Introduction
Asthma is the most common chronic disease of childhood, affecting 10-15% of all children [1]. Several different stimuli including allergens, tobacco smoke, certain drugs, and viral or bacterial infections are known to exacerbate asthma symptoms. Among these triggers, viruses are frequent inducers of asthma exacerbations with human rhinoviruses (HRV) as the most common in children and adults [2-11]. Moreover, HRV are associated with a significant burden of lower respiratory disease [2, 12-15] and this may contribute to the development of asthma during infancy [11, 16].
HRV history
Human rhinoviruses (HRV) are members of the Picornaviridae family (‘pico’ = small, ‘rna’ = ribonucleic acid genome) that were first identified in culture in 1956 [17, 18]. More than 100 serotypes of HRV have been identified to date [17, 19-21]. HRV are the most common cause of the common cold in both adults and children. Although once thought to cause only the common cold, it is now known that rhinoviruses are associated with lower respiratory illness [4, 5, 11-15, 23-25]. In addition, HRV is an important contributor to the etiology of bronchiolitis, pneumonia, and otitis in infants and school-aged children [27-29]. During recent years, in studies using the more sensitive reverse transcriptase-polymerase chain reaction (RT-PCR), HRV have been associated with a significant burden of disease in infants and young children [26 14]. In one United States population-based surveillance study of children <5 years of age who were hospitalized with respiratory symptoms or fever, rhinovirus was detected in nasal/throat swabs by RT-PCR in 26%, representing 5 hospitalizations per 1000 children. HRV-associated hospitalization was more frequent in children <6 months of age than in those 6-23 months or 24-59 months of age (17 versus 6 versus 2 hospitalizations per 1000, respectively) [14]. Rhinoviruses are best identified by RT-PCR performed on nasal secretions from infected individuals. However, not all rhinovirus infections are associated with symptoms. HRV has been detected by RT-PCR in an average of 15% of asymptomatic individuals [22].
HRV are distributed worldwide with no predictable pattern of infection by serotype. Multiple types may be present in a community or in a person at one time; and HRV strains belonging to the same genetic cluster may be isolated during consecutive epidemic seasons, suggesting persistence in a community over an extended period. In temperate climates the incidence of HRV infection peaks in fall, with another peak in spring, but HRV infections occur year-round [30]. Rhinoviruses are the major infectious trigger for asthma among young children, and studies have described a sharp increase in asthma exacerbations in this age group when school opens each fall (often referred to as the “September asthma epidemic”) [31]. Peak HRV incidence in the tropics occurs during the rainy season from June to October.
HRV and asthma
HRV are the most common trigger of asthma exacerbations in children and adults [2-11]. In one study, population-based rates of HRV in children <5 years of age hospitalized with respiratory symptoms or fever were more frequent in children with a history of wheezing or asthma than in those without such a history (25 versus 3 hospitalizations per 1000 children). More than 80% of pediatric asthma is diagnosed before five years of age [32]. Bronchiolitis, a lower respiratory infection in infants presenting with wheezing, rales, and respiratory distress, has typically been associated with respiratory syncytial virus (RSV) infection [15-16], but recent studies have identified HRV as another important cause of bronchiolitis [15, 17-19]. A study from Greece found that the presence of rhinovirus in bronchiolitis increased the risk for developing severe disease almost five-fold [27]. Bronchiolitis caused by RSV has been linked to the subsequent development of asthma [12-14]. HRV-related wheezing in infancy also has been hypothesized to play a causal role in the development of childhood asthma [11, 16]. One study demonstrated that infants of atopic families who wheeze with HRV in the first three years of life have an increased likelihood of developing subsequent childhood asthma at age six (OR = 25.6). In this study, 90% of children who wheezed with HRV in the third year of life had asthma at six years of age [11]. Another study implicated HRV as a causative agent for asthma based on bronchiolitis occurring during the HRV season and subsequent diagnosis of childhood asthma, but did not include viral testing [33]. The association of HRV with bronchiolitis [7, 12, 14] and asthma exacerbations suggests that HRV may be associated with the inception of asthma.
Ongoing studies are investigating the role of the host response in HRV-related illness and comparing it to the effects of viral pathogenicity on asthma exacerbations. Both allergen exposure and elevated IgE levels predispose patients with asthma to more severe respiratory symptoms in response to HRV infection. Studies suggest that abnormalities in the host cellular response to viral infection that result in impaired apoptosis and increased viral replication may be responsible for the severe and prolonged symptoms typical of asthmatic individuals. These mechanisms have yet to be elucidated.
HRVC--a new rhinovirus species
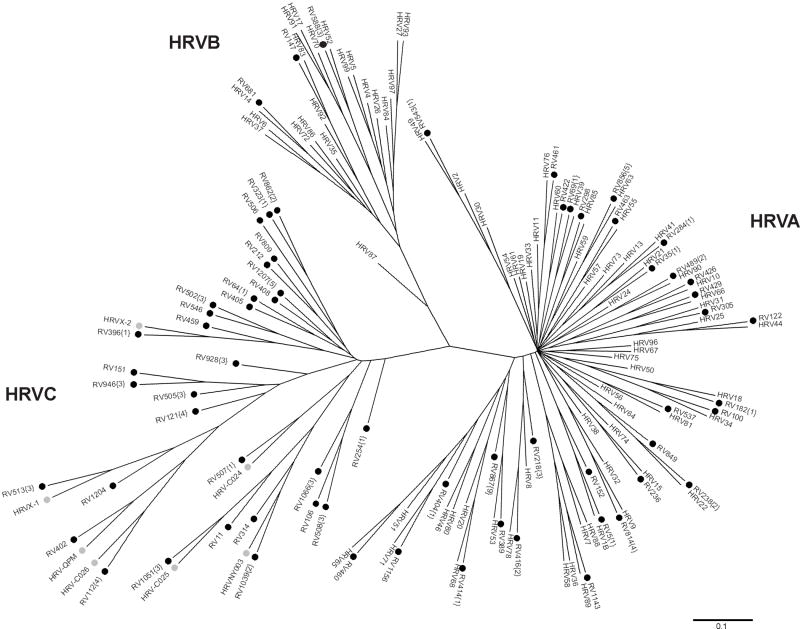
In the past, some HRV strains were difficult to detect by conventional methods because they do not grow well in culture. In fact, recent studies suggest that there are many rhinoviruses that are not cultivatable but have significant correlation with clinical illness. Traditional methods of virus typing using immune antisera have identified about 100 serotypes, classified into HRVA and HRVB species based on genetic sequence similarity [17, 19-21]. More recent PCR-based studies have discovered a novel group, HRVC (also called HRVA2, HRVNY, HRVQPM, and HRVX) [34-42]. HRVC has been proposed as a new HRV species within the genus Enterovirus by the International Committee on Taxonomy of Viruses (http://talk.ictvonline.org/media/p/1035.aspx). HRVC contains a distinct subgroup of strains. Since the discovery of this new species of HRV, other related viruses have been identified from different countries from Africa, Asia, Australia, Europe, and North America [34-42]. In 2009, Palmenburg et al. published complete genome sequences of all 99 previously known HRV serotypes and numerous novel strains, and sequence analysis demonstrates that the HRVC group comprises a genetically distinct species [43]. It also identified a potential new group, HRVD (or a subgroup of HRVA) [43]. Figure 1 illustrates the genetic diversity of rhinoviruses detected in one study of hospitalized children. The novel group HRVC contributed to a substantial burden of HRV-related illness.
Figure 1.
Phylogenetic tree depicting relationships between known HRV serotypes and novel HRV. Previously known HRV serotypes are designated by ‘HRV’. Novel sequences identified in this study are designated by ‘RV’ and a black circle. The numbers in parentheses after the label of these sequences indicates how many additional specimens contained each virus. Novel sequences identified in recent studies are designated by ‘HRV’ and a grey circle.
The identification of a novel group of HRV, and the finding that a significant proportion of HRV-related illness is attributable to these new HRVs, raises the question of why these viruses were not identified previously. One reason appears to be the extreme difficulty in recovering viable virus. Several groups have been unable thus far to recover these viruses in culture, despite numerous classical and non-classical approaches [15,17, 2].
It is likely that HRVC has been circulating for some time, and is not a newly emergent virus. An extensive search of HRV sequences published in GenBank prior to 2006 was unable to identify any that belonged to the HRVC group (Miller et al., unpublished data). One group reported detection of a genetically similar virus in a specimen taken in New York in 2004 [13] and another showed that these viruses have been circulating since at least 2002 [2]. Our group has detected HRVC in respiratory specimens that were collected 20 years ago (Miller et al. unpublished data).
HRVC frequency and symptomatology
During recent years, RT-PCR based studies of HRV have shown that they are associated with a substantial portion of upper and lower respiratory illness in outpatient and hospitalized adults and children, [7,24-33] with evidence that HRVC accounts for a large proportion of this HRV-associated illness. HRVC has been detected in patients with symptoms including acute upper or lower respiratory illness, wheezing, bronchiolitis, asthma exacerbations, COPD exacerbations, pneumonia, LRI, cold and flu-like illness, fever, cough, rhinitis, nasal congestion, bronchitis, retractions, crackle, bronchopneumonia, dyspnea, otitis media, gastroesophageal reflux disease, pericarditis, poor appetite, and apparent life-threatening events [55]. In published studies of adults and children with respiratory symptoms that were tested for HRVC, the proportion of HRV attributable to HRVC ranged from 8 to 81% [2, 35, 37, 42, 44-54]. Overall frequency of HRVC in published studies of adults and children with respiratory symptoms ranges from 2 to 20% [2, 35, 37, 42, 44-54]. (See Table 1.) Like HRVA and HRVB, HRVC has also been detected in asymptomatic individuals [52].
Table 1.
Summary of HRVC rates in studies of adults and children with acute respiratory illness, by inpatient versus outpatient status.
| HRV+/Total Specimens (%) | HRVC/HRV Total (%) | Rates of HRVC | |
|---|---|---|---|
| Adults | |||
| Inpatient | 32% | 17% | 5% |
| Outpatient | 45% | 14% | 6% |
| Children | |||
| Inpatient | 16-52% | 14-73% | 4-20% |
| Outpatient | 37% | 28% | 6% |
| Mixed | 13-22% | 8-81% | 2-10% |
| Adults and Children, Inpatient and Outpatient | 18% | 39% | 7% |
| Overall | 13-52% | 14-81% | 2-20%* |
HRVC seasonality
Among the studies that have tested for HRVC to date, the data on seasonality of HRVC is not conclusive. While many studies have detected HRVC as the predominant HRV species in the fall, suggesting it may play a role in the so-called September asthma epidemic [21-23], other studies in different regions and years have found otherwise. Han et al. reported HRVC to be more common in the spring and HRVA in the fall in South Korea in 2006, but co-circulation occurred during both seasons [56]. Consistent with our studies, Lau et al. found HRVA and HRVC to alternate as the most common HRV species at different times during the peak seasons [57]. Alternate disease activity by species and seasons suggests possible viral interference or serological cross-protection between HRVA and HRVC [57.
While one study reported HRVC year-round without peaks in the spring and fall [52], most studies do suggest that HRV species vary by season, year, and geographic location. This variation underscores the importance of studies over multiple years and seasons to understand fully the geographical and seasonal rhinovirus epidemiology.
Asymptomatic infection with HRVC
It is clear that HRV infection occurs in ill individuals and experimental inoculation of patients with HRV induces respiratory symptoms; however, asymptomatic HRV infection with HRV has been documented. The rates of asymptomatic infection have been little studied. One study that included asymptomatic controls identified HRVC more often in sick than in well patients, with HRV detected in only 3 of 93 asymptomatic individuals [52]. There is some evidence that HRVC may interfere with infection by other viruses [58].
HRVC and asthma/wheezing
Certain viruses may pose a greater risk for severe disease and asthma. There is much data to support a relationship of HRVC to wheezing/asthma (Table 2), but whether this differs from the symptoms produced by HRVA or HRVB strains is uncertain. In 2007, four retrospective studies showed a possible association of HRVC with wheezing or bronchiolitis [35, 37, 40, 42]. In those studies, however, sample size was insufficient to detect statistically significant clinical differences between HRVC and other species. In 2009, a large, prospective population-based study of young hospitalized children in two U.S. cities over two years found several clinically significant differences among patients infected with HRVC compared to HRVA, the other predominant HRV species. Of note, children with HRVC were twice as likely to have a discharge diagnosis of asthma than those with HRVA, and children with HRVA were more likely to present with fever [2]. In a similar study published in 2009, in a population of children less than five years of age hospitalized with fever or acute respiratory illness in Amman, Jordan, HRVC was significantly more often associated with wheezing and supplemental oxygen use, as compared with HRVA [53]. An unpublished asthma case-control study of 400 school-aged children in Argentina found slightly more HRVC in asthmatics with URI who wheezed than in asthmatics with URI and no wheezing (p = 0.036) (Miller et al, unpublished data). One retrospective study investigated hospitalized children <11 years of age and found a trend for HRVC to be detected more in patients with underlying asthma (p = 0.09) [46]. HRVC was the most prevalent HRV infection in this Thai study population and was detected in 22% of 50 children aged 4-23 months with first wheezing episode. There were also two patients in this study who had been admitted with five different episodes of acute respiratory infection and subsequent recurrent wheezing associated with HRVA and HRVC [47].
Table 2.
Summary of HRVC in patients with wheezing, bronchiolitis, or asthma.
| Study Type | Patient Age | Study Dates | Sample Size | Wheezing/Asthma Status | Different from HRVA/B (p<0.05) | Reference |
|---|---|---|---|---|---|---|
| Inpatient, Outpatient Retrospective | <80 yrs | 1/03-12/03 | 1244 | Wheeze, Persistent Cough | (39, 40) | |
| Inpatient | <14 yrs | 1/04-12/08 | 1555 | Asthma, Recurrent Wheeze, Bronchiolitis | No | (61) |
| Inpatient Retrospective | <18 yrs | 11/04-10/05 | 26 | Wheeze | (37) | |
| Inpatient, Outpatient | <18 yrs | 12/04-7/05 | 44 | Wheeze | (49) | |
| Prospective Case-Control | ≥2yrs | 3/03-2/04 | 29 | Asthma Exacerbation, Lower FEV | A and C in cases | (51) |
| Inpatient, Outpatient | <2 yrs | 1/04-12/04 | 447 (93 controls) | Wheezing | No | (52) |
| Inpatient Retrospective | <11 yrs | 2/06-2/07 | 289 | Bronchiolitis, Wheezing, Asthma | Trend | (46, 47) |
| Inpatient Prospective | <5 yrs | 10/01-9/03 | 1052 | Asthma | Yes | (41) |
| Inpatient PICU | <18 yrs | 02/07-10/07 | 43 | Wheeze | (54) | |
| Inpatient Retrospective | <14 yrs | 1/06-12/06 | 470 | Asthma exacerbation, Bronchiolitis | (56) | |
| Inpatient Retrospective | <12 yrs | 10/05-3/07 | 500 | Asthma, Bronchiolitis | (60) | |
| Inpatient Prospective | <5 yrs | 1/07-3/07 | 728 | Wheezing, Supplemental O2 | Yes | (53) |
| Inpatient | 2wks-5 yrs | 03-06 | 97 | Bronchiolitis | No | (42) |
| Inpatient | <18 years, adults | 04/04-03/05 | 1200 | Asthma, COPD, Febrile Wheeze | No | (57) |
| Outpatient | >18 yrs | Fall 01-12/04 | 83 | Asthma | (35) |
Other signs and symptoms associated with HRV-C in some studies but not included in this table include pneumonia, LRI, cold and flu-like illness, fever, cough, rhinitis, nasal congestion, bronchitis, retractions, crackle, bronchopneumonia, upper respiratory tract illness, dyspnea, otitis, gastroesophageal reflux disease, pericarditis, poor appetite, and apparent life-threatening events.
Other studies have reported HRVC in patients with wheezing, bronchiolitis, or asthma but either did not compare or did not find a significant difference between HRVC and HRVA or HRVB. McErlean et al. detected HRVC (then called HRVQPM) in 17/1244 (1.4%) of ill adult and pediatric patients. It was the sole virus detected in 62% of the patients, and those with HRVC infection alone were sicker than those with co-infections. Most patients with HRVC in this study were less than two years of age and 46% had lower respiratory illness, many with wheezing and bronchodilator use [40]. The same group later published an intensive chart review of 17 patients with HRVC and found 36% had bronchiolitis, 14% asthma, 7% COPD, and 21% persistent or hacking cough. Three subjects with HRVC did not have clinical data to assess. Of those with bronchiolitis or asthma, no other viruses were detected [39, 59].
Lau et al. studied 21 children with HRVC and found that wheezing or asthmatic exacerbations were common (16/21 cases, 76%), especially in those with a history of febrile wheeze or asthma [37]. Louie et al. studied 43 children admitted to the intensive care unit and detected HRV in 21:3 of the 10 sequenced had HRVC, many of the patients enrolled were noted to have wheezing [54]. Han et al. detected HRVC in 17 Korean children hospitalized with lower respiratory illness and the diagnoses were asthma exacerbation in eight patients, bronchiolitis in eight, and pneumonia in one [56]. Tan et al reported that 2/64 HRV-positive specimens tested had HRVC, both patients with asthma and bronchiolitis [60]. Another study determined HRVC to be associated with respiratory distress, hypoxia, and wheezing, but the numbers with HRVC were small [49].
Four studies have not detected a clinically significant difference between HRVC and HRVA or HRVB. In a retrospective study of virus-negative bronchiolitis, Renwick et al. found that the frequency of bronchiolitis with HRV A/B (11%) was comparable to that with HRV C (12%) [42]. Similarly, Piotrowska et al. studied 447 symptomatic children younger than two years of age and 93 asymptomatic controls and detected no associations between particular HRV genotypes and wheezing or asthma symptoms [52]. Khetsuriani et a.l performed a case-control study of asthmatics with acute exacerbation versus asthmatics without any respiratory symptoms, and found both HRVA and HRVC to be significantly associated with wheezing [51]. Calvo et al. detected HRV in 424/1555 (27%) hospitalized children. A subset was sequenced, and there was substantial wheezing in children with HRVA and HRVC, but the investigators did not detect a difference in wheezing between the species. Of note, 12% of healthy controls had HRV [61].
Several other studies have identified an association between HRVC and asthma or wheezing. Lau et al studied 1200 hospitalized adults and children with nasopharyngeal aspirate specimens negative for other viruses over a one-year period and found that of the 91 HRVC-positive specimens, 78 were detected in children, and 13 were detected in adults. Of note, in this study adults who had HRVC were more likely to present with lower respiratory illness than were children. Nonetheless, 33 (42%) of the 78 children with HRVC infection experienced asthma exacerbations or febrile wheeze. Sixty-two (68%) infections of adults or children occurred in patients with underlying diseases, with asthma or other chronic lung diseases being the most common. Wheezing episodes were more common in patients with HRVC (37%) and HRVA (20%) infection, compared with that in patients with HRVB (0%) infection [57].
Taken together, these data suggest that viruses in the HRVC group may be either more prone to stimulate asthma reactivity, or intrinsically more virulent. Further studies are required to determine whether these novel rhinoviruses have a greater propensity to cause the exacerbation of asthma and other respiratory illnesses. Moreover, while the role of HRV in the ontogeny or onset of asthma is a provocative area of research [16, 33], the question of whether HRVC may drive this relationship has not been studied. The discovery of an association between disease severity or asthma risk and particular groups or strains of HRV could help inform preventive and treatment measures.
Conclusions
It is well known that HRVs are a major trigger of asthma exacerbations in adults and children and are now under investigation for their potential involvement in the inception of asthma. It is also clear that the newly described HRVC species account for a large proportion of HRV-related illness, including asthma and wheezing exacerbations. HRVC is genetically diverse and appears to have seasonal variations. Whether HRVC or other as yet undiscovered divergent HRV species and strains, are consistently more pathogenic or ‘asthmagenic’ is uncertain, and further studies are warranted.
Should an association between HRVC or certain HRV strains and more severe disease and asthma development exist, focused vaccine development targeting these strains could offer benefit for asthmatic patients of all ages. While antigenic diversity complicates passive and active prophylactic interventions (i.e. antibodies or vaccines), further characterization of individual serotypes (and their neutralizing antigens) associated with severe disease could provide valuable information for developing potential preventive strategies in the future. In addition, the availability of rapid detection techniques for HRV could be implemented in the clinical setting to allow appropriate diagnosis and treatment of HRV-associated bronchiolitis and asthma.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin JA, Kochanek KD, Strobino DM, Guyer B, MacDorman MF. Annual summary of vital statistics--2003. Pediatrics. 2005;115:619–34. doi: 10.1542/peds.2004-2695. [DOI] [PubMed] [Google Scholar]
- 2.Miller EK, Edwards KM, Weinberg GA, Iwane MK, Griffin MR, Hall CB, et al. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol. 2009;123:98–104 e1. doi: 10.1016/j.jaci.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferreira A, Williams Z, Donninger H, van Schalkwyk EM, Bardin PG. Rhinovirus is associated with severe asthma exacerbations and raised nasal interleukin-12. Respiration. 2002;69:136–42. doi: 10.1159/000056316. [DOI] [PubMed] [Google Scholar]
- 4.Gern JE. Rhinovirus respiratory infections and asthma. Am J Med. 2002;112 6A:19S–27S. doi: 10.1016/s0002-9343(01)01060-9. [DOI] [PubMed] [Google Scholar]
- 5.Hayden FG. Rhinovirus and the lower respiratory tract. Rev Med Virol. 2004;14:17–31. doi: 10.1002/rmv.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jartti T, Lehtinen P, Vuorinen T, Osterback R, van den Hoogen B, Osterhaus AD, et al. Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerg Infect Dis. 2004;10:1095–101. doi: 10.3201/eid1006.030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotaniemi-Syrjanen A, Vainionpaa R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus-induced wheezing in infancy--the first sign of childhood asthma? J Allergy Clin Immunol. 2003;111:66–71. doi: 10.1067/mai.2003.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papadopoulos NG, Papi A, Psarras S, Johnston SL. Mechanisms of rhinovirus-induced asthma. Paediatr Respir Rev. 2004;5:255–60. doi: 10.1016/j.prrv.2004.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan WC. Viruses in asthma exacerbations. Curr Opin Pulm Med. 2005;11:21–6. doi: 10.1097/01.mcp.0000146781.11092.0d. [DOI] [PubMed] [Google Scholar]
- 10.Thumerelle C, Deschildre A, Bouquillon C, Santos C, Sardet A, Scalbert M, et al. Role of viruses and atypical bacteria in exacerbations of asthma in hospitalized children: a prospective study in the Nord-Pas de Calais region (France) Pediatr Pulmonol. 2003;35:75–82. doi: 10.1002/ppul.10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemanske RF, Jr, Jackson DJ, Gangnon RE, Evans MD, Li Z, Shult PA, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–7. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 12.Peltola V, Waris M, Osterback R, Susi P, Hyypia T, Ruuskanen O. Clinical effects of rhinovirus infections. J Clin Virol. 2008;43:411–4. doi: 10.1016/j.jcv.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 14.Miller EK, Lu X, Erdman DD, Poehling KA, Zhu Y, Griffin MR, et al. Rhinovirus-associated hospitalizations in young children. J Infect Dis. 2007;195:773–81. doi: 10.1086/511821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heymann PW, Carper HT, Murphy DD, Platts-Mills TA, Patrie J, McLaughlin AP, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004;114:239–47. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–72. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelon W, Mogabgab WJ, Phillips IA, Pierce WE. A cytopathogenic agent isolated from naval recruits with mild respiratory illnesses. Proc Soc Exp Biol Med. 1957;94:262–7. doi: 10.3181/00379727-94-22915. [DOI] [PubMed] [Google Scholar]
- 18.Bertino JS. Cost burden of viral respiratory infections: issues for formulary decision makers. Am J Med. 2002;112 6A:42S–9S. doi: 10.1016/s0002-9343(01)01063-4. [DOI] [PubMed] [Google Scholar]
- 19.Hamparian VV, Colonno RJ, Cooney MK, Dick EC, Gwaltney JM, Jr, Hughes JH, et al. A collaborative report: rhinoviruses--extension of the numbering system from 89 to 100. Virology. 1987;159:191–2. doi: 10.1016/0042-6822(87)90367-9. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan NM, Dove W, Abd-Eldayem SA, Abu-Zeid AF, Shamoon HE, Hart CA. Molecular epidemiology and disease severity of respiratory syncytial virus in relation to other potential pathogens in children hospitalized with acute respiratory infection in Jordan. J Med Virol. 2008;80:168–74. doi: 10.1002/jmv.21067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan NM, Dove W, Abu-Zeid AF, Shamoon HE, Abd-Eldayem SA, Hart CA. Evidence of human metapneumovirus infection in Jordanian children. Saudi Med J. 2006;27:1081–3. [PubMed] [Google Scholar]
- 22.Jartti T, Jartti L, Peltola V, Waris M, Ruuskanen O. Identification of respiratory viruses in asymptomatic subjects: asymptomatic respiratory viral infections. Pediatr Infect Dis J. 2008;27:1103–7. doi: 10.1097/INF.0b013e31817e695d. [DOI] [PubMed] [Google Scholar]
- 23.OJartti T, Lehtinen P, Vuorinen T, Osterback R, van den Hoogen B, Osterhaus AD, et al. Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerg Infect Dis. 2004;10:1095–101. doi: 10.3201/eid1006.030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venarske DL, Busse WW, Griffin MR, Gebretsadik T, Shintani AK, Minton PA, et al. The relationship of rhinovirus-associated asthma hospitalizations with inhaled corticosteroids and smoking. J Infect Dis. 2006;193:1536–43. doi: 10.1086/503809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnston NW, Johnston SL, Norman GR, Dai J, Sears MR. The September epidemic of asthma hospitalization: school children as disease vectors. J Allergy Clin Immunol. 2006;117:557–62. doi: 10.1016/j.jaci.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 26.Midulla F, Scagnolari C, Bonci E, Pierangeli A, Antonelli G, De Angelis D, et al. Respiratory syncytial virus, human bocavirus and rhinovirus bronchiolitis in infants. Arch Dis Child. 95:35–41. doi: 10.1136/adc.2008.153361. [DOI] [PubMed] [Google Scholar]
- 27.Papadopoulos NG, Moustaki M, Tsolia M, Bossios A, Astra E, Prezerakou A, et al. Association of rhinovirus infection with increased disease severity in acute bronchiolitis. Am J Respir Crit Care Med. 2002;165:1285–9. doi: 10.1164/rccm.200112-118BC. [DOI] [PubMed] [Google Scholar]
- 28.Tsolia MN, Psarras S, Bossios A, Audi H, Paldanius M, Gourgiotis D, et al. Etiology of community-acquired pneumonia in hospitalized school-age children: evidence for high prevalence of viral infections. Clin Infect Dis. 2004;39:681–6. doi: 10.1086/422996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blomqvist S, Roivainen M, Puhakka T, Kleemola M, Hovi T. Virological and serological analysis of rhinovirus infections during the first two years of life in a cohort of children. J Med Virol. 2002;66:263–8. doi: 10.1002/jmv.2140. [DOI] [PubMed] [Google Scholar]
- 30.Vesa S, Kleemola M, Blomqvist S, Takala A, Kilpi T, Hovi T. Epidemiology of documented viral respiratory infections and acute otitis media in a cohort of children followed from two to twenty-four months of age. Pediatr Infect Dis J. 2001;20:574–81. doi: 10.1097/00006454-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Johnston SL, Pattemore PK, Sanderson G, Smith S, Campbell MJ, Josephs LK, et al. The relationship between upper respiratory infections and hospital admissions for asthma: a time-trend analysis. Am J Respir Crit Care Med. 1996;154:654–60. doi: 10.1164/ajrccm.154.3.8810601. [DOI] [PubMed] [Google Scholar]
- 32.Yunginger JW, Reed CE, O'Connell EJ, Melton LJ, 3rd, O'Fallon WM, Silverstein MD. A community-based study of the epidemiology of asthma. Incidence rates, 1964-1983. Am Rev Respir Dis. 1992;146:888–94. doi: 10.1164/ajrccm/146.4.888. [DOI] [PubMed] [Google Scholar]
- 33.Carroll KN, Wu P, Gebretsadik T, Griffin MR, Dupont WD, Mitchel EF, et al. Season of infant bronchiolitis and estimates of subsequent risk and burden of early childhood asthma. J Allergy Clin Immunol. 2009;123:964–6. doi: 10.1016/j.jaci.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arden KE, McErlean P, Nissen MD, Sloots TP, Mackay IM. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J Med Virol. 2006;78:1232–40. doi: 10.1002/jmv.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kistler A, Avila PC, Rouskin S, Wang D, Ward T, Yagi S, et al. Pan-viral screening of respiratory tract infections in adults with and without asthma reveals unexpected human coronavirus and human rhinovirus diversity. J Infect Dis. 2007;196:817–25. doi: 10.1086/520816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamson D, Renwick N, Kapoor V, Liu Z, Palacios G, Ju J, et al. MassTag polymerase-chain-reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza-like illness in New York State during 2004-2005. J Infect Dis. 2006;194:1398–402. doi: 10.1086/508551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lau SK, Yip CC, Tsoi HW, Lee RA, So LY, Lau YL, et al. Clinical features and complete genome characterization of a distinct human rhinovirus (HRV) genetic cluster, probably representing a previously undetected HRV species, HRV-C, associated with acute respiratory illness in children. J Clin Microbiol. 2007;45:3655–64. doi: 10.1128/JCM.01254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee WM, Kiesner C, Pappas T, Lee I, Grindle K, Jartti T, et al. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS ONE. 2007;2:e966. doi: 10.1371/journal.pone.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McErlean P, Shackelton LA, Andrews E, Webster DR, Lambert SB, Nissen MD, et al. Distinguishing molecular features and clinical characteristics of a putative new rhinovirus species, human rhinovirus C (HRV C) PLoS One. 2008;3:e1847. doi: 10.1371/journal.pone.0001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McErlean P, Shackelton LA, Lambert SB, Nissen MD, Sloots TP, Mackay IM. Characterisation of a newly identified human rhinovirus, HRV-QPM, discovered in infants with bronchiolitis. J Clin Virol. 2007;39:67–75. doi: 10.1016/j.jcv.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller EK, Edwards KM, Weinberg GA, Iwane MK, Griffin MR, Hall CB, et al. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol. 2008 doi: 10.1016/j.jaci.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Renwick N, Schweiger B, Kapoor V, Liu Z, Villari J, Bullmann R, et al. A recently identified rhinovirus genotype is associated with severe respiratory-tract infection in children in Germany. J Infect Dis. 2007;196:1754–60. doi: 10.1086/524312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmenberg AC, Spiro D, Kuzmickas R, Wang S, Djikeng A, Rathe JA, et al. Sequencing and analyses of all known human rhinovirus genomes reveal structure and evolution. Science. 2009;324:55–9. doi: 10.1126/science.1165557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin Y, Yuan XH, Xie ZP, Gao HC, Song JR, Zhang RF, et al. Prevalence and clinical characterization of a newly identified human rhinovirus C species in children with acute respiratory tract infections. J Clin Microbiol. 2009;47:2895–900. doi: 10.1128/JCM.00745-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang T, Wang W, Bessaud M, Ren P, Sheng J, Yan H, et al. Evidence of recombination and genetic diversity in human rhinoviruses in children with acute respiratory infection. PLoS One. 2009;4:e6355. doi: 10.1371/journal.pone.0006355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linsuwanon P, Payungporn S, Samransamruajkit R, Posuwan N, Makkoch J, Theanboonlers A, et al. High prevalence of human rhinovirus C infection in Thai children with acute lower respiratory tract disease. J Infect. 2009;59:115–21. doi: 10.1016/j.jinf.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Linsuwanon P, Payungporn S, Samransamruajkit R, Theamboonlers A, Poovorawan Y. Recurrent human rhinovirus infections in infants with refractory wheezing. Emerg Infect Dis. 2009;15:978–80. doi: 10.3201/eid1506.081558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piralla A, Rovida F, Campanini G, Rognoni V, Marchi A, Locatelli F, et al. Clinical severity and molecular typing of human rhinovirus C strains during a fall outbreak affecting hospitalized patients. J Clin Virol. 2009;45:311–7. doi: 10.1016/j.jcv.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 49.Dominguez SR, Briese T, Palacios G, Hui J, Villari J, Kapoor V, et al. Multiplex MassTag-PCR for respiratory pathogens in pediatric nasopharyngeal washes negative by conventional diagnostic testing shows a high prevalence of viruses belonging to a newly recognized rhinovirus clade. J Clin Virol. 2008;43:219–22. doi: 10.1016/j.jcv.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Briese T, Renwick N, Venter M, Jarman RG, Ghosh D, Kondgen S, et al. Global distribution of novel rhinovirus genotype. Emerg Infect Dis. 2008;14:944–7. doi: 10.3201/eid1406.080271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khetsuriani N, Lu X, Teague WG, Kazerouni N, Anderson LJ, Erdman DD. Novel human rhinoviruses and exacerbation of asthma in children. Emerg Infect Dis. 2008;14:1793–6. doi: 10.3201/eid1411.080386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piotrowska Z, Vazquez M, Shapiro ED, Weibel C, Ferguson D, Landry ML, et al. Rhinoviruses are a major cause of wheezing and hospitalization in children less than 2 years of age. Pediatr Infect Dis J. 2009;28:25–9. doi: 10.1097/INF.0b013e3181861da0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller EK, Khuri-Bulos N, Williams JV, Shehabi AA, Faouri S, Al Jundi I, et al. Human rhinovirus C associated with wheezing in hospitalised children in the Middle East. J Clin Virol. 2009;46:85–9. doi: 10.1016/j.jcv.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Louie JK, Roy-Burman A, Guardia-Labar L, Boston EJ, Kiang D, Padilla T, et al. Rhinovirus associated with severe lower respiratory tract infections in children. Pediatr Infect Dis J. 2009;28:337–9. doi: 10.1097/INF.0b013e31818ffc1b. [DOI] [PubMed] [Google Scholar]
- 55.Arden KE, Mackay IM. Newly identified human rhinoviruses: molecular methods heat up the cold viruses. Rev Med Virol. 20:156–76. doi: 10.1002/rmv.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han TH, Chung JY, Hwang ES, Koo JW. Detection of human rhinovirus C in children with acute lower respiratory tract infections in South Korea. Arch Virol. 2009;154:987–91. doi: 10.1007/s00705-009-0383-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lau SK, Yip CC, Lin AW, Lee RA, So LY, Lau YL, et al. Clinical and molecular epidemiology of human rhinovirus C in children and adults in Hong Kong reveals a possible distinct human rhinovirus C subgroup. J Infect Dis. 2009;200:1096–103. doi: 10.1086/605697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wisdom A, Kutkowska AE, McWilliam Leitch EC, Gaunt E, Templeton K, Harvala H, et al. Genetics, recombination and clinical features of human rhinovirus species C (HRV-C) infections; interactions of HRV-C with other respiratory viruses. PLoS One. 2009;4:e8518. doi: 10.1371/journal.pone.0008518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arden KE, Faux CE, O'Neill NT, McErlean P, Nitsche A, Lambert SB, et al. Molecular characterization and distinguishing features of a novel human rhinovirus (HRV) C, HRVC-QCE, detected in children with fever, cough and wheeze during 2003. J Clin Virol. 47:219–23. doi: 10.1016/j.jcv.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan BH, Loo LH, Lim EA, Kheng Seah SL, Lin RT, Tee NW, et al. Human rhinovirus group C in hospitalized children, Singapore. Emerg Infect Dis. 2009;15:1318–20. doi: 10.3201/eid1508.090321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Calvo C, Casas I, Garcia-Garcia ML, Pozo F, Reyes N, Cruz N, et al. Role of Rhinovirus C Respiratory Infections in Sick and Healthy Children in Spain. Pediatr Infect Dis J. doi: 10.1097/INF.0b013e3181d7a708. [DOI] [PubMed] [Google Scholar]