The first recurrent translocation event in prostate cancer has been recently described.1 It results in the translocation of an ETS transcription factor (ERG or ETV1) to the TMPRSS2 promoter region, which contains androgen responsive elements.1 The TMPRSS:ERG genetic rearrangement has been reported to occur in approximately 40% of primary prostate tumors (ETV1 genetic rearrangements occurring at a much lower frequency) and results in an aberrant androgen regulated expression of ERG.1,2,3 While Tomlins et al.4 concluded that ETS genetic rearrangements are sufficient to initiate prostate neoplasia, we argue that ETS genetic rearrangements may in fact, represent progression events rather than initiation events in prostate tumorigenesis. To this end, we demonstrate that the prostate specific over-expression of ERG does not initiate prostate tumorigenesis.
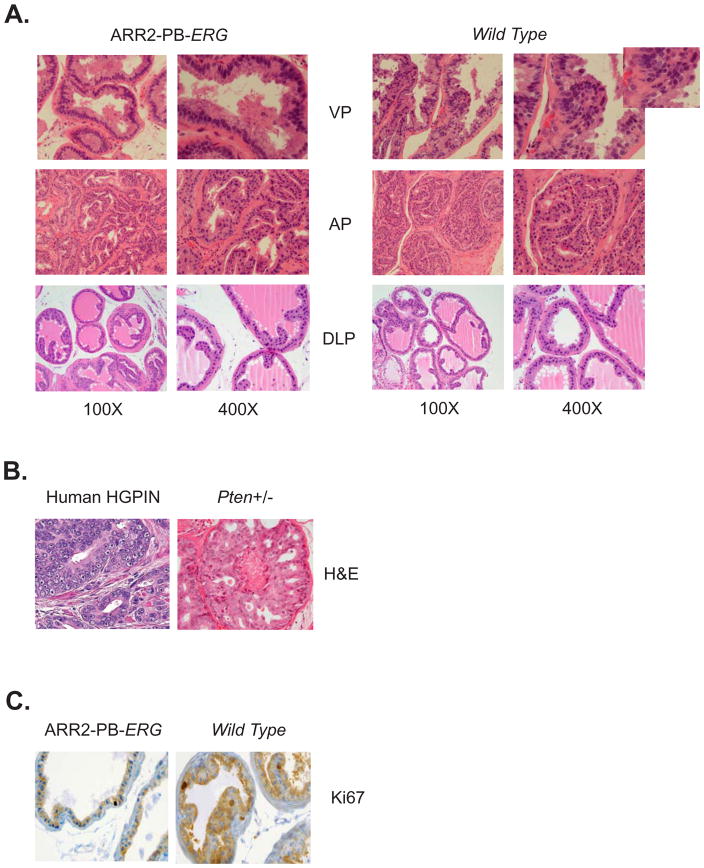
We have found that mice over-expressing ERG (expression confirmed by qRT-PCR, western blotting, and immunohistochemistry) under the control of the probasin (ARR2-PB, B6J background strain) promoter do not develop neoplasia, but only display a very subtle phenotype of nuclear atypia (prominent nucleoli) without an increase in cellular proliferation (Figure 1A). This is similar to what is shown by Tomlins et al. in their description of ETV1 transgenic mice.4 These subtle nuclear changes without an increase in cellular layers are not sufficient to be classified as prostatic intraepithelial neoplasia (PIN), and are in fact frequently observed also in the wild-type mouse prostate (Figure 1A). We observe no significant difference in the prostate phenotype across all prostatic lobes (anterior, ventral, and dorsal-lateral) between ERG transgenic and wild-type littermate mice. The subtle histologic changes observed in ETV1 and ERG transgenic mice is strikingly different from human high-grade PIN and the PIN lesions which develop in Pten heterozygous mice (Figure 1B). Additionally, we did not observe an increase in proliferative rate in the prostates of mice over-expressing ERG compared to wild-type controls. As measured by Ki67 staining, on average 1% of prostate epithelial cells in ARR2-PB-ERG mice were positive and 1% of prostate epithelial cells in wild-type mice were positive (Figure 1C). With analyses up to 18 months of age, no ARR2-PB-ERG mice have displayed any change in phenotype. While Tomlins et al. concluded that ETS genetic rearrangements are sufficient to initiate prostate neoplasia, we argue and present data that ETS genetic rearrangements may in fact, represent progression events rather than initiation events in prostate tumorigenesis, as there are no proliferative and pathological changes consistent with high-grade PIN found in either ERG or ETV1 mice.
Figure 1. Prostate specific over-expression of ERG does not induce high grade prostatic intra-epithelial neoplasia.
A total of 24 wild-type and 24 ERG transgenic mice were phenotypically characterized from one founding line after establishment that three independent founding lines produced a similar phenotype (A). Low (100X) and high (400X) power representative sections are shown for mice 6 months of age (ventral, anterior, and dorsal-lateral prostate lobes) demonstrating prominent nucleoli in wild-type mouse prostate glands. Representative histology of human high grade prostatic intra-epithelial neoplasia (HGPIN) and HGPIN in Pten heterozygous mice at 12 months of age (B). Immunohistochemical staining for Ki67 demonstrated no difference between wild-type mice and ERG transgenic mice (C).
Additionally, the ERG translocation is infrequently found in human high grade prostatic intra-epithelial neoplasia and only in a minority (approximately 10% to 20%) of patients who also have the translocation present in associated adenocarcinoma of the prostate. The majority of prostate cancer specimens with ERG genetic rearrangements do not display this rearrangement in the associated HGPIN. Therefore, the TMPRSS2:ERG translocation appears to be an early event in human prostate tumorigenesis, but one associated with progression from HGPIN to cancer.
Through mouse modeling, we have demonstrated that the aberrant expression of ERG is not sufficient to initiate neoplastic transformation but rather may cooperate with other genetic events to promote prostate cancer progression. We propose a working model whereby genetic initiating events conferring a proliferative advantage select for cooperating ETS genetic rearrangements which promote an invasive phenotype.
Methods
Mice (B6J background strain) expressing ERG under the control of the probasin (ARR2-PB) promoter were generated, genotyped, and examined for transgene expression by qRT-PCR, western blotting, and immunohistochemistry. We generated and analyzed 3 independent ERG lines. Founders were subsequently bred and 4 mice of each genotype were euthanized at 2, 4, 6, 8, 12, and 18 months of age. Prostate tissues were procured for formalin fixation, paraffin embedding, and frozen storage for future molecular analyses.
Acknowledgments
We would like to thank Drs. P. Romanienko and W. Mark from our genetically engineered mouse core facility for their assistance in generating and genotyping our prostate specific ERG mouse model. We acknowledge the Pathology, Genomic and Molecular Cytogenetics Core labs for their work with the human prostate cancer specimens. We would also like to thank Ms. R. Lester at our animal facility for caring for our mice on a daily basis, Ms. L. DiSantis for her editorial support, and Dr. C. Sawyers for stimulating discussions. We especially thank all members of the Pandolfi laboratory for their support and intellectual discussions regarding our study project. This work was funded through grants from the NIH (R01-CA82328, R01-CA84292, P50-CA92629), and MSKCC Research in Therapeutics Program in Prostate Cancer award to B.S.C. and P.P.P.
References
- 1.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–8. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 2.Tu JJ, Rohan S, Kao J, Kitabayashi N, Mathew S, Chen YT. Gene fusions between TMPRSS2 and ETS family genes in prostate cancer: frequency and transcript variant analysis by RT-PCR and FISH on paraffin-embedded tissues. Mod Pathol. 2007 doi: 10.1038/modpathol.3800903. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Perner S, Mosquera JM, Demichelis F, Hofer MD, Paris PL, Simko J, Collins C, Bismar TA, Chinnaiyan AM, De Marzo AM, Rubin MA. TMPRSS2-ERG fusion prostate cancer: an early molecular event associated with invasion. Am J Surg Pathol. 2007;31:882–8. doi: 10.1097/01.pas.0000213424.38503.aa. [DOI] [PubMed] [Google Scholar]
- 4.Tomlins SA, Bharathi L, Dhanasekaran SM, Helgeson BE, Cao X, Morris DS, Menon A, Jing A, Cao Q, Han B, Yu J, Wang L, Montie JE, Rubin MA, Pienta KJ, Roulston D, Shah RB, Varambally S, Mehra R, Chinnaiyan AM. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature. 2007;448:595–601. doi: 10.1038/nature06024. [DOI] [PubMed] [Google Scholar]