Abstract
Approximately 25% of patients with colorectal cancer develop metastases to the liver, and surgery is currently the best treatment available. But there are several patients who are unresectable, and isolated hepatic perfusion (IHP) offers a different approach in helping to treat these patients. IHP is a method used for isolating the liver and delivering high doses of chemotherapeutic agents. The efficacy of IHP has been improved by combining hyperthermia not only with chemotherapeutics but with other deliverable agents such as tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). In this study, we used human colorectal cancer CX-1 cells and treated them with hyperthermia and TRAIL, causing cytotoxicity. We were able to demonstrate that the numbers of live cells were significantly reduced with hyperthermia and 10 ng/ml of TRAIL combined. We also showed that the effect of hyperthermia on TRAIL in our studies was enhancement of the apoptotic pathway by the promotion of JNK and BimEL activity as well as PARP cleavage. We have also used our CX-1 cells to generate tumors in Balb/c nude mice. With intra-tumoral injections of TRAIL combined with hyperthermia at 42°C, we were able to show a delayed onset of tumor growth in our xenograft model.
Keywords: Hyperthermia, TRAIL, JNK, Bim, xenograft tumor
Introduction
Colorectal cancer is the second leading cause of cancer in the United States and death is usually attributed to metastatic liver disease [Ruers and Bleichrodt, 2002]. Surgical resection is currently the best form of treatment but surgery alone cannot treat the vast majority of these cases. Isolated hepatic perfusion (IHP), which involves complete vascular isolation of the liver to allow delivery of high doses of chemotherapeutic and biological agents as well as hyperthermia, has been able to treat the cases of metastatic liver disease that surgery cannot [Ridge et al., 1987a; Ridge et al., 1987b; Vahrmeijer et al., 2000]. The utilization of IHP involves a wide scope in various treatment regimens. It is essential to highlight one of its functions, hyperthermia. In the past 20 years, the biological effects of hyperthermia have been thoroughly investigated. The temperatures of interest for treating cancers are in the range of 40–46°C. This maximizes the tumor damage while preserving the surrounding normal tissue [Yoo and Lee, 2007]. Hyperthermia has a synergistic effect when combined with chemotherapeutic agents in treating cancer, but it also works just as well when combined with tumor necrosis factor related apoptosis-inducing ligand (TRAIL), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), or TNF-α + IFN-γ [Lee et al., 1993].
TRAIL consists of 281 and 291 amino acids in the human and murine forms, respectively. TRAIL is expressed as a type II integral membrane protein belonging to the tumor necrosis factor (TNF) superfamily (4-1BBL, APRIL, BAFF, CD27L, CD30L, CD40L, EDA1, EDA2, FasL, GITRL, LIGHT, lymphotoxin, OX40L, RANKL, TL1A, TNF, TWEAK, and TRAIL). Lymphotoxin and TRAIL are related most closely to a Fas/APO-1 ligand (FasL) among TNF superfamily members. Like Fas ligand (FasL) and TNF, the C-terminal extracellular region of TRAIL (amino acids 114-281) exhibits a homotrimeric subunit structure [Pitti, 1996].
TRAIL is well known to play a critical role as an inducer of apoptosis in a variety of cancer cells in vitro and has been shown to limit tumor growth efficiently in vivo, with minimal damage to normal tissues [Kelley et al., 2001; Ashkenazi et al., 1999; Walczak et al., 1999; Kato et al., 2007]. It is thought that TRAIL induces apoptosis by binding to the death receptors TRAIL-R1 (DR4) and TRAIL-R2 (DR5), members of the tumor necrosis factor (TNF) receptor superfamily, which results in conformational changes that expose a binding surface for Fas-associated death domain (FADD), an adaptor protein [Kischkel et al., 2000; Thomas et al., 2004; Park et al., 2005]. The adaptor molecule recruits the initiators caspase-8 and -10 to promote formation of the death-inducing signaling complex (DISC). The activation of caspases has been documented by several observations, providing evidence that caspase-8, an initiator caspase, cleaves Bid and triggers mitochondrial damage and subsequently induces the release of cytochrome c from the mitochondria [Li et al., 1997; Oh et al., 2005; Gong et al., 2004]. Cytochrome c in the cytoplasm binds to Apaf-1, which then permits recruitment of procaspase-9 to form the apoptosome protein complex. The formation of the apoptosome results in the activation of caspase-9. Caspase-9 cleaves and activates procaspase-3 [Slee et al., 1999]. This results in the activation of effector caspases such as caspase-3. Caspase-3 plays an important role in both death receptor- and mitochondria-mediated apoptosis [Sun et al., 1999]. Previous studies have shown that caspase-3 is required for the DNA fragmentation and membrane blebbing associated with apoptosis [Janicke et al., 1998]. MCF-7, a breast cancer cell line, is caspase-3 deficient [Janicke et al., 1998] and relatively insensitive to many chemotherapeutic agents [Yang et al., 2001]. Reconstitution of caspase-3 sensitizes MCF-7 cells to TRAIL [Lee et al., 2004].
We have demonstrated that hyperthermia has a synergistic effect with TRAIL in causing cytotoxicity in CX-1 human colorectal cancer cells [Yoo and Lee, 2007; Yoo and Lee, 2008]. It was observed that TRAIL-induced apoptotic death can be enhanced with mild hyperthermia (41 – 42oC) through caspase activation and cytochrome c release in mitochondria of CX-1 cancer cells. We also reported, in another study, that hyperthermia increases the efficacy of chemotherapeutic agents such as oxaliplatin or melphalan when used in combination with TRAIL, killing the CX-1 colorectal cancer cells more effectively [Yoo and Lee, 2008]. We are now reporting that hyperthermia combined with TRAIL decreases tumor cell survival and increases phosphorylation of Bim and JNK, on the contrary, by using a JNK-inhibitor we demonstrate the lack of phosphorylation of Bim and decreased phosphorylation in JNK in the face of TRAIL and hyperthermia. In addition, our in vivo study shows that combined mild hyperthermia with TRAIL leads to a significant decrease in tumor growth in Balb/c nude mice.
Materials and Methods
Cell Culture and Survival Assay
Human colorectal carcinoma CX-1 cells were cultured in RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (HyClone, Logan, UT, USA) and 26 mM sodium bicarbonate for monolayer cell culture. The dishes containing cells were kept in a 37°C humidified incubator with 5% CO2. One or 2 days prior to the experiment, cells were plated into 60-mm Petri-dishes. For the colony formation assay, CX-1 cells exposed to hyperthermia with or without TRAIL were trypsinized, counted, and plated at appropriate dilutions (200 to 1,000 cells/plate). After 10 days of culture at 37°C, colonies were fixed by 10% formalin and stained with 2% crystal violet. After the staining, all of the colonies were counted. Survival was normalized with plating efficiency. For trypan blue exclusion assay, trypsinized cells were pelleted and resuspended in 0.2 ml of medium, 0.5 ml of 0.4% trypan blue solution and 0.3 ml of phosphate-buffered saline solution (PBS). The samples were mixed thoroughly, incubated at room temperature for 15 min, and examined under a light microscope. At least 300 cells were counted for each survival determination.
Production of Recombinant TRAIL
A human TRAIL cDNA fragment (amino acids 114–281) obtained by RT-PCR was cloned into a pET-23d (Novagen, Madison, WI, USA) plasmid as described previously [Seol and Billiar, 1999]. The insertion sites and sequences were confirmed by using T7 primer. His-tagged TRAIL protein was purified using the Qiagen express protein purification system (Qiagen, Valencia, CA, USA).
Hyperthermia Treatment
Cells cultured in 60-mm dishes were sealed with parafilm and were placed in a circulating water bath (Heto, Thomas Scientific, Denmark) which was maintained within 0.028°C of the desired temperature. Control cells that were not in the water bath were kept at 5% CO2 in 60-mm dishes without parafilm.
Treatment with JNK Inhibitor
CX-1 colorectal cancer cell cultures were treated with 50 μg/ml of JNK Inhibitor II (Calbiochem, San Diego, CA, USA), also known as SP600125, 1 h prior to being treated with hyperthermia, 50 ng/ml of TRAIL and combination therapy involving hyperthermia with 50 ng/ml of TRAIL.
Protein Extracts and Polyacrylamide Gel Electrophoresis (PAGE) with Immunoblot Analysis
Cells were lysed with Laemmli lysis buffer (2.4 M glycerol, 0.14 M Tris, pH 6.8, 0.21 M sodium dodecyl sulfate (SDS), 0.3 mM bromophenol blue) and boiled for 10 min. Protein content was measured with BCA Protein Assay Reagent (Pierce, Rockford, IL, USA). The samples were diluted with lysis buffer containing 1.28 M β-mercaptoethanol, and equal amounts of protein were loaded on 8–12% SDS–polyacrylamide gels. SDS–PAGE analysis was performed according to Laemmli [1970] using a Hoefer gel apparatus.
Proteins were separated by SDS–PAGE and electrophoretically transferred to nitrocellulose membrane. The nitrocellulose membrane was blocked with 5% non-fat dry milk in PBSTween-20 (0.1%, v/v) at 4°C overnight. The membrane was incubated with primary antibody (diluted according to the manufacturer's instructions) for 2 h. Horseradish peroxidase conjugated anti-rabbit or anti-mouse IgG was used as the secondary antibody. Immunoreactive protein was visualized by the chemiluminescence protocol (ECL, Amersham, Arlington Heights, IL, USA).
Anti-JNK antibody was purchased from Santa Cruz (Santa Cruz, CA, USA), anti-phosphorylated JNK, anti-phospho-Bim and anti-Bim antibody were from Cell Signaling (Beverly, MA, USA), anti-PARP antibody from Biomol Research Laboratory (Plymouth Meeting, PA), and anti-actin antibody from ICN (Costa Mesa, CA, USA).
Densitometry analysis
The Personal Densitometer SI from Molecular Dynamics was used to analyze the bands performed in the immunoblotting technique described above. The ImageQuaNT program was used for the analysis.
Animal Model
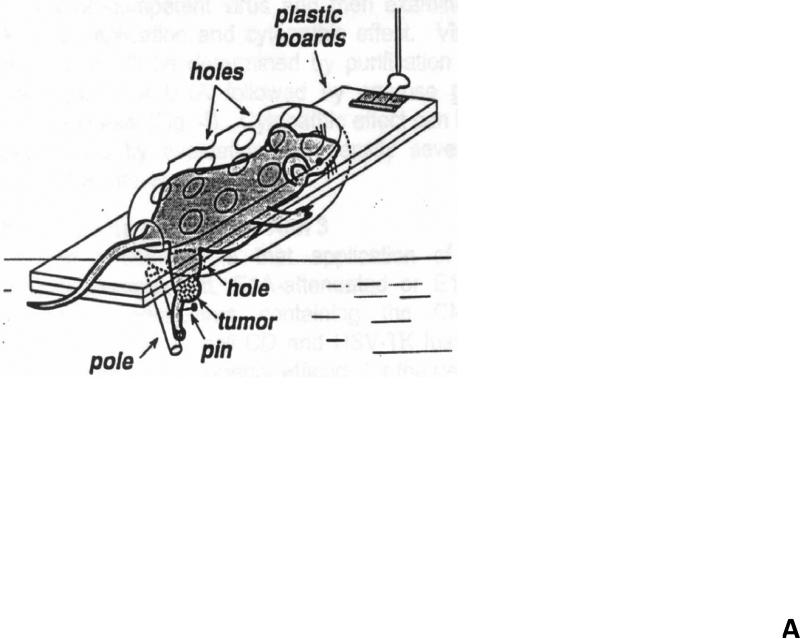
Human colon adenocarcinoma CX-1 tumors were established by subcutaneously injecting 106 cells into the right hind leg of 8 week old male nude mice (Balb/c nude) (Charles River Labs, Wilmington, MA, USA). Prior to treatment with hyperthermia and TRAIL, tumor size was measured 2-3 times per week until the volume reached approximately 50 mm3. Tumor volume was calculated as W2 × L × 0.52 where L is the largest diameter and W is the diameter perpendicular to L. After establishment of these tumor xenografts, mice were randomized into four groups of five mice per group. Mice were fed ad libitum and maintained in environments with controlled temperature of 22–24°C and 12 h light and dark cycles. For TRAIL treatment, TRAIL (1 mg/kg) was administrated by intra-tumoral injection. For hyperthermic treatment, tumor bearing legs were immersed in a water bath at 42°C for 1 h while the remainder of the mouse rested on a plastic board outside of the water bath (Fig 1A). All procedures involving the mice were in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
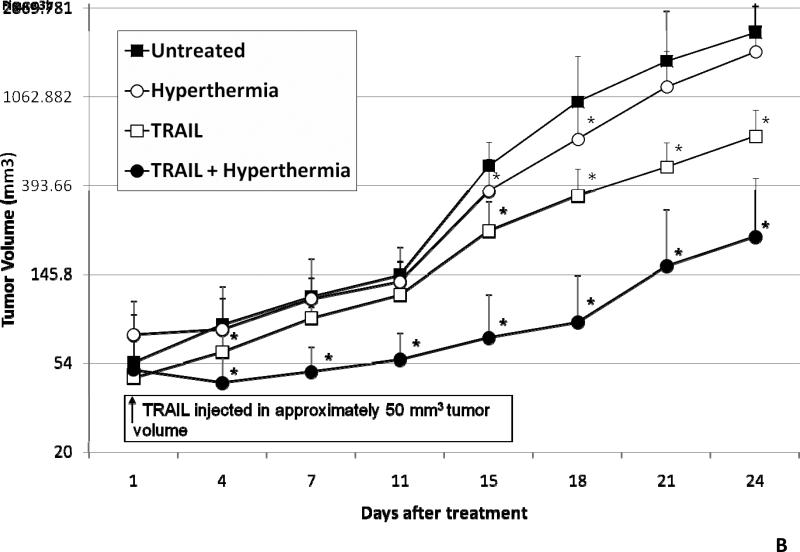
Figure 1. Schema of experimental model of heat treatment (A) and effect of hyperthermia, TRAIL, or hyperthermia in combination with TRAIL on growth of CX-1 xenograft (B).
(A) Heating of tumors at a mild hyperthermic temperature (42°C) for 1 hr was performed using water bath immersion of the tumor-bearing right leg. (B) TRAIL was injected into the subcutaneous tumors of mice once they were approximately 50 mm3 in tumor volume. Tumors were exposed to hyperthermic conditions as well as treated with and without TRAIL. Tumors were measured every three days. Error bars represent standard error of the mean from five mice. Asterisk * represents a statistically significant difference between control and hyperthermia alone, TRAIL alone, or hyperthermia in combination with TRAIL at P<0.05.
Statistical analysis
Statistical analysis was carried out using Graphpad InStat 3 software (GraphPad Software, Inc., San Diego, CA, USA). Results from the t-test were considered statistically significant at P<0.05 or P<0.01.
Results
Effect of hyperthermia, TRAIL, or hyperthermia in combination with TRAIL on growth of xenograft tumors
In vivo studies were performed to examine the effect of hyperthermia, TRAIL, and hyperthermia in combination with TRAIL on growth of xenograft tumors. Human colon adenocarcinoma CX-1 tumors were established as described in Materials and Methods. After treatment, tumor size was measured every three days. Figure 1B shows that there were complete disparities from 15 days after treatment between hyperthermia and TRAIL treated tumors compared to untreated and those treated with hyperthermia alone. TRAIL alone caused a statistically significantly decrease in tumor growth. Moreover, hyperthermia in combination with TRAIL caused a greater decrease of CX-1 xenograft tumor growth when compared to treatment with TRAIL alone, hyperthermia alone and the untreated control group. The effect of hyperthermia in combination with TRAIL on tumor growth was observed as early as 4 days after treatment. A slight difference, but not statistically significant, was observed in the heated group in comparison to the control group at 21 and 24 days after treatment.
Effect of hyperthermia on TRAIL-induced cytotoxicity
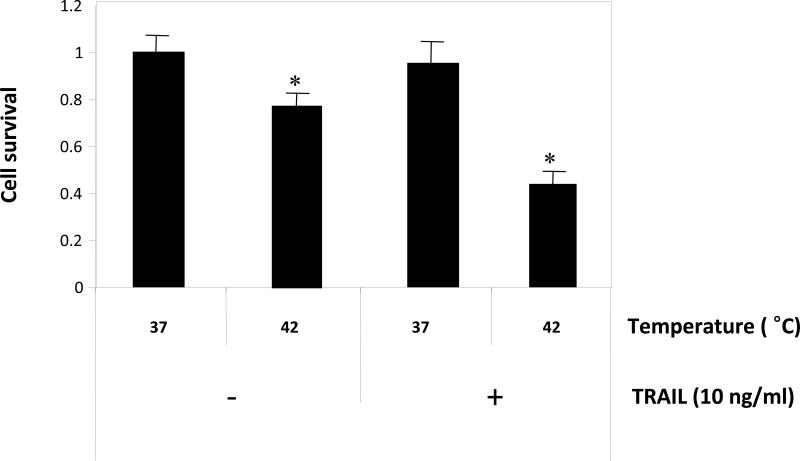
The technique of IHP exposes an individual to 1 h of hyperthermic conditions via an isolated vascular recirculating perfusion circuit [Yoo and Lee, 2007; Yoo and Lee, 2008]. To examine the effects of hyperthermia on TRAIL-induced cytotoxicity, CX-1 cells were treated with 10 ng/ml of TRAIL for 1 h at two different temperatures (37°C and 42°C) and then incubated for 3 h. We previously described the morphological features of these cells [Yoo and Lee, 2007] after exposure to hyperthermia and 50 ng/ml of TRAIL and observed a large number of them to be rounded and detached from the plate. For survival determination, cells were trypsinized, counted, and plated. After 10 days, colonies were counted and survival was determined as described in Materials and Methods. By doing a colony formation assay, we were able to demonstrate that the number of live cells was significantly smaller with hyperthermia and 10 ng/ml of TRAIL combined when compared to 37°C only, 42°C only and 37°C combined with 10 ng/ml of TRAIL (Fig. 2).
Figure 2. Effect of hyperthermia in combination with TRAIL on cell viability.
CX-1 tumor cells were exposed to hyperthermic (42°C) or normothermic (37°C) conditions for 1 h in conjunction with and without 10 ng/ml of TRAIL. After treatment, cells were then exposed to 37°C for 3 h in the presence of 10 ng/ml of TRAIL. After 3 h exposure, cell survival was determined by colony formation. For colony formation, cells were trypsinized, counted, and plated. After 10 days, colonies were counted and survival was determined as described in Materials and Methods. Error bars represent standard error from the mean (S.E.M.) for three separate experiments. Asterisk * represents a statistically significant difference between control (37°C only) and heat (42°C only) or heat + TRAIL-treated cells at P<0.01.
Effect of hyperthermia in combination with TRAIL on the JNK-Bim signal transduction pathway
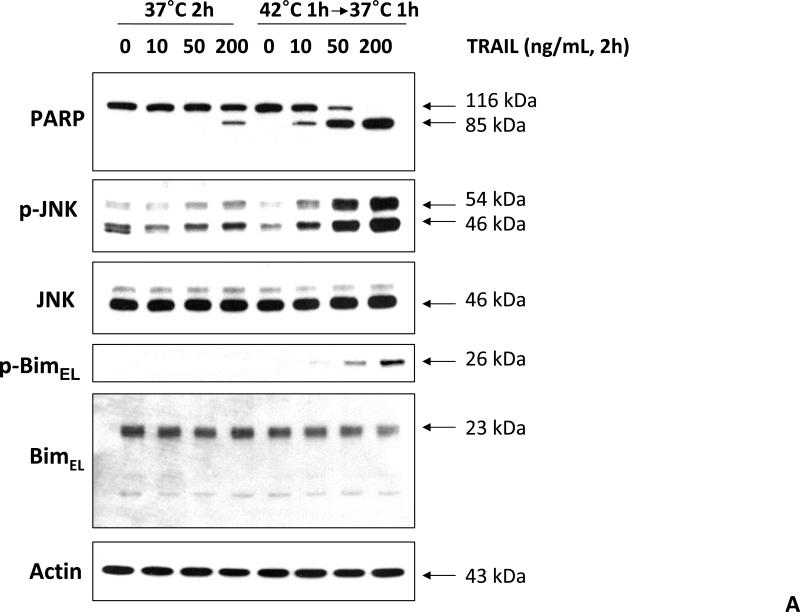
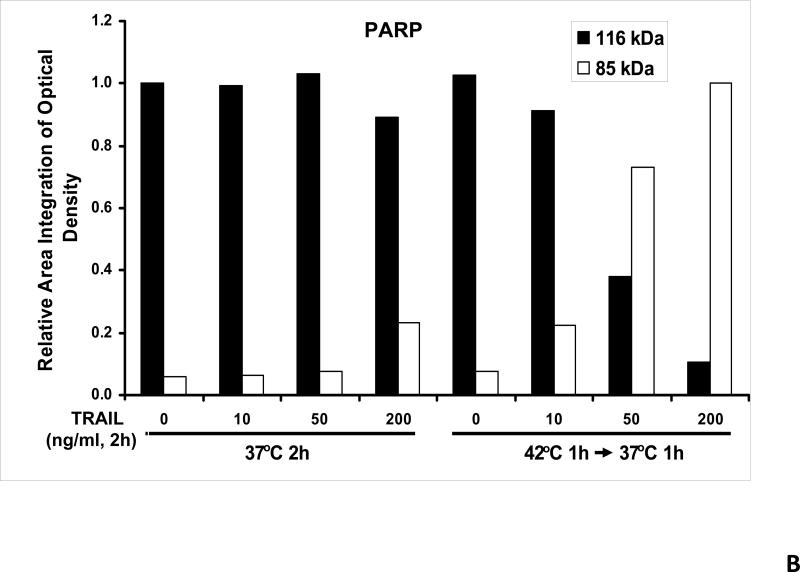
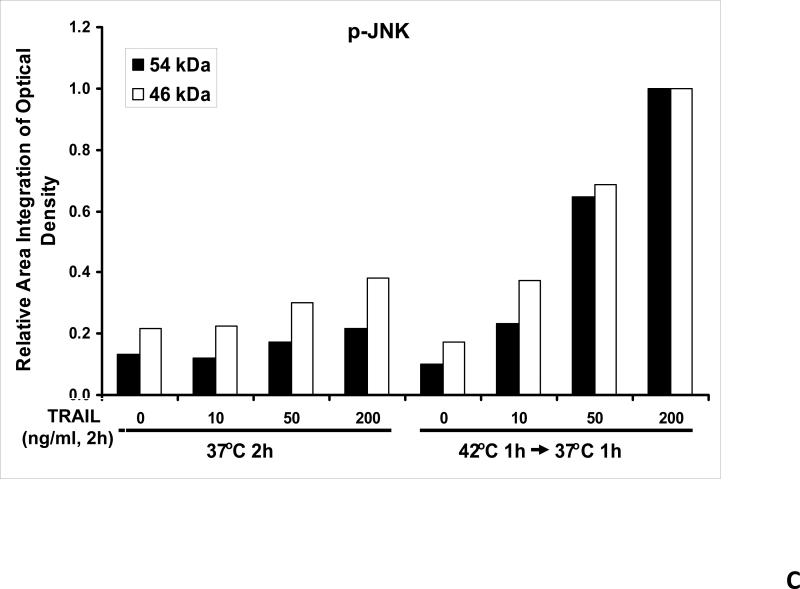
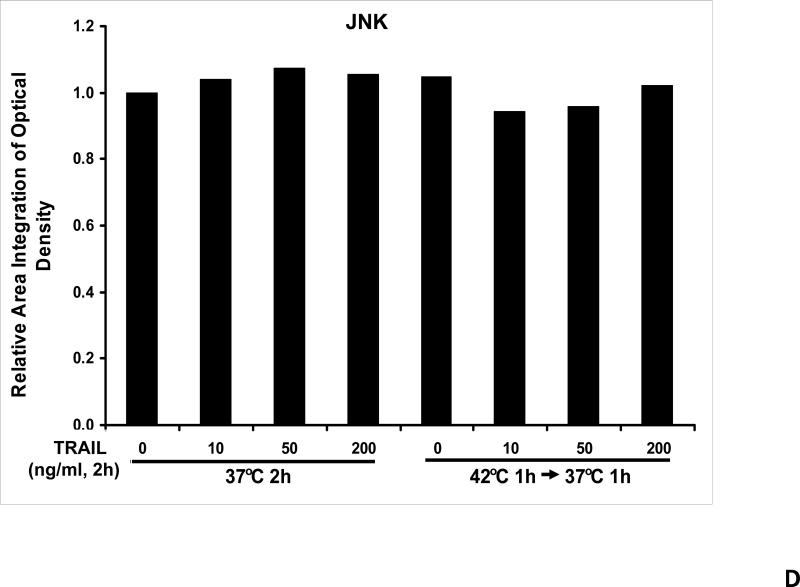
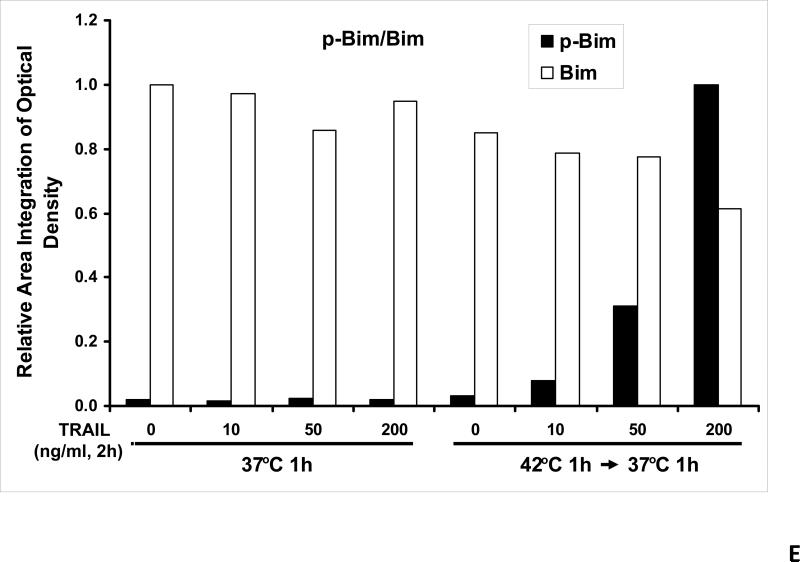
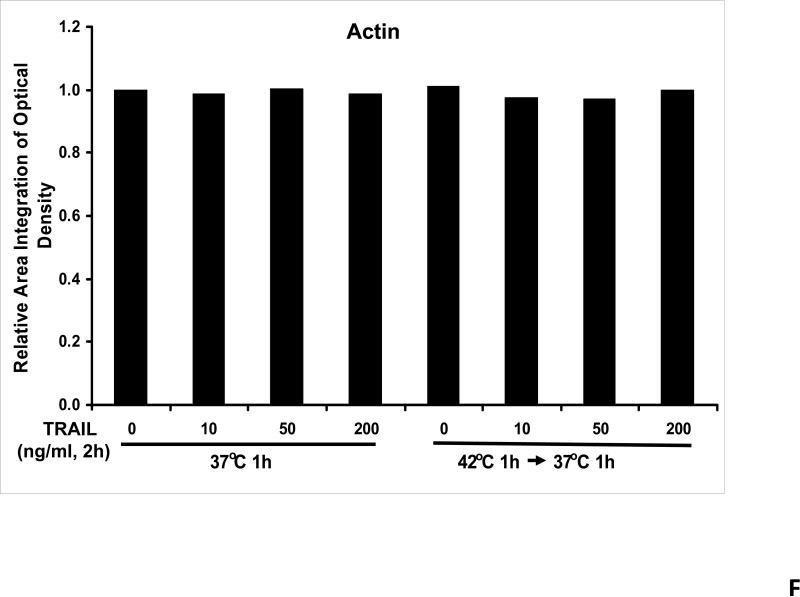
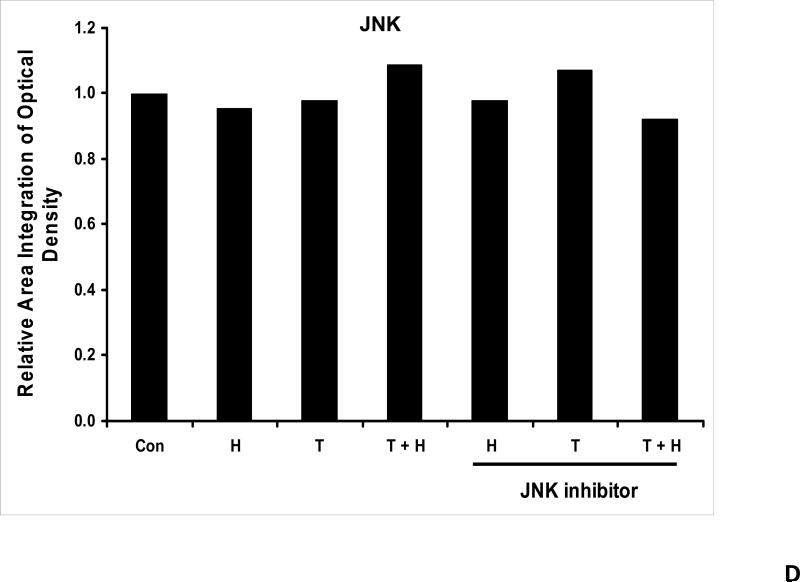
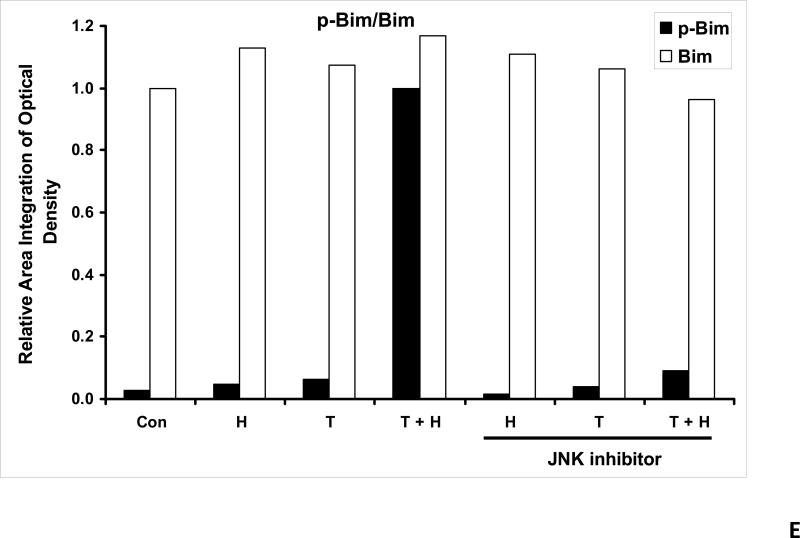
We previously reported that differential cleavage of Mst1 by caspase-7 and caspase-3 is responsible for TRAIL-induced activation of the MAPK superfamily [Song and Lee, 2008]. We hypothesized that hyperthermia facilitates TRAIL-induced JNK activation. JNK-mediated Bim phosphorylation potentiates Bax oligomerization and subsequent cytochrome c release [Putcha et al., 2003; Zhang et al., 2008]. To test the hypothesis, phosphorylation of JNK and BimEL was examined after heat shock at 42°C for 1 h in the presence of TRAIL (10-200 ng/ml) followed by TRAIL for 1 h at 37°C. Figure 3A shows that TRAIL treatment promotes JNK activity (phosphorylation). TRAIL-induced JNK activation and PARP cleavage were markedly enhanced by hyperthermia. Similar results were observed with phosphorylation of BimEL. Densitometry analysis of the bands in Figure 3A confirms that PARP cleavage is shown to increase with hyperthermia and TRAIL combined (Fig. 3B), as well as phosphorylated JNK (Fig. 3C) and phosphorylated Bim (Fig. 3E). The densitometry analysis for JNK (Fig 3D) and actin (Fig. 3F), however, show no significant changes.
Figure 3. Effect of hyperthermia in combination with TRAIL on the JNK-Bim signal transduction pathway.
(A) CX-1 cells were exposed to hyperthermic conditions (42°C) for 1 h in the presence of TRAIL (10-200 ng/ml) and then incubated for 1 h at 37°C in the presence of TRAIL. Equal amounts of protein (20 μg) from cell lysates were separated by SDS-PAGE and immunoblotted with anti-PARP, anti-phosphorylated JNK, anti-JNK, anti-phospho-Bim, or anti-Bim antibody. Actin was used to confirm the equal amount of proteins loaded in each lane. Hyperthermia combined with moderate to high doses of TRAIL (50 – 200ng/ml) shows an increase in activation of the JNK-Bim signaling transduction pathway demonstrating apoptotic cell death. (B, C, D, E, F) Densitometry analysis of the bands from the JNK-Bim signal pathway for PARP (B), p-JNK (C), JNK (D), pBim/Bim (E), and actin (F) was performed.
Effect of hyperthermia in combination with TRAIL on the JNK-Bim signal transduction pathway after treatment with JNK-inhibitor
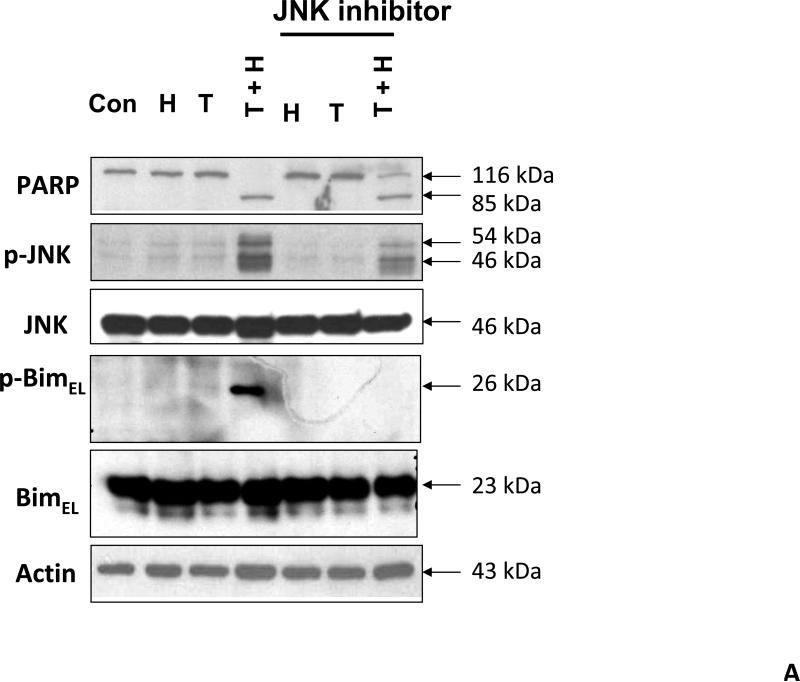
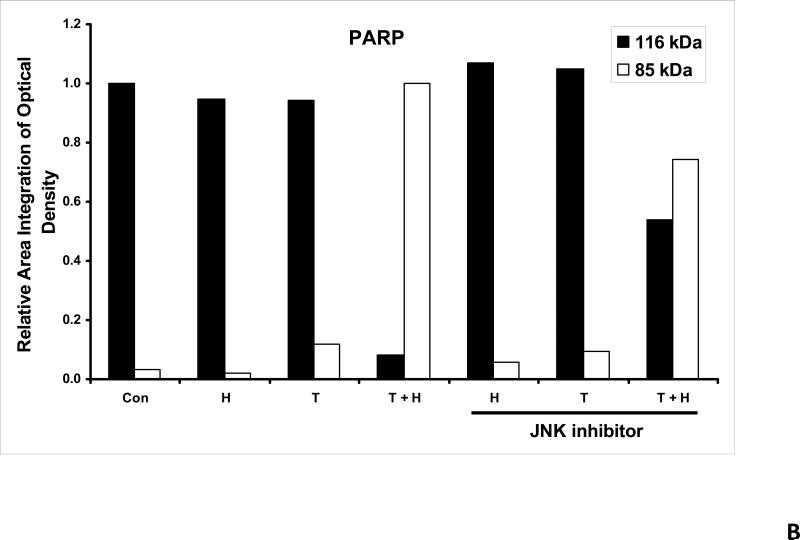
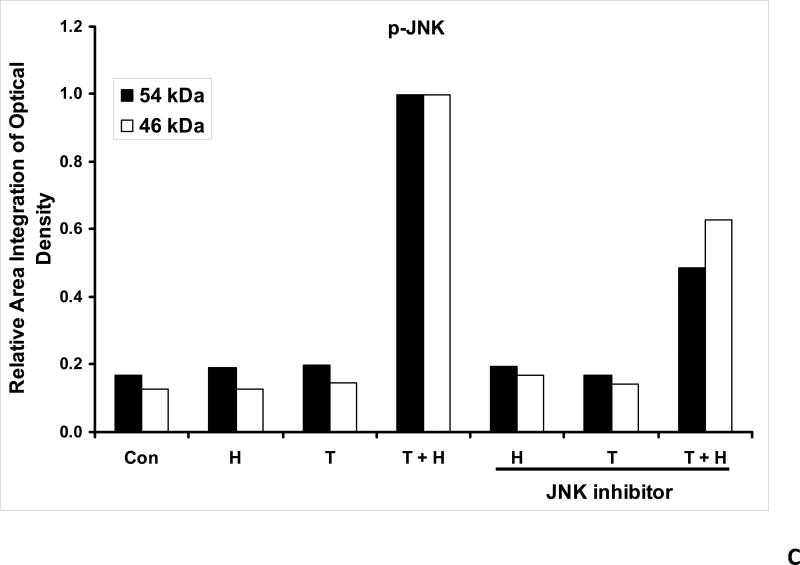
As we mentioned above, treatment with hyperthermia and TRAIL demonstrated that the JNK-Bim signaling transduction pathway was activated by the phosphorylation of JNK and BimEL. In order to prove that Bim is, in fact, directly related to JNK, we added a JNK inhibitor to our cell cultures prior to treating them. JNK inhibitor demonstrated decreases in the phosphorylation of JNK and resulted in no detection of phosphorylation of BimEL when treated with hyperthermia, TRAIL and combination of hyperthermia and TRAIL therapy (Fig. 4A). This provides evidence that phosphorylation of BimEL requires that JNK also be phosphorylated completely. Densitometry analysis of the bands in Figure 4A confirms that PARP cleavage is shown to be significantly less with JNK inhibitor added to the combined hyperthermia and TRAIL therapy when compared with hyperthermia and TRAIL without JNK inhibitor (Fig. 4B). The same observation is seen with phosphorylated JNK (Fig. 4C) and phosphorylated Bim (Fig. 4E). The densitometry analyses for JNK (Fig 4D) and actin (Fig. 4F), however, show no significant changes.
Figure 4. Effect of JNK inhibitor on hyperthermia in combination with TRAIL-activated JNK-Bim signal and apoptosis.
(A) JNK inhibitor II (50 μg/ml) was added to CX-1 cell cultures 1 h prior to exposing them to hyperthermic (42°C) conditions for 1 h in the presence of TRAIL (50 ng/ml) and then incubated for 1 h at 37°C in the presence of TRAIL. Immunoblotting assay was performed as described in Figure 3A. JNK inhibitor effectively inhibited the JNK-Bim signal and protected cells from apoptosis during treatment with hyperthermia in combination with TRAIL. (B, C, D, E, F) Densitometry analysis of the bands from the JNK-Bim signal pathway with/without JNK inhibitor for PARP (B), p-JNK (C), JNK (D), pBim/Bim (E), and actin (F) was performed.
Effect of JNK inhibitor on cell death from hyperthermia in combination with TRAIL
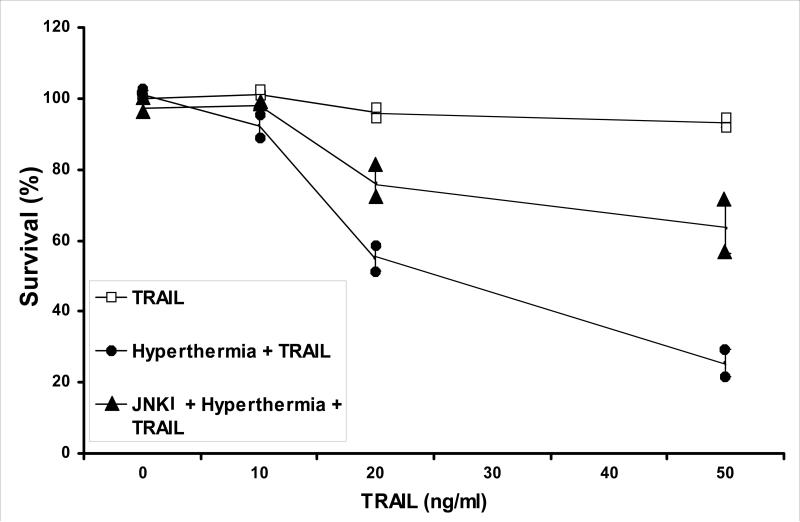
We further examined whether JNK inhibitor protects cells from hyperthermia in combination with TRAIL-induced cytotoxicity. For this study, cells preteated with JNK inhibitor II were then heated in the presence of TRAIL. As shown in Figure 5, JNK inhibitor II protected cells from hyperthermia in combination with TRAIL-induced cytotoxicity. For example, hyperthermic conditions at 42°C combined with 50 ng/ml of TRAIL has proven that this synergistic effect can kill nearly 75% of CX-1 colorectal cancer cells when compared to the nearly 5% killing effect that 50 ng/ml of TRAIL has alone (Fig. 5). Adding JNK inhibitor II to the hyperthermia / TRAIL combination therapy, we only see a killing effect of about 35% (Fig. 5). This demonstrates that the JNK inhibitor which inhibits the JNK-Bim pathway is protective of the synergistic effect of hyperthermia combined with TRAIL.
Figure 5. Effect of JNK inhibitor (JNKI) on hyperthermia in combination with TRAIL-induced cytotoxicity in CX-1 cells.
JNK inhibitor II (50 μg/ml) was added for 1 h prior to the treatment of hyperthermia (42°C) for 1 h in the presence of TRAIL (10 – 50 ng/ml). After treatment, cells were exposed to 37°C for 1 h in the presence of TRAIL (10 – 50 ng/ml). Cell survival was immediately determined by the trypan blue exclusion assay as described by Materials and Methods. Duplicate experiments were performed.
Discussion
Hyperthermia acts as a promoter in cytotoxicity of human cancer cells when combined with other modalities. Our previous studies have shown that mild hyperthermia (41-42°C) rather than acute hyperthermia (44-46°C) effectively promotes the effectiveness of TRAIL-induced cytotoxicity by facilitating activation of caspases through mitochondria-dependent cytochrome c release in human colorectal cancer cells [Yoo and Lee, 2007]. We have also demonstrated that hyperthermia works synergistically with chemotherapeutic agents like melphalan and oxaliplatin combined with TRAIL in killing cancer cells [Yoo and Lee, 2008]. In this study we were able to demonstrate that hyperthermia works synergistically with TRAIL by enhancing JNK activation. This provided similar results in cell death as well as showing a delay in growth in our in vivo mouse model of human colorectal tumors.
Several researchers reported that JNK activation leads to caspase activation and consequently apoptotic death [Fig. 3A, Chen et al., 1998; Lei, 1998]. We speculate that JNK-mediated Bim phosphorylation potentiates Bax-dependent apoptosis. Three isoforms of Bim -- BimS, BimL, and BimEL, are known to be involved in apoptosis. BimS is transiently expressed during apoptosis. In contrast, BimL and BimEL are constitutively expressed in cells. The apoptotic activity of these molecules is suppressed by binding to the dynein motor complex via an interaction with dynein light chain 1 (DLC1) [Putcha et al., 2001]. BimL and BimEL, but not BimS, contain a short peptide motif (DKSTQTP) which mediates the binding of Bim to DLC1. Previous studies revealed that the phosphorylation of threonine residue at QTP of BimL and BimEL by JNK causes the release of these proteins from dynein motor complexes [Lei and Davis, 2003]. Two main models have been proposed to explain the highly lethal activity of Bim. The released phosphorylated Bim may directly (direct model) or indirectly (displacement model) activate the Bax-dependent mitochondrial apoptotic pathway through Bax oligomerization. The apoptotic activation mechanism of Bax begins with a conformational change followed by oligomerization. The oligomerized Bax integrates in the outer mitochondria membrane, where it triggers cytochrome c release [Lee et al., 2008].
In this study, we demonstrated that hyperthermia increases TRAIL-induced apoptotic death through promoting JNK activation. A fundamental question which remains unanswered is how hyperthermia promotes JNK activation. Several researchers have revealed that hyperthermia induces an increase in reactive oxygen species (ROS) [Skibb et al., 1990; Hall et al., 1994; Frank et al., 1998; Flanagan et al., 1998; Venkataraman et al., 2004] and that increasing antioxidant enzyme levels results in protection of cells from oxidative stress [Venkataraman et al., 2004]. It is well known that ROS are generated through several intracellular sources including organellar sources, e.g., mitochondrial electron transport chain and peroxisomal cytochrome P-450 oxidases, as well as endogenous enzyme systems, e.g., plasma membrane NADPH oxidase and cytoplasmic xanthine oxidase [Gamaley and Klyubin, 1999]. Previous studies suggest that mitochondria are the main source of ROS generation during hyperthermia [Venkataraman et al., 2004]. During mitochondrial respiration, O2 acts as the terminal acceptor of electrons, with the 4- electron reduction of O2 yielding H2O. However, there exists a finite probability (which differs in different tissues) that a one-electron reduction of O2 to yield superoxide could occur [Boveris and Cadenas, 1982], probably at Site I (NADH-dehydrogenase) or Site III (ubiquinone-cytochrome b) of the electron transport chain [Boveris and Cadenas, 1982]. Superoxide then rapidly dismutes to form H2O2. It has been estimated that during normal respiration approximately 1-4% of O2 consumption results in the production of superoxide and hydrogen peroxide [Boveris and Cadenas, 1982], but during hyperthermia, disruption of the mitochondrial electron transport chain occurs and results in increase in the intracellular level of ROS. Hyperthermia in combination with TRAIL may facilitate the disruption of the mitochondrial electron transport chain and ROS generation. We previously observed that ROS can be sensed through thioredoxin (TRX) and glutaredoxin (GRX) and subsequently activate the ASK1 (Apoptosis signal-regulating kinase 1)-MEK (mitogen extracellular kinase)-MAPK (mitogen-activated protein kinase) signal transduction pathway [Lee et al., 1998; Song and Lee, 2003a; Song and Lee, 2003b]. TRX and GRX appear to act as physiological inhibitors of ASK1 by associating with the N-terminal and C-terminal portion of ASK1, respectively, and inhibiting ASK1 kinase activity [Saitoh et al., 1998; Song et al., 2002]. TRX and GRX contain two redoxactive half-cystine residues, -Cys-Gly-Pro-Cys- or -Cys-Pro-Tyr-Cys-, in an active catalytic center [Holmgren, 1989; Saitoh et al., 1998; Song et al., 2003a]. These sensor molecules may be converted to the intramolecular disulfide form of TRX-(S-S) and GRX-(S-S) during glucose deprivation. The oxidized form of TRX and GRX dissociates from ASK1 and consequently activates the ASK1-MEK-JNK signal transduction pathway [Saitoh et al., 1998; Song et al., 2002; Song et al., 2003b].
Previous studies show an elevation of the intracellular level of ROS during treatment with TRAIL [Perez-Cruz et al., 2007]. However, we could not observe the meaningful generation of ROS during TRAIL treatment (data not shown). This discrepancy needs to be clarified. Nonetheless, recently we observed that TRAIL can activate JNK in addition to caspases [Song and Lee, 2008]. TRAIL-induced JNK activation is dependent on caspase activation, and mammalian sterile 20-like kinase 1 (Mst1) functions as a mediator between caspase activation and JNK activation [Song and Lee, 2008]. Mst1 is cleaved by caspase-mediated proteolysis in response to TRAIL treatment. The C-terminus regulatory region of Mst1 contains two distinct functional domains, which are required for homo- and/or hetero-dimerization and the regulation of kinase activity. It is possible that hyperthermia promotes caspase enzyme activity and subsequently enhances JNK activity through promoting Mst1 cleavage (activation) and TRAIL-induced apoptotic death. We believe that many critical questions still remain to be answered to understand the mechanism of the promotion of JNK activation during hyperthermia in combination with TRAIL. However, this model will provide a framework for future studies.
Based on these studies, we now know that these two modalities, hyperthermia and TRAIL, delays the onset of tumor growth in subcutaneous tumors. We also know that cell survival is decreased when those therapies are administered. The combination of hyperthermia and TRAIL offer a great advantage in killing human colorectal cancer cells and decreasing tumor growth. They need to be applied in conjunction with specific delivery vehicles and infused directly into the liver, perhaps by way of hepatic perfusion which leads to regional therapy, in metastatic animal models. Gannon et al [2007 and 2008] with the use of gold nanoparticles and the external radiofrequency concept provides another approach in which this combination therapy could be applied. The effects of external hyperthermia along with TRAIL could lend another approach to our future studies. We have come to the conclusion that our data will serve as the backdrop to these future studies.
Acknowledgments
2This work was supported by the following grants: NCI grant funds (CA95191, CA96989, CA121395, and CA140554: K.P., J.Y., D.H.L, Y.J. L.) and (CA113263: M.A.A., D.L.B., Y.J.L.)
Abbreviations used in this paper
- DLC1
dynein light chain 1
- DISC
death-inducing signaling complex
- DR4
TRAIL-R1
- DR5
TRAIL-R2
- FADD
Fas-associated death domain
- FasL
Fas/APO-1 ligand
- IHP
isolated hepatic perfusion (IHP)
- JNK
c-Jun N-terminal Kinase
- PAGE
polyacrylamide gel electrophoresis
- PARP
poly (ADP-ribose) polymerase
- PBS
phosphate-buffered saline
- SDS
sodium dodecyl sulfate
- TNF
tumor necrosis factor
- TRAIL
tumor necrosis factor-related apoptosis-inducing ligand
References
- Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett DL, Libutti SK, Figg WD, Fraker DL, Alexander HR. Isolated hepatic perfusion for unresectable hepatic metastases from colorectal cancer. Surgery. 2001;129:176–187. doi: 10.1067/msy.2001.110365. [DOI] [PubMed] [Google Scholar]
- Boveris A, Cadenas E. Production of superoxide radicals and hydrogen peroxide in mitochondria. In: Oberley LW, editor. Superoxide Dismutase. II. CRC Press Inc.; Boca Raton, FL: 1982. pp. 15–30. [Google Scholar]
- Chen YR, Wang W, Kong AN, Tan TH. Molecular mechanisms of c-Jun N-terminal kinase-mediated apoptosis induced by anticarcinogenic isothiocyanates. J Biol Chem. 1998;2733:1769–1775. doi: 10.1074/jbc.273.3.1769. [DOI] [PubMed] [Google Scholar]
- Erkmen K, Egorin MJ, Reyno LM, Morgan R, Jr., Doroshow JH. Effects of storage on the binding of carboplatin to plasma proteins. Cancer Chemother Pharmacol. 1995;35:254–256. doi: 10.1007/BF00686557. [DOI] [PubMed] [Google Scholar]
- Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein E, Rosen GM, Rauckman EJ. Spin trapping of superoxide and hydroxyl radical: practical aspects. Arch. Biochem. Biophys. 1980;200:1–16. doi: 10.1016/0003-9861(80)90323-9. [DOI] [PubMed] [Google Scholar]
- Finkelstein E, Rosen GM, Rauckman EJ, Paxton J. Spin trapping of superoxide. Mol. Pharmacol. 1979;16:676–685. [PubMed] [Google Scholar]
- Finkelstein E, Rosen GM, Rauckman EJ. Production of hydroxyl radical by decomposition of superoxide spin-trapped adducts. Mol Pharmacol. 1982;21:262–265. [PubMed] [Google Scholar]
- Flanagan SW, Moseley PL, Buettner GR. Increased flux of free radicals in cells subjected to hyperthermia: detection by electron paramagnetic resonance spin trapping. FEBS Lett. 1998;431:285–286. doi: 10.1016/s0014-5793(98)00779-0. [DOI] [PubMed] [Google Scholar]
- Frank J, Kelleher DK, Pompella A, Thews O, Biesalski HK, Vaupel P. Enhancement of oxidative cell injury and antitumor effects of localized 44°C hyperthermia upon combination with respiratory hyperoxia and xanthine oxidase. Cancer Res. 1998;58:2693–2698. [PubMed] [Google Scholar]
- Gamaley IA, Klyubin IV. Roles of reactive oxygen species: Signaling and regulation of cellular functions. Int Rev Cytol. 1999;188:203–238. doi: 10.1016/s0074-7696(08)61568-5. [DOI] [PubMed] [Google Scholar]
- Gannon CJ, Patra CR, Bhattacharya R, Mukherjee P, Curley SA. Intracellular gold nanoparticles enhance non-invasive radiofrequency thermal destruction of human gastrointestinal cancer cells. J Nanobiotechnol. 2008;6:2. doi: 10.1186/1477-3155-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon CJ, Cherukuri P, Yakobson BI, Cognet L, Kanzius JS, Kittrell C, Weisman RB, Pasquali M, Schmidt HK, Smalley RE, Curley SA. Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field. Cancer. 2007;110:2654–2665. doi: 10.1002/cncr.23155. [DOI] [PubMed] [Google Scholar]
- Gong XM, Choi J, Franzin CM, Zhai D, Reed JC, Marassi FM. Conformation of membrane-associated proapoptotic tBid. J Biol Chem. 2004;279:28954–28960. doi: 10.1074/jbc.M403490200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MR, Hill HA, Okolow-Zubkowska MJ, Segal AW. The production of hydroxyl and superoxide radicals by stimulated human neutrophils- measurements by EPR spectroscopy. FEBS Lett. 1979;100:23–26. doi: 10.1016/0014-5793(79)81123-0. [DOI] [PubMed] [Google Scholar]
- Hagen TL, Eggermont AM. Tumor vascular therapy with TNF: critical review on animal models. Methods Mol Med. 2004;98:227–246. doi: 10.1385/1-59259-771-8:227. 2004. [DOI] [PubMed] [Google Scholar]
- Hall DM, Buettner GR, Matthes RD, Gisolfi CV. Hyperthermia stimulates nitric oxide formation: electron paramagnetic resonance detection of.NO-heme in blood. J Appl Physiol. 1994;77:548–553. doi: 10.1152/jappl.1994.77.2.548. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989;264:13963–13966. [PubMed] [Google Scholar]
- Ishii S, Mizoi T, Kawano K, Cay O, Thomas P, Nachman A, Ford R, Shoji Y, Kruskal JB, Steele G, Jr, Jessup JM. Implantation of human colorectal carcinoma cells in the liver studied by in vivo fluorescence videomicroscopy. Clin Exp Metastasis. 1996;14:153–164. doi: 10.1007/BF00121212. [DOI] [PubMed] [Google Scholar]
- Janicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem. 1998;273:9357–9360. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- Kato S, Sadarangani A, Lange S, Villalon M, Branes J, Brosens JJ, Owen GI, Cuello M. The oestrogen metabolite 2-methoxyoestradiol alone or in combination with tumour necrosis factor-related apoptosis-inducing ligand mediates apoptosis in cancerous but not healthy cells of the human endometrium. Endocr Relat Cancer. 2007;14:351–368. doi: 10.1677/ERC-07-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley SK, Harris LA, Xie D, Deforge L, Totpal K, Bussiere J, Fox JA. Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. J Pharmacol Exp Ther. 2001;299:31–38. [PubMed] [Google Scholar]
- Kim WJ, Rajasekaran B, Brown KD. MLH1- and ATM-dependent MAPK signaling is activated through c-Abl in response to the alkylator N-methyl-N'-nitro-N'-nitrosoguanidine. J Biol Chem. 2007;282:32021–32031. doi: 10.1074/jbc.M701451200. [DOI] [PubMed] [Google Scholar]
- Kischkel FC, Lawrence DA, Chuntharapai A, Schow P, Kim KJ, Ashkenazi A. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity. 2000;12:611–620. doi: 10.1016/s1074-7613(00)80212-5. [DOI] [PubMed] [Google Scholar]
- Kwon SJ, Song JJ, Lee YJ. Signal pathway of hypoxia-inducible factor-1alpha phosphorylation and its interaction with von Hippel-Lindau tumor suppressor protein during ischemia in MiaPaCa-2 pancreatic cancer cells. Clin Cancer Res. 2005;11:7607–7613. doi: 10.1158/1078-0432.CCR-05-0981. [DOI] [PubMed] [Google Scholar]
- Lee DH, Kim C, Zhang L, Lee YJ. Role of p53, PUMA, and Bax in wogonin-induced apoptosis in human cancer cells. Biochem Pharmacol. 2008;75:2020–2033. doi: 10.1016/j.bcp.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Froelich CJ, Fujita N, Tsuruo T, Kim JH. Reconstitution of caspase-3 confers low glucose-enhanced tumor necrosis factor-related apoptosis-inducing ligand cytotoxicity and Akt cleavage. Clin Cancer Res. 2004;10:1894–1900. doi: 10.1158/1078-0432.ccr-03-0136. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Galoforo SS, Berns CM, Chen JC, Davis BH, Sim JE, Corry PM, Spitz DR. Glucose deprivation-induced cytotoxicity and alterations in mitogen-activated protein kinase activation are mediated by oxidative stress in multidrug-resistant human breast carcinoma cells. J Biol Chem. 1998;273:5294–5299. doi: 10.1074/jbc.273.9.5294. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Hou Z, Curetty L, Cho JM, Corry PM. Synergistic effects of cytokine and hyperthermia on cytotoxicity in HT-29 cells are not mediated by alteration of induced protein levels. J Cell Physiol. 1993;155:27–35. doi: 10.1002/jcp.1041550105. [DOI] [PubMed] [Google Scholar]
- Lei K, Nimnual A, Zong WX, Kennedy NJ, Flavell RA, Thompson CB, Bar-Sagi D, Davis RJ. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH(2)-terminal kinase. Mol Cell Biol. 2002;22:4929–4942. doi: 10.1128/MCB.22.13.4929-4942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci USA. 2003;100:2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W, Yu R, Mandlekar S, Kong AN. Induction of apoptosis and activation of interleukin 1beta-converting enzyme/Ced-3 protease (caspase-3) and c-Jun NH2-terminal kinase 1 by benzo(a)pyrene. Cancer Res. 1998;58:2102–2106. [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnermri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Maroney AC, Glickman MA, Basma AN, Walton KM, Knight E, Jr, Murphy CA, Bartlett BA, Finn JP, Angeles T, Matsuda Y, Neff NT, Dionne CA. Motoneuron apoptosis is blocked by CEP-1347 (KT 7515), a novel inhibitor of the JNK signaling pathway. J Neurosci. 1998;18:104–111. doi: 10.1523/JNEUROSCI.18-01-00104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh KJ, Barbuto S, Meyer N, Kim RS, Collier RJ, Korsmeyer SJ. Conformational changes in BID, a pro-apoptotic BCL-2 family member, upon membrane binding. A site-directed spin labeling study. J Biol Chem. 2005;280:753–767. doi: 10.1074/jbc.M405428200. [DOI] [PubMed] [Google Scholar]
- Park SM, Schickel R, Peter ME. Nonapoptotic functions of FADD-binding death receptors and their signaling molecules. Curr Opin Cell Biol. 2005;17:610–616. doi: 10.1016/j.ceb.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Perez-Cruz I, Cárcamo JM, Golde DW. Caspase-8 dependent TRAIL-induced apoptosis in cancer cell lines is inhibited by vitamin C and catalase. Apoptosis. 2007;12:225–234. doi: 10.1007/s10495-006-0475-0. [DOI] [PubMed] [Google Scholar]
- Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo2 Ligand, a new member of the tumor necrosis factor receptor family. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- Putcha GV, Le S, Frank S, Besirli CG, Clark K, Chu B, Alix S, Youle RJ, LaMarche A, Maroney AC, Johnson EM., Jr JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron. 2003;38:899–914. doi: 10.1016/s0896-6273(03)00355-6. [DOI] [PubMed] [Google Scholar]
- Putcha GV, Moulder KL, Golden JP, Bouillet P, Adams JA, Strasser A, Johnson EM. Induction of BIM, a proapoptotic BH3-only BCL-2 family member, is critical for neuronal apoptosis. Neuron. 2001;29:615–628. doi: 10.1016/s0896-6273(01)00238-0. [DOI] [PubMed] [Google Scholar]
- Ridge JA, Bading JR, Gelbard AS, Benua RS, Daly JM. Perfusion of colorectal hepatic metastases. Relative distribution of flow from the hepatic artery and portal vein. Cancer 1. 1987;59(9):1547–1553. doi: 10.1002/1097-0142(19870501)59:9<1547::aid-cncr2820590903>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Rietbroek RC, van de Vaart PJ, Haveman J, Blommaert FA, Geerdink A, Bakker PJ, Veenhof CH. Hyperthermia enhances the cytotoxicity and platinum-DNA adduct formation of lobaplatin and oxaliplatin in cultured SW 1573 cells. J Cancer Res Clin Oncol. 1997;123:6–12. doi: 10.1007/BF01212608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge JA, Sigurdson ER, Daly JM. Distribution of fluorodeoxyuridine uptake in the liver and colorectal hepatic metastases of human beings after arterial infusion. Surg Gynecol Obstet. 1987;164(4):319–323. [PubMed] [Google Scholar]
- Rosen GM, Freeman BA. Detection of superoxide generated by endothelial cells. Proc Natl Acad Sci USA. 1984;81:7269–7273. doi: 10.1073/pnas.81.23.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruers T, Bleichrodt RP. Treatment of liver metastases, an update on the possibilities and results. Eur J Cancer. 2002;38(7):1023–1033. doi: 10.1016/s0959-8049(02)00059-x. [DOI] [PubMed] [Google Scholar]
- Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seol DW, Billiar TR. A caspase-9 variant missing the catalytic site is an endogenous inhibitor of apoptosis. J Biol Chem. 1999;274:2072–2076. doi: 10.1074/jbc.274.4.2072. [DOI] [PubMed] [Google Scholar]
- Skibba JL, Powers RH, Stadnicka A, Kalbfleisch JH. Lipid peroxidation caused by hyperthermic perfusion of rat liver. Biochem Pharmacol. 1990;40:1411–1414. doi: 10.1016/0006-2952(90)90411-d. [DOI] [PubMed] [Google Scholar]
- Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri ES, Green DR, Martin SJ. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–92. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JJ, Lee YJ. Differential role of glutaredoxin and thioredoxin in metabolic oxidative stress-induced activation of apoptosis signal-regulating kinase 1. Biochem J. 2003a;73:845–853. doi: 10.1042/BJ20030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JJ, Lee YJ. Catalase, but not MnSOD, inhibits glucose deprivation-activated ASK1-MEK-MAPK signal transduction pathway and prevents relocalization of Daxx: hydrogen peroxide as a major second messenger of metabolic oxidative stress. J Cell Biochem. 2003b;90:304–314. doi: 10.1002/jcb.10619. [DOI] [PubMed] [Google Scholar]
- Song JJ, Lee YJ. Daxx deletion mutant (amino acids 501-625)-induced apoptosis occurs through the JNK/p38-Bax-dependent mitochondrial pathway. J Cell Biochem. 2004;92:1257–1270. doi: 10.1002/jcb.20155. [DOI] [PubMed] [Google Scholar]
- Song JJ, Lee YJ. Differential cleavage of Mst1 by caspase-7/-3 is responsible for TRAIL-induced activation of the MAPK superfamily. Cell Signal. 2008;20:892–906. doi: 10.1016/j.cellsig.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JJ, Rhee JG, Suntharalingam M, Walsh SA, Spitz DR, Lee YJ. Role of glutaredoxin in metabolic oxidative stress. Glutaredoxin as a sensor of oxidative stress mediated by H2O2. J Biol Chem. 2002;277:46566–46575. doi: 10.1074/jbc.M206826200. [DOI] [PubMed] [Google Scholar]
- Song YK, Billiar TR, Lee YJ. Role of Galectin-3 in Breast Cancer Metastasis: Involvement of Nitric Oxide. Am J Pathol. 2002;160:1069–1075. doi: 10.1016/S0002-9440(10)64927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan JM, Fajardo LF, Hahn GM. Mechanism of antitumor activity of tumor necrosis factor alpha with hyperthermia in a tumor necrosis factor alpha-resistant tumor. J Natl Cancer Inst. 1990;82:1904–1910. doi: 10.1093/jnci/82.24.1904. [DOI] [PubMed] [Google Scholar]
- Sun XM, MacFarlane M, Zhuang J, Wolf BB, Green DR, Cohen GM. Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. J Biol Chem. 1999;274:5053–5060. doi: 10.1074/jbc.274.8.5053. [DOI] [PubMed] [Google Scholar]
- Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, Iwakura Y, Yagita H, Okumura K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat. Med. 2001;7:94–100. doi: 10.1038/83416. [DOI] [PubMed] [Google Scholar]
- Thomas LR, Henson A, Reed JC, Salsbury FR, Thorburn A. Direct binding of Fas-associated death domain (FADD) to the tumor necrosis factor-related apoptosis-inducing ligand receptor DR5 is regulated by the death effector domain of FADD. J Biol Chem. 2004;279:32780–32785. doi: 10.1074/jbc.M401680200. [DOI] [PubMed] [Google Scholar]
- Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita KI, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- Vahrmeijer AL, van Dierendonck JH, Keizer HJ, Beijnen JH, Tollenaar RA, Pijl ME, Marinelli A, Kuppen PJ, van Bockel JH, Mulder GJ, van de Velde CJ. Increased local cytostatic drug exposure by isolated hepatic perfusion: A phase I clinical and pharmacologic evaluation of treatment with high dose melphalan in patients with colorectal cancer confined to the liver. Br J Cancer. 2000;82(9):1539–1546. doi: 10.1054/bjoc.2000.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varfolomeev E, Maecker H, Sharp D, Lawrence D, Renz M, Vucic D, Ashkenazi A. Molecular determinants of kinase pathway activation by Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand. J Biol Chem. 2005;280:40599–40608. doi: 10.1074/jbc.M509560200. [DOI] [PubMed] [Google Scholar]
- Venkataraman S, Wagner BA, Jiang X, Wang HP, Schafer FQ, Ritchie JM, Patrick BC, Oberley LW, Buettner GR. Overexpression of manganese superoxide dismutase promotes the survival of prostate cancer cells exposed to hyperthermia. Free Radic Res. 2004;38:1119–1132. doi: 10.1080/10715760400010470. [DOI] [PubMed] [Google Scholar]
- Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin RG, Rauch CT, Schuh JCL, Lynch DH. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N, Timour MS, Gerhart MJ, Schooley KA, Smith CA, Goodwin RG, Rauch CT. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 1997;16:5386–5397. doi: 10.1093/emboj/16.17.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wang M, Kar S, Carr BI. Involvement of ATM-mediated Chk1/2 and JNK kinase signaling activation in HKH40A-induced cell growth inhibition. J Cell Physiol. 2009 doi: 10.1002/jcp.21844. In press. [DOI] [PubMed] [Google Scholar]
- Woynarowski JM, Faivre S, Herzig MC, Arnett B, Chapman WG, Trevino AV, Raymond E, Chaney SG, Vaisman A, Varchenko M, Juniewicz PE. Mol Pharmacol. 2000;58:920–927. doi: 10.1124/mol.58.5.920. [DOI] [PubMed] [Google Scholar]
- Xiao D, Lew KL, Kim Y-A, Zeng Y, Hahm E-R, Dhir R, Singh SV. Diallyl trisulfide suppresses growth of PC-3 human prostate cancer xenograft in vivo in association with Bax and Bak induction. Clin. Cancer Res. 2006;12:6836–6843. doi: 10.1158/1078-0432.CCR-06-1273. [DOI] [PubMed] [Google Scholar]
- Yang XH, Sladek TL, Liu X, Butler BR, Froelich CJ, Thor AD. Reconstitution of caspase-3 sensitizes MCF-7 breast cancer cells to doxorubicin- nd etoposide-induced apoptosis. Cancer Res. 2001;61:348–354. [PubMed] [Google Scholar]
- Yoo J, Kim HR, Lee YJ. Hyperthermia enhances tumour necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in human cancer cells. Int J Hyperthermia. 2006;22:713–728. doi: 10.1080/02656730601074052. [DOI] [PubMed] [Google Scholar]
- Yoo J, Lee Y. Effect of hyperthermia on TRAIL-induced apoptotic death in human colon cancer cells: development of a novel strategy for regional therapy. J Cell Biochem. 2007;101:619–630. doi: 10.1002/jcb.21203. [DOI] [PubMed] [Google Scholar]
- Yoo L, Lee Y. Effect of hyperthermia and chemotherapeutic agents on TRAIL-induced cell death in human colon cancer cells. J Cell Biochem. 2008;103:98–109. doi: 10.1002/jcb.21389. [DOI] [PubMed] [Google Scholar]
- Zamboni WC, Gervais AC, Egorin MJ, Schellens JH, Hamburger DR, Delauter BJ, et al. Inter- and intratumoral disposition of platinum in solid tumors after administration of cisplatin. Clin Cancer Res. 2002;8:2992–2999. [PubMed] [Google Scholar]
- Zeh HJ, III, Brown CK, Holtzman MP, Egorin MJ, Holleran JL, Potter DM, Bartlett DL. A phase I study of hyperthermic isolated hepatic perfusion with oxaliplatin in the treatment of unresectable liver metastases from colorectal cancer. Ann Surg Oncol. 2009;16:385–394. doi: 10.1245/s10434-008-0179-5. [DOI] [PubMed] [Google Scholar]
- Zhang L, Xing D, Chen M. Bim(L) displacing Bcl-x(L) promotes Bax translocation during TNFalpha-induced apoptosis. Apoptosis. 2008;13:950–958. doi: 10.1007/s10495-008-0226-5. [DOI] [PubMed] [Google Scholar]