Summary
Tumor vasculature is derived from sprouting of local vessels (angiogenesis) and bone marrow (BM)-derived circulating cells (vasculogenesis). By using a model system of transplanting tumors into an irradiated normal tissue to prevent angiogenesis, we found that tumors were unable to grow in matrix metalloproteinase-9 (MMP-9) knockout mice, but tumor growth could be restored by transplantation of wild-type BM. Endothelial progenitor cells did not contribute significantly to this process. Rather, CD11b positive myelomonocytic cells from the transplanted BM were responsible for tumor growth and the development of immature blood vessels in MMP-9 knockout mice receiving wild-type BM. Our results suggest that MMP-9 could be an important target for adjunct therapy to enhance the response of tumors to radiotherapy.
Introduction
Tumor growth depends on the formation of new blood vessels for the supply of oxygen and nutrients through processes known as angiogenesis and vasculogenesis. Angiogenesis occurs primarily by endothelial migration and sprouting from preexisting blood vessels, while vasculogenesis involves the formation of blood vessels in situ by recruitment of precursor cells such as bone marrow (BM)-derived endothelial progenitor cells (EPCs) from the circulation. Although the contribution of EPCs to vasculogenesis has been demonstrated in several models including hind limb ischemia (Takahashi et al., 1999), vascular trauma (Gill et al., 2001), and tumor growth (Lyden et al., 2001), the extent to which EPCs are incorporated into newly formed blood vessels in tumors varies significantly with the tumor model (De Palma et al., 2003; Gothert et al., 2004; Lyden et al., 2001).
However, regardless of the different extent of the involvement of EPCs, investigators have commonly observed BM-derived vascular endothelial growth factor receptor-1 (VEGFR-1) positive myelomonocytic cells associated with the tumor vasculature (De Palma et al., 2003; Lyden et al., 2001). Believed to be derived from a common precursor cell known as the hemangioblast (Rafii et al., 2002), myelomonocytic cells share many characteristics with EPCs. Phenotypically, these cells share a number of surface markers with EPCs including platelet-endothelial cell adhesion molecule -1 (PECAM-1; CD31), Tie-2, endoglin, and integrate lectin and acetylated low density lipoprotein (Fujiyama et al., 2003; Rafii et al., 2002; Rohde et al., 2006). Functionally, BM-derived myelomonocytic cells have been shown to mimic EPCs in improving neovascularization in models of normal tissue injury (Capoccia et al., 2006; Fujiyama et al., 2003; Moldovan et al., 2000). These myelomonocytic cells are often observed in the perivascular regions of the endothelium (De Palma et al., 2003; Moldovan et al., 2000) or co-localized with endothelial cells (Bailey et al., 2006; Capoccia et al., 2006; Ruzinova et al., 2003) and have been shown to stabilize the tumor vasculature (Lyden et al., 2001). Moreover, when these cells are depleted either by using the Id1+/−Id3−/− mouse model where mobilization of VEGFR-1 and VEGFR-2 BM cells are genetically impaired (Lyden et al., 2001), or by using a suicide gene therapy approach (De Palma et al., 2003), tumor growth is markedly inhibited. Overall, these studies indicate that BM-derived myelomonocytic cells play an important role in tumor vasculogenesis.
The BM-derived myelomonocytic cells that infiltrate tumors and differentiate into macrophages are commonly referred to as tumor-associated macrophages (TAMs). Clinical evidence indicates that the presence of large numbers of TAMs correlates with poor prognosis in cancers (Pollard, 2004). TAMs are a polarized population of macrophages (Sica et al., 2006) and release many angiogenic factors including vascular endothelial growth factor (VEGF), interleukin-8, tumor necrosis factor-α, and matrix metalloproteinase-9 (MMP-9) (Dirkx et al., 2006; Lewis and Pollard, 2006; Yang et al., 2004).
MMP-9 is a member of a family of zinc containing endopeptidases that is involved in degradation of extracellular matrix (ECM) and in vascular remodeling (Heissig et al., 2003). As other members of the MMP family, MMP-9 is synthesized as an inactive zymogen (pro-MMP-9) that is activated by proteolysis or autolysis (Bergers and Coussens, 2000; Galis and Khatri, 2002). MMP-9 is involved in mobilizing EPCs and other progenitor cells from the BM niche (Heissig et al., 2002), liberating growth factors including VEGF (Bergers et al., 2000) and transforming growth factor-β (Yu and Stamenkovic, 2000) from the matrix-bound forms, and recruiting the BM-derived leukocytes to the tumor vasculature (Jodele et al., 2005). MMP-9 provided by BM-derived cells has been shown to initiate the angiogenic switch leading to tumor growth and progression in K14-HPV16 epithelial squamous carcinoma in mice (Coussens et al., 2000; Giraudo et al., 2004).
Radiotherapy is one of the most important treatment modalities for cancer. However, many patients treated with radiotherapy relapse in the irradiated field (Liang et al., 1991). It is unclear how this occurs because even though all of the tumor cells may not be killed by radiation, it is unlikely that a sufficient number of endothelial cells to allow for subsequent tumor growth could survive the large doses delivered in radiotherapy (Itasaka et al., 2007; Tsai et al., 2005). One possibility to account for this is that a subset of BM-derived cells could enter the irradiated tumor and restore the vasculature by vasculogenesis. EPCs have recently been shown to rescue tumor growth following treatment by vascular disrupting agents (Shaked et al., 2006), but it remains to be determined whether this could also apply following irradiation.
In the present study we investigate the role of the BM-derived CD11b positive (CD11b+) myelomonocytic cells expressing MMP-9 in the growth of tumors that are irradiated or transplanted into a tissue that has been irradiated prior to transplantation. Tumors grown in previously irradiated tissues generally show an increased latency period and a reduced growth rate because of impaired neovascularization resulted from radiation-induced injury to the host vasculature and connective tissue (a phenomenon known as the ‘tumor bed effect’) (Milas et al., 1986). Such tumors also have an increased metastatic potential, reduced blood perfusion and oxygen tension, and resistance to treatments such as ionizing radiation and cytotoxic agents, thereby mimicking human primary tumors that recur following radiotherapy (Rofstad et al., 2005). We therefore hypothesized that the vasculature of tumors grown in previously irradiated tissues would derive from cells circulating in the blood stream and in particular the BM. We show that tumors did not grow in pre-irradiated subcutaneous tissues of MMP-9 knockout (KO) mice and that MMP-9 expressing CD11b+ myelomonocytic cells in the tumors from transplanted BM cells restored tumor growth in these mice. We further provide evidence that MMP-9 expressing BM-derived myelomonocytic cells are essential to the process of vasculogenesis and could be an important target for adjunct therapy to high dose radiotherapy.
Results
BM-derived cells infiltrating tumors are not EPCs
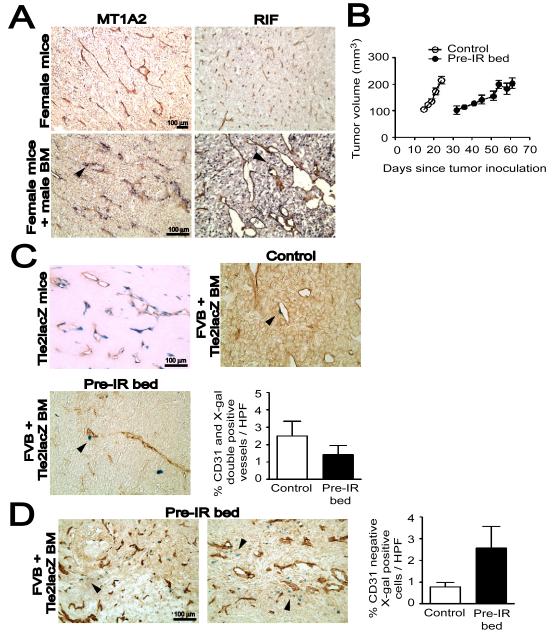
We examined the BM-derived infiltrates in MT1A2 mouse mammary carcinoma in FVB mice or radiation-induced fibrosarcoma (RIF) in C3H mice by in situ hybridization for a Y-specific DNA probe in female mice that had received syngeneic male BM cells. Consistent with the fact that both the MT1A2 (Guy et al., 1992) and RIF (see Experimental Procedures) tumors are of female origin, we found no staining for the Y-probe in these tumors grown in female mice (Figure 1A). However, when grown in female mice transplanted with male BM we saw significant infiltration of male-derived cells that was different for the two tumors: MT1A2 tumors showed BM-derived infiltrates mainly in perivascular regions, whereas RIF tumors showed a more diffusely distributed pattern (Figure 1A).
Figure 1. BM-derived EPCs contribute minimally to tumor vasculogenesis.
A: MT1A2 and RIF tumors grown in female mice (upper panel) or in female mice that had received syngeneic male BM cells (lower panel). The tumors were stained for CD31 (brown) and Y-DNA probe by in situ hybridization (dark blue). Arrow heads (black) in the lower panel indicate cells positively stained for the Y-probe. B: Growth kinetics of MT1A2 tumors grown in non-irradiated (control; open symbols) or pre-irradiated (pre-IR bed; filled symbols) subcutaneous tissues of FVB mice. C: MT1A2 tumors grown in Tie2lacZ transgenic mice (Tie2lacZ mice) or in FVB mice that had received BM cells from Tie2lacZ mice (FVB + Tie2lacZ BM). Tumors in FVB + Tie2lacZ BM were grown in non-irradiated (control) or pre-irradiated (pre-IR bed) tissues. Vessels were stained with CD31 (brown) and X-gal (blue). Arrow heads (black) indicate some of the BM-derived LacZ positive cells. The graph shows quantification of CD31 and X-gal double positive vessels cells as a percentage of the total CD31 positive cells in high power field (HPF). The difference between control and pre-IR bed was not statistically significant (P > 0.05). D: A significant number of X-gal positive (blue) but CD31 (brown) negative cells were observed in the tumors grown in the pre-irradiated tissues (pre-IR bed) of FVB + Tie2lacZ BM (indicated with black arrow heads). Quantification of X-gal positive but CD31 negative cells counted per HPF is shown on the right. The data was not however significantly different between control and pre-IR bed (P > 0.05). Symbols and error bars in B, C, and D are the mean ± SEM for n ≥ 5 mice per group.
Because the dose of irradiation (IR) used in this study (20 Gy) would be expected to sterilize essentially all of the endothelial cells in the irradiated tissue prior to tumor transplantation and hence abrogate local angiogenesis (Udagawa et al., 2007), we hypothesized that the BM-derived infiltrates may be EPCs and that the tumors grown in the irradiated bed might incorporate more EPCs from the BM to establish the tumor vessels by vasculogenesis. First, we examined whether 20 Gy of IR to subcutaneous tissues of the lower back of the mice would cause ulceration to the skin. We found that there was no difference in the histology of the skin between irradiated and non-irradiated mice (Figure S1A) and that there were infiltrating cells present only when the tumor was implanted on the irradiated skin (Figure S1B). We therefore quantified the BM-derived EPCs in MT1A2 tumors grown in the pre-irradiated tissues (‘pre-IR bed’) of mice that had received BM cells from Tie2lacZ transgenic mice, which express the lacZ reporter gene driven by the endothelial receptor tyrosine kinase promoter Tie2 (Schlaeger et al., 1997). EPCs were detected by double label immunostaining for X-gal and CD31. The growth kinetics showed that tumors grew significantly slower in pre-irradiated tissues (Figures 1B and S3), as reported by others (Milas et al., 1986; Rofstad et al., 2005). We confirmed that Tie2 marks endothelial cells in MT1A2 tumors by growing the tumors in Tie2lacZ transgenic mice (Figure 1C). However, when grown in mice that had received BM cells from Tie2lacZ mice, the tumors showed only rare incorporation of EPCs in the vessels (Figure 1C). Tumors grown in pre-irradiated tissues of these BM transplanted mice also showed very low levels of EPCs in the tumor vasculature (Figure 1C), and the proportion of CD31 and X-gal double positive tumor vessels did not differ significantly between control tumors without IR and tumors grown in pre-irradiated tissues (Figure 1C). However, in the tumors grown in pre-irradiated tissues we observed a significant number of Tie2 expressing cells in stromal regions of the tumors that were not positive for CD31 staining (Figure 1D).
To determine if the minimal incorporation of BM-derived EPCs in the tumors was a general phenomenon, we examined a number of different tumor models: TG1-1 mouse mammary carcinoma or 6780 lymphoma in FVB mice with BM cells from Tie2lacZ mice; and the Lewis lung carcinoma (LLC) or B16F1 melanoma in C57Bl/6 with BM cells from either Tie2GFP or Rosa26 transgenic mice. Double label immunostaining for BM-derived EPCs again showed that there was only rare incorporation of EPCs in the tumor vasculature of all control tumors and tumors grown in pre-irradiated tissues (Figure S2).
Overall the results suggest that BM-derived EPCs contribute only to a small extent to tumor blood vessels and that they are not a major component of the BM-derived infiltrates in the tumors used in this study.
CD11b+ myelomonocytic cells account for most of the BM-derived infiltrates in the tumors and are increased by IR
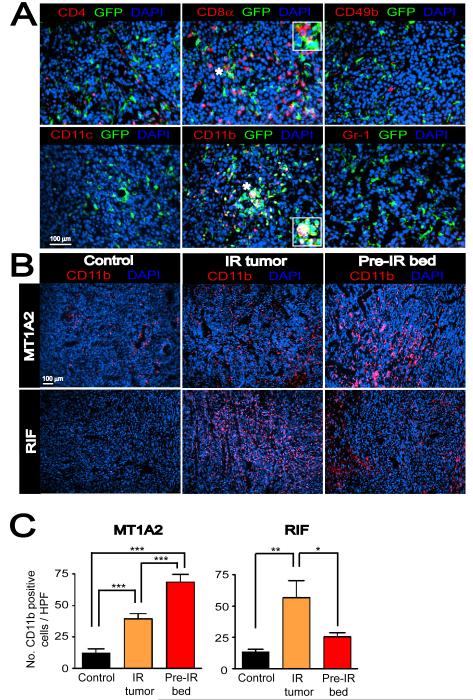
To ascertain the nature of the BM-derived infiltrates in the tumors, we examined MT1A2 tumors grown in pre-IRradiated tissues of mice that had received BM cells from mice ubiquitously expressing green fluorescent protein (GFP). The GFP-expressing BM-derived infiltrates in the tumors were examined for markers of inflammatory cells including cytotoxic T-cells (CD8α), helper T-cells (CD4), natural killer cells (CD49b), monocytes/macrophages (CD11b), dendritic cells (CD11c), and granulocytes/neutrophils (Gr-1). Double label immunofluorescent staining showed that a significant number of GFP-expressing cells co-localized with CD11b (Figure 2A). Although there were some CD4 or CD8α positive cells detected in the tumors, they did not co-localized with GFP (Figure 2A), indicating that these cells are not derived from the BM but possibly from the thymus or spleen. We did not observe any detectable numbers of CD11c, Gr-1, or CD49b positive cells in the tumors that were also positive for GFP (Figure 2A).
Figure 2. BM-derived CD11b+ myelomonocytic cells are recruited to the tumors with IR.
A: Immunostaining of MT1A2 tumors grown in the pre-irradiated tissues of FVB mice that had received BM cells from mice ubiquitously expressing GFP. Tumor sections were stained for CD4 (red), CD8α (red), CD49b (red), CD11c (red), CD11b (red), or Gr-1 (red), and anti-GFP (green), counterstained with DAPI (blue). Co-localization of red, green, and blue is shown in white. Insets in the middle panels show magnified regions of the microphotograph where indicated with asterisks (*). B: Immunostaining for CD11b (red) in tumors with no IR (control), irradiated tumors (IR tumor), or tumors grown in the irradiated bed (pre-IR bed) for MT1A2 (upper panel) and RIF (lower panel). Nucleus staining with DAPI is shown in blue. C: Quantification of CD11b+ myelomonocytic cells in B for MT1A2 (left) and RIF (right) tumors. Symbols and error bars represent the mean ± SEM for n ≥ 4 animals per group. *, **, and *** indicates P values of < 0.05, < 0.01, and < 0.001, respectively, determined by one-way ANOVA.
We next determined how the BM-derived CD11b+ myelomonocytic cells in the tumors were affected by IR. Tumors were grown either without IR (control) or in pre-irradiated tissues (pre-IR bed) as previously. We also tested a clinically relevant IR model by delivering a single dose of IR (20 Gy) to already established tumors at approximately 200 mm3 and allowing the tumors to re-grow beyond the volume at which they were irradiated (IR tumor). Immunostaining showed that both IR tumors and tumors grown in pre-irradiated tissues had significantly more CD11b+ cells compared to control MT1A2 tumors of the same size (Figures 2B and 2C). In RIF tumors, we also observed more CD11b+ cells in the IR tumors although tumors grown in pre-irradiated tissues showed similar numbers of CD11b+ cells to controls (Figures 2B and 2C). The latter was because there were only small proportions of viable cells present in RIF tumors grown pre-irradiated tissues.
CD11b+ myelomonocytic cells express MMP-9
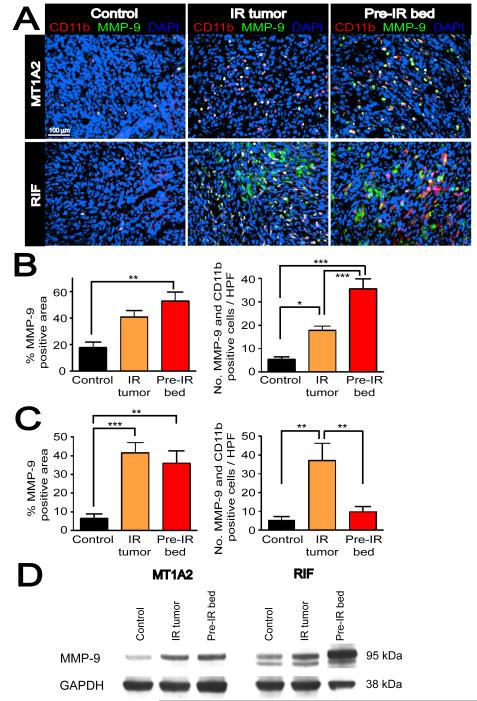
Because of the known role of monocytes/macrophages in promoting tumor angiogenesis (Coussens et al., 2000; Giraudo et al., 2004; Lin et al., 2006) we hypothesized that CD11b+ myelomonocytic cells contribute to the vasculogenesis of the tumors grown in the irradiated bed by remodeling the ECM. We tested this hypothesis by examining the expression of MMP-9, one of the key players in remodeling ECM, in CD11b+ cells using double label immunofluorescent staining. Histological examination showed that most of the CD11b+ cells were also MMP-9 positive in MT1A2 tumors (Figure 3A). Moreover, irradiated tumors (IR tumors) and tumors grown in the irradiated bed (pre-IR bed) showed an increase in MMP-9 positive areas and in the numbers of CD11b and MMP-9 double positive cells (Figure 3B).
Figure 3. CD11b+ myelomonocytic cells express MMP-9 in the tumors.
A: Double label immunofluorescent staining for CD11b (red) and MMP-9 (green) in MT1A2 (upper panel) and RIF (lower panel) tumors with no IR (control), irradiated tumors (IR tumor), or grown in pre-irradiated tissues (pre-IR bed). Co localization of CD11b and MMP-9 is shown in white with DAPI counterstaining. B: Quantification of MMP-9 positive area determined by the point-count method (left) and the number of CD11b and MMP-9 double positive cells in non necrotic regions (right) of MT1A2 tumors. C: RIF tumors as in B. Symbols and error bars in B and C are mean ± SEM for n ≥ 4 animals per group. *, **, and *** denote for P values < 0.05, < 0.01, < 0.001, respectively, by one-way ANOVA. D: Western blot for MMP-9 in MT1A2 (left panel) and RIF (right panel) tumors. GAPDH was used as the loading control.
In RIF tumors, we similarly observed an increase in MMP-9 positive areas in ‘IR tumors’ and ‘pre-IR bed’, and increased numbers of CD11b and MMP-9 positive cells in ‘IR tumors’ (Figure 3C). However, we also observed numerous CD11b negative tumor cells that strongly expressed MMP-9 (Figures 3A and S4A), suggesting that RIF tumor cells themselves are a significant source of MMP-9.
To confirm the histological observations of MMP-9 expression, we performed immunoblots with tumor lysates. We found that IR, either of the tumors directly (‘IR tumors’) or of the transplantation site (‘pre-IR bed’) increased the expression of MMP-9 in both the MT1A2 and RIF tumors (Figure 3D), consistent with the histological observations. The higher expression of MMP-9 in RIF tumors especially from ‘pre-IR bed’ compared to that in MT1A2 tumors is likely due to the strong expression of MMP-9 by the RIF tumor cells.
Genetic depletion of MMP-9 abrogates tumor vasculogenesis
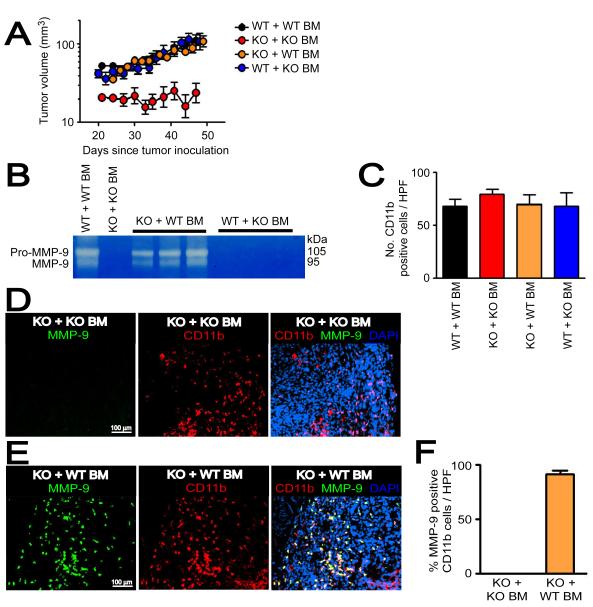
To address the role of MMP-9 in vasculogenesis, we used MMP-9 knockout (KO) mice with tumors grown in pre-irradiated tissues to inhibit angiogenesis. We selected MT1A2 tumors over RIF tumors because MT1A2 tumors are syngeneic to MMP-9 KO and wild type (WT) mice, and the source of MMP-9 in the tumors is largely restricted to CD11b+ myelomonocytic cells allowing us to investigate the role of the BM-derived CD11b+ myelomonocytic cells in vasculogenesis. We first examined the growth of MT1A2 tumors in MMP-9 KO and WT mice, and found that the tumors grew progressively in the MMP-9 KO mice although a little slower than in the WT mice (Figure S5A). However, tumor growth in pre-irradiated tissues was severely impaired in MMP-9 KO mice (Figure S5B) or in MMP-9 KO mice that had received BM cells from MMP-9 KO mice (MMP-9 KO mice + KO BM) (Figure 4A) compared to their WT counterparts (Figures S5B and 4A).
Figure 4. Functional BM cells restore tumor growth in pre-irradiated tissues of MMP-9 KO mice.
A: MT1A2 tumor growth in pre-irradiated tissues of WT mice that had received the BM cells from WT mice (WT + WT BM), MMP-9 KO mice receiving BM cells from MMP-9 KO mice (KO + KO BM), MMP-9 KO mice receiving BM cells from WT mice (KO + WT BM), or WT mice that had received the BM cells from MMP-9 KO mice (WT + KO BM). Note that tumor growth was severely impaired in KO + KO BM, and was restored in KO + WT BM. B: Zymography was used to determine the efficiency of the BM reconstitution. Whole BM cells of the recipients were isolated and analyzed for MMP-9 activity at ≥ 4 weeks post BM transplantation. Each lane represents one mouse. C: Quantification of CD11b+ myelomonocytic cells in HPF from tumors in A. D: Immunostaining of the tumors from KO + KO BM for CD11b myelomonocytic cells (red) and MMP-9 (green), counterstained with DAPI (blue). E: Immunostaining of the tumors from KO + WT BM as in D. F: Quantification of MMP-9 and CD11b double positive cells in tumors from KO + KO BM or KO+ WT BM. Symbols in A, C, and F are the mean ± SEM for n ≥ 5 mice per group.
We next determined whether functional BM could restore tumor growth in pre-irradiated tissues of MMP-9 KO mice by transplanting BM cells from WT mice into MMP-9 KO mice (MMP-9 KO mice + WT BM). The efficiency of BM reconstitution was confirmed by zymography at the time of tumor implantation (≥ 4 weeks post-BM transplantation), which showed that the activity of MMP-9 in the BM could be restored in MMP-9 KO mice or lost in WT mice by transplanting BM cells (Figure 4B). Reconstitution of the BM in MMP-9 KO mice with WT BM completely restored the growth of the tumors in the pre-irradiated site to that of WT mice (Figure 4A). However, WT mice receiving BM cells from MMP-9 KO mice (WT mice + KO BM) showed no difference in tumor growth in pre-irradiated tissues compared to WT mice + WT BM (Figure 4A), indicating that non-BM-derived cells can compensate for the lack of MMP-9 in BM cells. We examined CD11b+ myelomonocytic cells in the tumors of all 4 groups and found that there was no significant difference in the number of CD11b+ cells in the tumors from the different groups (Figure 4C). When examined for MMP-9 in tumors grown in pre-irradiated tissues of MMP-9 KO mice + WT BM, we found that the majority of MMP-9 positive cells (91 ± 3 %) were positive for CD11b (Figures 4E and 4F), while there were no MMP-9 positive CD11b myelomonocytic cells in those tumors from MMP-9 KO mice + KO BM (Figures 4D and 4F) as expected, or from WT mice + KO BM (Figure 6D). In the tumors from WT mice + KO BM, some MMP-9 positive areas were observed with morphology of smooth muscle- or fibroblast-like cells (Figure S5C). Overall, the results suggest that CD11b+ myelomonocytic cells are the main source of MMP-9 and that these cells can restore tumor growth in irradiated tissues of MMP-9 KO mice.
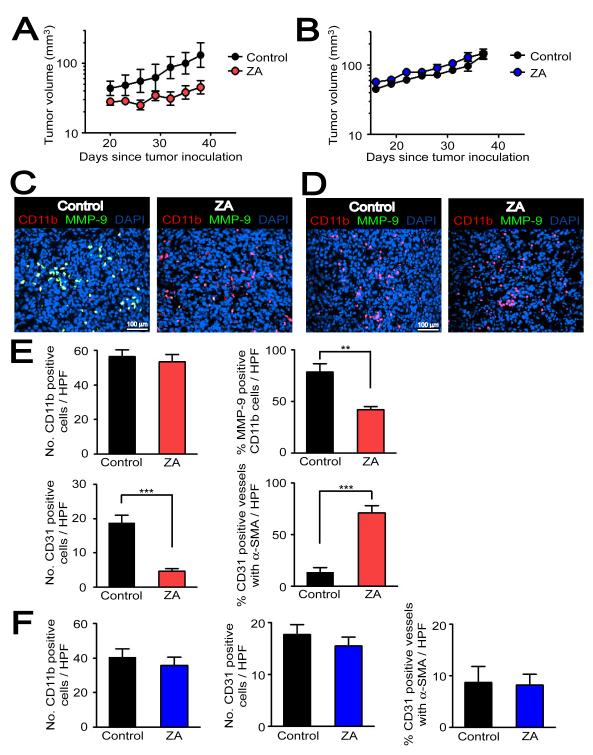
Figure 6. Pharmacological targeting of MMP-9 in CD11b+ myelomonocytic cells by ZA inhibits tumor growth in the pre-irradiated site.
A: MT1A2 tumor growth with (ZA, n = 5) or without ZA (control, n = 4) in pre-irradiated tissues of MMP-9 KO mice that had received BM cells from WT mice. B: MT1A2 tumor growth in pre-irradiated tissues of WT mice receiving BM cells from MMP-9 KO mice. n = 5 animals per group. Symbols and error bars in A and B are the mean ± SEM. C: Immunostaining of the tumors from A for MMP-9 (green), CD11b (red), and nuclei (blue). D: Immunostaining of the tumors from B as in C. E: Quantification of CD11b+ cells (upper left), percentage of MMP-9 expressing CD11b+ cells (upper right), CD31 positive endothelial cells (lower left), and CD31 positive vessels that were associated with α-SMA (lower right) in the tumors from A. F: Quantification of the parameters as in E but from tumors in B. Error bars in E and F are SEM. ** and *** denote for P-values < 0.01 and < 0.001, respectively, determined by two-tailed Student’s t-test.
To determine whether the restored tumor growth by functional BM was associated with vasculogenesis, we first tested a possibility that CD11b+ myelomonocytic cells differentiate into endothelial cells in tumors. By immunostaining, we observed in tumors of all 4 groups that CD11b+ cells did not co-localize with CD31 (Figure S5E), suggesting that CD11b+ myelomonocytic cells did not differentiate to the tumor endothelium. This also indicates that CD11b+ myelomonocytic cells indirectly contributed to vasculogenesis. We examined vessel density and maturity in the tumors, determined by the number of CD31 positive vessels and the proportion of CD31 labeled endothelial cells surrounded by pericytes, identified by α-smooth muscle actin (α-SMA) immunostaining. Without IR, most of the vessels in tumors grown in WT (87 ± 2 %) or MMP-9 KO (87 ± 2 %) mice were associated with α-SMA (Figures 5A and 5B). In sharp contrast, the vasculature of the tumor growing in the pre-irradiated site of WT + WT BM was sparsely covered by α-SMA (Figure 5C). This is consistent with our hypothesis of a different etiology of the vessels in the two situations: in the unirradiated tissue most of the vessels arise from angiogenesis (local sprouting), whereas in pre-irradiated bed they can only arise from vasculogenesis (circulating cells). On the other hand, the majority of the vessels in the very small (and non-growing) tumors in the irradiated site of MMP-9 KO mice + KO BM had a mature appearance with extensive α-SMA coverage (Figure 5C). Moreover, while there was little or no difference in the density and maturity of the CD31 positive endothelial cells between the WT and MMP-9 KO mice without IR (Figure 5B), the vessel density was significantly lower in the very small non-growing tumors growing in the irradiated site of the MMP-9 KO mice and this was restored to WT levels by transplantation of WT BM cells (Figure 5D).
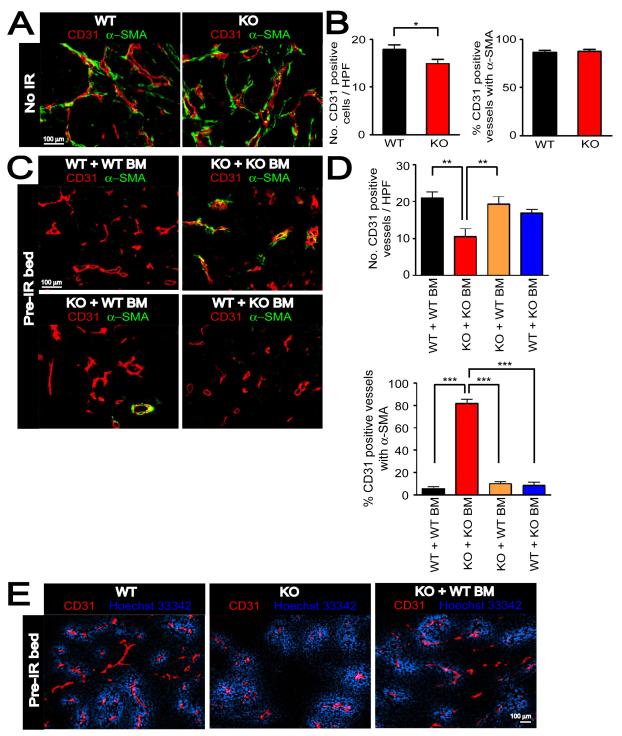
Figure 5. Immature vessels are developed in MMP-9 KO mice + WT BM to support tumor growth in pre-irradiated tissues.
A: Immunostaining of CD31 (red) and α-SMA (green) in MT1A2 tumors grown in the wild-type (WT) or MMP-9 KO (KO) mice. B: Number of CD31 positive vessels per HPF (left) and proportions of CD31 positive vessels associated with α-SMA (right) in tumors from A. C: Immunostaining of CD31 (red) and α-SMA (green) for tumors from Figure 4A. Group names are as in Figure 4A. D: Quantification of CD31 positive vessels per HPF (upper panel) and proportions of CD31 positive vessels associated with α-SMA (lower panel) in the tumors from C. Symbols in B and D are the mean ± SEM for n ≥ 5 animals per group. *, **, and *** denote for P-values < 0.05, < 0.01, < 0.001, respectively, determined by one-way ANOVA. E: Functional blood vessels of tumors grown in pre-irradiated tissues of WT, KO, or KO + WT BM examined by intravenous infusion with Hoechst 33342 (blue). Tumor sections were stained with CD31 (red).
We further examined whether the impaired tumor growth in pre-irradiated tissues of MMP-9 KO mice was due to an impairment in the function of the tumor vessels. To investigate this we intravenously injected Hoechst 33342 into WT mice, MMP-9 KO mice, or MMP-9 KO mice + WT BM, all of which had tumors implanted in pre-irradiated tissues. We found that the vessels were well-perfused in the tumors grown in pre-irradiated tissues of MMP-9 KO mice and that there was no difference in functionality of vessels in the tumors between WT mice, MMP-9 KO mice, or MMP-9 KO mice + WT BM (Figure 5E).
Taken together these data suggest that the failure of the tumors to grow in the pre-irradiated site of MMP-9 KO mice was the result of abrogation of both angiogenesis (by irradiation) and of vasculogenesis (by lack of MMP-9 in BM-derived cells), and that the blood vessels within the tumors (Figure 5) and around the periphery (Figure S5D) of the small tumors in the pre-irradiated tissue of MMP-9 KO mice were mature and well-perfused normal vessels, probably co-opted by growth of the tumor into surrounding normal tissue. Overall, it suggests that restoration of tumor growth in the irradiated tissue by WT BM allowed immature vessels to form by MMP-9 expressing CD11b+ myelomonocytic cells that arose from the transplanted BM.
Pharmacological inhibition of CD11b+ myelomonocytic cells expressing MMP-9 significantly reduces tumor growth in pre-irradiated tissues
Based on the above results, we hypothesized that selective inhibition of CD11b+ myelomonocytic cells expressing MMP-9 would inhibit BM-derived vasculogenesis and tumor growth in pre-irradiated tissues. To test this, we used the aminobisphosphonate zolendronic acid (ZA, Zometa®), a clinically available agent to ameliorate bone metastases and recently reported to selectively target MMP-9 expressing macrophages in K14-HPV16 cervical carcinoma in mice (Giraudo et al., 2004). MT1A2 tumors were grown in the pre-irradiated tissues in MMP-9 KO mice that had received BM cells from WT mice (MMP-9 KO mice + WT BM) as previously described. Hence tumors grown in these mice, the major source of MMP-9 would come from the BM-derived CD11b+ myelomonocytic cells. In order to determine the specific activity of ZA in targeting MMP-9 from BM-derived cells but not from other tissues, we also tested ZA in WT mice receiving BM cells from MMP-9 KO mice (WT mice + KO BM). Because WT mice + KO BM do not have MMP-9 expressing CD11b+ myelomonocytic cells, the tumor growth in pre-irradiated tissues of these mice should not be affected by the ZA treatment. Treatment with ZA at 100 μg/kg intraperitoneally once per day for up to 6 weeks produced a significant inhibition of tumor growth in the irradiated site of MMP-9 KO mice + WT BM (Figure 6A). In contrast, there was no effect of ZA on tumor growth of WT mice + KO BM (Figure 6B), as predicted. Histological examinations showed that ZA-treated tumors in MMP-9 KO mice + WT BM had similar numbers of CD11b+ myelomonocytic cells but a smaller fraction that were MMP-9 positive (Figures 6C and 6E). In addition, ZA-treated tumors showed significantly fewer numbers of CD31 positive vessels than the control tumors (Figure 6E). When examined for vessel maturity, ZA-treated tumors had significantly more vessels associated with α-SMA than the control tumors (Figure 6E) in agreement with the data obtained with MMP-9 KO mice + KO BM (Figure 5). In WT mice + KO BM, we did not observe any CD11b+ myelomonocytic cells that were MMP-9 positive (Figure 6D), and there was no significant difference in the number of CD11b+ cells between ZA treated and control tumors (Figure 6F). As found earlier, the percentage of CD31 positive tumor vessels and those associated with α-SMA was low in the WT mice + KO BM and this was not affected by ZA (Figure 6F). In summary, ZA efficiently targeted MMP-9 expressed in the BM-derived CD11b+ myelomonocytic cells in the tumor, inhibited the growth of tumors in the pre-irradiated tissues and reduced the numbers of immature (tumor like) vessels in the tumors.
Conditional ablation of CD11b+ myelomonocytic cells further abrogates tumor growth in pre-irradiated tissues of MMP-9 KO mice + functional BM cells
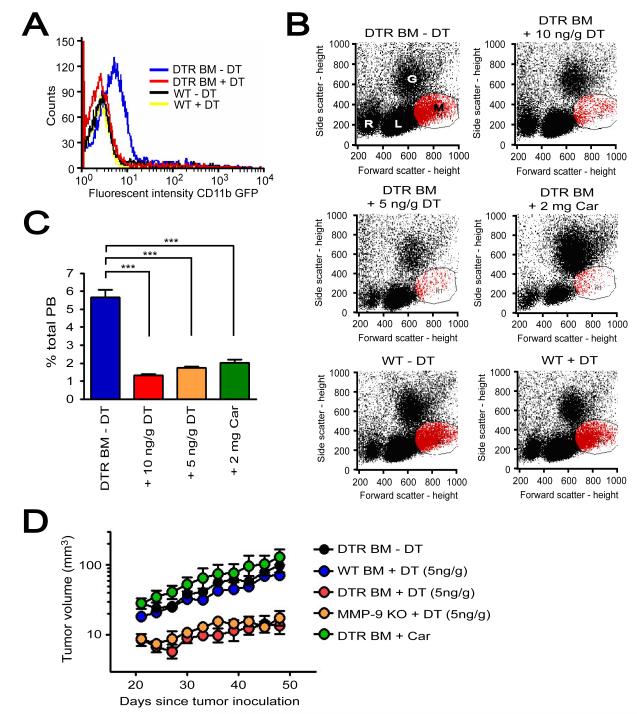
We further examined the role of CD11b+ myelomonocytic cells in restoring tumor growth in pre-irradiated tissues by conditionally ablating CD11b+ cells. To do this, we transplanted BM cells from transgenic mice expressing human diphtheria toxin receptor (DTR) and GFP driven by the CD11b promoter (Stoneman et al., 2007) into lethally irradiated MMP-9 KO mice (MMP-9 KO mice + DTR BM). Treatment by diphtheria toxin (DT, 10 ng/g) of MMP-9 KO mice + DTR BM effectively depleted GFP positive CD11b+ cells in the peripheral blood at 24 hr post-administration (Figure 7). In contrast, administration of DT in WT mice showed no depletion of CD11b+ myelomonocytic cells (Figures 7A and 7B). However, repeated administration of DT 10 ng/g once in every two days in MMP-9 KO mice + DTR BM resulted in mortality in 20 days (Figure S6A). We therefore lowered the dose of DT to 5 ng/g once every two days and still observed effective depletion of CD11b+ myelomonocytic populations in the peripheral blood of MMP-9 KO mice + DTR BM (Figures 7B and 7C). The depleted CD11b+ cells rebounded to untreated levels at 48 hr (data not shown). We also tested another approach to deplete CD11b+ cells by using carrageenan, a compound that eliminates macrophages (Li et al., 2007). Although carrageenan at 2 mg/mouse showed effective depletion of CD11b+ myelomonocytic cells at 24 hr (Figures 7B and 7C) it also resulted in a significant expansion of granulocyte populations (Figure 7B). Moreover, carrageenan treated mice showed significant body weight loss and some early mortality (Figures S6A and S6B). The dose of carrageenan was therefore lowered to 1 mg/mouse but once per week treatment was ineffective in maintaining low levels of CD11b+ myelomonocytic cells (data not shown).
Figure 7. Depletion of CD11b+ cells expressing MMP-9 by DT further abrogates tumor growth in pre-irradiated tissues of MMP-9 KO + DTR BM.
A: Peripheral blood of MMP-9 KO mice that had received BM cells from transgenic mice expressing diphtheria toxin receptor (DTR) and GFP driven by CD11b promoter (DTR BM) or of WT mice without BM transplantation (WT). These mice were treated with or without diphtheria toxin (DT, 10 ng/g). Blood was analyzed for GFP fluorescent intensity as an indicator of DTR levels at 24 hr post treatment. Note that treatment with DT 10 ng/g in DTR BM mice resulted in GFP signal similar to that in WT mice with or without DT. B: Peripheral blood of MMP-9 KO mice + DTR BM (DTR BM) treated without DT (-DT), with 10 ng/g DT, 5 ng/g DT, or 2 mg/g carrageenan (Car) at 24 hr post treatment. Blood of WT mice (WT) with and without DT 10 ng/g is also shown. Abbreviations are: R, red blood cells; L, lymphocytes; G, granulocytes; M, monocytes. C: Quantification of monocytes gated in red from B. Symbols and error bars indicate the mean ± SEM for n ≥ 5 mice per group. *** indicates P < 0.001 analyzed by one way ANOVA. D: MT1A2 tumor growth in pre-irradiated tissues of MMP-9 KO mice + DTR BM (DTR BM) without DT (−DT, n = 4), with DT (5 ng/g, n = 5), or with Car (n = 4). Tumors were also grown in pre-irradiated tissues of WT mice receiving BM cells from WT mice (WT BM, n = 5) or MMP-9 KO mice (MMP-9 KO, n = 5) that were treated with DT (5 ng/g). Error bars indicate the SEM for the number of animals indicated in the parentheses.
When tumors were implanted in pre-irradiated tissues of these mice, tumor growth was significantly inhibited in MMP-9 KO mice + DTR BM receiving DT (5 ng/g), mimicking the lack of tumor growth in MMP-9 KO mice (Figure 7D). Treatment with DT in WT mice + WT BM showed no effect in tumor growth (Figure S6E). Carrageenan on the other hand did not inhibit tumor growth (Figure 7D), likely due to the rebound of CD11b+ myelomonocytic cells resulted from the once a week treatment regimen. Overall, these results further demonstrate that MMP-9 expressing CD11b+ myelomonocytic cells play an essential role in promoting tumor growth in pre-irradiated tissues.
Discussion
In this study, we show the crucial role played by the ECM degrading enzyme MMP-9 provided by BM-derived CD11b+ myelomonocytic cells in allowing tumors to grow in irradiated normal tissues of the mice. We used the irradiated tumor bed model to abrogate local angiogenesis so as to examine the role of BM-derived cells in tumor growth. We demonstrate that CD11b+ myelomonocytic cells are recruited into irradiated tumors and into tumors growing in pre-irradiated tissues, and these cells restored tumor growth in MMP-9 KO mice by allowing immature blood vessels to develop. Further, when MMP-9 or a major source of MMP-9 expressing cells are genetically absent, ablated, or chemically inhibited, tumors in pre-irradiated tissues fail to grow beyond a very small size and are composed of normal, mature blood vessels.
Given the role of MMP-9 in degrading and remodeling the ECM, CD11b+ myelomonocytic cells expressing MMP-9 are likely to be important in reorganizing stromal compartments of tumors. In particular, by degrading the ECM they could provide a means for endothelial cells to enter or migrate to the tumor when the existing endothelial cells in and adjacent to the tumor cannot proliferate because of the local irradiation they have received. MMP-9 is involved in cleaving fibrillar type I collagen, the major constituent of the extracellular matrix to which endothelial cells are exposed in an injured tissue, allowing growth-factor induced angiogenesis to occur in chick chorioallantoic membrane assay (Seandel et al., 2001). Moreover MMP-9 provided by BM-derived macrophages has been shown to be essential in capillary branching in ischemia-induced revascularization of normal tissues (Johnson et al., 2004). MMP-9 may also enhance local angiogenesis in a spatiotemporal manner due to its ability to cleave membrane-bound VEGF thereby increasing bioavailable levels of VEGF (Bergers et al., 2000), a growth factor critical for survival and growth of endothelial cells (Ferrara et al., 2003). Macrophages themselves can also express VEGF and these cells are observed in poorly vascularized areas of human breast carcinoma (Lewis et al., 2000). Other functions of macrophages such as inducible nitric oxide synthase, arginase, and cyclooxygenase-2 have been reported to be important in growth of irradiated tumors in mice (Tsai et al., 2007).
CD11b+ myelomonocytic cells, including a subset of CD11b+Gr-1+ myeloid suppressor cells, are increasingly recognized for their roles in promoting tumor progression. Studies have shown that these cells enhance tumor angiogenesis (Yang et al., 2004), prepare the pre-metastatic niche in the lung (Hiratsuka et al., 2006), and are responsible for the refractoriness of tumors to anti-VEGF treatment (Shojaei et al., 2007). Our study is consistent with these reports by showing that they promote tumor growth in pre-irradiated tissues of mice when local angiogenesis is inhibited.
Increased leukocyte infiltration, especially by CD68 positive macrophages, is observed in biopsy samples of rectal cancer patients after high-dose (and short term) and low-dose (and long term) fractionated radiotherapy (Baeten et al., 2006). The authors of this study proposed that an increased expression of adhesion molecules such as intracellular adhesion molecule-1, vascular cell adhesion molecule, and E-selectin on tumor endothelium after radiotherapy is responsible for stimulating leukocyte infiltration in the tumors. However, other factors including VEGF and stromal-cell derived factor-1 (SDF-1) have been also reported to be essential in recruiting BM-derived myelomonocytic cells to tumors (Grunewald et al., 2006; Petit et al., 2007). VEGF and SDF-1 are downstream targets of hypoxia-inducible factor -1 (HIF-1), a transcription factor induced by hypoxia due to stabilization under hypoxic conditions (Ceradini et al., 2004; Semenza, 2003). HIF-1 levels are likely to increase in tumors regrowing after irradiation or tumors grown in pre-irradiated tissues due to the increased levels of hypoxia (Kim et al., 1993; Teicher et al., 1994). Furthermore, recent studies have shown that irradiation increases HIF-1 activity in tumors (Moeller et al., 2004) and this occurs by recruited macrophages in irradiated tumors producing nitric oxide, which in turn nitrosylates cysteine residues of the oxygen-dependent degradation domain of HIF-1α thereby stabilizing it (Li et al., 2007).
Overall, there is strong evidence that BM-derived myelomonocytic cells promote tumor growth and they do so by forming a positive feedback loop with many other components of the tumor microenvironment. Indeed several investigators have reported that targeting TAMs produces significant antitumor activity (Allavena et al., 2005; Lin et al., 2001; Luo et al., 2006; Zeisberger et al., 2006). However, it is evident that tumors show large variations in recruiting and utilizing these BM-derived cells, and our study demonstrates that the pattern of BM-derived infiltrates and reliance for the source of MMP-9 varies widely between RIF and MT1A2 tumors. Hence prospective knowledge of tumor cytokines and their role in the recruitment of BM-derived cells will be important to derive maximum antitumor activity from therapies targeting these cells.
We observed a minimal contribution of BM-derived EPCs to the vasculature of tumors grown in pre-irradiated tissues. This raises the question as to the source of the additional endothelial cells. Studies have shown that there are mature circulating endothelial cells derived from vessel wall turnover and these cells are increased in patients with some types of cancer (Bertolini et al., 2006). Recently, Aicher and colleagues reported that there are non-BM-derived circulating progenitor cells from organs such as the small intestine and liver and that these cells incorporate into sites of neovascularization (Aicher et al., 2007). However further studies are needed to determine the exact source of those new endothelial cells that promote tumor growth following high dose radiotherapy.
Our results also suggest that BM-derived cells expressing MMP-9 are sufficient but not essential for tumor vasculogenesis. This is evident from the similar tumor growth in pre-irradiated tissues of WT mice + MMP-9 KO BM compared to WT mice + WT BM. These data indicate that non-BM cells of the host that are still proficient in MMP-9, such as fibroblasts and smooth muscle cells, can compensate for the deficiency of MMP-9 from BM cells. MMP-9 in fibroblasts has been shown to promote mitogenic induction of breast cancer cells by enhancing endothelial cell survival and function in an in vitro co-culture model (Shekhar et al., 2001). This supports our observation that other sources of MMP-9 could also play a role in promoting tumor growth and angiogenesis. It also strengthens the rationale that MMP-9 is an important target for adjunct therapy to radiotherapy.
Clinical trials with MMP inhibitors have been uniformly disappointing. Although MMP-9 expression has been shown to correlate with tumor response in patients (Unsal et al., 2007), MMP inhibitors when given alone or in combination with cytotoxic agents showed no gain in clinical efficacy (Coussens et al., 2002). However, none of these trials have been performed in conjunction with radiotherapy, a therapy that can selectively inhibit local angiogenesis and make tumor growth dependent on vasculogenesis. Even though currently available MMP-9 inhibitors lack specificity for MMP-9 by also inhibiting the closely related MMP-2, a recent pre-clinical study showed that the MMP-2 and MMP-9 inhibitor Metastat significantly potentiated the antitumor efficacy of irradiation (Kaliski et al., 2005), supporting the rationale of combining MMP inhibitors with radiotherapy. We believe that our data point the way to further studies of MMP-9 inhibitors with radiation.
Experimental Procedures
Mice
All animal procedures were approved by Stanford’s Administrative Panel on Laboratory Animal Care (APLAC). All mice except C3H (FVB/N TgN(TIE2-lacZ)182Sato; B6.Cg Tg(TIE2GFP)287Sato/1J; B6;129S Gt(ROSA)26Sor/J; FVB-Tg(ITGAM DTR/EGFP)34Lan/J; FVB.Cg-Tg(GFPU)5Nagy/J; FVB.Cg-Mmp9tm1Tvu/J;FVB/NJ; and C57Bl/6J) were purchased from the Jackson laboratory (Bar Harbor, ME). C3H mice were obtained from the breeding facility at Stanford University’s Research Animal Facility. Mice were maintained in a germ-free environment and had access to food and water available ad libitum.
Bone marrow transplantation
Six- to twelve-week old mice were used as BM recipients and donors. BM cells from the donors were harvested from both femurs and tibias by flushing the bone cavity with Hank’s balanced salt solution (Invitrogen, Carlsbad, CA) using 25 gauge needles (BD, Franklin Lakes, NJ). The recipient mice were lethally irradiated 24 hr prior to the BM transplantation. The IR doses used were 9 Gy for FVB, MMP-9 KO, and C3H mice, and 9.5 Gy for C57Bl/6 mice. The lethally irradiated mice received > 2 × 106 BM cell suspensions intravenously and were allowed to recover for a minimum of 4 weeks.
Cell lines
The MT1A2 mouse mammary carcinoma cell line was obtained from Dr. Frank Graham (McMaster University, Canada), the TG1-1 mouse mammary carcinoma cell line was from Dr. Rakish Jain (Harvard University, MA), the 6780 lymphoma cell line was from Dr. Dean Felsher (Stanford University, CA), and the B16F1 melanoma cell line was from Dr. Garth Nicolson (UC Irvine, CA). LLC cell line was purchased from American Tissue Culture Collection (Manassas, VA). MT1A2, TG1-1, RIF, B16F1, and LLC cells were maintained in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA) supplemented with 10 % fetal bovine serum (FBS, Mediatech, Inc., Herndon, VA), penicillin-streptomycin (1%). 6780 cells were grown in RPMI (Invitrogen) supplemented with 10 % FBS, and penicillin-streptomycin (1%). RIF cells were constantly passaged in vitro-in vivo by implanting in syngeneic female C3H mice as described previously (Twentyman et al., 1980).
Tumor implantation, irradiation, measurement, and perfusion
The MT1A2 and TG1-1 cells were inoculated at 1.5 × 106 cells/mouse, 6780 cells at 5 × 106 cells/mouse, and RIF, B16F1, or LLC cells at 5 × 105 cells/mouse intradermally on the back of the mouse approximately 1 cm proximal to the base of the tail. Unanaesthetized mice are placed in lead jigs through which the tumor implantation site or established tumors (at approximately 200 mm3 in volume) was protruded for irradiation to an area of approximately 2 cm diameter. Irradiation was performed with a Phillips X-ray unit operated at 200 kVp with the dose rate of 1.21 Gy/min (20 mÅ with added filtration of 0.5 mm copper, the distance from X-ray source to the target of 31 cm and a half value layer of 1.3 mm copper). For the tumors grown in the pre-irradiated site the tumors were implanted 5 days after irradiation. Tumor volume (V) was calculated using the formula for a spheroid: V = π/6 × (width)2 × (length). When tumor volumes reached approximately 200 mm3 for control tumors and slightly more than 200 mm3 for irradiated tumors and tumors grown in the irradiated bed, cardiac perfusion was performed in asphyxiated tumor bearing mice with 4 % paraformaldehyde in phosphate buffered saline (PBS; Invitrogen). Tumors were removed, embedded in optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA), and frozen in −80 °C until cryosectioning and immunostaining.
Immunostaining
Primary antibodies for immunofluorescent staining were a rat monoclonal CD31/PECAM-1 (MEC13.3; BD Pharmingen, San Diego, CA), a rabbit polyclonal GFP antibody (Invitrogen, Eugene, OR), a rabbit polyclonal MMP-9 (Abcam, Cambridge, MA), a biotinylated CD11b/macrophage-associated antigen-1α (M1/70; BD Pharmingen), a biotinylated CD11c (HL3; BD Pharmingen), and a biotinylated Gr-1/Ly-6C (RB6-8C5; BD Pharmingen). Primary antibodies were detected by using secondary antibodies of anti-rat AlexaFluor 594 (Invitrogen), anti-rabbit AlexaFluor 488 (Invitrogen), anti-rabbit AlexaFluor 647 (Invitrogen), or streptavidin AlexaFluor 555 (Invitrogen). FITC-conjugated anti-mouse alpha-smooth muscle actin (α-SMA) antibody (Sigma, St. Louis, MO) was used to detect pericytes, and Phycoerythrin (PE) –conjugated antibodies for CD4 (StemCell Technologies, Vancouver, BC, Canada), CD8α/Ly-2 (53-6.7; BD Pharmingen), and CD49b (StemCell Technologies) were used to detect CD4, CD8α T cells, and NK cells, respectively. 8 μm frozen sections of tumors were dried in air, hydrated with PBS, blocked with 5 % goat serum in PBS (containing 0.03 % Triton X-100) for 30 min, and incubated with primary antibodies for 2 hr at room temperature (RT). Sections were washed three times in PBS, followed by secondary antibody for 1 hr at RT. After washing in PBS, sections were mounted with anti-fade reagent with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen) and viewed with Leica DMRA2 microscope (Wetzlar, Germany) using Plan 20×/0.40 and 40×/0.65 objective lenses with HC PLAN s 10×/22 eyepieces. Images were acquired with a Hamamatsu ORCA-ER camera and Improvision OpenLab software.
Histological assessment
MMP-9 positive area in the tumors was determined by the point-count method (Gray, 1996). Briefly, the proportions were calculated as the number of points directly over MMP-9 positive staining divided by the total number of points examined using a six-point grid in a 15 × eyepieces at a 10 × objective.
Quantitative analysis for CD11b and MMP-9 positive cells was done by counting the number of cells in the photographed fields where the most CD11b were observed in non-necrotic regions using a 40 × objective with 10 × eyepieces of the DMRA2 fluorescent microscope. X-gal positive cells were counted in 5 random fields per tumor with a 20 × objective and 10 × eyepieces of the DMLB microscope. Quantification was made on 3-5 independent specimens per tumor, 4-5 animals per group.
Drug treatment
ZA (Zometa; Novartis Pharma AG) was dissolved in sterile water and stored long term at −80 °C and at 4 °C for short-term storage as reported previously (Giraudo et al., 2004). Mice were treated every day with ZA or water from the first day of the local irradiation at the lower back. The animals were monitored during the treatment for their body weight to assess side effects and did not show any significant loss in weight (less than 10 % of body weight).
DTR (List biological laboratories Inc., CA) was prepared in sterile water containing 1 % of bovine serum albumin (BSA, Sigma). Carrageenan (Sigma) was dissolved in saline at 10 mg/ml. Mice were treated with DT or vehicle (1 % BSA in water) once in two days or carrageenan once per week by intraperitoneal injections from the first day of the local irradiation. The treated animals received water containing antibiotics (neomycin and polymyxin B) throughout the study.
Statistical analysis
Statistical comparisons of data sets were performed by a two tailed Student’s t-test or one-way ANOVA with Tukey post test (V4.00 GraphPad Inc., CA). The data were considered to be significantly different when probability values of P < 0.05.
Significance.
Tumors have an absolute requirement for neovasculature to grow beyond a very small size. This vasculature can arise by proliferation and migration of nearby blood vessels (angiogenesis) or from colonization by circulating endothelial and other cells primarily derived from the BM (vasculogenesis). Using transplantable tumor models in irradiated tissues (a model that simulates tumors recurring after high dose radiotherapy), we demonstrate that MMP-9, an enzyme involved in degrading extracellular matrix, is required for vasculogenesis but not for angiogenesis. The BM-derived CD11b positive myelomonocytic cells expressing MMP-9 are the major contributors to tumor vasculogenesis. This study suggests that MMP-9 from the BM-derived cells could be an important target for adjunct therapy to improve the therapeutic benefit for patients receiving radiotherapy.
Supplementary Material
Acknowledgements
We would like to thank Dr. Frank Graham (McMaster University, Canada) for providing the MT1A2 mouse mammary carcinoma cell line, Dr. Rakish Jain (Harvard University) for the TG1-1 mouse mammary carcinoma cell line, Dr. Dean Felsher (Stanford University) for the 6780 lymphoma cell line, Dr. Mary Jo Dorie for culturing RIF cells, Mr. Doug Menke for intravenous injection of BM cells, and Ms. Pauline Chu for frozen sections and H&E staining. Support was provided by the Stanford University Ludwig Translational Program in Cancer Research and a Translational Cancer Research Award from the Stanford Comprehensive Cancer Center. We disclose that there are no financial conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aicher A, Rentsch M, Sasaki K, Ellwart JW, Fandrich F, Siebert R, Cooke JP, Dimmeler S, Heeschen C. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ Res. 2007;100:581–589. doi: 10.1161/01.RES.0000259562.63718.35. [DOI] [PubMed] [Google Scholar]
- Allavena P, Signorelli M, Chieppa M, Erba E, Bianchi G, Marchesi F, Olimpio CO, Bonardi C, Garbi A, Lissoni A, et al. Anti-inflammatory properties of the novel antitumor agent yondelis (trabectedin): inhibition of macrophage differentiation and cytokine production. Cancer Res. 2005;65:2964–2971. doi: 10.1158/0008-5472.CAN-04-4037. [DOI] [PubMed] [Google Scholar]
- Baeten CIM, Castermans K, Lammering G, Hillen F, Wouters BG, Hillen HFP, Griffioen AW, Baeten CGM. Effects of radiotherapy and chemotherapy on angiogenesis and leukocyte infiltration in rectal cancer. Int J Rad Oncol Biol Phys. 2006;66:1219–1227. doi: 10.1016/j.ijrobp.2006.07.1362. [DOI] [PubMed] [Google Scholar]
- Bailey AS, Willenbring H, Jiang S, Anderson DA, Schroeder DA, Wong MH, Grompe M, Fleming WH. Myeloid lineage progenitors give rise to vascular endothelium. Proc Natl Acad Sci U S A. 2006;103:13156–13161. doi: 10.1073/pnas.0604203103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Coussens LM. Extrinsic regulators of epithelial tumor progression: metalloproteinases. Curr Opin Genet Dev. 2000;10:120–127. doi: 10.1016/s0959-437x(99)00043-x. [DOI] [PubMed] [Google Scholar]
- Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer. 2006;6:835–845. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- Capoccia BJ, Shepherd RM, Link DC. G-CSF and AMD3100 mobilize monocytes into the blood that stimulate angiogenesis in vivo through a paracrine mechanism. Blood. 2006;108:2438–2445. doi: 10.1182/blood-2006-04-013755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma M, Venneri MA, Roca C, Naldini L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med. 2003;9:789–795. doi: 10.1038/nm871. [DOI] [PubMed] [Google Scholar]
- Dirkx AE, Egbrink M. G. Oude, Wagstaff J, Griffioen AW. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukoc Biol. 2006;80:1183–1196. doi: 10.1189/jlb.0905495. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Fujiyama S, Amano K, Uehira K, Yoshida M, Nishiwaki Y, Nozawa Y, Jin D, Takai S, Miyazaki M, Egashira K, et al. Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ Res. 2003;93:980–989. doi: 10.1161/01.RES.0000099245.08637.CE. [DOI] [PubMed] [Google Scholar]
- Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251–262. [PubMed] [Google Scholar]
- Gill M, Dias S, Hattori K, Rivera ML, Hicklin D, Witte L, Girardi L, Yurt R, Himel H, Rafii S. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res. 2001;88:167–174. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest. 2004;114:623–633. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothert JR, Gustin SE, van Eekelen JA, Schmidt U, Hall MA, Jane SM, Green AR, Gottgens B, Izon DJ, Begley CG. Genetically tagging endothelial cells in vivo: bone marrow-derived cells do not contribute to tumor endothelium. Blood. 2004;104:1769–1777. doi: 10.1182/blood-2003-11-3952. [DOI] [PubMed] [Google Scholar]
- Gray T. Quantitation in histopathology. In: Bancroft JD, Gamble M, editors. Theory and practice of histological techniques. Churchill-Livingstone; New York: 1996. pp. 641–663. [Google Scholar]
- Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissig B, Hattori K, Friedrich M, Rafii S, Werb Z. Angiogenesis: vascular remodeling of the extracellular matrix involves metalloproteinases. Curr Opin Hematol. 2003;10:136–141. doi: 10.1097/00062752-200303000-00007. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- Itasaka S, Komaki R, Herbst RS, Shibuya K, Shintani T, Hunter NR, Onn A, Bucana CD, Milas L, Ang KK, O’Reilly MS. Endostatin improves radioresponse and blocks tumor revascularization after radiation therapy for A431 xenografts in mice. Int J Rad Oncol Biol Phys. 2007;67:870–878. doi: 10.1016/j.ijrobp.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodele S, Chantrain CF, Blavier L, Lutzko C, Crooks GM, Shimada H, Coussens LM, DeClerck YA. The contribution of bone marrow-derived cells to the tumor vasculature in neuroblastoma is matrix metalloproteinase-9 dependent. Cancer Res. 2005;65:3200–3208. doi: 10.1158/0008-5472.CAN-04-3770. [DOI] [PubMed] [Google Scholar]
- Johnson C, Sung HJ, Lessner SM, Fini ME, Galis ZS. Matrix metalloproteinase-9 is required for adequate angiogenic revascularization of ischemic tissues. Potential role in capillary branching. Circ Res. 2004;94:262–268. doi: 10.1161/01.RES.0000111527.42357.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliski A, Maggiorella L, Cengel KA, Mathe D, Rouffiac V, Opolon P, Lassau N, Bourhis J, Deutsch E. Angiogenesis and tumor growth inhibition by a matrix metalloproteinase inhibitor targeting radiation-induced invasion. Mol Cancer Ther. 2005;4:1717–1728. doi: 10.1158/1535-7163.MCT-05-0179. [DOI] [PubMed] [Google Scholar]
- Kim IH, Lemmon MJ, Brown JM. The influence of irradiation of the tumor bed on tumor hypoxia: measurements by radiation response, oxygenation electrodes, and nitroimidazole binding. Radiat Res. 1993;135:411–417. [PubMed] [Google Scholar]
- Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- Lewis JS, Landers RJ, Underwood JCE, Harris AL, Lewis CE. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol. 2000;192:150–158. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH687>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Li F, Sonveaux P, Rabbani ZN, Liu S, Yan B, Huang Q, Vujaskovic Z, Dewhirst MW, Li CY. Regulation of HIF-1α stability through S-nitrosylation. Mol Cell. 2007;26:63–74. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang BC, Thornton AF, Jr., Sandler HM, Greenberg HS. Malignant astrocytomas: focal tumor recurrence after focal external beam radiation therapy. J Neurosurg. 1991;75:559–563. doi: 10.3171/jns.1991.75.4.0559. [DOI] [PubMed] [Google Scholar]
- Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C, Markowitz D, Wu W, Liu C, Reisfeld RA, Xiang R. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest. 2006;116:2132–2141. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, et al. Impaired recruitment of bone marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- Milas L, Ito H, Hunter N, Jones S, Peters LJ. Retardation of tumor growth in mice caused by radiation-induced injury of tumor bed stroma: Dependency on tumor type. Cancer Res. 1986;46:723–727. [PubMed] [Google Scholar]
- Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Moldovan NI, Goldschmidt-Clermont PJ, Parker-Thornburg J, Shapiro SD, Kolattukudy PE. Contribution of monocytes/macrophages to compensatory neovascularization: the drilling of metalloelastase-positive tunnels in ischemic myocardium. Circ Res. 2000;87:378–384. doi: 10.1161/01.res.87.5.378. [DOI] [PubMed] [Google Scholar]
- Petit I, Jin D, Rafii S. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol. 2007;28:299–307. doi: 10.1016/j.it.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002;2:826–835. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- Rofstad EK, Mathiesen B, Henriksen K, Kindem K, Galappathi K. The tumor bed effect: increased metastatic dissemination from hypoxia-induced up-regulation of metastasis promoting gene products. Cancer Res. 2005;65:2387–2396. doi: 10.1158/0008-5472.CAN-04-3039. [DOI] [PubMed] [Google Scholar]
- Rohde E, Malischnik C, Thaler D, Maierhofer T, Linkesch W, Lanzer G, Guelly C, Strunk D. Blood monocytes mimic endothelial progenitor cells. Stem Cells. 2006;24:357–367. doi: 10.1634/stemcells.2005-0072. [DOI] [PubMed] [Google Scholar]
- Ruzinova MB, Schoer RA, Gerald W, Egan JE, Pandolfi PP, Rafii S, Manova K, Mittal V, Benezra R. Effect of angiogenesis inhibition by Id loss and the contribution of bone-marrow-derived endothelial cells in spontaneous murine tumors. Cancer Cell. 2003;4:277–289. doi: 10.1016/s1535-6108(03)00240-x. [DOI] [PubMed] [Google Scholar]
- Schlaeger TM, Bartunkova S, Lawitts JA, Teichmann G, Risau W, Deutsch U, Sato TN. Uniform vascular-endothelial-cell-specific gene expression in both embryonic and adult transgenic mice. Proc Natl Acad Sci U S A. 1997;94:3058–3063. doi: 10.1073/pnas.94.7.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seandel M, Noack-Kunnmann K, Zhu D, Aimes RT, Quigley JP. Growth factor-induced angiogenesis in vivo requires specific cleavage of fibrillar type I collagen. Blood. 2001;97:2323–2332. doi: 10.1182/blood.v97.8.2323. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Shaked Y, Ciarrocchi A, Franco M, Lee CR, Man S, Cheung AM, Hicklin DJ, Chaplin D, Foster FS, Benezra R, Kerbel RS. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313:1785–1787. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- Shekhar MPV, Werdell J, Santner SJ, Pauley RJ, Tait L. Breast stroma plays a dominant regulatory role in breast epithelial growth and differentiation: Implications for tumor development and progression. Cancer Res. 2001;61:1320–1326. [PubMed] [Google Scholar]
- Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, Fuh G, Gerber HP, Ferrara N. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;8:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–727. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Stoneman V, Braganza D, Figg N, Mercerm J, Lang R, Goddard M, Bennett M. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ Res. 2007;100:884–893. doi: 10.1161/01.RES.0000260802.75766.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- Teicher BA, Dupuis NP, Robinson MF, Kusumoto T, Liu M, Menon K. Reduced oxygenation in a rat mammary carcinoma post-radiation and reoxygenation with a perflubron emulsion/carbogen breathing. In Vivo. 1994;8:125–131. [PubMed] [Google Scholar]
- Tsai CS, Chen FH, Wang CC, Huang HL, Jung SM, Wu CJ, Lee CC, McBride WH, Chiang CS, Hong JH. Macrophages from irradiated tumors express higher levels of iNOS, arginase-I and COX-2, and promote tumor growth. Int J Rad Oncol Biol Phys. 2007;68:499–507. doi: 10.1016/j.ijrobp.2007.01.041. [DOI] [PubMed] [Google Scholar]
- Tsai J, Makonnen S, Feldman M, Sehgal CM, Maity A, Lee WM. Ionizing radiation inhibits tumor neovascularization by inducing ineffective angiogenesis. Cancer Biol Ther. 2005;4:1395–1400. doi: 10.4161/cbt.4.12.2331. [DOI] [PubMed] [Google Scholar]
- Twentyman PR, Brown JM, Gray JW, Franko AJ, Scoles MA, Kallman RF. A new mouse tumor model system (RIF-1) for comparison of end-point studies. J Natl Cancer Inst. 1980;64:595–604. [PubMed] [Google Scholar]
- Udagawa T, Birsner AE, Wood M, D’Amato RJ. Chronic suppression of angiogenesis following radiation exposure is independent of hematopoietic reconstitution. Cancer Res. 2007;67:2040–2045. doi: 10.1158/0008-5472.CAN-06-2877. [DOI] [PubMed] [Google Scholar]
- Unsal D, Uner A, Akyurek N, Erpolat P, Dursun A, Pak Y. Matrix metalloproteinase-9 expression correlated with tumor response in patients with locally advanced rectal cancer undergoing preoperative chemoradiotherapy. Int J Rad Oncol Biol Phys. 2007;67:196–203. doi: 10.1016/j.ijrobp.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin C. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006;95:272–281. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.