Abstract
The use of Abs that induce tumor cell death together with immunostimulatory reagents to activate innate and adaptive immune cells has emerged as a potent approach for the treatment of cancer. We have previously demonstrated that the use of three mAbs (anti-DR5, anti-CD40, anti-CD137) termed TriMab can induce rejection in a majority of mice with established experimental or carcinogen-induced tumors. However, given the potential toxicity of CD40 agonists in the clinic, we tested an alternative approach to directly activate/mature APCs using anti-CD1d mAbs. In this study, we used a combination of three mAbs (anti-DR5, anti-CD137, anti-CD1d) that we termed 1DMab and demonstrated that this approach suppressed and/or eradicated established experimental renal, breast, and colon carcinomas in mice. Tumor suppression induced by 1DMab therapy required CD8+ T cells, IFN-γ, and CD1d, while NK cells and IL-12 were partially required. Interestingly 1DMab therapy was more effective than TriMab in tumor models regulated by CD1d-restricted type II NKT cells, but less efficacious against tumors where T regulatory cells were critical. Anti-CD1d mAbs could also be relatively effective in combination with anti-CD137 and conventional chemotherapeutics. This is the first study to illustrate the antitumor activity of CD1d-reactive mAbs in combination and our results strongly suggest that rational combination chemoimmunotherapies based on tumor immunoregulation may improve the efficacy of treatment.
The use of agents that induce immunogenic tumor cell death along with immunomodulating mAbs is emerging as a potent combination approach for the treatment of established cancer. We previously demonstrated that a rational combination of three mAbs termed TriMab induced rejection in a majority of mice with established experimental or carcinogen-induced tumors and this was dependent on CD8+ T cells and the cytokine IFN-γ (1). TriMab therapy consists of the agonistic mAb targeting TRAIL receptor 2 (DR5) inducing TRAIL-sensitive tumor apoptosis, anti-CD40 mAb to mature dendritic cells (DCs),3 and anti-CD137 mAb (4-1BB) to costimulate/activate CD8+ T cells. Importantly, given reports of CD40 agonist toxicities in clinical trials (2, 3), we felt it was critical to examine whether replacing anti-CD40 mAb in the combination therapy could be achieved with other agents capable of activating/maturing DCs.
Type I NKT cells express an invariant TCR Vα14Jα18 (in mice) and are functionally defined by reactivity with CD1d loaded with the glycolipid α-galactosylceramide (α-GC) (4). Type I NKT cells have been reported to play a protective role in natural and α-GC-induced tumor immunity (5, 6). Recently, we reported that CD1d-reactive glycolipid ligands that activate type I NKT cells can effectively substitute for anti-CD40 mAb in the combination therapy approach (7). The combination of α-GC or a more potent analog with anti-DR5 mAb and anti-CD137 mAb (termed NKTMab therapy) eradicated established tumors and metastases in a majority of mice. Similar to TriMab, tumor rejection induced by NKTMab therapy required CD8+ T cells and IFN-γ. However, unlike TriMab therapy, host CD4+ T cells and type I NKT cells were also critical.
Although the use of CD1d-reactive glycolipid ligands and invariant NKT cells was one effective alternative to anti-CD40 mAb, reduced numbers and defective function of type I NKT cells in tumor-bearing mice and advanced cancer patients (8–11) suggest that poor host type I NKT cell responsiveness might limit the success of this approach. Furthermore, another type of CD1d-restricted NKT cell, termed type II, appears to immunosuppress effector T cell responses, including those reactive with tumor (12). Type II NKT cells do not express the invariant TCR Vα14Jα18 and are poorly characterized due to a lack of definitive surface markers or reactive glycolipid ligands that identify them (13). Importantly, this differential activity of type I and II NKT cells raised the possibility that specific targeting of the CD1d molecule may have the ability to modulate tumor growth.
CD1d is expressed prominently and constitutively on professional APCs such as DCs, macrophages, and B cells (14). In addition, CD1d expression can be detected on some solid tissue cells and non-Ag-presenting hemopoietic cells (14). Several years ago, we demonstrated that anti-human CD1d mAbs alone were capable of maturing human peripheral blood monocytes and immature DCs in vitro as measured by the secretion of IL-12p70 (15). More recently, we demonstrated that anti-mouse CD1d mAbs induced specific release of IL-12p70 from F4/80+ and class II+CD1d+ APCs, but not B or T cells, and serum IL-12, IFN-γ, and IFN-α were detected after anti-CD1d administration (16). Interestingly, anti-mouse CD1d mAb also demonstrated modest antitumor activity against experimental s.c. tumors, but surprisingly this activity was enhanced against tumors where type II CD1d-restricted T cells were postulated to suppress the effector response (16). Given the known requirement of IL-12 for activation of NK cells, type I NKT cells, and T cells downstream of DCs (17), we reasoned that anti-CD1d mAb may sufficiently mature DCs in vivo, while potentially interfering with the function of type II CD1d-restricted NKT cells.
To determine whether these properties of anti-CD1d mAb were beneficial in combination therapy, we compared the antitumor efficacy of anti-CD1d mAb in combination with anti-DR5 and anti-CD137 (termed 1DMab) against TriMab therapy in three different established s.c. tumor models; R331 renal carcinoma, 4T1 mammary carcinoma, and CT26L5 colon adenocarcinoma. These were chosen because they do not express CD1d and type II NKT cells are known to play a role in immune suppression in the 4T1 and CT26L5 tumor models (12), whereas regulatory T cells suppress natural immune responses to R331 tumors. Herein, we demonstrated that anti-mouse CD1d mAb, in 1DMab therapy, effectively substituted for anti-CD40 mAb to induce rejection of established tumors. Furthermore, 1DMab therapy was specifically more efficacious than TriMab therapy in the eradication of 4T1 and CT26L5 tumors, as opposed to R331 tumors. 1DMab-induced tumor rejection was completely dependent on CD8+ T cells, IFN-γ, and CD1d and partially dependent on NK cells and IL-12. Tailoring rational combination immunotherapies based on tumor immunoregulation may improve the efficacy of treatment.
Materials and Methods
Mice
Inbred BALB/c mice were either bred at the Peter MacCallum Cancer Centre (Peter Mac) or purchased from the Walter and Eliza Hall Institute of Medical Research. BALB/c CD1d gene-targeted (CD1d−/−) (12) and Jα18 (Jα18−/−) gene-targeted mice (12) were bred and maintained at the Peter Mac. Both strains of gene-targeted mice were backcrossed onto the BALB/c background for at least 10 generations and genotyped by PCR and phenotyped using flow cytometry. Mice >6 wk old were used in all experiments. Female mice were used for all experiments with the 4T1 tumor and all experiments were performed in accordance with guidelines set out by the Peter Mac Animal Experimentation Ethics Committee.
Tumor cell lines
BALB/c-derived CD1d-negative TRAIL-sensitive 4T1 mammary carcinoma (18, 19), the TRAIL-sensitive Renca variant R331 (18, 20), and the carcinogen-induced colon carcinoma line Colon-26 (CT26L5) (12) were maintained as described previously.
Antibodies
We prepared and purified agonistic mAb to mouse DR5 (MD5.1), agonistic mAb to mouse CD40 (FGK45; provided by A. Rolink, University of Basel, Basel, Switzerland), agonistic mAb to mouse 4-1BB (CD137; 3H3), and mAb reactive with mouse CD1d (1B1) (21). Depleting anti-mouse CD4 (GK1.5) and anti-CD8 (53–6.72) mAb, neutralizing mAb to mouse IL-12 (C17.8) and IFN-γ (H22) were prepared and used as previously described (7). Anti-asialo GM1 (ASGM1) for depletion of NK cells was obtained from Wako Pure Chemical. All Abs used were from eBioscience unless otherwise stated. Abs used for flow cytometry included PE-anti-CD25 (PC61.5), PE-anti-CD62L (MEL-14; BD Pharmingen), PE-anti-CD8a (53–6.7), allophycocyanin-anti-CD8a (53–6.7), and allophycocyanin-Alexa Fluor 750-anti-CD4 (RM4-5). For FOXP3 staining, cells were first stained with Abs to the appropriate markers, followed by staining for intracellular FOXP3 with FITC-anti-FOXP3 (FJK-16a) according to the manufacturer’s instructions (eBioscience). Flow cytometry was performed using a FACSCanto and analyzed on FCS Express (BD Biosciences).
Flow cytometry and intracellular cytokine staining
Groups of BALB/c mice were inoculated s.c. with 4T1 tumors (2 × 105) and treated with TriMab, 1DMab therapy, or control Ig (cIg) at days 7 and 11 after tumor inoculation. Four days after the second treatment, we harvested the draining inguinal and opposite inguinal lymph nodes from individual mice. Single-cell suspensions were generated and incubated with plate-bound CD3-specific mAb (clone 145-2C11; 0.5 μg/well of 24-well tissue culture plate; BD Pharmingen) for 16 h. GolgiStop (BD Pharmingen) was added for the last 2 h of culture. Then we analyzed intracellular IFN-γ using allophycocyanin-conjugated mouse IFN-γ-specific mAb according to the manufacturer’s instructions (BD Pharmingen) on electronically gated CD8+ or CD4+ T cells. To assess the activation status of T cells following TriMab or 1DMab therapy or cIg, a similar experiment was set up as above. Single-cell suspensions were generated and stained with Abs to the appropriate surface markers as indicated. In some samples, cells were also stained intracellularly for FOXP3.
Therapy of transplanted tumors
Groups of five BALB/c wild-type (WT), BALB/c Jα18−/−, or BALB/c CD1d−/− mice were inoculated s.c. with either 5 × 105 R331 tumor cells, 1 × 105 CT26L5, or 2 × 105 4T1 tumor cells on day 0. On the days indicated after tumor inoculation, groups of mice were treated i.p. with the following: cIg (300 μg), TriMab therapy (100 μg each of anti-DR5, anti-CD40, and anti-CD137 mAbs), NKTMab therapy (100 μg each of anti-DR5, anti-CD40 mAbs, and 100 ng of α-c-galactosylceramide), 1DMab therapy (100 μg each of anti-DR5 and anti-CD137, and the indicated dose of anti-CD1d) or a combination of anti-CD137 and anti-DR5 mAbs. In some experiments, mice were depleted of CD4+ (GK1.5; 100 μg), CD8+ (53–6.7; 100 μg), a combination of CD4+ and CD8+ T cells (100 μg each), or NK cells (anti-ASGM1; 100 μg) on days as indicated (days 7, 14, and 21 or 10, 17, and 24). In some experiments, IFN-γ or IL-12 were neutralized with mAbs (250 μg each) starting on day 7 or 10 and then given twice weekly until day 24 or 26, respectively. In some experiments, mice were either injected with a single dose of anti-DR5 mAb alone (100 μg), a single dose of gemcitabine (GEM; 60 mg/kg i.v.; Lilly Research Laboratories) alone, a single dose of doxorubicin (Dox; 2 mg/kg i.v.; Pharmacia) alone, or 5-fluorouracil (5-FU; 40 mg/kg i.p; Commonwealth Serum Laboratories) for the first 5 consecutive days alone or each in combination with anti-CD1d (200 μg, three times) and anti-CD137 (100 μg, three times). Tumor size was measured using a caliper and recorded as the product of two perpendicular diameters (cm2). Sera were harvested from 4T1 tumor-bearing mice 1 day after treatment as previously described and serum transaminase was measured (7).
Statistical analysis
Statistical significance of tumor growth or tumor-free survival was assessed using a Mann-Whitney U test or log-rank test, respectively (p < 0.05).
Results
1DMab therapy induces the rejection of established R331 tumors
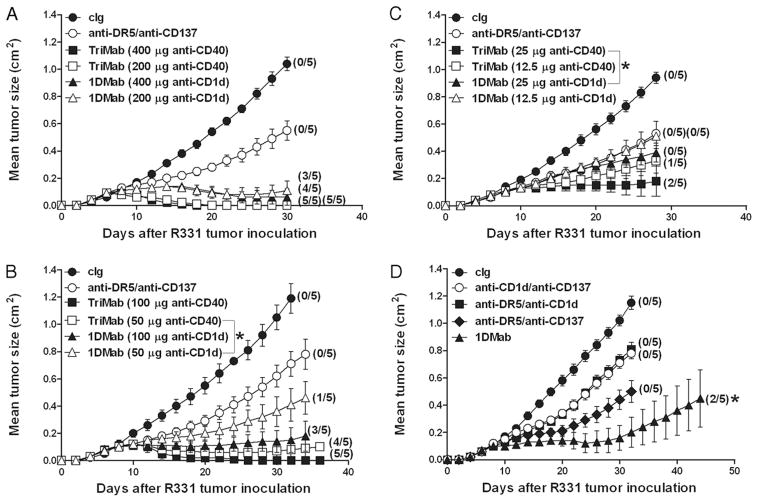
Recently, we demonstrated that the IgG anti-CD1d mAb (1B1) activated class II+ DCs and F4/80+ macrophages, stimulated an increase in serum IL-12, IFN-γ, and IFN-α levels, and modestly inhibited established tumor growth as a single agent in several different experimental tumor models (16). Based on the clear agonistic activity of anti-mouse CD1d mAbs, we substituted original anti-CD40 in the TriMab (anti-DR5/anti-CD40/anti-CD137) for anti-CD1d and called this new therapy 1DMab (anti-DR5/anti-CD1d/anti-CD137). To compare their agonistic activities in the combination therapy, we titrated the doses of anti-CD1d or anti-CD40 (400-12.5 μg) while maintaining a constant dose of anti-DR5 and anti-CD137 at 100 μg/injection (three times for each mAb). We began comparing the antitumor efficacy of 1DMab and TriMab therapy in groups of BALB/c mice with established day 7 s.c. R331 renal carcinomas (~0.1 cm2; Fig. 1, A–C).
FIGURE 1.
TriMab is more effective than 1DMab against day 7 established R331 tumors. Groups of BALB/c mice (n = 5) were inoculated s.c. with the renal carcinoma cell line R331 (5 × 105). On days 7, 11, and 15 after tumor inoculation, mice were i.p. treated with either 1DMab (anti-DR5/anti-CD137/anti-CD1d; ▲ and △ ), or TriMab (anti-DR5/anti-CD137/anti-CD40; ■ and □) therapy, or with anti-DR5/anti-CD137 (○), or cIg (●). Decreasing doses of anti-CD1d or anti-CD40 (400, 200, 100, 50, 25, and 12.5 μg) were used in the 1DMab or TriMab therapy (A–C). One hundred microgram each of anti-DR5 and anti-CD137 mAbs was used in either TriMab or 1DMab therapy. Statistical differences in survival between 1DMab-treated mice or TriMab-treated mice containing the corresponding dose of anti-CD1d or anti-CD40 mAbs were determined by log-rank test (*, p < 0.05). D, On days 7, 11, and 15 after tumor inoculation, groups of mice were treated with either 1DMab (▲), or anti-CD1d (200 μg)/anti-CD137 (○), or anti-DR5/anti-CD1d (200 μg; ■), or anti-DR5/anti-CD137 (◆), or cIg (●). Statistical differences in tumor sizes between mice treated with 1DMab or any two combinations of mAb from the 1DMab therapy were determined by Mann-Whitney U test (*, p < 0.05). Tumor sizes are represented as the mean ± SEM of all mice and tumor rejection rates are indicated in parentheses. Representative of two independent experiments.
Using a 400-μg dose of anti-CD1d or anti-CD40 mAb, 1DMab therapy induced a relatively similar rate of tumor eradication as TriMab therapy (80% vs 100%; Fig. 1A). Similar results were obtained using a 200-μg dose of anti-CD1d or anti-CD40 mAb. Treatment with anti-DR5, anti-CD1d, or anti-CD137 alone (data not shown; previously in Teng et al. (7)) or in anti-DR5/anti-CD137 in combination (Fig. 1, A–C), using the same dose and regimen, had a comparatively minor effect (0% cures). Subsequent titration of anti-CD1d mAb (100 –50 μg) in the 1DMab therapy still resulted in strong tumor inhibition and eradication compared with mice treated with anti-DR5/anti-CD137 (20–60% vs 0%; Fig. 1B). However, TriMab therapy containing similar doses of anti-CD40 (100 or 50 μg) induced a greater proportion of cures (80–100%; Fig. 1B). Further titration of anti-CD1d (25 or 12.5 μg) in the 1DMab therapy of tumor-bearing mice only resulted in a similar rate of tumor growth inhibition as mice treated with anti-DR5/anti-CD137 (Fig. 1C). By contrast, TriMab therapies containing 25 or 12.5 μg of anti-CD40 mAbs were still able to induce eradication of tumors in 20 – 40% of mice (Fig. 1C). At a dose of 200 μg of anti-CD1d mAb, 1DMab induced 40% R331 tumor rejection, whereas any dual combination of the constituents failed to cure mice (Fig. 1D). Clearly, these data suggested that anti-CD40 mAb was more effective than anti-CD1d when used in combination for the treatment of established s.c. R331 renal carcinomas. Given these dose titration result, and that higher doses of anti-CD1d mAb were not practical to easily manufacture, we decided in all subsequent experiments to use 1DMab therapy containing 200 μg of anti-CD1d to compare against TriMab therapy.
TriMab is more efficacious than 1DMab therapy against more established R331 tumors
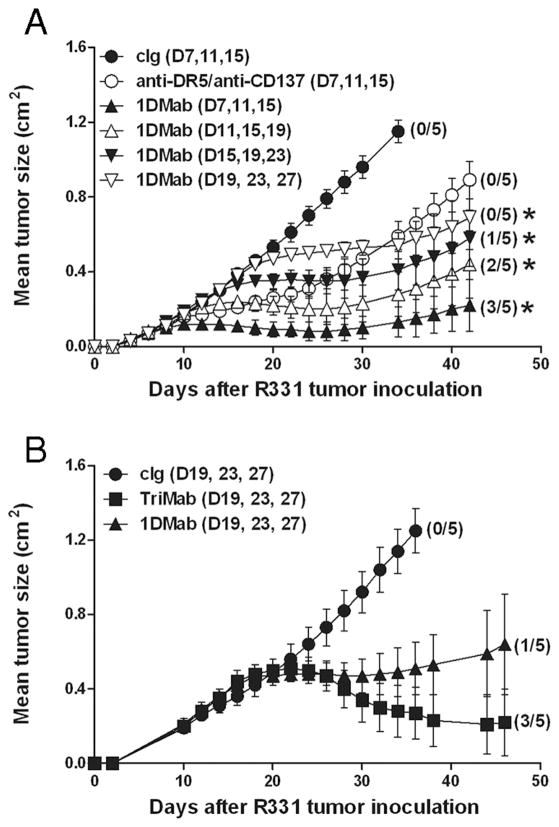
Since day 7 (0.1-cm2) s.c. R331 tumors were still relatively small, we next examined the antitumor efficacy of 1DMab therapy against larger s.c. R331 tumors (Fig. 2). 1DMab therapy commencing at day 7 resulted in 60% tumor rejection as previously demonstrated, whereas later treatments commencing at day 11 or day 15 resulted in 40 or 20% tumor rejection, respectively (Fig. 2A). Further delay of treatment of tumor-bearing mice until day 19 resulted in no cures, but rather only suppression of tumor growth similar to groups treated early (day 7) with anti-DR5/anti-CD137 mAb (Fig. 2A). When comparing the antitumor efficacy of 1DMab and TriMab therapy against established day 19 R331 tumors (~0.5 cm2), TriMab therapy cured 60% of tumor-bearing mice compared with 1DMab therapy that caused only 20% tumor rejection (Fig. 2B). Delaying of TriMab therapy until day 27 (~0.7 cm2) further illustrated its greater tumor suppression than 1DMab therapy (data not shown). Notably, in all experiments, tumor-bearing mice cured after treatment with 1DMab or TriMab therapy remained tumor free for >150 days.
FIGURE 2.
TriMab is more effective against larger R331 tumors than 1DMab therapy. Groups of BALB/c mice (n = 5) were inoculated s.c. with the renal carcinoma cell line R331 (5 × 105). A and B, After tumor inoculation, mice were i.p. treated either with TriMab therapy (100 μg each of anti-DR5, anti-CD137, and 200 μg of anti-CD40) (■), or 1DMab therapy (100 μg each of anti-DR5, anti-CD137, and 200 μg of anti-CD1d; ▲, △, ▼, and ▽), or anti-DR5/anti-CD137 (○), or cIg (●) at the time points indicated in parentheses. Tumor sizes are represented as the mean ± SEM of all mice and tumor rejection rates are indicated in parentheses. Representative of two (A) or three (B) independent experiments. Statistical differences in tumor sizes between either cIg-treated mice or 1DMab-treated mice or 1DMab-treated mice and TriMab-treated mice were determined by Mann-Whitney U test (*, p < 0.05).
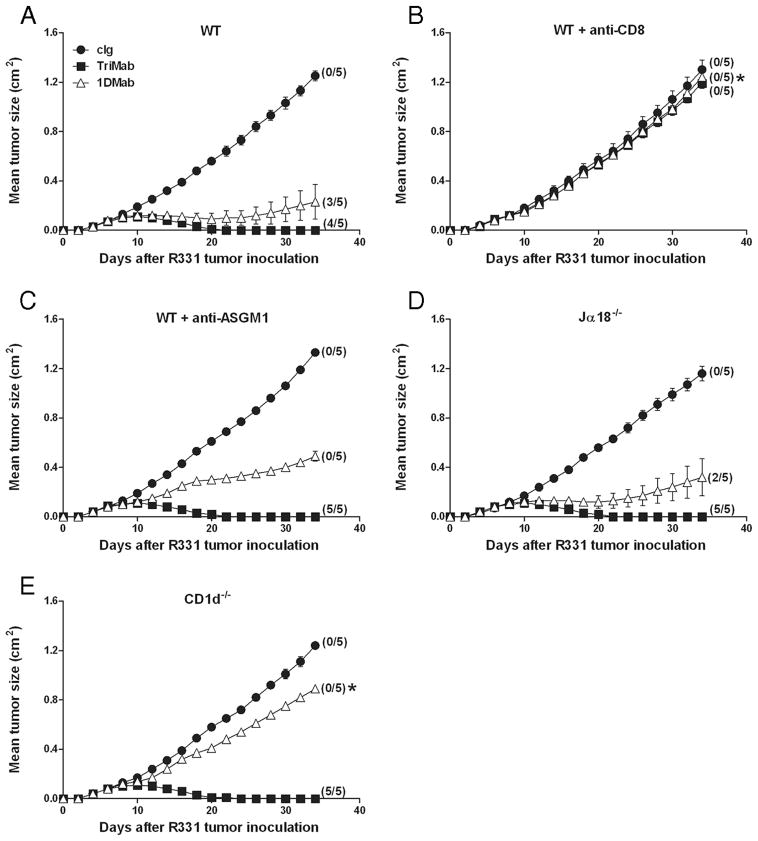
1DMab-mediated R331 tumor rejection is dependent on CD8+ T cells, CD1d, and IFN-γ
Given that we demonstrated that 1DMab induced R331 tumor rejection, albeit at a lower efficacy than TriMab therapy, we next wanted to determine whether there were any differences in the cell subsets and cytokines required for effective 1DMab vs TriMab therapy. Groups of BALB/c WT mice (Fig. 3, A–C), BALB/c Jα18−− (Fig. 3D), or CD1d−− mice (Fig. 3E) were inoculated s.c. with R331 tumor cells. In some groups of WT mice, anti-CD8 (Fig. 3B)- or anti-ASGM1 (Fig. 3C)-depleting Abs were administered on days 7, 14, and 21. Seven days after tumor inoculation, mice were then treated with cIg, TriMab therapy, or 1DMab therapy. 1DMab significantly suppressed R331 tumor growth and cured 60% of the treated group. By contrast, in CD8 T cell-depleted tumor-bearing mice, 1DMab therapy had no effect on tumor growth ( p = 0.0112; Fig. 3B), whereas in NK cell-depleted tumor-bearing mice, a very substantial level of tumor suppression was achieved, but no mice were cured ( p = 0.3976; Fig. 3C). These data demonstrated that 1DMab-mediated rejection of R331 tumors was completely dependent on CD8+ T cells and partially dependent on NK cells. It was not possible to assess CD4+ T cell depletion since both R331 and its parental Renca are tumors whose growth is completely dependent on CD4+ regulatory T cells (our unpublished data). 1DMab therapy induced similar rates of tumor rejection in BALB/c Jα18−− and WT tumor-bearing mice, demonstrating that type I NKT cells were not critical ( p = 0.5038; Fig. 3D). Not surprisingly, 1DMab treatment of CD1d−− tumor- bearing mice did not result in any tumor eradication, demonstrating the requirement for CD1d ( p = 0.0112; Fig. 3E). Consistent with our previous reports (1, 7), TriMab therapy was dependent on CD8+ T cells, but did not require NK cells, type I NKT cells, or CD1d (Fig. 3).
FIGURE 3.
Rejection of R331 tumors by 1DMab therapy requires CD8+ T cells and CD1d. Groups of BALB/c WT (A–C), BALB/c Jα18−− (D), or BALB/c CD1d−− (E) mice (n = 5) were inoculated with the renal carcinoma cell line, R331 (5 × 105). On days 7, 11, and 15 after tumor inoculation, mice were treated with either 1DMab therapy (containing 200 μg of anti-CD1d; △ ), TriMab (containing 200 μg of anti-CD40; ■) therapy, or cIg (●). Additionally, some groups of mice were also i.p. injected on days 0, 7, 14, and 21 with either CD8-depleting mAbs (B) or anti-ASGM1 Ab (C). Tumor sizes are represented as the mean ± SEM of all mice and tumor rejection rates are indicated in parentheses. Representative of two independent experiments. Statistical differences in tumor sizes between WT mice injected with 1DMab therapy or gene-targeted or mAb-treated mice injected with 1DMab therapy were determined by Mann-Whitney U test (*, p < 0.05).
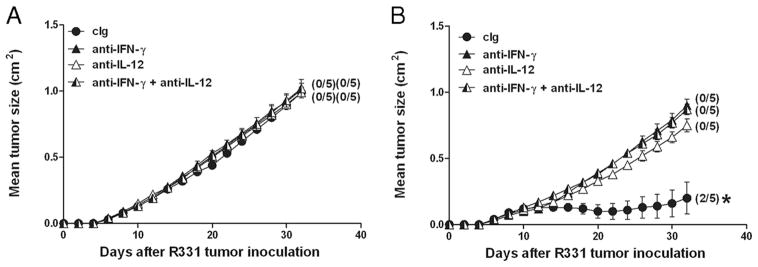
We next wanted to assess the critical cytokines for 1DMab-mediated rejection of R331 tumors (Fig. 4). Groups of R331 tumor-bearing mice were injected with either neutralizing anti-IFN-γ, anti-IL-12, anti- IFN-γ, and IL-12 or cIg mAbs on day 7 before being treated with cIg (Fig. 4A) or 1DMab therapy (Fig. 4B). Neither IFN-γ and/or IL-12 neutralization affected established R331 tumor growth (Fig. 4A). Both IFN-γ and IL-12 were critical for tumor rejection by 1DMab therapy since no cures were observed in tumor-bearing mice neutralized with either: anti-IFN-γ, anti-IL-12, or both mAbs (p = 0.0119, 0.0119, and 0.0119, respectively). 1DMab therapy of tumor-bearing mice not injected with cytokine- neutralizing mAbs resulted in 60% tumor rejection as previously demonstrated.
FIGURE 4.
Requirement for IL-12 and IFN-γ in 1DMab-induced rejection of R331 tumors. Groups of BALB/c mice (n = 5) were inoculated with the renal carcinoma cell line R331 (5 × 105). On days 7, 11, and 15 after tumor inoculation, mice were treated with cIg (A; ●) or 1DMab therapy (B) (containing 200 μg of anti-CD1d mAbs; ▲ and △ ). Additionally, some groups of mice were also i.p. injected on day 7 and then twice weekly with either anti-IL-12, anti- IFN-γ, or anti-IL-12 and anti- IFN-γ-neutralizing Abs or cIg. Tumor sizes are represented as the mean ± SEM of all mice and tumor rejection rates are indicated in parentheses. Representative of two independent experiments. Statistical differences in tumor sizes between WT mice injected with 1DMab therapy or mAb-treated mice injected with 1DMab therapy were determined by Mann-Whitney U test (*, p < 0.05).
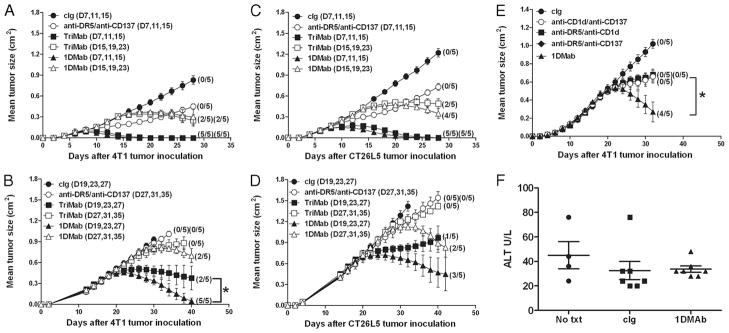
1DMab therapy is more effective against established 4T1 and CT26L5 tumors
We next compared the antitumor efficacy of 1DMab vs TriMab therapy against established 4T1 and CT26L5 tumors (Fig. 5). Whereas the growth of R331 tumors is controlled by regulatory T cells, the growth of 4T1 and CT26L5 tumors is known to be regulated by type II NKT cells (12). Given that the 1B1 anti-mouse CD1d mAb displayed agonistic activity, but can also block CD1d and therefore potentially inhibit NKT cell activation, we hypothesized that 1DMab might have superior antitumor efficacy in tumors where type II NKT cells were controlling tumor growth. Interestingly, treatment of mice with day 7 or day 15 established 4T1 tumors with either 1DMab or TriMab therapy resulted in similar cure rates (Fig. 5A) and, strikingly, tumor rejection rates of 100% were still achieved when 1DMab therapy was delayed until day 19 in contrast to only a 40% tumor rejection rate in similar groups of tumor-bearing mice treated with TriMab therapy (Fig. 5B). Impressively, further delay of 1DMab therapy until day 27 (~0.78 cm2) still induced 40% tumor rejection compared with no cures following TriMab therapy (Fig. 5B). To determine whether this improved effect translated to another tumor model, we then compared the antitumor efficacy of 1DMab vs TriMab therapy against established s.c. CT26L5 tumors (Fig. 5, C and D). Tumor-bearing mice treated with either 1DMab or TriMab therapy at day 7 resulted in 100% cures while delaying of treatment until day 15 resulted in 80 or 40% cures, respectively (Fig. 5C). 1DMab therapy commencing at day 19 resulted in 60% tumor eradication compared with TriMab therapy that induced 20% cures (Fig. 5D). Impressively, 1DMab therapy commencing at day 27 (~1 cm2) still eradicated tumors in 40% of mice, whereas TriMab therapy commencing at day 27 was largely ineffective. When comparing the antitumor efficacy of 1DMab therapy (against established day 19 4T1) to any two agents, 1DMab therapy enabled cures (80% tumor rejection), whereas each dual combination displayed only moderate tumor suppression and no cures (p < 0.05; Fig. 5E). In addition to being more effective against larger tumors, no significant toxicities were observed in mice following 1DMab therapy against 4T1 tumors as measured by both observing the mice and measuring serum alanine transferase (Fig. 5F) and aspartate aminotransferase (data not shown) levels.
FIGURE 5.
1DMab therapy is more effective than TriMab therapy against established 4T1 and CT26L5 tumors. Groups of BALB/c mice (n = 5) were inoculated with either the mammary carcinoma cell line 4T1 (2 × 105; A, B, E, and F) or the colon carcinoma cell line CT26L5 (1 × 105; C and D). After tumor inoculation, mice were i.p. treated either with TriMab therapy (containing 200 μg of anti-CD40; ■ and □), 1DMab therapy (containing 200 μg of anti-CD1d; ▲ and △ ), or anti-DR5/anti-CD137 (○) or with cIg (●) at the time points indicated in parentheses. Statistical differences in survival between 1DMab-treated mice and TriMab-treated mice receiving the corresponding dose of anti-CD1d or anti-CD40 mAbs were determined by log-rank test (*, p < 0.05). E, On days 19, 23, and 27 after tumor inoculation groups of mice were treated with either 1DMab (▲), or anti-CD1d (200 μg)/anti-CD137 (○), or anti-DR5/anti-CD1d (200 μg; ■), or anti-DR5/anti-CD137 (◆), or cIg (●). Statistical differences in survival between mice treated with 1DMab or any two combinations of mAb from the 1DMab therapy were determined by log-rank test (*, p < 0.05). Tumor sizes are represented as the mean ± SEM of all mice and tumor rejection rates are indicated in parentheses. F, Serum was harvested 1 day later from untreated (No txt), cIg-treated, or 1DMab-treated mice bearing 4T1 tumors and alanine transferase (U/L) was measured. Results show the mean ± SEM of four mice per group.
Rejection of 4T1 or CT26L5 tumors by 1DMab requires CD8+ T cells and IFN-γ
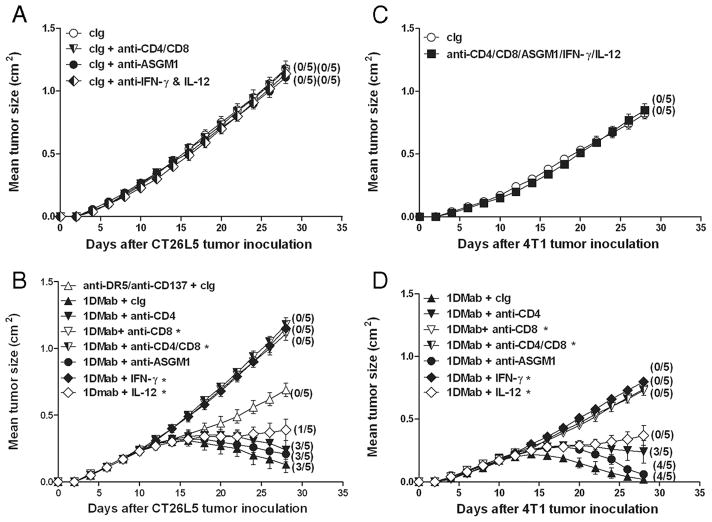
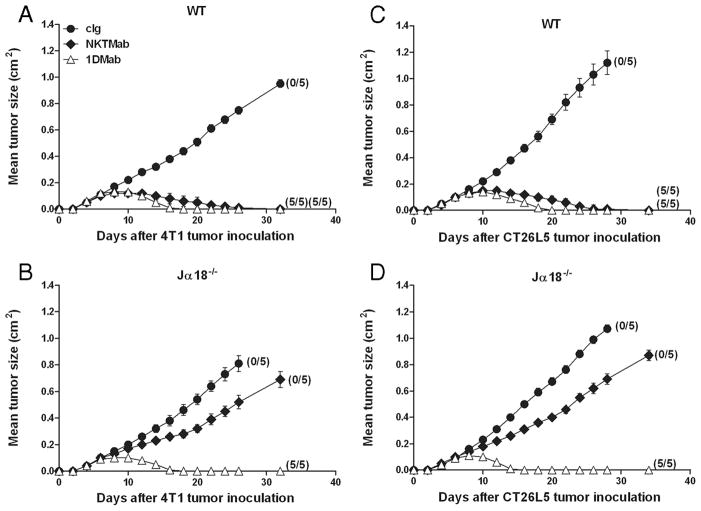
Given that the therapeutic efficacy of 1DMab therapy was more efficacious against 4T1 and CT26L5 tumors compared with R331 tumors, we next examined whether the cell subsets and cytokines involved in rejection of all three tumors by 1DMab therapy were similar (Fig. 6). Groups of BALB/c mice were inoculated with CT26L5 (Fig. 6, A and B) or 4T1 (Fig. 6, C and D) tumors. Before 1DMab therapy commencing on day 11, groups of tumor-bearing mice were depleted of either NK cells, CD4+, CD8+, or CD4+ and CD8+ T cells or were injected with neutralizing Abs to IFN-γ, IL-12, or cIg. CT26L5 or 4T1 tumor-bearing mice depleted of CD4+ and CD8+ T cells, NK cells, and/or neutralized for IFN-γ and IL-12 displayed similar tumor growth curves to those groups of mice that had received cIg alone (Fig. 6, A and C). 1DMab therapy of CT26L5 or 4T1 tumor-bearing mice depleted of either CD8+ T cells or neutralized for IFN-γ resulted in complete loss of tumor suppression and no eradication (p = 0.0119, 0.0119; Fig. 6, B and D). Thus, CD8+ T cells and IFN-γ were critical for 1DMab-induced rejection of CT26L5 and 4T1 tumors, just as they were key for 1DMAb-mediated rejection of R331 tumors. However, unlike R331 tumors, 1DMab therapy of CT26L5 and 4T1 tumors did not require NK cells ( p = 0.2652; Fig. 6, B and D). In addition, while IL-12 was absolutely required for 1DMab-induced rejection of R331 tumors, 1DMab therapy of CT26L5 and 4T1 tumor-bearing mice neutralized for IL-12 still resulted in tumor growth suppression but no cures ( p = 0.0362), demonstrating in some tumor models that IL-12 was not as critical. Depletion of CD4+ T cells played a minor role, since 1DMab therapy induced reduced levels of CT26L5 and 4T1 tumor suppression compared with cIg-treated tumor-bearing mice treated with 1DMab (Fig. 6, B and D). Similar to the R331 tumor model, 1DMab therapy of 4T1 or CT26L5 tumors did not require type I NKT cells (Fig. 7). These data demonstrated that 1DMab therapy was more effective than TriMab therapy against 4T1 and CT26L5 tumors and that only CD8+ T cells and the cytokine IFN-γ were critical for tumor eradication.
FIGURE 6.
Rejection of 4T1 and CT26L5 tumors by 1DMab therapy requires CD8+ T cells and IFN-γ. Groups of BALB/c mice (n = 5) were inoculated with either the colon carcinoma cell line CT26L5 (1 × 105; A and B) or the mammary carcinoma cell line 4T1 (2 × 105; C and D). On days 11, 15, and 19 after tumor inoculation, mice were treated with either 1DMab therapy (containing 200 μg of anti-CD1d; B and D), anti-DR5/anti-CD137 (B), or cIg (A and C). Additionally, some groups of mice were also i.p. injected on day 10 and then twice weekly with either anti-CD4 or anti-CD8 or anti-CD4/ CD8-depleting mAbs, or ASGM1 Ab, or anti-IL-12- and anti- IFN-γ-neutralizing mAbs, or a combination of these Abs or cIg. Tumor sizes are represented as the mean ± SEM of all mice and tumor rejection rates are indicated in parentheses. Statistical differences in tumor sizes between mice injected with 1DMab therapy or mAb-treated mice injected with 1DMab therapy were determined by Mann-Whitney U test (*, p < 0.05).
FIGURE 7.
4T1 and CT26L5 tumor rejection by 1DMab therapy does not require type I NKT cells. Groups of BALB/c WT (A and C) and BALB/c Jα18−− (B and D) mice (n = 5) were inoculated with either the mammary carcinoma cell line 4T1 (2 × 105; A and B) or the colon carcinoma cell line CT26L5 (1 × 105; C and D). On days 7, 11, and 15 after tumor inoculation, groups of mice were treated with either 1DMab (△), NKTMab (containing 100 ng α-c-galactosylceramide; ◆), or cIg (●). Tumor sizes are represented as the mean ± SEM of all mice and tumor rejection rates are indicated in parentheses.
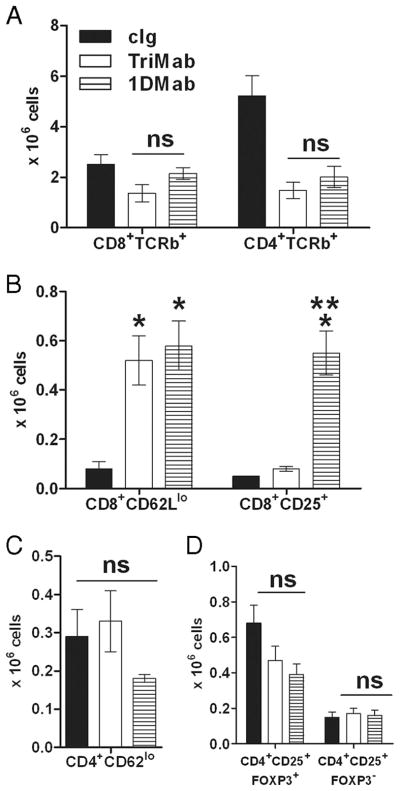
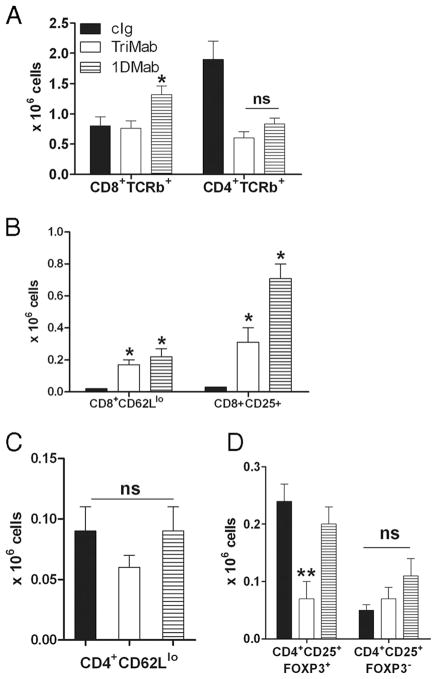
One possibility was that 1DMab therapy effectively increased the number or activation status of CD8+ T cells, even more than that induced by TriMab therapy (1). We chose to assess the immune response in the tumor-draining lymph nodes (DLN), since the tumors in cIg, 1DMab, and TriMab treatment groups rapidly differed in size and the harvest of infiltrating leukocytes was not reproducible. To measure activated T cells in the tumor DLN, groups of 4T1 tumor-bearing mice were treated with 1DMab, TriMab, or cIg on days 7 and 11 after tumor inoculation (Fig. 8). Single-cell suspensions were generated from tumor DLN of 4T1 tumor-bearing mice 4 days after the second treatment with 1DMab, TriMab, or cIg. Although the number of CD8+TCRβ+ and CD4+TCRβ+ T cells was relatively similar in TriMab- or 1DMab-treated mice (Fig. 8A), the number of CD8+ T cells that were CD62Llow significantly increased in both TriMab- and 1DMab-treated mice compared with cIg-treated mice (Fig. 8B) and, indeed, 1DMab therapy generated a significantly greater number of activated CD8+CD25+ T cells than TriMab therapy ( p < 0.05). This increase in activated T cells may be at least one explanation as to why 1DMab therapy was more effective than TriMab therapy in the 4T1 tumor model. In addition to CD8+ T cells, we assessed the number of CD4+CD62Llow and CD4+CD25+FOXP3+/− cells following TriMab, 1DMab, or cIg therapy, but observed no significant differences between groups (Fig. 8, C and D). Comparatively, when examining R331-inoculated mice (Fig. 9) following TriMab therapy, there was a more obvious increase in CD8+ CD25+ cells (Fig. 9B) and decrease in CD4+CD25+FOXP3+ cells (Fig. 9D). These alterations in the R331 model may partially explain why TriMab was more effective than 1DMAb in this setting.
FIGURE 8.
Increase in numbers of CD8+CD25+ T cells in 4T1 tumor-bearing mice after 1DMab therapy. Groups of BALB/c WT mice (n = 4–5) were inoculated s.c. with the mammary carcinoma cell line 4T1 (2 × 105). On days 7 and 11 after tumor inoculation, groups of mice were treated with TriMab (□), 1DMab therapy (▤), or cIg (■). Four days after the second treatment, tumor DLN were harvested and single-cell suspensions were generated. Cells were stained for surface expression of CD8, CD4, and TCR-β (A); CD8, CD62L, and CD25 (B); CD4, CD62L, and CD25 (C); and CD4, CD25, and intracellularly for FOXP3 (D). Cell numbers are represented as the mean ± SEM. Similar results were obtained in two independent experiments. Statistical differences in cell numbers between mice treated with cIg and mice treated with either 1DMab therapy or TriMab (*) or cell numbers between mice treated with 1DMab therapy or TriMab therapy (**) were determined by Mann-Whitney U test (p < 0.05). ns, Not significant.
FIGURE 9.
Decrease in numbers of CD4+CD25+FOXP3+ T cells in R331 tumor-bearing mice after 1DMab therapy. Groups of BALB/c WT mice (n = 4–5) were inoculated s.c. with the renal carcinoma cell line R331 (5 × 105). On days 7 and 11 after tumor inoculation, groups of mice were treated with TriMab (□), 1DMab therapy (▤), or cIg (■). Four days after the second treatment, tumor DLN were harvested and single-cell suspensions were generated. Cells were stained for surface expression of CD8, CD4, and TCR-β (A); CD8, CD62L, and CD25 (B); CD4, CD62L, and CD25 (C); and CD4, CD25, and intracellularly for FOXP3 (D). Cell numbers are represented as the mean ± SEM. Statistical differences in cell numbers between mice treated with cIg and mice treated with either 1DMab therapy or TriMab (*) or cell numbers between mice treated with 1DMab therapy or TriMab therapy (**) were determined by Mann-Whitney U test (p < 0.05). ns, Not significant.
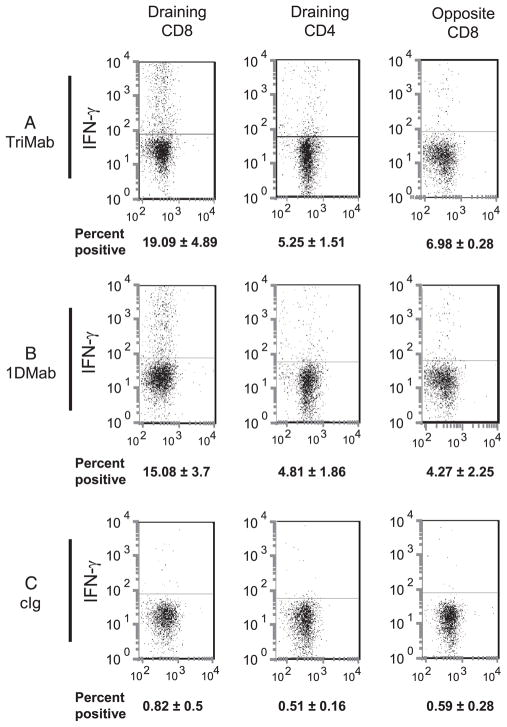
Another possibility was that 1DMab therapy effectively enhanced the level of IFN-γ production of CD8+ T cells. To measure production of IFN-γ by T cells in the tumor DLN, groups of 4T1 tumor-bearing mice were treated with 1DMab, TriMab, or cIg on days 7 and 11 after tumor inoculation (Fig. 10). Four days after the last treatment, tumor DLN and corresponding opposite lymph nodes were examined. Following overnight restimulation with CD3-specific mAb, we detected similar increases in the levels of intracellular IFN-γ in the tumor DLN of TriMab (Fig. 10A)- or 1DMab-treated mice (Fig. 10B) compared with cIg-treated mice (Fig. 10C). Similar data were obtained in mice with R331 tumors (data not shown). This suggested that the improvement in antitu-mor efficacy of 1DMAb compared with TriMab therapy in the 4T1 model was not due to enhanced production of IFN-γ. CD4+ T cells from the tumor DLN of both 1DMab- and TriMab-treated mice produced lower levels of IFN-γ equivalent to the levels observed in CD8+ T cells from the opposite nondraining lymph nodes of similarly treated mice. Consistent with our previous report (1), only background levels of IFN-γ were detected in both the DLN and nondraining lymph nodes cIg-treated mice (Fig. 10C).
FIGURE 10.
Induction of IFN-γ production by CD8+ T cells in 4T1 tumor-bearing mice after 1DMab therapy. Groups of BALB/c WT mice (n = 6–8) were inoculated s.c. with the mammary carcinoma cell line 4T1 (2 × 105). On days 7 and 11 after tumor inoculation, groups of mice were treated with TriMab (A), 1DMab therapy (B), or cIg (C). Four days after the second treatment, tumor-draining and opposite inguinal lymph nodes were harvested and intracellular IFN-γ was examined in the indicated T cell population after ex vivo CD3-mediated restimulation. The percentage of IFN-γ+ cells stained by IFN-γ-specific mAb in the indicated T cell subsets is represented as the mean ± SEM (numbers shown on x-axis) with one representative plot shown for each group. Data presented are representative of two experiments.
Anti-CD1d mAb is effective in some other combination chemoimmunotherapies
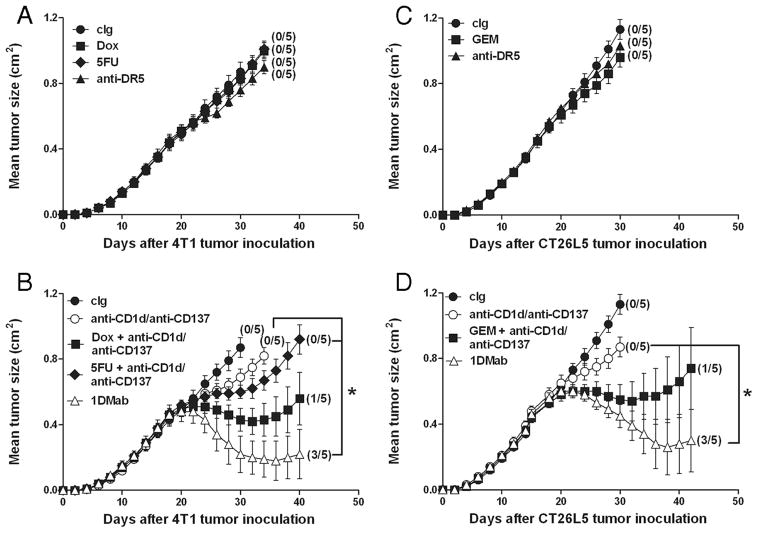
Since not all tumors are TRAIL sensitive, we wanted to examine whether anti-CD1d mAbs would also be effective against established tumors when used in combination with other broadly applicable chemotherapeutics and anti-CD137. GEM, Dox, and 5-FU are all routinely used chemotherapeutics in human cancer patients and we have previously shown the antitumor activity of GEM against CT26 tumors (1) and Dox and 5-FU against 4T1 mammary tumors (22). The experiment was designed such that the single agents anti-DR5, Dox, 5-FU, and GEM were largely without effect (Fig. 11, A and C). Although 5-FU did not appear to synergize particularly effectively with anti-CD1d and anti-CD137 mAbs ( p = 0.074; Fig. 11B), both Dox and GEM significantly improved the antitumor activity of the anti-CD1d/anti-CD137 combination ( p = 0.0212 (Dox) and 0.0362 (GEM); Fig. 11, B and D). Interestingly, in both TRAIL-sensitive tumor models, the 1DMab combination (anti-DR5/anti-CD1d/anti-CD137) was the most effective combination chemoimmunotherapy (Fig. 11, B and D).
FIGURE 11.
1DMab therapy is more effective against larger tumors compared with other combination chemoimmunotherapies. Groups of BALB/c mice (n = 5) were inoculated with either the mammary carcinoma cell line 4T1 (2 × 105; A and B) or the colon carcinoma cell line CT26L5 (1 × 105; C and D). On days 19, 23, and 27 after tumor inoculation, groups of mice were treated with either cIg (A–D), 1DMab therapy or anti-CD1d/anti-CD137 (B and D), or anti-DR5 alone (A and C), or Dox (A), 5-FU (A), GEM (C) alone, or in combination with anti-CD1d/anti-CD137 (B and D). Mice were either injected with a single dose of anti-DR5 mAb alone (100 μg), a single dose of GEM i.v. (60 mg/kg) alone, a single dose of Dox i.v. (2 mg/kg) alone, or 5-FU i.p. alone (40 mg/kg) for the first 5 consecutive days, or each in combination with anti-CD1d (200 μg on days 19, 23, and 27) and anti-CD137 (100 μg on days 19, 23, and 27). Tumor sizes are represented as the mean ± SEM of all mice and tumor rejection rates are indicated in parentheses. Statistical differences in tumor sizes between mice treated with either 1DMab therapy and mice treated with anti-CD1d/anti-CD137, Dox, 5-FU. or GEM in combination with anti-CD1d/anti-CD137 therapy were determined by Mann-Whitney U test (*, p < 0.05).
Discussion
We have made the observation that anti-CD1d mAbs may be useful antitumor agents when used in combination with chemoimmunotherapies and in the context of large established s.c. tumors, like 4T1 and CT26L5, that are controlled by regulatory type II NKT cells. 1DMab (anti-DR5/anti-CD1d/anti-CD137) therapy was more efficacious than TriMab therapy in the eradication of CT26L5 and 4T1 tumors, but less effective against R331 tumors. In this manner, anti-CD1d mAbs are a very effective substitute for anti-CD40 mAbs, particularly when tumors are regulated by CD1d and type II NKT cells. There were no adverse toxicities detected after 1DMab therapy. 1DMab-mediated tumor suppression was dependent on CD8+ T cells, IFN-γ, and CD1d in all three tumor models examined. In seeking an explanation as to why 1DMab was more effective than TriMab in the 4T1 and CT26L5 tumor models, we revealed that although 1DMab and TriMab therapy yielded similarly increased proportions of CD8+ T cells in the tumor DLN producing IFN-γ, the number of activated CD8+CD62Llow or CD8+CD25+ T cells in the tumor DLN was far greater after 1DMab therapy.
There were subtle differences in the requirements for NK cells and IL-12 depending upon the tumor model examined. In the R331 tumor model, IL-12 was completely required and NK cells were largely required for 1DMab-induced tumor suppression. By contrast, in the CT26L5 and 4T1 tumor models, IL-12 was only minimally required, while NK cells were not critical. These findings were consistent with our hypothesis that in the absence of type II NKT cell activity (i.e., R331 tumor model), only the agonistic activity of the anti-CD1d mAb was operational and thus the mechanism of tumor suppression was more dependent upon IL-12 release and effector cells that would quickly respond, such as activated CD8+ T cells and NK cells. By contrast, in the 4T1 and CT26L5 models, blockade of CD1d and type II NKT cell regulatory activity possibly more effectively releases CD8+ T effector cells and the release of IL-12 from APCs becomes less critical. Our findings suggest that incorporating anti-CD1d and anti-CD137 mAb into combinations with anti-DR5 or some conventional chemotherapeutics may enable better therapeutic outcomes in cancers regulated by type II NKT cells. Given that the type II NKT cell is so poorly studied in mice and humans, particularly compared with conventional regulatory T cells, our data provide further impetus to understand this important subset of regulatory cells. Certainly, the humanization of anti-human CD1d mAbs is now attractive, so that potential activities and toxicities can be properly assessed.
One unresolved question of mechanism of action of 1DMab therapy is what role CD1d+ DCs, macrophages, and B cells may each play. Although it is clear that only DCs and macrophages produce active IL-12p70 after anti-CD1d mAb administration (16), currently effective and selective depletion of particular CD1d+ subsets of leukocytes is not feasible in the mouse. Furthermore, we have not characterized a C57BL/6 mouse tumor model where type II NKT cells are the dominant regulatory cell and thus we have not been able to use potentially useful tools such as the μMT gene-targeted mice that lack functional B cells or CD11c-DTR-transgenic mice where CD11c+ DCs can be conditionally depleted with diphtheria toxin (23). Future studies will also be designed to determine whether the agonistic activities of anti-CD1d are largely mediated via DCs and/or B cells in a C57BL/6 tumor model.
A few other questions about the mechanism of action of 1DMab therapy are raised by this study. We know that anti-CD1d mAbs can stimulate IFN-α in addition to IL-12 and IFN-γ, but it is not clear what role type 1 IFN plays in the antitumor activity of anti-CD1d mAb. We expect that anti-CD1d mAbs are active independently of CD40L/CD40 ligation but this has not yet been formally tested. Furthermore, although type I NKT cells did not appear critical in the antitumor activity of 1DMab therapy, it is not clear whether anti-CD1d mAbs might also neutralize the activity of type I NKT cells. It may be interesting to now examine whether the 1B1 anti-CD1d mAb we have used can inhibit or potentiate glycolipid ligand-mediated antitumor activity in the same tumor models.
To simplify our mechanism studies and to concentrate on common solid malignancies, the tumor models we studied herein were by design CD1d negative. However, a large proportion of hematopoietic tumors in mice and humans do express CD1d. Whereas this is an attractive feature when considering loading such tumors with glycolipid Ags to create a viable vaccine strategy (24, 25), the ability of anti-CD1d to stimulate CD1d itself maybe very undesirable if indeed it promotes a growth advantage for the CD1d-positive neoplasm. This issue is common to any immunomodulating mAb, including anti-CD40, since a number of tumor cell types and tumor endothelium can respond directly to CD40 ligation (26). On the other hand, CD1d mAb could lead to direct depletion of CD1d+ tumors. Future study of the efficacy of 1DMab against mouse hematopoietic tumors that express CD1d will begin to determine whether agonistic activities of anti-CD1d mAb are sometimes limiting.
Some further experimental manipulation revealed that some conventional chemotherapeutics like GEM and Dox could be combined with anti-CD1d/anti-CD137 to produce active combination chemoimmunotherapies; however, 5-FU was not useful in this regard. Although anti-DR5 appeared more potent in combination than the chemotherapeutics, clearly many human tumors are relatively TRAIL resistant (27) and additional first-line therapies might be the first choice in combining with anti-CD1d mAbs.
Acknowledgments
We thank Michelle Stirling for technical assistance and Dr. Michael Kershaw for critical reading of our manuscript.
Footnotes
Disclosures
The authors have no financial conflict of interest.
Abbreviations used in this paper: DC, dendritic cell; α-GC, α-galactosylceramide; ASGM1, asialo GM1; cIg, control Ig; WT, wild type; GEM, gemcitabine; Dox, doxorubicin; 5-FU-, 5-fluoracil; Treg, regulatory T cell; DLN, draining lymph node.
M.J.S. was supported by a National Health and Medical Research Council of Australia Senior Principal Research Fellowship and a Cancer Council of Victoria Project Grant. M.W.L.T. was supported by a National Health and Medical Research Council Doherty Fellowship and the Cancer Council of Victoria. M.A.E. was supported by National Institutes of Health Grant DK66917.
References
- 1.Uno T, Takeda K, Kojima Y, Yoshizawa H, Akiba H, Mittler RS, Gejyo F, Okumura K, Yagita H, Smyth MJ. Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med. 2006;12:693–698. doi: 10.1038/nm1405. [DOI] [PubMed] [Google Scholar]
- 2.Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, Sullivan P, Mahany JJ, Gallagher M, Kramer A, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25:876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 3.Vonderheide RH, Dutcher JP, Anderson JE, Eckhardt SG, Stephans KF, Razvillas B, Garl S, Butine MD, Perry VP, Armitage RJ, et al. Phase I study of recombinant human CD40 ligand in cancer patients. J Clin Oncol. 2001;19:3280–3287. doi: 10.1200/JCO.2001.19.13.3280. [DOI] [PubMed] [Google Scholar]
- 4.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 5.Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M, Kawano T, Pelikan SB, Crowe NY, Godfrey DI. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661–668. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smyth MJ, Crowe NY, Pellicci DG, Kyparissoudis K, Kelly JM, Takeda K, Yagita H, Godfrey DI. Sequential production of interferon-γ by NK1.1+ T cells and natural killer cells is essential for the antimetastatic effect of α-galactosylceramide. Blood. 2002;99:1259–1266. doi: 10.1182/blood.v99.4.1259. [DOI] [PubMed] [Google Scholar]
- 7.Teng MW, Westwood JA, Darcy PK, Sharkey J, Tsuji M, Franck RW, Porcelli SA, Besra GS, Takeda K, Yagita H, et al. Combined natural killer T-cell based immunotherapy eradicates established tumors in mice. Cancer Res. 2007;67:7495–7504. doi: 10.1158/0008-5472.CAN-07-0941. [DOI] [PubMed] [Google Scholar]
- 8.Tahir SM, Cheng O, Shaulov A, Koezuka Y, Bubley GJ, Wilson SB, Balk SP, Exley MA. Loss of IFN-γ production by invariant NK T cells in advanced cancer. J Immunol. 2001;167:4046–4050. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- 9.Dhodapkar MV, Geller MD, Chang DH, Shimizu K, Fujii S, Dhodapkar KM, Krasovsky J. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J Exp Med. 2003;197:1667–1676. doi: 10.1084/jem.20021650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yanagisawa K, Exley MA, Jiang X, Ohkochi N, Taniguchi M, Seino K. Hyporesponsiveness to natural killer T-cell ligand α -galactosylceramide in cancer-bearing state mediated by CD11b+Gr-1+ cells producing nitric oxide. Cancer Res. 2006;66:11441–11446. doi: 10.1158/0008-5472.CAN-06-0944. [DOI] [PubMed] [Google Scholar]
- 11.Fujii S, Shimizu K, Klimek V, Geller MD, Nimer SD, Dhodapkar MV, Chang DH, Dhodapkar KM, Krasovsky J. Severe and selective deficiency of interferon-γ -producing invariant natural killer T cells in patients with myelodysplastic syndromes. Br J Haematol. 2003;122:617–622. doi: 10.1046/j.1365-2141.2003.04465.x. [DOI] [PubMed] [Google Scholar]
- 12.Terabe M, Swann J, Ambrosino E, Sinha P, Takaku S, Hayakawa Y, Godfrey DI, Ostrand-Rosenberg S, Smyth MJ, Berzofsky JA. A nonclassical non-Vα 14Jα18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med. 2005;202:1627–1633. doi: 10.1084/jem.20051381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terabe M, Berzofsky JA. NKT cells in immunoregulation of tumor immunity: a new immunoregulatory axis. Trends Immunol. 2007;28:491–496. doi: 10.1016/j.it.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 15.Yue SC, Shaulov A, Wang R, Balk SP, Exley MA. CD1d ligation on human monocytes directly signals rapid NF-κ B activation and production of bioactive IL-12. Proc Natl Acad Sci USA. 2005;102:11811–11816. doi: 10.1073/pnas.0503366102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teng MWL, Yue S, Sharkey J, Exley MA, Smyth MJ. CD1d activation and blockade: a new antitumor strategy. J Immunol. 2009;182:3366–3371. doi: 10.4049/jimmunol.0802964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujii S, Shimizu K, Hemmi H, Steinman RM. Innate Vα 14+ natural killer T cells mature dendritic cells, leading to strong adaptive immunity. Immunol Rev. 2007;220:183–198. doi: 10.1111/j.1600-065X.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 18.Takeda K, Yamaguchi N, Akiba H, Kojima Y, Hayakawa Y, Tanner JE, Sayers TJ, Seki N, Okumura K, Yagita H, Smyth MJ. Induction of tumor-specific T cell immunity by anti-DR5 antibody therapy. J Exp Med. 2004;199:437–448. doi: 10.1084/jem.20031457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol. 2002;168:1356–1361. doi: 10.4049/jimmunol.168.3.1356. [DOI] [PubMed] [Google Scholar]
- 20.Seki N, Hayakawa Y, Brooks AD, Wine J, Wiltrout RH, Yagita H, Tanner JE, Smyth MJ, Sayers TJ. Tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis is an important endogenous mechanism for resistance to liver metastases in murine renal cancer. Cancer Res. 2003;63:207–213. [PubMed] [Google Scholar]
- 21.Brossay L, Jullien D, Cardell S, Sydora BC, Burdin N, Modlin RL, Kronenberg M. Mouse CD1 is mainly expressed on hemopoietic-derived cells. J Immunol. 1997;159:1216–1224. [PubMed] [Google Scholar]
- 22.Kershaw MH, Jackson JT, Haynes NM, Teng MW, Moeller M, Hayakawa Y, Street SE, Cameron R, Tanner JE, Trapani JA, et al. Gene-engineered T cells as a superior adjuvant therapy for metastatic cancer. J Immunol. 2004;173:2143–2150. doi: 10.4049/jimmunol.173.3.2143. [DOI] [PubMed] [Google Scholar]
- 23.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimizu K, Kurosawa Y, Taniguchi M, Steinman RM, Fujii S. Cross-presentation of glycolipid from tumor cells loaded with α -galactosylcer-amide leads to potent and long-lived T cell mediated immunity via dendritic cells. J Exp Med. 2007;204:2641–2653. doi: 10.1084/jem.20070458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung Y, Qin H, Kang CY, Kim S, Kwak LW, Dong C. An NKT-mediated autologous vaccine generates CD4 T-cell dependent potent anti-lymphoma immunity. Blood. 2007;110:2013–2019. doi: 10.1182/blood-2006-12-061309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiodoni C, Iezzi M, Guiducci C, Sangaletti S, Alessandrini I, Ratti C, Tiboni F, Musiani P, Granger DN, Colombo MP. Triggering CD40 on endothelial cells contributes to tumor growth. J Exp Med. 2006;203:2441–2450. doi: 10.1084/jem.20060844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smyth MJ, Takeda K, Hayakawa Y, Peschon JJ, van den Brink MR, Yagita H. Nature’s TRAIL: on a path to cancer immunotherapy. Immunity. 2003;18:1–6. doi: 10.1016/s1074-7613(02)00502-2. [DOI] [PubMed] [Google Scholar]