.7 (9.2) | 1.16 | 18 | ns |
| Range | 55-88 | 55-88 | 59-85 | |||
| Social Cognition | ||||||
| Mean (SD) | 75.4 (13.1) | 81.8 (12.0) | 67.4 (10.1) | 2.85 | 18 | 0.01 |
| Range | 52-106 | 64-106 | 52-81 | |||
| Social Communication | ||||||
| Mean (SD) | 71.3 (11.1) | 75.1 (11.2) | 66.7 (9.5) | 1.78 | 18 | ns |
| Range | 52-93 | 55-93 | 52-84 | |||
| Social Motivation | ||||||
| Mean (SD) | 61.1 (11.6) | 63.2 (11.1) | 58.6 (12.2) | .886 | 18 | ns |
| Range | 40-82 | 43-82 | 40-80 | |||
| Autistic Mannerisms | ||||||
| Mean (SD) | 87.7 (20.2) | 99.2 (18.8) | 73.7 (11.3) | 3.57 | 18 | 0.002 |
| Range | 58-123 | 61-123 | 58-94 | |||
| Total T-Score1 | ||||||
| Mean (SD) | 76.6 (12.8) | 82.6 (12.2) | 69.2 (9.7) | 2.65 | 18 | 0.02 |
| Range | 56-100 | 60-100 | 56-82 | |||
| Total raw score2 | ||||||
| Mean (SD) | 80.8 (21.4) | 86.2 (21.7) | 74.2 (20.1) | 1.27 | 18 | ns |
| Range | 46-118 | 46-118 | 47-100 | |||
| SRS Classification3 | ||||||
| Normal | 2 | 0 | 2 | |||
| Mild-Moderate (60-75) | 7 | 3 | 4 | |||
| Severe (≥76) | 11 | 8 | 3 | |||
T-scores: Mild/moderate (60-75); Severe range (76 or greater)
Unpaired t-test (significance p<.05)
Classification based on Total T-score by gender: Chi-square f = 4.26; p=ns.
Total raw score cut points (quantitative measure of symptomatology): males >70; females >65 total.
Fig. 1. Gender comparison of SRS total and subscale T-scores (n=20).
Comparison of SRS subscales and total T-scores by gender. Females demonstrate higher t-scores for all five subscales; however, significant gender differences were found for only two subscales, Social Cognition (p=.01) and Autistic Mannerisms (p=.002), and total T-score (p=.02) by unpaired t-test.
Gender-specific cut points for SRS total raw scores are used when screening for autism spectrum disorders (PDD-NOS, Asperger's or Autistic Disorder) in school or other general population groups where the prevalence may be 1/150 with test sensitivity of .77; specificity of .75. SRS raw scores above gender cut points were observed for 55% of males (>70) and 90% of females (>65). Thus, 75% of the total sample had raw SRS scores above gender cut point.
Social Communication Questionnaire (SCQ)
The SCQ was analyzed for all subjects age 4 years and older (n=26; 15F/11M) with results for both the Current (SCQ-C) and Lifetime (SCQ-L) scales summarized in Table 3. Scoring for SCQ-L was incomplete for one male participant and was excluded from the analysis. The group mean SCQ-C score (12.6 SD 5.9) was within the normal range (cut score <15). There were no differences by gender. The group mean SCQ-Lifetime score was 14.8 SD 5.7 with 14 (10F/4M) individuals above the score cutoff (>15). Although the mean SCQ-L was higher and above the cutoff in males (16.2 SD 6.8) as compared to females (13.87 SD 4.86) these differences were not statistically significant. No correlation between SCQ-L score and age (Z=.243; p=ns) or gender (t=-1.004; p=ns) was found.
Table III. SCQ Current and Lifetime scales for SMS subjects ages 4.2-49.9yr.
| SMS Group | Females | Males | t (df) | Significance1 | ||
|---|---|---|---|---|---|---|
| SCQ-Current (n=26) | n=26 | n=15 | n=11 | |||
| Mean (SD) | 12.6 (5.9) | 11.5 (4.5) | 14.0 (7.5) | -1.049 | ns | |
| Range | 2-27 | 2-20 | 5-27 | (24) | ||
| % Above Cutoff | 35% | 15% | 19% | |||
| SCQ-Lifetime (n=25) | n=25 | n=15 | n=10 | |||
| Mean (SD) | 14.8 (5.7) | 13.9 (4.9) | 16.2 (6.8) | -1.004 | ns | |
| Range | 3-28 | 3-22 | 7-28 | (23) | ||
| % Above Cutoff | 54% | 38% | 15% | |||
Unpaired t-test
IQ Scores
Intelligence quotient scores were available for 16/20 subjects with SRS scores and 22/25 with both SCQ-L and IQ scores. We conducted two one-way ANOVAs to examine the relationship between IQ and SRS or IQ and SCQ-L scores. IQ was not found to be predictive of scores on the SCQ-L or SRS (p=ns). Similarly, age was not predictive of scores on the SCQ-L or SRS (p=ns).
Discussion
The assessment of specific mental disorders as a part of a phenotype that has known genotypic abnormalities (heterozygous RAI1 mutation or deletion at chromosome 17p11.2) has significant potential to further the mechanistic understanding of gene to behavior and gene to syndrome interactions. The genomic and developmental behavioral characterization of autistic-like features in specific populations, such as individuals with SMS, could be beneficial to both autism and SMS research.
Two previous studies have documented findings that support ASD in SMS using the Childhood Rating Scale (CARS)[Schopler et al., 1980] As part of our ongoing natural history study of SMS that began in 2001 (NIH protocol 01-HG-0109), we prospectively evaluated the neurodevelopment of children < 3 years of age with SMS. The group mean CARS total score was in the normal range; however, further analysis comparing infants to toddlers revealed more severe autistic-like behaviors for toddlers than compared to mild-moderate autistic range for infants, suggesting an age related progression [Wolters et al., 2009]. Compared to normal ratings in infants, toddlers rated mild-moderately abnormal in five areas: imitation, emotional response, object use, verbal communication and general impression. However, when the CARS was used to ascertain older SMS patients, the age difference disappeared [Martin et al., 2006]. The results reported herein expand our previous work by further characterizing the range of autism spectrum symptoms in individuals with SMS. The diagnosis of ASD is based upon clinical and behavioral review and direct observation for presence versus absence of specific features, as to date no one genetic region has been identified, and genotype-phenotype correlation studies are still underway. As such, it is imperative to use the most objective measures of the behavioral features available. Currently, the Autistic Diagnostic Interview (ADI) and the Autistic Diagnostic Observation Scale (ADOS) are considered to be the most robust and sensitive of those instruments. Unfortunately, due to the inherent expense of the instruments, (extensive administrator training and certification required, and the time and inconvenience for the participant and their families in commuting to a testing location), it is often prudent to utilize an instrument that can ascertain autistic-like features via parent-report. The Social Communication Questionnaire-Lifetime (SCQ-L) has been demonstrated to be very highly correlated (.71; p<.01) with the ADI-R [Rutter et al., 2003]. The SCQ and SRS are two easily administered, well-validated instruments, frequently used in autism research. Instruments were chosen based on their availability, psychometrics and previous experience with this population; however, it is important to keep in mind that none of these instruments have been validated in SMS.
Given the paucity of data surrounding psychiatric aspects of SMS, an over inclusive strategy has been chosen to capture the subtleties of symptoms and syndromes. Redundancy in information gathering will reinforce reliability of findings. The data reported herein were derived from a relatively small convenience sample that included deletion cases only (one mutation case available was excluded). However, in light of the SMS prevalence our sample is one of the largest available worldwide.
These results suggest the majority of SMS patients may meet criteria for autism spectrum disorders at some point in their lifetime. However, what would seem to remain constant are the social communication deficits as evidenced by the SRS scores that are at or above the moderate range for this scale. Moreover, 55% of participants were in the severe range. We also found gender differences in global scores and in the social cognition and autistic mannerisms subscales with females showing more impairment than males. These findings suggest that while all SMS patients would benefit from interventions to enhance their social skills, females, may require additional emphasis on social cognition (ability to interpret social cues) and autistic mannerisms (stereotypic behavior and restricted interests).
Another interesting cluster of symptoms shared by SMS and ASD is sleep disturbance. In SMS, sleep is characterized by fragmented and decreased sleep duration, with frequent and prolonged nocturnal awakenings, along with excessive daytime sleepiness and napping [Greenberg et al., 1991; Smith et al., 1998b]. In children with ASD, sleep is characterized by a marked decrease in REM, reduced efficiency, and increased fragmentation due to night-time arousals, obstructive sleep apnea, and insomnias [Johnson and Malow, 2008]. Unlike in ASD, sleep disorders in SMS are associated with an inverted circadian rhythm of melatonin, with present or high levels during the day and decreased or undetectable levels at night [De Leersnyder et al., 2001]. However, treatment with melatonin has proven helpful in both SMS [Laje,et al., unpublished data; De Leersnyder et al., 2003; Gropman et al., 2006;Smith et al., 1998b] and ASD [Wirojanan et al., 2009; Wright et al., 2010]. It is possible that the mechanisms involved in sleep disturbances in SMS may have shared pathways with ASD.
These results also suggest that, from a clinical perspective, a large majority of SMS cases may meet criteria for an axis I diagnosis of pervasive developmental disorders [DSM-IV-TR, 2000; (APA, 2000)]. This could help clinicians in the community to determine treatment strategies and services to address this population's needs. Due to the relative frequency of SMS in relation to ASD (1/15,000 versus 1/110), our results support consideration of inclusion of SMS in the differential diagnosis of those suspected of having ASD. Although SMS children have a specific and known underlying genetic etiology, their phenotype fits very clearly with ASD such that services beneficial to ASD may also prove beneficial to the SMS population. On the one hand, this is particularly important considering the clinical factors that may be present in SMS and not fully appreciated in younger children who have only received a diagnosis of ASD. On the other hand, there are well-established guidelines for clinical evaluation of individuals with SMS that are development-dependent [Smith et al., 2010; Smith and Gropman, 2010]. Having both diagnoses of ASD and SMS may help families in receiving the best care/assistance for their child, especially as ASD is more recognized and treated than most rare diseases with a genetic etiology. Moreover, the known etiology of SMS offers not only a lead in the ascertainment of the developmental role of genes within the deletion, but a model to further our understanding of ASD.
To conclude, our results suggest that a majority of patients with SMS have concomitant symptoms of autism spectrum disorders. The consistency of the deletion that leads to haploinsufficiency of RAI1 and contiguous genes makes SMS a human hemizygous molecular model, with the potential to lead to new insights into the biology of behavioral traits, therefore in general terms, presents a “human knock-out model.” Thus, the study of SMS offers an opportunity to understand the developmental effect of genes and, through neuroimaging techniques, to further characterize chemical and functional changes in the brain. The mechanisms by which the deletion of RAI1 and contiguous genes cause psychopathology remain unknown, but behavioral phenotyping in conjunction with a known gene deletion provides a solid starting point for future studies of gene-brain-behavior interactions.
Acknowledgments
This study was supported by the Intramural Research Programs of the National Institute of Mental Health and the National Human Genome Research Institute, NIH, USDHHS. The content of this publication does not necessarily reflect the views or policies of the DHHS, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The author's gratefully recognize PRISMS (Parents and Researchers Interested in Smith-Magenis syndrome) for partial sponsorship of a doctoral student fellowship (RM).
Biographies
Gonzalo Laje, M.D. M.H.Sc. is an Associate Clinical Investigator at the Intramural Research Program at the National Institute of Mental Health, NIH. He is board certified in General Psychiatry and holds a Master of Health Sciences in Clinical Research. His research interests include pharmacogenetics and psychiatric management of genetic disorders. He has been the recipient of multiple awards and he serves as member of the NIH SMS Research Team.
Rebecca S. Morse, M.A., is an applied developmental psychology doctoral student at George Mason University in Virginia. She has spent the past eight years at the NIH working with families of children and adults with Smith-Magenis syndrome. Her research interests include maladaptive and self-injurious behaviors, intellectual disabilities, family functioning, and issues of grief and bereavement.
Jonathan W. Ball and William Richter, both special volunteers in the Office of the Clinical Director, NHGRI/NIH worked with the SMS Research Team between 2007-2009. Mr. Richter received a B.S. in Biology and Society from Cornell University and worked for five years as a behavioral therapist with children with autism; he is entering his 2nd year of medical school at Georgetown University, Washington, DC. Jonathan Ball received a B.S. in Psychology from Towson University, Towson, MD and, after completing his pre-health post-baccalaureate studies at University Maryland, College Park, MD, he hopes to pursue a medical degree.
Maryland Pao, M.D., is the Clinical Director of the National Institute of Mental Health. She serves as Chief of the Psychiatry Consultation Liaison Service in the Hatfield Clinical Research Center at NIH and is the NIMH Clinical Fellowship Training Director. Board certified in Pediatrics, General Psychiatry, Child and Adolescent Psychiatry and Psychosomatic Medicine, her core research interests are in the complex interactions between somatic and psychiatric illnesses.
Ann C.M. Smith, M.A., D.Sc.(Hon), is a board certified genetic counselor in the Office of the Clinical Director, Division of Intramural Research of the National Human Genome Research Institute. In collaboration with an interdisciplinary team of intramural investigators at the NIH Hatfield Clinical Research Center, she is adjunct principal investigator of two protocols studying Smith-Magenis syndrome (SMS), a syndrome she described in the early 1980's. As a senior genetic counselor member of the NHGRI/NIH medical genetics consult service, she provides support to NIH investigators on issues related to medical genetics, genetic counseling, and molecular genetic testing.
Footnotes
Disclosures: The authors have no conflict of interest to report.
References
- Allen CW, Silove N, Williams K, Hutchins P. Validity of the social communication questionnaire in assessing risk of autism in preschool children with developmental problems. J Autism Dev Disord. 2007;37:1272–1278. doi: 10.1007/s10803-006-0279-7. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition-Text Revision. Washington: American Psychological Association; 2000. [Google Scholar]
- Constantino JN, Bruber CP. Social Responsiveness Scale (SRS) Manual. Los Angeles, CA: Western Psychological Services, Publishers; 2005. [Google Scholar]
- De Leersnyder H, Bresson JL, de Blois MC, Souberbielle JC, Mogenet A, Delhotal-Landes B, Salefranque F, Munnich A. Beta 1-adrenergic antagonists and melatonin reset the clock and restore sleep in a circadian disorder, Smith-Magenis syndrome. J Med Genet. 2003;40:74–78. doi: 10.1136/jmg.40.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leersnyder H, De Blois MC, Claustrat B, Romana S, Albrecht U, Von Kleist-Retzow JC, Delobel B, Viot G, Lyonnet S, Vekemans M, et al. Inversion of the circadian rhythm of melatonin in the Smith-Magenis syndrome. J Pediatr. 2001;139:111–116. doi: 10.1067/mpd.2001.115018. [DOI] [PubMed] [Google Scholar]
- Duncan W, Gropman A, Morse R, Krasnewich D, Smith ACM. Good Babies Sleeping Poorly: Insufficient Sleep in Infants with Smith-Magenis Syndrome (SMS) Am J Hum Genet. 2003;73(suppl):A896. [Google Scholar]
- Dykens EM, Finucane BM, Gayley C. Brief report: cognitive and behavioral profiles in persons with Smith-Magenis syndrome. J Autism Dev Disord. 1997;27:203–211. doi: 10.1023/a:1025800126086. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Smith AC. Distinctiveness and correlates of maladaptive behaviour in children and adolescents with Smith-Magenis syndrome. J Intellect Disabil Res. 1998;42(Pt 6):481–9. doi: 10.1046/j.1365-2788.1998.4260481.x. [DOI] [PubMed] [Google Scholar]
- Elsea SH, Girirajan S. Smith-Magenis syndrome. Eur J Hum Genet. 2008;16:412–421. doi: 10.1038/sj.ejhg.5202009. [DOI] [PubMed] [Google Scholar]
- Finucane B, Dirrigl KH, Simon EW. Characterization of self-injurious behaviors in children and adults with Smith-Magenis syndrome. Am J Ment Retard. 2001;106:52–58. doi: 10.1352/0895-8017(2001)106<0052:COSIBI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Finucane BM, Konar D, Haas-Givler B, Kurtz MB, Scott CI., Jr The spasmodic upper-body squeeze: a characteristic behavior in Smith-Magenis syndrome. Dev Med Child Neurol. 1994;36:78–83. doi: 10.1111/j.1469-8749.1994.tb11770.x. [DOI] [PubMed] [Google Scholar]
- Greenberg F, Guzzetta V, Montes de Oca-Luna R, Magenis RE, Smith AC, Richter SF, Kondo I, Dobyns WB, Patel PI, Lupski JR. Molecular analysis of the Smith-Magenis syndrome: a possible contiguous-gene syndrome associated with del(17)(p11.2) Am J Hum Genet. 1991;49:1207–1218. [PMC free article] [PubMed] [Google Scholar]
- Gropman AL, Duncan WC, Smith AC. Neurologic and developmental features of the Smith-Magenis syndrome (del 17p11.2) Pediatr Neurol. 2006;34:337–350. doi: 10.1016/j.pediatrneurol.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Johnson KP, Malow BA. Sleep in children with autism spectrum disorders. Curr Treat Options Neurol. 2008;10:350–359. doi: 10.1007/s11940-008-0038-5. [DOI] [PubMed] [Google Scholar]
- Martin SC, Wolters PL, Smith AC. Adaptive and maladaptive behavior in children with Smith-Magenis Syndrome. J Autism Dev Disord. 2006;36:541–552. doi: 10.1007/s10803-006-0093-2. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. The Social Communication Questionnaire (SCQ) Manual. Los Angeles, CA: Western Psychological Services, Publishers; 2003. [Google Scholar]
- Schopler E, Reichler RJ, DeVellis RF, Daly K. Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS) J Autism Dev Disord. 1980;10:91–103. doi: 10.1007/BF02408436. [DOI] [PubMed] [Google Scholar]
- Smith AC, Dykens E, Greenberg F. Behavioral phenotype of Smith-Magenis syndrome (del 17p11.2) Am J Med Genet. 1998a;81:179–185. doi: 10.1002/(sici)1096-8628(19980328)81:2<179::aid-ajmg10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Smith AC, Dykens E, Greenberg F. Sleep disturbance in Smith-Magenis syndrome (del 17 p11.2) Am J Med Genet. 1998b;81:186–191. [PubMed] [Google Scholar]
- Smith AC, McGavran L, Robinson J, Waldstein G, Macfarlane J, Zonona J, Reiss J, Lahr M, Allen L, Magenis E. Interstitial deletion of (17)(p11.2p11.2) in nine patients. Am J Med Genet. 1986;24:393–414. doi: 10.1002/ajmg.1320240303. [DOI] [PubMed] [Google Scholar]
- Smith ACM, Boyd K, Elsea SH, Finucane BM, Haas-Givler B, Gropman A, Johnson KP, Lupski JR, Magenis E, Potocki L, et al. Smith-Magenis Syndrome GeneReviews. 2010 http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=sms.
- Smith ACM, Gropman A. Smith-Magenis Syndrome. In: Cassidy SB, Allanson JE, editors. Management of Genetic Syndromes. 3rd. Hoboken NJ: Wiley-Liss; 2010. pp. 739–768. [Google Scholar]
- Smith ACM, McGavran L, Waldstein G, Robinson J. Deletion of the 17 short arm in two patients with facial clefts. Amer J Hum Genet. 1982;34:410A. [Google Scholar]
- Wirojanan J, Jacquemont S, Diaz R, Bacalman S, Anders TF, Hagerman RJ, Goodlin-Jones BL. The efficacy of melatonin for sleep problems in children with autism, fragile X syndrome, or autism and fragile X syndrome. J Clin Sleep Med. 2009;5:145–150. [PMC free article] [PubMed] [Google Scholar]
- Wolters PL, Gropman AL, Martin SC, Smith MR, Hildenbrand HL, Brewer CC, Smith AC. Neurodevelopment of children under 3 years of age with Smith-Magenis syndrome. Pediatr Neurol. 2009;41:250–258. doi: 10.1016/j.pediatrneurol.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright B, Sims D, Smart S, Alwazeer A, Alderson-Day B, Allgar V, Whitton C, Tomlinson H, Bennett S, Jardine J, et al. Melatonin Versus Placebo in Children with Autism Spectrum Conditions and Severe Sleep Problems Not Amenable to Behaviour Management Strategies: A Randomised Controlled Crossover Trial. J Autism Dev Disord. 2010 doi: 10.1007/s10803-010-1036-5. [DOI] [PubMed] [Google Scholar]