Abstract
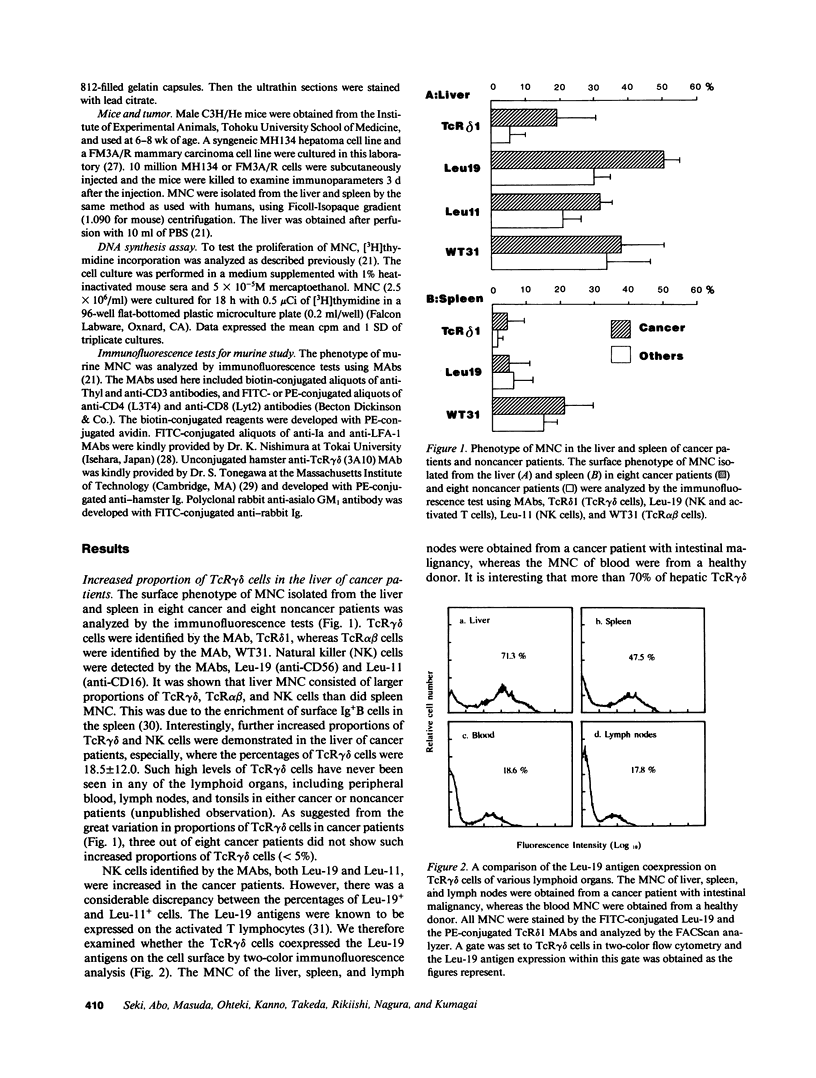
T cell receptor (TcR)gamma delta cells are known to be a minor population of T lymphocytes in the blood (less than 10%) and other peripheral lymphoid organs in healthy donors. We demonstrated here that a large proportion of TcR gamma delta cells, i.e., up to 30% of mononuclear cells (MNC) were detectable in the liver, but not other lymphoid organs of cancer patients. More importantly, the majority of such TcR gamma delta cells (greater than 70%) were shown to be lymphoblastic by electron microscopy. An activation marker of T lymphocytes, Leu-19 (CD56) was also highly expressed on the hepatic TcR gamma delta cells. The possibility of hepatic TcR gamma delta cells being activated was further examined in mice. C3H/He mice injected with syngeneic tumor cells were demonstrated to have an increased number of liver MNC; such MNC showed an ability to proliferate in vitro. These mice eventually had a considerable proportion of TcR gamma delta cells in the liver, showing activation markers, the Ia and LFA-1 antigens. These results suggest that the liver may be an important organ for activation and probably expansion of TcR gamma delta cells especially in tumor bearing hosts.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Miller C. A., Balch C. M. Characterization of human granular lymphocyte subpopulations expressing HNK-1 (Leu-7) and Leu-11 antigens in the blood and lymphoid tissues from fetuses, neonates and adults. Eur J Immunol. 1984 Jul;14(7):616–623. doi: 10.1002/eji.1830140707. [DOI] [PubMed] [Google Scholar]
- Abo T., Miller C. A., Gartland G. L., Balch C. M. Differentiation stages of human natural killer cells in lymphoid tissues from fetal to adult life. J Exp Med. 1983 Jan 1;157(1):273–284. doi: 10.1084/jem.157.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abo T., Sugawara S., Amenomori A., Itoh H., Rikiishi H., Moro I., Kumagai K. Selective phagocytosis of gram-positive bacteria and interleukin 1-like factor production by a subpopulation of large granular lymphocytes. J Immunol. 1986 May 1;136(9):3189–3197. [PubMed] [Google Scholar]
- Augustin A., Kubo R. T., Sim G. K. Resident pulmonary lymphocytes expressing the gamma/delta T-cell receptor. Nature. 1989 Jul 20;340(6230):239–241. doi: 10.1038/340239a0. [DOI] [PubMed] [Google Scholar]
- Bluestone J. A., Cron R. Q., Cotterman M., Houlden B. A., Matis L. A. Structure and specificity of T cell receptor gamma/delta on major histocompatibility complex antigen-specific CD3+, CD4-, CD8- T lymphocytes. J Exp Med. 1988 Nov 1;168(5):1899–1916. doi: 10.1084/jem.168.5.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneville M., Ito K., Krecko E. G., Itohara S., Kappes D., Ishida I., Kanagawa O., Janeway C. A., Murphy D. B., Tonegawa S. Recognition of a self major histocompatibility complex TL region product by gamma delta T-cell receptors. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5928–5932. doi: 10.1073/pnas.86.15.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneville M., Janeway C. A., Jr, Ito K., Haser W., Ishida I., Nakanishi N., Tonegawa S. Intestinal intraepithelial lymphocytes are a distinct set of gamma delta T cells. Nature. 1988 Dec 1;336(6198):479–481. doi: 10.1038/336479a0. [DOI] [PubMed] [Google Scholar]
- Borst J., Wicherink A., Van Dongen J. J., De Vries E., Comans-Bitter W. M., Wassenaar F., Van Den Elsen P. Non-random expression of T cell receptor gamma and delta variable gene segments in functional T lymphocyte clones from human peripheral blood. Eur J Immunol. 1989 Sep;19(9):1559–1568. doi: 10.1002/eji.1830190907. [DOI] [PubMed] [Google Scholar]
- Borst J., van de Griend R. J., van Oostveen J. W., Ang S. L., Melief C. J., Seidman J. G., Bolhuis R. L. A T-cell receptor gamma/CD3 complex found on cloned functional lymphocytes. Nature. 1987 Feb 19;325(6106):683–688. doi: 10.1038/325683a0. [DOI] [PubMed] [Google Scholar]
- Brenner M. B., Trowbridge I. S., McLean J., Strominger J. L. Identification of shared antigenic determinants of the putative human T lymphocyte antigen receptor. J Exp Med. 1984 Aug 1;160(2):541–551. doi: 10.1084/jem.160.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M. B., Trowbridge I. S., Strominger J. L. Cross-linking of human T cell receptor proteins: association between the T cell idiotype beta subunit and the T3 glycoprotein heavy subunit. Cell. 1985 Jan;40(1):183–190. doi: 10.1016/0092-8674(85)90321-6. [DOI] [PubMed] [Google Scholar]
- Bucy R. P., Chen C. L., Cihak J., Lösch U., Cooper M. D. Avian T cells expressing gamma delta receptors localize in the splenic sinusoids and the intestinal epithelium. J Immunol. 1988 Oct 1;141(7):2200–2205. [PubMed] [Google Scholar]
- Bucy R. P., Chen C. L., Cooper M. D. Tissue localization and CD8 accessory molecule expression of T gamma delta cells in humans. J Immunol. 1989 May 1;142(9):3045–3049. [PubMed] [Google Scholar]
- Crispe I. N., Moore M. W., Husmann L. A., Smith L., Bevan M. J., Shimonkevitz R. P. Differentiation potential of subsets of CD4-8- thymocytes. Nature. 1987 Sep 24;329(6137):336–339. doi: 10.1038/329336a0. [DOI] [PubMed] [Google Scholar]
- Falini B., Flenghi L., Pileri S., Pelicci P., Fagioli M., Martelli M. F., Moretta L., Ciccone E. Distribution of T cells bearing different forms of the T cell receptor gamma/delta in normal and pathological human tissues. J Immunol. 1989 Oct 15;143(8):2480–2488. [PubMed] [Google Scholar]
- Faure F., Jitsukawa S., Triebel F., Hercend T. Characterization of human peripheral lymphocytes expressing the CD3-gamma/delta complex with anti-receptor monoclonal antibodies. J Immunol. 1988 Nov 15;141(10):3357–3360. [PubMed] [Google Scholar]
- Ferrini S., Prigione I., Bottino C., Ciccone E., Tambussi G., Mammoliti S., Moretta L., Moretta A. Monoclonal antibodies which react with the T cell receptor gamma/delta recognize different subsets of CD3+WT31- T lymphocytes. Eur J Immunol. 1989 Jan;19(1):57–61. doi: 10.1002/eji.1830190110. [DOI] [PubMed] [Google Scholar]
- Goodman T., Lefrançois L. Expression of the gamma-delta T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 1988 Jun 30;333(6176):855–858. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- Groh V., Porcelli S., Fabbi M., Lanier L. L., Picker L. J., Anderson T., Warnke R. A., Bhan A. K., Strominger J. L., Brenner M. B. Human lymphocytes bearing T cell receptor gamma/delta are phenotypically diverse and evenly distributed throughout the lymphoid system. J Exp Med. 1989 Apr 1;169(4):1277–1294. doi: 10.1084/jem.169.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayday A. C., Saito H., Gillies S. D., Kranz D. M., Tanigawa G., Eisen H. N., Tonegawa S. Structure, organization, and somatic rearrangement of T cell gamma genes. Cell. 1985 Feb;40(2):259–269. doi: 10.1016/0092-8674(85)90140-0. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Singer K. H., Denning S. M., Martin M. E. Analysis of expression of CD2, CD3, and T cell antigen receptor molecules during early human fetal thymic development. J Immunol. 1988 Dec 1;141(11):3776–3784. [PubMed] [Google Scholar]
- Itoh H., Abo T., Sugawara S., Kanno A., Kumagai K. Age-related variation in the proportion and activity of murine liver natural killer cells and their cytotoxicity against regenerating hepatocytes. J Immunol. 1988 Jul 1;141(1):315–323. [PubMed] [Google Scholar]
- Kamps W. A., Timens W., De Boer G. J., Spanjer H. H., Poppema S. In situ study of haemopoiesis in human fetal liver. Scand J Immunol. 1989 Oct;30(4):399–408. doi: 10.1111/j.1365-3083.1989.tb02443.x. [DOI] [PubMed] [Google Scholar]
- Kaneda K. Liver-associated large granular lymphocytes: morphological and functional aspects. Arch Histol Cytol. 1989 Dec;52(5):447–459. doi: 10.1679/aohc.52.447. [DOI] [PubMed] [Google Scholar]
- Kishihara K., Yoshikai Y., Matsuzaki G., Mak T. W., Nomoto K. Functional alpha and beta T cell chain receptor messages can be detected in old but not in young athymic mice. Eur J Immunol. 1987 Apr;17(4):477–482. doi: 10.1002/eji.1830170407. [DOI] [PubMed] [Google Scholar]
- Koide J., Rivas A., Engleman E. G. Natural killer (NK)-like cytotoxic activity of allospecific T cell receptor-gamma,delta+ T cell clones. Distinct receptor-ligand interactions mediate NK-like and allospecific cytotoxicity. J Immunol. 1989 Jun 15;142(12):4161–4168. [PubMed] [Google Scholar]
- Koning F., Stingl G., Yokoyama W. M., Yamada H., Maloy W. L., Tschachler E., Shevach E. M., Coligan J. E. Identification of a T3-associated gamma delta T cell receptor on Thy-1+ dendritic epidermal Cell lines. Science. 1987 May 15;236(4803):834–837. doi: 10.1126/science.2883729. [DOI] [PubMed] [Google Scholar]
- Kuziel W. A., Takashima A., Bonyhadi M., Bergstresser P. R., Allison J. P., Tigelaar R. E., Tucker P. W. Regulation of T-cell receptor gamma-chain RNA expression in murine Thy-1+ dendritic epidermal cells. Nature. 1987 Jul 16;328(6127):263–266. doi: 10.1038/328263a0. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Civin C. I., Loken M. R., Phillips J. H. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986 Jun 15;136(12):4480–4486. [PubMed] [Google Scholar]
- Lanier L. L., Weiss A. Presence of Ti (WT31) negative T lymphocytes in normal blood and thymus. Nature. 1986 Nov 20;324(6094):268–270. doi: 10.1038/324268a0. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Fitzgerald K. A., Hussey R. E., Hodgdon J. C., Schlossman S. F., Reinherz E. L. Clonotypic structures involved in antigen-specific human T cell function. Relationship to the T3 molecular complex. J Exp Med. 1983 Feb 1;157(2):705–719. doi: 10.1084/jem.157.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T., Itoh T. Higher level expression of lymphocyte function-associated antigen-1 (LFA-1) on in vivo natural killer cells. Eur J Immunol. 1988 Dec;18(12):2077–2080. doi: 10.1002/eji.1830181231. [DOI] [PubMed] [Google Scholar]
- Pardoll D. M., Fowlkes B. J., Bluestone J. A., Kruisbeek A., Maloy W. L., Coligan J. E., Schwartz R. H. Differential expression of two distinct T-cell receptors during thymocyte development. Nature. 1987 Mar 5;326(6108):79–81. doi: 10.1038/326079a0. [DOI] [PubMed] [Google Scholar]
- Pardoll D. M., Fowlkes B. J., Lechler R. I., Germain R. N., Schwartz R. H. Early genetic events in T cell development analyzed by in situ hybridization. J Exp Med. 1987 Jun 1;165(6):1624–1638. doi: 10.1084/jem.165.6.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll D. M., Fowlkes B. J., Lew A. M., Maloy W. L., Weston M. A., Bluestone J. A., Schwartz R. H., Coligan J. E., Kruisbeek A. M. Thymus-dependent and thymus-independent developmental pathways for peripheral T cell receptor-gamma delta-bearing lymphocytes. J Immunol. 1988 Jun 15;140(12):4091–4096. [PubMed] [Google Scholar]
- Preffer F. I., Kim C. W., Fischer K. H., Sabga E. M., Kradin R. L., Colvin R. B. Identification of pre-T cells in human peripheral blood. Extrathymic differentiation of CD7+CD3- cells into CD3+ gamma/delta+ or alpha/beta+ T cells. J Exp Med. 1989 Jul 1;170(1):177–190. doi: 10.1084/jem.170.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulet D. H., Garman R. D., Saito H., Tonegawa S. Developmental regulation of T-cell receptor gene expression. Nature. 1985 Mar 7;314(6006):103–107. doi: 10.1038/314103a0. [DOI] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. Complete primary structure of a heterodimeric T-cell receptor deduced from cDNA sequences. 1984 Jun 28-Jul 4Nature. 309(5971):757–762. doi: 10.1038/309757a0. [DOI] [PubMed] [Google Scholar]
- Sawada H., Abo T., Sugawara S., Kumagai K. Prerequisite for the induction of lymphokine-activated killer cells from T lymphocytes. J Immunol. 1988 May 15;140(10):3668–3673. [PubMed] [Google Scholar]
- Sperling A. I., Wortis H. H. CD4-, CD8- gamma/delta cells from normal mice respond to a syngeneic B cell lymphoma and can induce its differentiation. Int Immunol. 1989;1(4):434–442. doi: 10.1093/intimm/1.4.434. [DOI] [PubMed] [Google Scholar]
- Tsuchiya Y., Igarashi M., Suzuki R., Kumagai K. Production of colony-stimulating factor by tumor cells and the factor-mediated induction of suppressor cells. J Immunol. 1988 Jul 15;141(2):699–708. [PubMed] [Google Scholar]
- Winoto A., Baltimore D. Separate lineages of T cells expressing the alpha beta and gamma delta receptors. Nature. 1989 Mar 30;338(6214):430–432. doi: 10.1038/338430a0. [DOI] [PubMed] [Google Scholar]
- Yachie A., Ueno Y., Takano N., Miyawaki T., Taniguchi N. Developmental changes of double-negative (CD3+ 4-8-) T cells in human peripheral blood. Clin Exp Immunol. 1989 May;76(2):258–261. [PMC free article] [PubMed] [Google Scholar]
- Yoshikai Y., Reis M. D., Mak T. W. Athymic mice express a high level of functional gamma-chain but greatly reduced levels of alpha- and beta-chain T-cell receptor messages. Nature. 1986 Dec 4;324(6096):482–485. doi: 10.1038/324482a0. [DOI] [PubMed] [Google Scholar]




