Abstract
Objective
Osteopontin (OPN), a pleiotropic extracellular matrix glycoprotein, has been reported to be protective against ischemic lesions, but effects of OPN on vascular functions have not been investigated. The aim of this study was to assess whether recombinant OPN (r-OPN) could prevent cerebral vasospasm after subarachnoid hemorrhage (SAH) in rats.
Methods
r-OPN was administered intraventricularly to rats undergoing SAH by the endovascular perforation, and its protective effects were evaluated by measuring the diameter of cerebral arteries and neurobehavioral testing. Western blotting and immunofluorescence were performed to explore the underlying mechanisms. An integrin receptor antagonist GRGDSP or mitogen-activated protein kinase (MAPK) phosphatase (MKP)-1 small interfering RNA (siRNA) was also administered to r-OPN-treated SAH rats, and those effects were evaluated.
Results
Pre-SAH administration of r-OPN prevented vasospasm and neurological impairments at 24–72 hours post-SAH. r-OPN enhanced an endogenous MAPK inhibitor, MKP-1, and suppressed the phosphorylation of MAPKs, caldesmon and heat shock protein 27 in the spastic cerebral arteries at 24 hours post-SAH. Immunofluorescence revealed that MKP-1 was induced in the arterial smooth muscle layer. GRGDSP prevented r-OPN-induced MKP-1 upregulation, and MKP-1 siRNA abolished both MAPK inactivation and anti-vasospastic effects by r-OPN. Post-SAH r-OPN treatment also prevented vasospasm.
Interpretation
r-OPN induced MKP-1 in the spastic cerebral arteries via binding to L-arginyl-glycyl-L-aspartate-dependent integrin receptors and prevented vasospasm after SAH. Therapeutic induction of MKP-1 may be a novel approach for the prevention and treatment of cerebral vasospasm.
Aneurysmal subarachnoid hemorrhage (SAH)is a common and devastating neurological disorder.1 Cerebral vasospasm, the delayed narrowing of large capacitance arteries at the base of the brain, has been believed to be a leading cause of mortality and morbidity and to be the most preventable complication secondary to SAH.2 Recent randomized clinical trials, however, showed that currently available anti-vasospastic drugs are not enough to achieve a better outcome.3,4 Thus, further research efforts are needed to develop the new therapy against vasospasm.
Osteopontin (OPN) is a multifunctional extracellular matrix glycoprotein that is known to be protective against ischemic injuries involving the brain and other organs.5,6 However, no studies have investigated the effect of OPN on vascular functions that might contribute to the protective effect. Considering pleiotropic effects of OPN,6 it would be interesting to examine if OPN modulates the vessel diameter in a pathological condition. In fact, OPN is reported to affect some signaling pathways such as protein kinase C (PKC) that is potentially involved in vascular smooth muscle contraction.7,8 We recently reported that recombinant OPN (r-OPN) suppressed interleukin (IL)-1β-induced nuclear factor (NF)-κB activation and matrix metalloproteinase (MMP)-9 induction without affecting IL-1β levels, preventing blood-brain barrier disruption after SAH in rats.9 It is reported that IL-1β activates three types of mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase (ERK)1/2, p38, and c-Jun N-Terminal kinase (JNK) as well as NF-κB, all of which mediate MMP-9 induction.10 Thus, we hypothesized that r-OPN inhibits MAPK activation that may be implicated in the pathogenesis of cerebral vasospasm after SAH.11 The present study is the first to demonstrate that r-OPN induces an endogenous MAPK inhibitor MAPK phosphatase (MKP)-1 in the spastic cerebral arteries and prevents cerebral vasospasm.
Methods
All procedures were approved by Loma Linda University animal care committee.
SAH Model and Study Protocol
The endovascular perforation model of SAH was produced in male adult Sprague-Dawley rats (300–370g, Harlan, Indianapolis, IN) as previously described.9 Under 2–3% isoflurane anesthesia, a sharpened 4–0 monofilament nylon suture was advanced rostrally into the left internal carotid artery (ICA) from the external carotid artery stump to perforate the bifurcation of the left anterior (ACA) and middle cerebral arteries (MCA). Blood pressure and blood gas were measured via the left femoral artery. Rectal temperature was kept at 37°C during surgery. Sham-operated rats underwent the identical procedures except that the suture was withdrawn without puncture.
First, 94 rats were randomly divided into 5 groups and effects of pre-SAH intracerebroventricular infusion (ICV) of 2 dosages of r-OPN on vasospasm were evaluated using neurobehavioral tests, India ink angiography, Western blotting and immunofluorescence at 24–72 hours post-SAH (Fig 1A).
Fig 1.
Experimental designs. Experiment 1 (A) is designed to examine effects of pre-subarachnoid hemorrhage (SAH) treatment of 2 dosages of recombinant osteopontin (OPN) on vasospasm; experiments 2 (B) and 3 (C), to examine if an L-arginyl-glycyl-L-aspartate-dependent integrin receptor antagonist GRGDSP or MKP-1 small interfering RNA (siRNA) blocks the effects of OPN treatment, respectively; and experiment 4 (D), to examine effects of post-SAH OPN treatment on vasospasm. All treatments are performed by the intracerebroventricular infusion (ICV). Angio., angiography.
Secondly, 18 SAH rats were used, and whether L-arginyl-glycyl-L-aspartate (RGD) motif-containing hexapeptide GRGDSP blocked r-OPN-induced MKP-1 upregulation was evaluated at 24 hours post-SAH (Fig 1B).
Thirdly, 44 rats were randomly divided into 4 groups, and whether MKP-1 small interfering RNA (siRNA) blocked r-OPN-induced anti-vasospastic effects was evaluated at 24 hours post-SAH (Fig 1C).
Lastly, 22 rats were randomly divided into 3 groups and effects of post-SAH ICV of r-OPN on vasospasm were evaluated at 24 hours post-SAH (Fig 1D).
Neurobehavioral Test
Neurological impairments were blindly evaluated using 2 methods. Neurological scores (3–18) were assessed by summing up six test scores (spontaneous activity; spontaneous movement of four limbs; forepaw outstretching; climbing; body proprioception; and response to whisker stimulation) as previously described.12 Beam balance test investigated the animal’s ability to walk on a narrow wooden beam (2.25cm diameter and columnar) for 60 seconds: four points, walking ≥20cm; three points, walking ≥10cm but <20cm; two points, walking ≥10cm but falling; one point, walking <10cm; and zero points, falling with walking <10cm. The mean score of three consecutive trials in a 5-minute interval was calculated.
Severity of SAH
The severity of SAH was blindly assessed at each sacrifice as previously described.12 The basal cistern was divided into six segments, and each segment was allotted a grade from 0 to 3 depending on the amount of SAH. The animals received a total score ranging from 0 to 18 by summing up the scores.
Intracerebroventricular Infusion (ICV)
The needle of a 10-μL Hamilton syringe (Hamilton Company, Reno, NV) was inserted into the left lateral ventricle using the following coordinates relative to bregma: 1.5mm posterior; 1.0mm lateral; 3.2mm below the horizontal plane of bregma.9 Sterile phosphate-buffered saline (PBS) vehicle (1μL) or mouse r-OPN (0.02 or 0.1μg in 1μL; EMD Chemicals, La Jolla, CA) was infused at a rate of 0.5μL/minute irrespective of the animal’s body weight at one hour pre-surgery (as a pre-treatment) or at 5 hours post-surgery (as a post-treatment). GRGDSP (Sigma-Aldrich, St. Louis, MO) was dissolved in 0.1N acetate and was further diluted in PBS (final concentration of acetate=1.73%). GRGDSP (100pmol in 1μL) or the vehicle was infused together with r-OPN (0.1μg/1μL in PBS). MKP-1 siRNA (sense, 5′-CCACCAUCUGCCUUGCUUA[dT][dT]-3′, and antisense, 5′-UAAGCAAGGCAGAUGGUGG[dT][dT]-3′; Sigma-Aldrich, St. Louis, MO) or irrevalent control siRNA (Dharmacon/Thermo Fisher Scientific, Lafayette, CO) at 500pmol/2μL in sterile PBS was injected at a rate of 0.5 μL/minute at 48 hours pre-surgery. In rats having a sham ICV, a burr hole was performed on the skull at the same position, but neither needle insertion nor drug infusion was received. The needle was removed 10 minutes after an infusion, and the burr hole was quickly plugged with a bone wax.
India Ink Angiography
Gelatin-India ink solution was made by dissolving gelatin powder (7g) in 100mL PBS and mixing with 100mL India ink (Sanford Co, Bellwood, IL).13 The ascending aorta was cannulated with a blunted 16-gauge needle attached to flexible plastic tubing, which was connected to a pressure transducer (Micro-Med Inc, Louisville, KY) and a syringe on an automatic infusion pump (Harvard Apparatus, Holliston, MA). After an incision was made in the right atrium to allow for the outflow of perfusion solutions, 100mL of PBS, 15 minutes of 10% formalin, and 10 minutes of 3.5% gelatin-India ink solution were infused through the closed circuit. All perfusates were passed through a 0.2-μm pore size filter and delivered at 60–80mmHg.13 The rat was refrigerated at 4°C for 24 hours to allow gelatin solidification. The brains were harvested and high-resolution pictures of the circle of Willis and basilar arteries (BAs) were taken with a scale before and after the removal of a subarachnoid clot. The brain was stored in 10% neutral buffered formalin.
An experienced person who was unaware of the treatment groups measured the smallest lumen diameter within each vascular segment of intracranial cerebral arteries (sphenoidal segment of the MCA, precommunicating segment of the ACA, intradural ICA, and BA) three times using Image J software (National Institutes of Health, Bethesda, MD) and determined a mean value per segment.
Western Blot Analyses
Western blot analysis was performed as previously described.14 The circle of Willis and BAs were harvested under a microscope and homogenized. Equal amounts of protein samples (10μg) were loaded on a Tris glycine gel, electrophoresed, and transferred to a nitrocellulose membrane. Membranes were blocked with a blocking solution, followed by incubation overnight at 4°C with the rabbit anti-MKP-1 (1:5000, Sigma-Aldrich, St. Louis, MO), anti-phospho-ERK1/2, anti-phospho-caldesmon, mouse anti-phospho-JNK, anti-phospho-p38 (1:200, Santa Cruz Biotechnology, Santa Cruz, CA), and rabbit anti-phospho-heat shock protein (HSP) 27 (1:400, R&D Systems, Minneapolis, MN) antibodies. Blot bands were detected with a chemiluminescence reagent kit (ECL Plus; Amersham Bioscience, Arlington Heights, IL) and quantified by densitometry with Image J software. β-Tubulin (1:200, Santa Cruz Biotechnology, Santa Cruz, CA) was blotted on the same membrane as a loading control.
Immunofluorescence
Double fluorescence labeling was performed as described previously.15 Among the stored brains after India ink angiography, 4 brains were randomly used from sham+vehicle, SAH+vehicle and SAH+0.1μg of r-OPN groups, respectively. The intracranial ICA was sectioned every 200μm, and 10μm-thick coronal sections cut by a cryostat were incubated overnight at 4°C with the goat anti-MYH11 (1:50, Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit anti-MKP-1 (1:500, Sigma-Aldrich, St. Louis, MO) antibodies, followed by incubation with appropriate fluorescence dye-conjugated secondary antibodies (Jackson Immunoresearch, West Grove, PA). The sections were visualized with a fluorescence microscope, and pictographs were analyzed with MagnaFire SP 2.1B software (Olympus, Melville, NY).
Statistics
Neurological and beam balance scores were expressed as median±25th-75th percentiles, and were analyzed using Mann-Whitney U tests or Kruskal-Wallis tests, followed by Steel-Dwass multiple comparisons. Other values were expressed as mean±standard deviation, and unpaired t, chi-square tests and one-way analysis of variance (ANOVA) with Tukey-Kramer post hoc tests were used as appropriate. The P<0.05 was considered significant.
Results
Pre-SAH r-OPN Prevents Vasospasm
Comparisons of physiological parameters revealed no significant differences among the groups (data not shown). None of the sham-operated rats died within the 72-hour observation period. The mortality of SAH rats was not significantly different among the PBS (20.8%, 5 of 24 rats), 0.02μg (18.2%, 4 of 22 rats) and 0.01μg (13.6%, 3 of 22 rats) of r-OPN treatment groups. The average SAH grading score was similar among the groups in each analysis at both 24 and 72 hours post-SAH (Supplementary Fig 1).
Pre-SAH administration of 0.1μg of r-OPN significantly improved neurological scores at 24 (n=19; P<0.01) and 48 (n=7; P<0.05) hours and beam balance scores at 48 (P<0.05) and 72 (n=7; P<0.01) hours post-SAH compared with the PBS-treated SAH groups (n=19, 7 and 7 at 24, 48 and 72 hours post-SAH, respectively; Kruskal-Wallis tests; Supplementary Fig 2). Both dosages of r-OPN significantly attenuated vasospasm in the bilateral ICA, left MCA and BA, and 0.1μg of r-OPN prevented it in the left ACA at 24 hours post-SAH (n=6; P<0.05, ANOVA, respectively; Fig 2). r-OPN also prevented vasospasm in the left ICA at 72 hours post-SAH (n=7; P<0.05, ANOVA; Fig 2B).
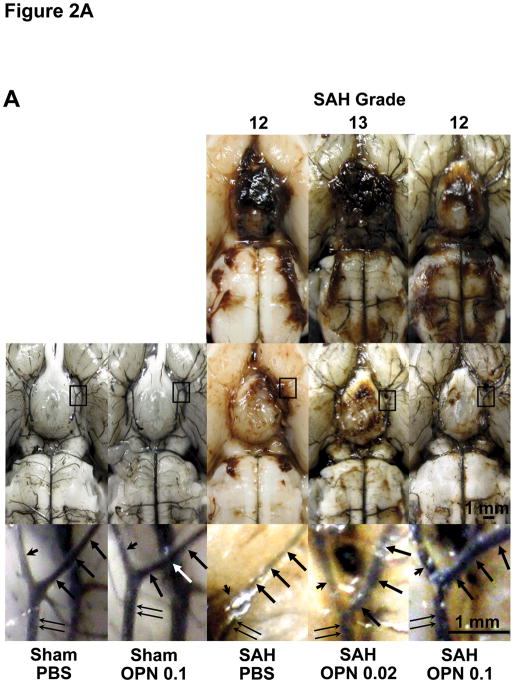
Fig 2.
Effects of pre-subarachnoid hemorrhage (SAH) recombinant osteopontin (OPN) treatment on vasospasm. A, representative India ink angiograms; B–E, vessel diameter of each cerebral artery at 24 and 72 hours post-surgery. Pre-SAH OPN treatment significantly prevents vasospasm in the bilateral internal carotid arteries (B), left middle cerebral artery (C), left anterior cerebral artery (D) and basilar artery (E) associated with the improvement of neurobehavioral scores (ANOVA). Arrow, left middle cerebral artery; arrow head, left anterior cerebral artery; double arrow, left internal carotid artery; P, O 0.02, O 0.1, rats treated with phosphate-buffered saline (PBS), OPN (0.02μg) or OPN (0.1μg), respectively; n=6 per group except for n=7 in PBS- and 0.1μg of OPN-treated SAH rats at 72 hours post-SAH. Data are expressed as mean±standard deviation. ANOVA, *P<0.05, #P<0.05 vs. sham-operated rats treated with PBS or 0.1μg of OPN, respectively.
Effects of r-OPN on Protein Expression Changes in Cerebral Arteries
Western blot analysis showed that 0.1μg of r-OPN significantly induced MKP-1 in the spastic arteries (n=6; P<0.05, ANOVA) but not in the normal arteries (n=4; Fig 3A-B). SAH significantly increased phosphorylated JNK (P<0.05) and p38 (P<0.01) but not ERK1/2 levels, all of which were significantly suppressed by 0.1μg of r-OPN (n=6; P<0.05, ANOVA, respectively; Fig 3C-E). Phosphorylation of caldesmon and HSP27, substrates of MAPKs, was also increased after SAH and suppressed by 0.1μg of r-OPN (n=6; P<0.01, ANOVA, respectively; Fig 3F-G). Immunofluorescence showed that MKP-1 was induced in the smooth muscle layer of the arteries in the 0.1μg of r-OPN-treated SAH rats (n=4; Fig 4).
Fig 3.
Representative Western blots (A) and effects of pre-subarachnoid hemorrhage (SAH) recombinant osteopontin (OPN) treatment on mitogen-activated protein kinase phosphatase-1 (MKP-1; B), phosphorylated c-Jun N-Terminal kinase (p-JNK; C), p38 (p-p38; D), extracellular signal-regulated kinase1/2 (p-ERK1/2; E), caldesmon (p-caldesmon; F) or heat shock protein 27 (p-HSP27; G) levels in cerebral arteries at 24 hours post-SAH. Pre-SAH 0.1μg of OPN significantly induces MKP-1 while reduces phosphorylated JNK, p38, ERK1/2, caldesmon and HSP27 levels in the spastic cerebral arteries associated with the improvement of vasospasm (ANOVA). Expression levels of each protein are expressed as a ratio of β-tubulin levels for normalization and as mean±standard deviation. N=4 in phosphate-buffered saline (PBS)- or OPN (0.1μg)-treated sham-operated rats; n=6 in PBS- or OPN (0.02 or 0.1μg)-treated SAH rats.
Fig 4.
Immunofluorescence for smooth muscle cells (MYH11) and mitogen-activated protein kinase phosphatase-1 (MKP-1) in the intracranial internal carotid artery at 24 hours after subarachnoid hemorrhage (SAH). Pre-SAH 0.1μg of osteopontin (OPN) induces MKP-1 in the smooth muscle layer of the post-SAH arteries, in which vasospasm is improved. Sham-PBS, Sham-operated rats treated with phosphate-buffered saline (PBS); SAH-PBS or -OPN0.1, SAH rats treated with PBS or 0.1μg of OPN.
GRGDSP Inhibits r-OPN-Induced MKP-1 Upregulation
The mortality was significantly higher in the r-OPN+GRGDSP-treated SAH rats (45.5%, 5 of 11 rats) compared with the r-OPN+vehicle-treated SAH rats (0%, 0 of 7 rats; P<0.05, chi-square tests). The average SAH grading score was similar between the 2 groups (Supplementary Fig 3A). GRGDSP abolished r-OPN-induced improvement of neurological scores (Supplementary Fig 3B-C) and MKP-1 upregulation (Fig 5).
Fig 5.

Representative Western blots (A) and effects of GRGDSP treatment on mitogen-activated protein kinase phosphatase-1 (MKP-1) levels (B) in cerebral arteries in subarachnoid hemorrhage (SAH) rats treated with 0.1μg of osteopontin (SAH-OPN0.1) at 24 hours post-SAH. An L-arginyl-glycyl-L-aspartate-dependent integrin receptor antagonist GRGDSP abolishes osteopontin-induced MKP-1 upregulation (unpaired t tests) irrespective of similar severity of SAH. MKP-1 levels are expressed as a ratio of β-tubulin levels for normalization and as mean±standard deviation. PBS, phosphate-buffered saline.
MKP-1 siRNA Abolishes Anti-vasospastic Effects of r-OPN
MKP-1 upregulation by r-OPN (n=7) was significantly inhibited after MKP-1 siRNA treatment (n=8; P<0.05), which was associated with increased phosphorylation of JNK (P<0.05), p38 (P<0.01) and ERK1/2 (P<0.05) in the cerebral arteries 24 hours post-SAH (unpaired t tests, respectively; Supplementary Fig 4). MKP-1 siRNA significantly aggravated neurological and beam balance scores (P<0.05, Kruskal-Wallis tests), although the mortality and SAH grading scores were not significantly different between the MKP-1 (n=16) and control (n=15) siRNA groups (Supplementary Fig 5). MKP-1 inhibition (n=8) resulted in significantly worse vasospasm in all cerebral arteries in the r-OPN-treated SAH rats (P<0.05, ANOVA, respectively; Fig 6).
Fig 6.

Effects of mitogen-activated protein kinase phosphatase-1 (M) small interfering RNA (siRNA) treatment on cerebral vasospasm in subarachnoid hemorrhage (SAH) rats treated with 0.1μg of osteopontin (SAH-OPN) at 24 hours post-SAH. MKP-1 siRNA abolishes osteopontin-induced improvement of vasospasm in all cerebral arteries (ANOVA; A, internal carotid artery; B, middle cerebral artery; C, anterior cerebral artery; D, basilar artery) irrespective of similar severity of SAH. Data are expressed as mean±standard deviation. C, control; sham ICV, sham intracerebroventricular infusion (burr hole only).
Post-SAH r-OPN Prevents Vasospasm
The mortality and SAH grading scores were similar between the PBS-treated (n=8) and r-OPN-treated (n=7) SAH groups (Supplementary Fig 6). Post-SAH r-OPN treatment significantly prevented vasospasm in all cerebral arteries (P<0.05, ANOVA, respectively), although neurological and beam balance scores were improved but did not reach significance compared with the PBS-treated SAH groups (Supplementary Fig 7–8).
Discussion
In this study, pre-SAH ICV of r-OPN demonstrated significant prevention against cerebral vasospasm associated with an increase in MKP-1 levels and a decrease in phosphorylated MAPK, caldesmon and HSP27 levels in the major cerebral artery. An RGD-dependent integrin receptor antagonist GRGDSP blocked r-OPN-induced MKP-1 upregulation, and selective MKP-1 inhibition using MKP-1 siRNA abolished r-OPN-induced MAPK inactivation and anti-vasospastic effects. Post-SAH r-OPN treatment was also effective for preventing vasospasm. These findings show that r-OPN prevents post-SAH vasospasm in rats via the integrin-mediated MKP-1 upregulation.
OPN, a secreted pleiotropic extracellular matrix glycoprotein, engages a number of receptors, including RGD-dependent or RGD-independent integrins and certain variant forms of CD44, which activate intracellular signaling pathways and mediate OPN’s variable biological functions.6 However, the receptors and intracellular pathways mediating OPN’s effects are not well established, although the primary receptors for OPN are considered to be those integrins that bind to the RGD motif.16 Actually, the signaling pathways activated by OPN are highly variable, and often seemingly contradictory depending on the biological scenario, explaining OPN’s diverse functions.7,16 For example, OPN activates NF-κB and PKC-mediated pathways in cancer progression,7 while OPN provided protection against IL-1β-mediated cytotoxic effects by suppressing NF-κB and PKC pathways in non-tumor cells or tissues.8,17 In contrast, OPN alone had no effect on the signaling pathways in non-tumor culture cells or sham-operated animal models.8,9,17 OPN also activates MAPK pathways in cancer progression,7 but a negative regulatory role of OPN on MAPK has not been reported. However, OPN was reported to inhibit IL-1β-induced PKC-ζ activation, upstream of NF-κB, ERK1/2 and JNK, preventing MMP-2 and -9 induction in rat cardiac fibroblasts.8,18 The inhibitory effects of OPN on MMPs were abolished by inhibitors of RGD-dependent integrins.18 Moreover, OPN prevented proinflammatory cytokine-induced upregulation of inducible nitric oxide synthase and MMP-9 in non-tumor tissues or diseases,9,17,19 one of whose important mediators is MAPK.10,18,20 These previous studies are consistent with our findings that r-OPN prevented MAPK activation in the spastic cerebral artery after SAH via binding to RGD-dependent integrins.
MAPKs (ERK1/2, p38 and JNK) are present in vascular smooth muscle cells, and existing evidence supports a role for MAPK in cerebral vasospasm after SAH.11 Activation of G protein-coupled receptors by spasmogens such as endothelin-1, adenosine triphosphate or bilirubin oxidation product leads to intracellular Ca2+ mobilization and PKC activation, which activate protein tyrosine kinase, eventually leading to the activation of MAPK.21,22 Growth factors such as platelet-derived growth factor or epidermal growth factor released during vasospasm may activate receptor tyrosine kinase and then MAPK.23 Oxyhemoglobin and bilirubin oxidation product generate free radicals and lipid peroxide and activate the phosphatidylcholine and phosphatidylethanolamine pool to sustain a prolonged elevation of diacylglycerol, leading to PKC activation and then MAPK activation.24 Free radicals also promote Ras activation, causing MAPK activation.25 Moreover, cytokines, such as IL-1β or tumor necrosis factor-α, have been shown to activate MAPK with or without PKC activation.26,27 Thus, MAPK may be an important final common pathway for the signaling transduction during cerebral vasospasm, but the downstream of MAPK and the role of endogenous MAPK inhibitors MKPs have not been investigated in vasospasm.
The potential mediators for MAPK to induce sustained vascular smooth muscle contraction are caldesmon, calponin and HSP27,28,29 although the link between MAPK and caldesmon, calponin or HSP27 has not been investigated in cerebral vasospasm. Both caldesmon and calponin block myosin binding to actin, and these inhibitory effects are reversed on the phosphorylation by MAPK.30–32 Phosphorylation of HSP27, downstream of p38, enhances actin polymerization that is an important event in the mechanism of force maintenance during smooth muscle contraction.28,33 Phosphorylated HSP27 also prevents both cyclic adenosine monophosphate and cyclic guanosine monophosphate-dependent vasorelaxation.34 In addition to sustained vasocontraction and impaired vasorelaxation, MAPK is involved in tissue proliferation, apoptosis and inflammation development, all of which are key features of cerebral vasospasm.24 These mechanisms may be involved in a complex pathological process of cerebral vasospasm characterized by persistent contraction of arterial smooth muscle and morphological changes in the arterial wall. This is the first report demonstrating that r-OPN-mediated MAPK deactivation prevented vasospasm with close linkage to the dephosphorylation of caldesmon and HSP27, suggesting that caldesmon and HSP27 are downstream of MAPK to induce vasospasm.
MAPK pathways are activated through phosphorylation. Therefore, dephosphorylation of MAPKs mediated by phosphatases represents a highly efficient mode of kinase deactivation. In mammalian cells, the dual-specificity protein phosphatases, which are often referred to as MKPs, are the primary phosphatases responsible for dephosphorylation or deactivation of MAPKs.35 To date, at least 10 MKPs have been identified in mammalian cells, with MKP-1 being the archetype.36,37 MKP-1 deactivates all MAPKs (ERK1/2, p38 and JNK)36,37 and may be an ideal therapeutic target for vasospasm, because all MAPKs may be involved in the pathogenesis of vasospasm while no available drugs can deactivate all MAPKs.11 This study showed that MKP-1 levels were unchanged in the cerebral arteries after SAH, and that r-OPN induced MKP-1, which in turn deactivated MAPK, preventing vasospasm. Therapeutic induction of MKP-1 may be a novel approach for the prevention and treatment of cerebral vasospasm.
The cause of neurological impairment after SAH consists of many factors including cerebral vasospasm and early brain injury. Animal studies usually focus on one factor among them, and the difference in endpoints may cause lack of translation of treatments effective in animals to humans. Thus, functional outcome has become a key parameter to determine the efficacy of therapeutic interventions.38 In this study, pre-SAH r-OPN improved neurological scores at 24 and 48 hours while beam balance scores at 48 and 72 hours post-SAH associated with the attenuation of vasospasm. This discrepancy might be due to that neurological scores evaluate sensorimotor disturbance while beam balance scores assess motor and vestibular functions. On the other hand, post-SAH r-OPN treatment prevented vasospasm but failed to improve neurological and beam balance scores at 24 hours post-SAH. These findings suggest that the improvement of vasospasm does not necessarily lead to the improvement of functional outcome, and that other factors such as early brain injury also cause neurological impairment in our model. This study thus confirms the importance of the assessment of functional outcome in animal studies and suggests that two or more different methods should be used if possible.
In conclusion, we demonstrated for the first time that r-OPN treatment prevented pathological vasoconstriction, in our case cerebral vasospasm. This study also showed MKP-1 as a novel target of r-OPN action, representing the underlying mechanism for r-OPN to prevent vasospasm.
Supplementary Material
Severity of subarachnoid hemorrhage (SAH) at 24 (A) and 72 (B) hours post-SAH. SAH grade is not significantly different among SAH rats receiving pre-SAH intracerebroventricular infusion of the vehicle (phosphate-buffered saline, PBS), 0.02μg of recombinant osteopontin (OPN0.02) and 0.1μg of recombinant osteopontin (OPN0.1; ANOVA). Data are expressed as mean±standard deviation.
Effects of pre-subarachnoid hemorrhage (SAH) intracerebroventricular infusion of recombinant osteopontin (OPN) treatment on neurological (A) and beam balance scores (B) at 24–72 hours post-SAH. Pre-SAH 0.1μg of OPN treatment significantly improves neurological scores at 24 and 48 hours and beam balance scores at 48 and 72 hours post-SAH compared with the vehicle (phosphate-buffered saline, PBS)-treated SAH rats (SAH-PBS) irrespective of similar severity of SAH (Kruskal-Wallis tests, *P<0.01, #P<0.05 vs. SAH-PBS). Data are expressed as median±25th-75th percentiles. Sham-PBS, Sham-operated rats treated with PBS, n=16 (pre-SAH, 24 hrs post-SAH) or 6 (48, 72 hrs post-SAH); Sham-OPN0.1, Sham-operated rats treated with 0.1μg of OPN, n=10 (pre-SAH, 24 hrs post-SAH); SAH-PBS, -OPN0.02 or -OPN0.1, SAH rats treated with PBS, 0.02 or 0.1μg of OPN, n=24, 22 or 22 (pre-SAH), n=19, 18 or 19 (24 hrs post-SAH), n=7, 6 or 7 (48, 72 hrs post-SAH), respectively.
Severity of subarachnoid hemorrhage (SAH; A), and effects of GRGDSP treatment on neurological (B) and beam balance scores (C) in SAH rats treated with 0.1μg of osteopontin (SAH-OPN0.1) at 24 hours post-SAH. An L-arginyl-glycyl-L-aspartate-dependent integrin receptor antagonist GRGDSP abolishes osteopontin-induced improvement of neurological scores (Mann-Whitney U tests) irrespective of similar severity of SAH (unpaired t tests). Data are expressed as mean±standard deviation (A) or median±25th-75th percentiles (B, C).
Representative Western blots (A) and effects of mitogen-activated protein kinase phosphatase-1 (MKP-1) small interfering RNA (siRNA) treatment on MKP-1 (B), phosphorylated c-Jun N-Terminal kinase (p-JNK; C), p38 (p-p38; D) or extracellular signal-regulated kinase1/2 (p-ERK1/2; E) levels in cerebral arteries in subarachnoid hemorrhage (SAH) rats treated with 0.1μg of osteopontin (SAH-OPN0.1) at 24 hours post-SAH. Osteopontin-induced MKP-1 upregulation is significantly inhibited after MKP-1 siRNA treatment, which is associated with increased p-JNK, p-p38 and p-ERK1/2 levels in the spastic cerebral arteries (unpaired t tests). Expression levels of each protein are expressed as a ratio of β-tubulin levels for normalization and as mean±standard deviation. Sham-PBS, Sham-operated rats treated with phosphate-buffered saline.
Severity of subarachnoid hemorrhage (SAH; A), and effects of mitogen-activated protein kinase phosphatase-1 (M) small interfering RNA(siRNA) treatment on neurological (B) and beam balance scores (C) in SAH rats treated with 0.1μg of osteopontin (SAH-OPN) at 24 hours post-SAH. MKP-1 siRNA abolishes osteopontin-induced improvement of neurological and beam balance scores (Kruskal-Wallis tests) irrespective of similar severity of SAH (unpaired t tests). Data are expressed as mean±standard deviation (A) or median±25th-75th percentiles (B, C). C, control; sham ICV, sham intracerebroventricular infusion (burr hole only).
Severity of subarachnoid hemorrhage (SAH) in SAH rats treated with 0.1μg of recombinant osteopontin (OPN0.1) or the vehicle (phosphate-buffered saline, PBS) as a post-treatment at 24 hours post-SAH. There is no significant difference in SAH grade between the 2 groups (unpaired t tests). Data are expressed as mean±standard deviation.
Effects of 0.1μg of recombinant osteopontin (OPN0.1) as a post-treatment on neurological (A) and beam balance scores (B) at 24 hours after subarachnoid hemorrhage (SAH). Post-SAH osteopontin treatment fails to improve neurological and beam balance scores significantly compared with the vehicle (phosphate-buffered saline, PBS) treatment in SAH rats (Kruskal-Wallis tests). Data are expressed as median±25th-75th percentiles. Sham ICV, sham intracerebroventricular infusion (burr hole only).
Effects of 0.1μg of recombinant osteopontin (OPN0.1) as a post-treatment on cerebral vasospasm at 24 hours after subarachnoid hemorrhage (SAH). Post-SAH osteopontin treatment significantly prevents vasospasm in all cerebral arteries (ANOVA; A, internal carotid artery; B, middle cerebral artery; C, anterior cerebral artery; D, basilar artery) in contrast with neurological and beam balance scores. Data are expressed as mean±standard deviation. PBS, phosphate-buffered saline; sham ICV, sham intracerebroventricular infusion (burr hole only).
Acknowledgments
This study was partially supported by grants (NS053407) from the National Institutes of Health to J.H.Z.
References
- 1.Suarez JI, Tarr RW, Selman WR. Aneurysmal subarachnoid hemorrhage. N Engl J Med. 2006;354:387–396. doi: 10.1056/NEJMra052732. [DOI] [PubMed] [Google Scholar]
- 2.Bederson JB, Connolly ES, Jr, Batjer HH, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 3.Vajkoczy P, Meyer B, Weidauer S, et al. Clazosentan (AXV-034343), a selective endothelin A receptor antagonist, in the prevention of cerebral vasospasm following severe aneurysmal subarachnoid hemorrhage: results of a randomized, double-blind, placebo-controlled, multicenter phase IIa study. J Neurosurg. 2005;103:9–17. doi: 10.3171/jns.2005.103.1.0009. [DOI] [PubMed] [Google Scholar]
- 4.Macdonald RL, Kassell NF, Mayer S, et al. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke. 2008;39:3015–3021. doi: 10.1161/STROKEAHA.108.519942. [DOI] [PubMed] [Google Scholar]
- 5.Meller R, Stevens SL, Minami M, et al. Neuroprotection by osteopontin in stroke. J Cereb Blood Flow Metab. 2005;25:217–225. doi: 10.1038/sj.jcbfm.9600022. [DOI] [PubMed] [Google Scholar]
- 6.Xie Y, Sakatsume M, Nishi S, et al. Expression, roles, receptors, and regulation of osteopontin in the kidney. Kidney Int. 2001;60:1645–1657. doi: 10.1046/j.1523-1755.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- 7.Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol. 2006;16:79–87. doi: 10.1016/j.tcb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Xie Z, Singh M, Siwik DA, et al. Osteopontin inhibits interleukin-1β-stimulated increases in matrix metalloproteinase activity in adult rat cardiac fibroblasts: role of protein kinase C-ζ. J Biol Chem. 2003;278:48546–48552. doi: 10.1074/jbc.M302727200. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki H, Ayer R, Sugawara T, et al. Protective effects of recombinant osteopontin on early brain injury after subarachnoid hemorrhage in rats. Crit Care Med. 2010;38:612–618. doi: 10.1097/CCM.0b013e3181c027ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu CY, Hsieh HL, Jou MJ, Yang CM. Involvement of p42/p44 MAPK, p38 MAPK, JNK and nuclear factor-kappa B in interleukin-1beta-induced matrix metalloproteinase-9 expression in rat brain astrocytes. J Neurochem. 2004;90:1477–1488. doi: 10.1111/j.1471-4159.2004.02682.x. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki H, Hasegawa Y, Kanamaru K, Zhang JH. Mitogen-activated protein kinases in cerebral vasospasm after subarachnoid hemorrhage: a review. Acta Neurochir Suppl. 2010 doi: 10.1007/978-3-7091-0353-1_23. in press. [DOI] [PubMed] [Google Scholar]
- 12.Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods. 2008;167:327–334. doi: 10.1016/j.jneumeth.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parra A, McGirt MJ, Sheng H, et al. Mouse model of subarachnoid hemorrhage associated cerebral vasospasm: methodological analysis. Neurol Res. 2002;24:510–516. doi: 10.1179/016164102101200276. [DOI] [PubMed] [Google Scholar]
- 14.Kusaka G, Ishikawa M, Nanda A, et al. Signaling pathways for early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2004;24:916–925. doi: 10.1097/01.WCB.0000125886.48838.7E. [DOI] [PubMed] [Google Scholar]
- 15.Sugawara T, Ayer R, Jadhav V, et al. Simvastatin attenuation of cerebral vasospasm after subarachnoid hemorrhage in rats via increased phosphorylation of Akt and endothelial nitric oxide synthase. J Neurosci Res. 2008;86:3635–3643. doi: 10.1002/jnr.21807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kazanecki CC, Uzwiak DJ, Denhardt DT. Control of osteopontin signaling and function by post-translational phosphorylation and protein folding. J Cell Biochem. 2007;102:912–924. doi: 10.1002/jcb.21558. [DOI] [PubMed] [Google Scholar]
- 17.Arafat HA, Katakam AK, Chipitsyna G, et al. Osteopontin protects the islets and β-cells from interleukin-1β-mediated cytotoxicity through negative feedback regulation of nitric oxide. Endocrinology. 2007;148:575–584. doi: 10.1210/en.2006-0970. [DOI] [PubMed] [Google Scholar]
- 18.Xie Z, Singh M, Singh K. Differential regulation of matrix metalloproteinase-2 and -9 expression and activity in adult rat cardiac fibroblasts in response to interleukin-1β. J Biol Chem. 2004;279:39513–39519. doi: 10.1074/jbc.M405844200. [DOI] [PubMed] [Google Scholar]
- 19.Guo H, Wai PY, Mi Z, et al. Osteopontin mediates Stat1 degradation to inhibit iNOS transcription in a cecal ligation and puncture model of sepsis. Surgery. 2008;144:182–188. doi: 10.1016/j.surg.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pannu R, Singh I. Pharmacological strategies for the regulation of inducible nitric oxide synthase: neurodegenerative versus neuroprotective mechanisms. Neurochem Int. 2006;49:170–182. doi: 10.1016/j.neuint.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Zubkov AY, Ogihara K, Tumu P, et al. Mitogen-activated protein kinase mediation of hemolysate-induced contraction in rabbit basilar artery. J Neurosurg. 1999;90:1091–1097. doi: 10.3171/jns.1999.90.6.1091. [DOI] [PubMed] [Google Scholar]
- 22.Zhang JH. Role of protein kinase C in cerebral vasospasm: past and future. Neurol Res. 2000;22:369–378. doi: 10.1080/01616412.2000.11740686. [DOI] [PubMed] [Google Scholar]
- 23.Berk BC, Corson MA. Angiotensin signal transduction in vascular smooth muscle: role of tyrosine kinase. Circ Res. 1997;80:607–616. doi: 10.1161/01.res.80.5.607. [DOI] [PubMed] [Google Scholar]
- 24.Laher I, Zhang JH. Protein kinase C and cerebral vasospasm. J Cereb Blood Flow Metab. 2001;21:887–906. doi: 10.1097/00004647-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Lander HM, Ogiste JS, Teng KK, Novogrodsky A. p21ras as a common signal target of reactive free radicals and cellular redox stress. J Biol Chem. 1995;270:21195–21198. doi: 10.1074/jbc.270.36.21195. [DOI] [PubMed] [Google Scholar]
- 26.Yan CM, Luo SF, Wang CC, et al. Tumour necrosis factor-alpha- and interleukin-1beta-stimulated cell proliferation through activation of mitogen-activated protein kinase in canine tracheal smooth muscle cell. Br J Pharmacol. 2000;130:891–899. doi: 10.1038/sj.bjp.0703359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schutze S, Berkovic D, Tomsing O, et al. Tumor necrosis factor induces rapid production of 1′2′diacylglycerol by a phosphatidylcholine-specific phospholipase C. J Exp Med. 1991;174:975–988. doi: 10.1084/jem.174.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamboliev IA, Hedges JC, Mutnick JLM, et al. Evidence for modulation of smooth muscle force by the p38 MAP kinase/HSP27 pathway. Am J Physiol Heart Circ Physiol. 2000;278:1899–1907. doi: 10.1152/ajpheart.2000.278.6.H1899. [DOI] [PubMed] [Google Scholar]
- 29.Zubkov AY, Nanda A, Zhang JH. Signal transduction pathways in cerebral vasospasm. Pathophysiology. 2003;9:47–61. doi: 10.1016/s0928-4680(02)00055-x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Moreland S, Moreland RS. Regulation of vascular smooth muscle contraction: myosin light chain phosphorylation dependent and independent pathways. Can J Physiol Pharmacol. 1994;72:1386–1391. doi: 10.1139/y94-200. [DOI] [PubMed] [Google Scholar]
- 31.Menice CB, Hulvershorn J, Adam LP, et al. Calponin and mitogen-activated protein kinase signaling in differentiated vascular smooth muscle. J Biol Chem. 1997;272:25157–25161. doi: 10.1074/jbc.272.40.25157. [DOI] [PubMed] [Google Scholar]
- 32.Hedges JC, Oxhorn BC, Carty M, et al. Phosphorylation of caldesmon by ERK MAP kinase in smooth muscle. Am J Physiol Cell Physiol. 2000;278:C718–C726. doi: 10.1152/ajpcell.2000.278.4.C718. [DOI] [PubMed] [Google Scholar]
- 33.Gerthoffer W, Gunst SJ. Focal adhesion and small heat shock proteins in the regulation of actin remodeling and contractility in smooth muscle. J Appl Physiol. 2001;91:963–972. doi: 10.1152/jappl.2001.91.2.963. [DOI] [PubMed] [Google Scholar]
- 34.McLemore EC, Tessier DJ, Thresher J, et al. Role of the small heat shock proteins in regulating vascular smooth muscle tone. J Am Coll Surg. 2005;201:30–36. doi: 10.1016/j.jamcollsurg.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Liu Y. Regulation of innate immune response by MAP kinase phosphatase-1. Cell Signal. 2007;19:1372–1382. doi: 10.1016/j.cellsig.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- 37.Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev. 2008;27:253–261. doi: 10.1007/s10555-008-9123-1. [DOI] [PubMed] [Google Scholar]
- 38.Jeon H, Ai J, Sabri M, et al. Neurological and neurobehavioral assessment of experimental subarachnoid hemorrhage. BMC Neurosci. 2009;10:103. doi: 10.1186/1471–2202–10–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Severity of subarachnoid hemorrhage (SAH) at 24 (A) and 72 (B) hours post-SAH. SAH grade is not significantly different among SAH rats receiving pre-SAH intracerebroventricular infusion of the vehicle (phosphate-buffered saline, PBS), 0.02μg of recombinant osteopontin (OPN0.02) and 0.1μg of recombinant osteopontin (OPN0.1; ANOVA). Data are expressed as mean±standard deviation.
Effects of pre-subarachnoid hemorrhage (SAH) intracerebroventricular infusion of recombinant osteopontin (OPN) treatment on neurological (A) and beam balance scores (B) at 24–72 hours post-SAH. Pre-SAH 0.1μg of OPN treatment significantly improves neurological scores at 24 and 48 hours and beam balance scores at 48 and 72 hours post-SAH compared with the vehicle (phosphate-buffered saline, PBS)-treated SAH rats (SAH-PBS) irrespective of similar severity of SAH (Kruskal-Wallis tests, *P<0.01, #P<0.05 vs. SAH-PBS). Data are expressed as median±25th-75th percentiles. Sham-PBS, Sham-operated rats treated with PBS, n=16 (pre-SAH, 24 hrs post-SAH) or 6 (48, 72 hrs post-SAH); Sham-OPN0.1, Sham-operated rats treated with 0.1μg of OPN, n=10 (pre-SAH, 24 hrs post-SAH); SAH-PBS, -OPN0.02 or -OPN0.1, SAH rats treated with PBS, 0.02 or 0.1μg of OPN, n=24, 22 or 22 (pre-SAH), n=19, 18 or 19 (24 hrs post-SAH), n=7, 6 or 7 (48, 72 hrs post-SAH), respectively.
Severity of subarachnoid hemorrhage (SAH; A), and effects of GRGDSP treatment on neurological (B) and beam balance scores (C) in SAH rats treated with 0.1μg of osteopontin (SAH-OPN0.1) at 24 hours post-SAH. An L-arginyl-glycyl-L-aspartate-dependent integrin receptor antagonist GRGDSP abolishes osteopontin-induced improvement of neurological scores (Mann-Whitney U tests) irrespective of similar severity of SAH (unpaired t tests). Data are expressed as mean±standard deviation (A) or median±25th-75th percentiles (B, C).
Representative Western blots (A) and effects of mitogen-activated protein kinase phosphatase-1 (MKP-1) small interfering RNA (siRNA) treatment on MKP-1 (B), phosphorylated c-Jun N-Terminal kinase (p-JNK; C), p38 (p-p38; D) or extracellular signal-regulated kinase1/2 (p-ERK1/2; E) levels in cerebral arteries in subarachnoid hemorrhage (SAH) rats treated with 0.1μg of osteopontin (SAH-OPN0.1) at 24 hours post-SAH. Osteopontin-induced MKP-1 upregulation is significantly inhibited after MKP-1 siRNA treatment, which is associated with increased p-JNK, p-p38 and p-ERK1/2 levels in the spastic cerebral arteries (unpaired t tests). Expression levels of each protein are expressed as a ratio of β-tubulin levels for normalization and as mean±standard deviation. Sham-PBS, Sham-operated rats treated with phosphate-buffered saline.
Severity of subarachnoid hemorrhage (SAH; A), and effects of mitogen-activated protein kinase phosphatase-1 (M) small interfering RNA(siRNA) treatment on neurological (B) and beam balance scores (C) in SAH rats treated with 0.1μg of osteopontin (SAH-OPN) at 24 hours post-SAH. MKP-1 siRNA abolishes osteopontin-induced improvement of neurological and beam balance scores (Kruskal-Wallis tests) irrespective of similar severity of SAH (unpaired t tests). Data are expressed as mean±standard deviation (A) or median±25th-75th percentiles (B, C). C, control; sham ICV, sham intracerebroventricular infusion (burr hole only).
Severity of subarachnoid hemorrhage (SAH) in SAH rats treated with 0.1μg of recombinant osteopontin (OPN0.1) or the vehicle (phosphate-buffered saline, PBS) as a post-treatment at 24 hours post-SAH. There is no significant difference in SAH grade between the 2 groups (unpaired t tests). Data are expressed as mean±standard deviation.
Effects of 0.1μg of recombinant osteopontin (OPN0.1) as a post-treatment on neurological (A) and beam balance scores (B) at 24 hours after subarachnoid hemorrhage (SAH). Post-SAH osteopontin treatment fails to improve neurological and beam balance scores significantly compared with the vehicle (phosphate-buffered saline, PBS) treatment in SAH rats (Kruskal-Wallis tests). Data are expressed as median±25th-75th percentiles. Sham ICV, sham intracerebroventricular infusion (burr hole only).
Effects of 0.1μg of recombinant osteopontin (OPN0.1) as a post-treatment on cerebral vasospasm at 24 hours after subarachnoid hemorrhage (SAH). Post-SAH osteopontin treatment significantly prevents vasospasm in all cerebral arteries (ANOVA; A, internal carotid artery; B, middle cerebral artery; C, anterior cerebral artery; D, basilar artery) in contrast with neurological and beam balance scores. Data are expressed as mean±standard deviation. PBS, phosphate-buffered saline; sham ICV, sham intracerebroventricular infusion (burr hole only).