Abstract
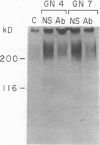
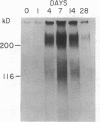
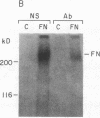
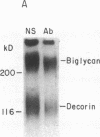
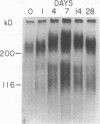
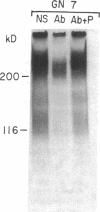
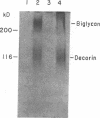
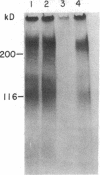
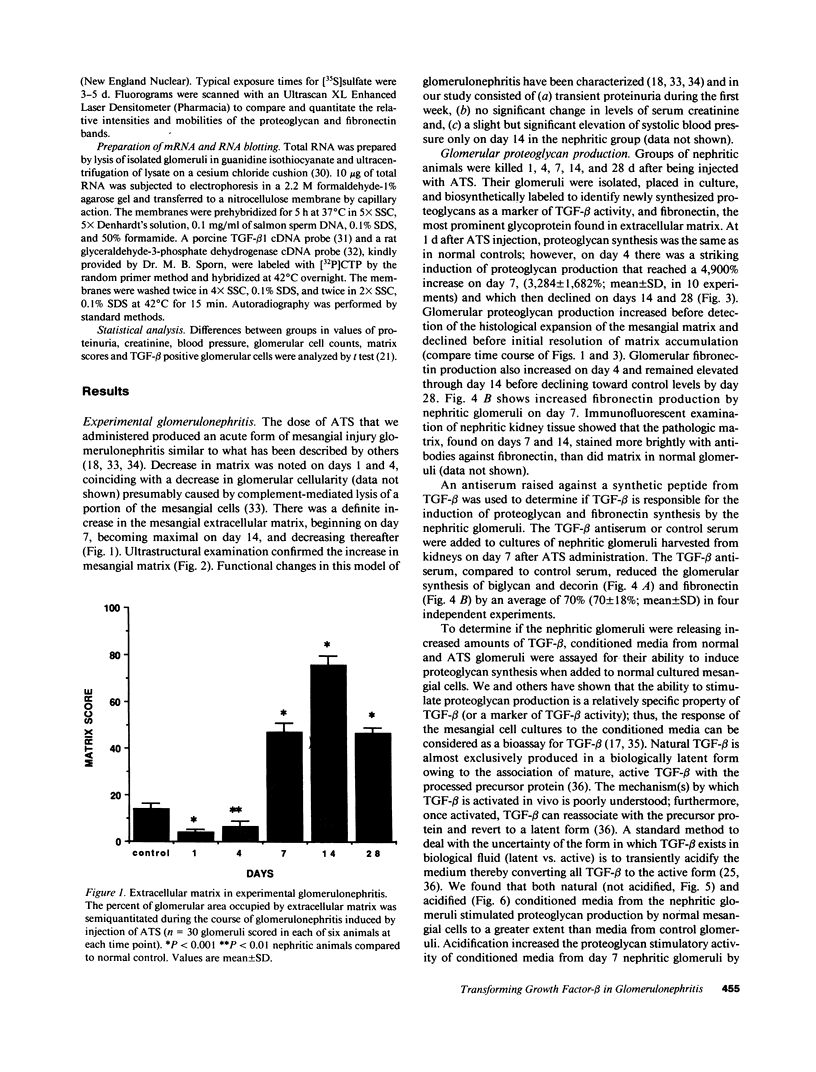
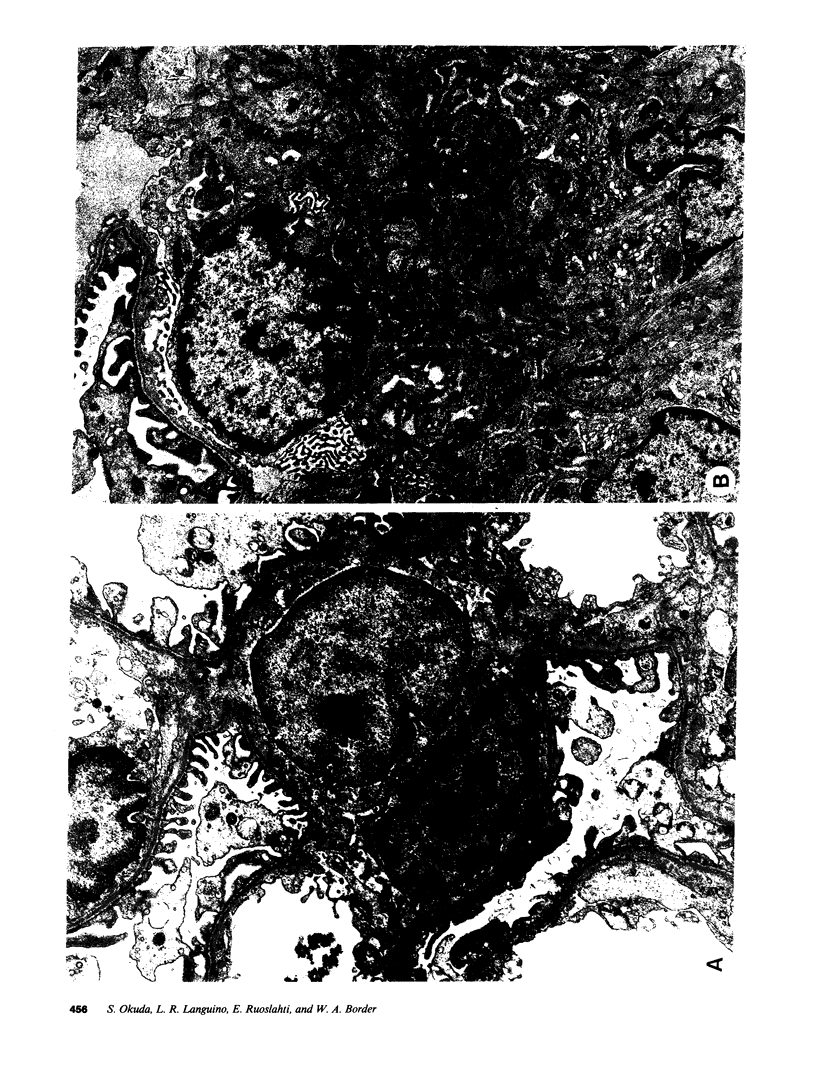
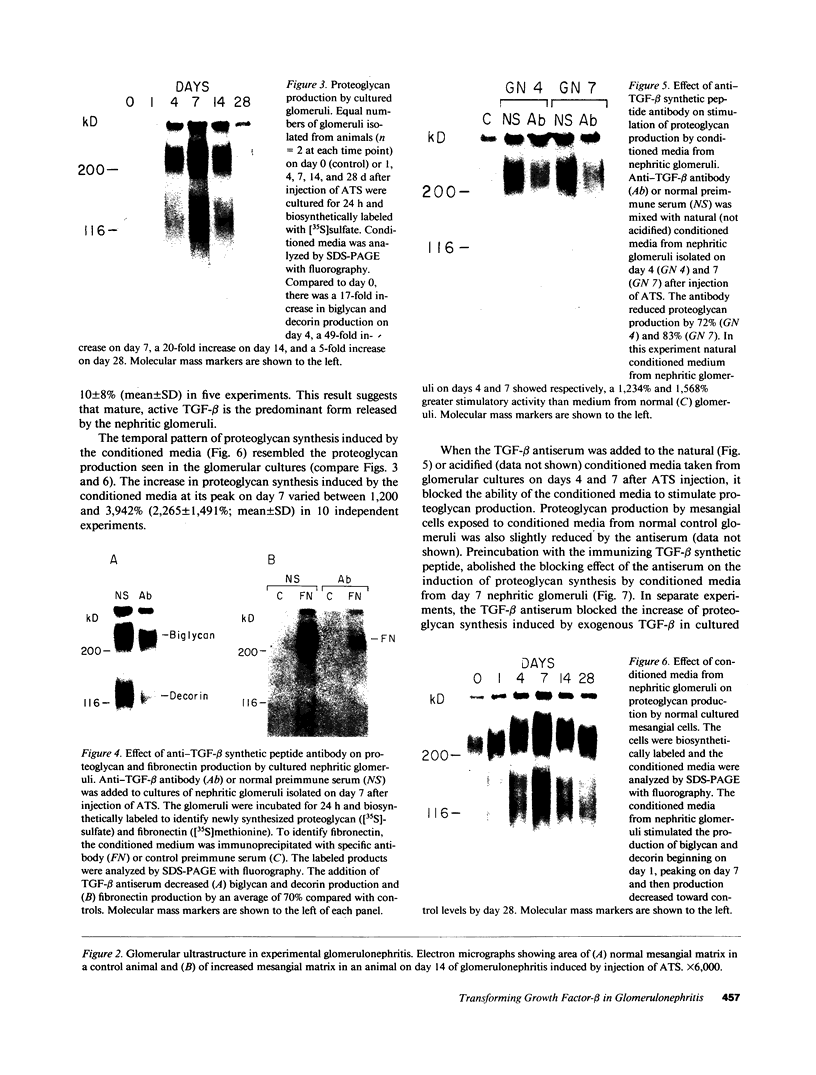
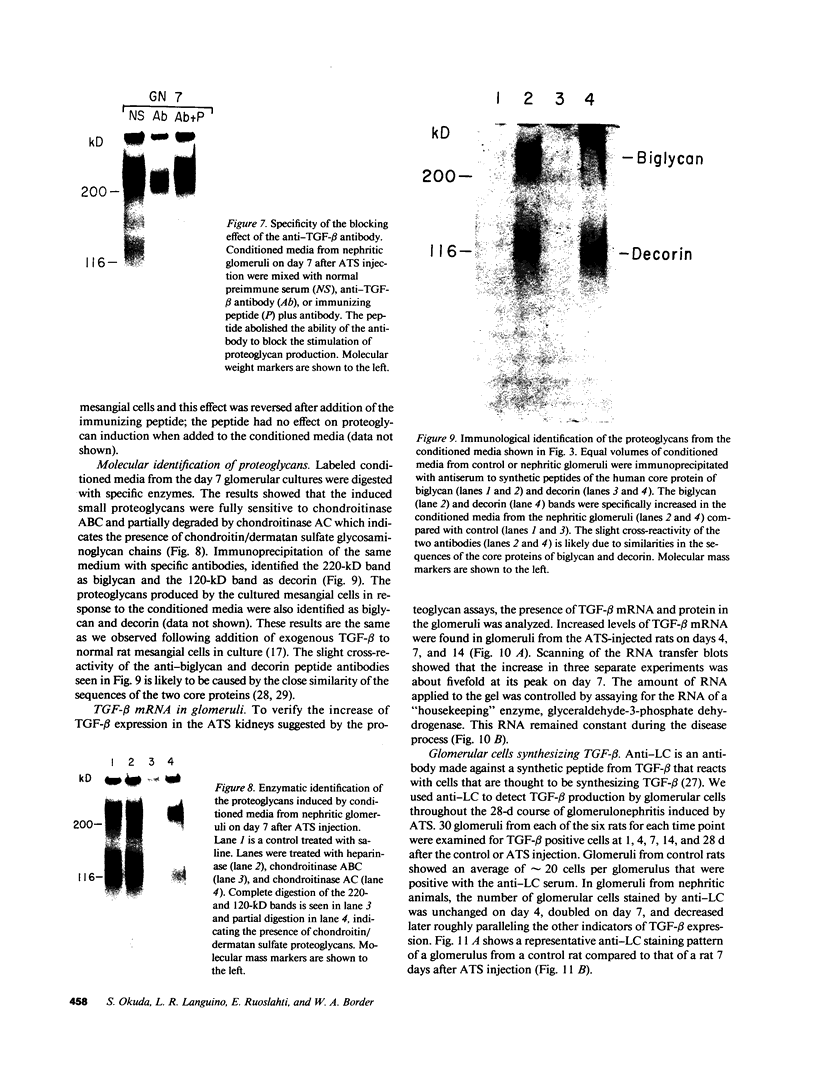
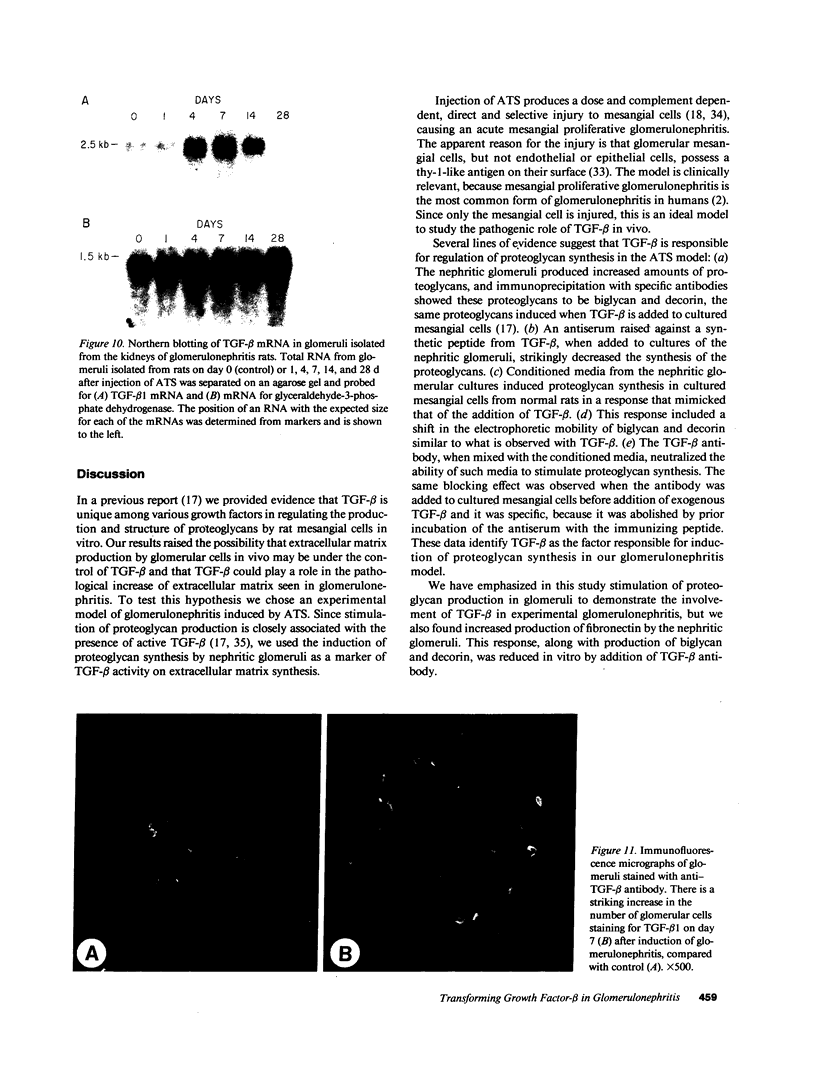
Glomerular accumulation of extracellular matrix is a prominent feature of progressive glomerulonephritis. Previously, we have shown that transforming growth factor-beta (TGF-beta) is unique among growth factors in regulating the production of the proteoglycans biglycan and decorin by glomerular mesangial cells in vitro. We now provide evidence of an elevated expression of TGF-beta, proteoglycans, and fibronectin in glomerulonephritis induced in rats by injection of anti-thymocyte serum (ATS). Glomeruli were cultured from rat kidneys at 1, 4, 7, 14, and 28 d after ATS administration. Increased proteoglycan synthesis was detected beginning on day 4, which peaked at a 4,900% increase compared with control on day 7, and returned toward control levels by day 28. The increased proteoglycan synthesis by cultured nephritic glomeruli, as well as that of fibronectin, were greatly reduced by addition of antiserum raised against a synthetic peptide from TGF-beta. Conditioned media from ATS glomerular cultures, when added to normal cultured mesangial cells, induced elevated proteoglycan synthesis that also peaked on day 7 and that mimicked the response to added exogenous TGF-beta. The stimulatory activity of the conditioned media was blocked by addition of TGF-beta antiserum. Prior addition of the immunizing peptide to the antiserum abolished the blocking effect. The main induced proteoglycans were identified as biglycan and decorin by immunoprecipitation with antiserum made against synthetic peptides from the proteoglycan core proteins. Glomerular histology showed mesangial matrix expansion in a time course that roughly paralleled both the elevated proteoglycan synthesis by the ATS glomeruli and the ability of the conditioned media from these glomeruli to induce proteoglycan synthesis. At the same time there was an increased expression of TGF-beta mRNA and TGF-beta protein in the glomeruli. These results suggest a central role for TGF-beta in the accumulation of pathological extracellular matrix in glomerulonephritis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagchus W. M., Hoedemaeker P. J., Rozing J., Bakker W. W. Glomerulonephritis induced by monoclonal anti-Thy 1.1 antibodies. A sequential histological and ultrastructural study in the rat. Lab Invest. 1986 Dec;55(6):680–687. [PubMed] [Google Scholar]
- Bassols A., Massagué J. Transforming growth factor beta regulates the expression and structure of extracellular matrix chondroitin/dermatan sulfate proteoglycans. J Biol Chem. 1988 Feb 25;263(6):3039–3045. [PubMed] [Google Scholar]
- Border W. A. Distinguishing minimal-change disease from mesangial disorders. Kidney Int. 1988 Sep;34(3):419–434. doi: 10.1038/ki.1988.197. [DOI] [PubMed] [Google Scholar]
- Border W. A., Okuda S., Languino L. R., Ruoslahti E. Transforming growth factor-beta regulates production of proteoglycans by mesangial cells. Kidney Int. 1990 Feb;37(2):689–695. doi: 10.1038/ki.1990.35. [DOI] [PubMed] [Google Scholar]
- Border W. A., Ward H. J., Kamil E. S., Cohen A. H. Induction of membranous nephropathy in rabbits by administration of an exogenous cationic antigen. J Clin Invest. 1982 Feb;69(2):451–461. doi: 10.1172/JCI110469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Border W. A., Wilson C. B., Dixon F. J. Failure of heparin to affect two types of experimental glomerulonephritis in rabbits. Kidney Int. 1975 Sep;8(3):140–148. doi: 10.1038/ki.1975.93. [DOI] [PubMed] [Google Scholar]
- Boswell J. M., Yui M. A., Burt D. W., Kelley V. E. Increased tumor necrosis factor and IL-1 beta gene expression in the kidneys of mice with lupus nephritis. J Immunol. 1988 Nov 1;141(9):3050–3054. [PubMed] [Google Scholar]
- Chen J. K., Hoshi H., McKeehan W. L. Transforming growth factor type beta specifically stimulates synthesis of proteoglycan in human adult arterial smooth muscle cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5287–5291. doi: 10.1073/pnas.84.15.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Courtoy P. J., Kanwar Y. S., Hynes R. O., Farquhar M. G. Fibronectin localization in the rat glomerulus. J Cell Biol. 1980 Dec;87(3 Pt 1):691–696. doi: 10.1083/jcb.87.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtoy P. J., Timpl R., Farquhar M. G. Comparative distribution of laminin, type IV collagen, and fibronectin in the rat glomerulus. J Histochem Cytochem. 1982 Sep;30(9):874–886. doi: 10.1177/30.9.7130672. [DOI] [PubMed] [Google Scholar]
- Czaja M. J., Weiner F. R., Flanders K. C., Giambrone M. A., Wind R., Biempica L., Zern M. A. In vitro and in vivo association of transforming growth factor-beta 1 with hepatic fibrosis. J Cell Biol. 1989 Jun;108(6):2477–2482. doi: 10.1083/jcb.108.6.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. R., Murphy G., Reynolds J. J., Whitham S. E., Docherty A. J., Angel P., Heath J. K. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987 Jul;6(7):1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher L. W., Termine J. D., Young M. F. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J Biol Chem. 1989 Mar 15;264(8):4571–4576. [PubMed] [Google Scholar]
- Flanders K. C., Roberts A. B., Ling N., Fleurdelys B. E., Sporn M. B. Antibodies to peptide determinants in transforming growth factor beta and their applications. Biochemistry. 1988 Jan 26;27(2):739–746. doi: 10.1021/bi00402a037. [DOI] [PubMed] [Google Scholar]
- Flanders K. C., Thompson N. L., Cissel D. S., Van Obberghen-Schilling E., Baker C. C., Kass M. E., Ellingsworth L. R., Roberts A. B., Sporn M. B. Transforming growth factor-beta 1: histochemical localization with antibodies to different epitopes. J Cell Biol. 1989 Feb;108(2):653–660. doi: 10.1083/jcb.108.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolik C. A., Wakefield L. M., Smith D. M., Sporn M. B. Characterization of a membrane receptor for transforming growth factor-beta in normal rat kidney fibroblasts. J Biol Chem. 1984 Sep 10;259(17):10995–11000. [PubMed] [Google Scholar]
- Harper P. A., Robinson J. M., Hoover R. L., Wright T. C., Karnovsky M. J. Improved methods for culturing rat glomerular cells. Kidney Int. 1984 Dec;26(6):875–880. doi: 10.1038/ki.1984.231. [DOI] [PubMed] [Google Scholar]
- House P. D., Poulis P., Weidemann M. J. Isolation of a plasma-membrane subfraction from rat liver containing an insulin-sensitive cyclic-AMP phosphodiesterase. Eur J Biochem. 1972 Jan 21;24(3):429–437. doi: 10.1111/j.1432-1033.1972.tb19703.x. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Cell adhesion protein receptors as targets for transforming growth factor-beta action. Cell. 1987 Oct 23;51(2):189–197. doi: 10.1016/0092-8674(87)90146-2. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Johnson R. J., Garcia R. L., Pritzl P., Alpers C. E. Platelets mediate glomerular cell proliferation in immune complex nephritis induced by anti-mesangial cell antibodies in the rat. Am J Pathol. 1990 Feb;136(2):369–374. [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S. Biophysiology of glomerular filtration and proteinuria. Lab Invest. 1984 Jul;51(1):7–21. [PubMed] [Google Scholar]
- Klahr S., Schreiner G., Ichikawa I. The progression of renal disease. N Engl J Med. 1988 Jun 23;318(25):1657–1666. doi: 10.1056/NEJM198806233182505. [DOI] [PubMed] [Google Scholar]
- Kondaiah P., Van Obberghen-Schilling E., Ludwig R. L., Dhar R., Sporn M. B., Roberts A. B. cDNA cloning of porcine transforming growth factor-beta 1 mRNAs. Evidence for alternate splicing and polyadenylation. J Biol Chem. 1988 Dec 5;263(34):18313–18317. [PubMed] [Google Scholar]
- Krusius T., Ruoslahti E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7683–7687. doi: 10.1073/pnas.83.20.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiho M., Saksela O., Keski-Oja J. Transforming growth factor-beta induction of type-1 plasminogen activator inhibitor. Pericellular deposition and sensitivity to exogenous urokinase. J Biol Chem. 1987 Dec 25;262(36):17467–17474. [PubMed] [Google Scholar]
- Lawrence W. T., Norton J. A., Sporn M. B., Gorschboth C., Grotendorst G. R. The reversal of an Adriamycin induced healing impairment with chemoattractants and growth factors. Ann Surg. 1986 Feb;203(2):142–147. doi: 10.1097/00000658-198602000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett D. H., Sterzel R. B. Cell culture approaches to the analysis of glomerular inflammation. Kidney Int. 1986 Aug;30(2):246–254. doi: 10.1038/ki.1986.176. [DOI] [PubMed] [Google Scholar]
- MacKay K., Striker L. J., Stauffer J. W., Doi T., Agodoa L. Y., Striker G. E. Transforming growth factor-beta. Murine glomerular receptors and responses of isolated glomerular cells. J Clin Invest. 1989 Apr;83(4):1160–1167. doi: 10.1172/JCI113996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. The TGF-beta family of growth and differentiation factors. Cell. 1987 May 22;49(4):437–438. doi: 10.1016/0092-8674(87)90443-0. [DOI] [PubMed] [Google Scholar]
- Morales T. I., Roberts A. B. Transforming growth factor beta regulates the metabolism of proteoglycans in bovine cartilage organ cultures. J Biol Chem. 1988 Sep 15;263(26):12828–12831. [PubMed] [Google Scholar]
- Oomura A., Nakamura T., Arakawa M., Ooshima A., Isemura M. Alterations in the extracellular matrix components in human glomerular diseases. Virchows Arch A Pathol Anat Histopathol. 1989;415(2):151–159. doi: 10.1007/BF00784353. [DOI] [PubMed] [Google Scholar]
- Raij L., Azar S., Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int. 1984 Aug;26(2):137–143. doi: 10.1038/ki.1984.147. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Meyers C. A., Wideman J., Blacher R., Pan Y. C., Stein S., Lehrman S. R., Smith J. M., Lamb L. C. Purification and properties of a type beta transforming growth factor from bovine kidney. Biochemistry. 1983 Dec 6;22(25):5692–5698. doi: 10.1021/bi00294a002. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Flanders K. C., Kondaiah P., Thompson N. L., Van Obberghen-Schilling E., Wakefield L., Rossi P., de Crombrugghe B., Heine U., Sporn M. B. Transforming growth factor beta: biochemistry and roles in embryogenesis, tissue repair and remodeling, and carcinogenesis. Recent Prog Horm Res. 1988;44:157–197. doi: 10.1016/b978-0-12-571144-9.50010-7. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Structure and biology of proteoglycans. Annu Rev Cell Biol. 1988;4:229–255. doi: 10.1146/annurev.cb.04.110188.001305. [DOI] [PubMed] [Google Scholar]
- Schmidt G., Robenek H., Harrach B., Glössl J., Nolte V., Hörmann H., Richter H., Kresse H. Interaction of small dermatan sulfate proteoglycan from fibroblasts with fibronectin. J Cell Biol. 1987 Jun;104(6):1683–1691. doi: 10.1083/jcb.104.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., Assoian R. K. Transforming growth factor-beta: biological function and chemical structure. Science. 1986 Aug 1;233(4763):532–534. doi: 10.1126/science.3487831. [DOI] [PubMed] [Google Scholar]
- Vogel K. G., Paulsson M., Heinegård D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984 Nov 1;223(3):587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield L. M., Smith D. M., Broz S., Jackson M., Levinson A. D., Sporn M. B. Recombinant TGF-beta 1 is synthesized as a two-component latent complex that shares some structural features with the native platelet latent TGF-beta 1 complex. Growth Factors. 1989;1(3):203–218. doi: 10.3109/08977198908997997. [DOI] [PubMed] [Google Scholar]
- Wakefield L. M., Smith D. M., Flanders K. C., Sporn M. B. Latent transforming growth factor-beta from human platelets. A high molecular weight complex containing precursor sequences. J Biol Chem. 1988 Jun 5;263(16):7646–7654. [PubMed] [Google Scholar]
- Wakefield L. M., Smith D. M., Masui T., Harris C. C., Sporn M. B. Distribution and modulation of the cellular receptor for transforming growth factor-beta. J Cell Biol. 1987 Aug;105(2):965–975. doi: 10.1083/jcb.105.2.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werber H. I., Emancipator S. N., Tykocinski M. L., Sedor J. R. The interleukin 1 gene is expressed by rat glomerular mesangial cells and is augmented in immune complex glomerulonephritis. J Immunol. 1987 May 15;138(10):3207–3212. [PubMed] [Google Scholar]
- Yamaguchi Y., Ruoslahti E. Expression of human proteoglycan in Chinese hamster ovary cells inhibits cell proliferation. Nature. 1988 Nov 17;336(6196):244–246. doi: 10.1038/336244a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Wilson C. B. Complement dependence of antibody-induced mesangial cell injury in the rat. J Immunol. 1987 Jun 1;138(11):3758–3765. [PubMed] [Google Scholar]
- Yamamoto T., Wilson C. B. Quantitative and qualitative studies of antibody-induced mesangial cell damage in the rat. Kidney Int. 1987 Oct;32(4):514–525. doi: 10.1038/ki.1987.240. [DOI] [PubMed] [Google Scholar]