Abstract
Consistent with the theory that individuals with hypofunctioning reward circuitry overeat to compensate for a reward deficit, obese versus lean humans have fewer striatal D2 receptors and show less striatal response to palatable food intake. Low striatal response to food intake predicts future weight gain in those at genetic risk for reduced signaling of dopamine-based reward circuitry. Yet animal studies indicate that intake of palatable food results in downregulation of D2 receptors, reduced D2 sensitivity, and decreased reward sensitivity, implying that overeating may contribute to reduced striatal responsivity. Thus, we tested whether overeating leads to reduced striatal responsivity to palatable food intake in humans using repeated-measures functional magnetic resonance imaging. Results indicated that women who gained weight over a 6 month period showed a reduction in striatal response to palatable food consumption relative to weight-stable women. Collectively, results suggest that low sensitivity of reward circuitry increases risk for overeating and that this overeating may further attenuate responsivity of reward circuitry in a feedforward process.
Introduction
The striatum plays a key role in encoding reward from food intake. Feeding is associated with dopamine (DA) release in the dorsal striatum and the degree of DA release correlates with the amount of pleasure from eating (Szczypka et al., 2001; Small et al., 2003). The dorsal striatum responds to ingestion of chocolate in lean humans and is sensitive to its devaluation by feeding beyond satiety (Small et al., 2001).
Obese humans show less striatal D2 receptor availability than lean humans (Wang et al., 2001; Volkow et al., 2008) and obese rats have lower basal DA levels and reduced D2 receptor availability than lean rats (Orosco et al., 1996; Fetissov et al., 2002). Obese versus lean humans show less activation of striatal DA target regions (caudate, putamen) in response to palatable food intake (Stice et al., 2008a,b), yet show greater striatal activation in response to pictures of food (Rothemund et al., 2007; Stoeckel et al., 2008; Stice et al., 2010), suggesting a dissociation between consummatory food reward and the incentive salience of food cues. Critically, humans that exhibited weaker striatal activation in response to food intake who had an A1 TaqIA allele, which is associated with lower D2 striatal receptor availability (Noble et al., 1991; Ritchie and Noble, 2003; Tupala et al., 2003) and reduced striatal resting metabolism (Noble et al., 1997), showed elevated future weight gain (Stice et al., 2008a). Collectively, these findings accord with the theory that individuals with lower signaling capacity in reward circuitry overeat to compensate for this reward deficit (Blum et al., 1996; Wang et al., 2002).
However, there is evidence that consumption of palatable food leads to downregulation of DA signaling. Regular intake of high-fat and high-sugar foods that results in weight gain leads to downregulation of postsynaptic D2 receptors, decreased D2 sensitivity, and reduced reward sensitivity in rodents (Colantuoni et al., 2001; Bello et al., 2002; Kelley et al., 2003; Johnson and Kenny, 2010). Because these data imply that overeating may contribute to a further attenuation of striatal responsivity to food, we conducted a prospective repeated-measures functional magnetic resonance imaging (fMRI) study to directly test whether overeating is associated with reduced striatal activation in response to palatable foods in humans.
Materials and Methods
Participants
Participants were 26 overweight and obese young women (mean age, 21.0 ± 1.11 years; mean body mass index [BMI], 27.8 ± 2.45). The sample consisted of 7% Asian/Pacific Islander, 2% African Americans, 77% European Americans, 5% Native Americans, and 9% mixed racial heritage. Participants provided written consent. The local ethics review panel approved this study. Those who reported binge eating or compensatory behaviors in the past 3 months, current use of psychotropic medications or illicit drugs, head injury with a loss of consciousness, or current Axis I psychiatric disorder were excluded. Data were collected at baseline and at a 6 month follow-up.
Measures
Body mass.
The BMI (kg/m2) was used to reflect adiposity (Dietz and Robinson, 1998). After removal of shoes and coats, height was measured to the nearest millimeter using a stadiometer and weight was assessed to the nearest 0.1 kg using a digital scale. Two measures of each were obtained and averaged. Participants were asked to refrain from eating for 3 h before completing anthropomorphic measures for standardization purposes. BMI correlates with direct measures of total body fat such as dual energy x-ray absorptiometry (r = 0.80–0.90) and with health measures such as blood pressure, adverse lipoprotein profiles, atherosclerotic lesions, serum insulin levels, and diabetes mellitus (Dietz and Robinson, 1998).
fMRI paradigm.
Participants were asked to consume their regular meals, but to refrain from eating or drinking (including caffeinated beverages) for 4–6 h preceding their imaging session for standardization. We selected this deprivation period to capture the hunger state that most individuals experience as they approach their next meal, which is a time when individual differences in food reward would logically impact caloric intake. Participants completed the paradigm between 11:00 and 13:00 or 16:00 and 18:00. Although we attempted to conduct baseline and follow-up scans at the same time of day, because of scheduling limitations only 62% of participants conducted their second scan within 3 h of the time they completed their baseline scan (mean difference in time of scans, 3.0 h; range, 0.5–6.0 h). Participants were familiarized with the fMRI paradigm through practice on a separate computer before scanning.
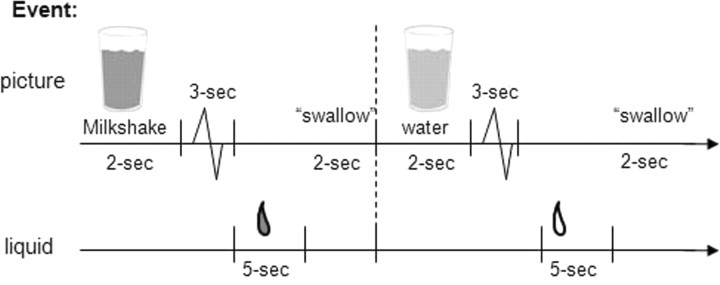
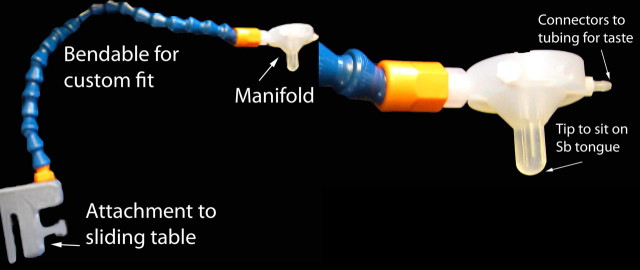
The milkshake paradigm was designed to examine activation in response to consumption and anticipated consumption of palatable food (Fig. 1), though this report focused solely on the former. Stimuli were presented in five separate scanning runs. Stimuli consisted of two images (glass of milkshake and glass of water) that signaled the delivery of either 0.5 ml of a chocolate milkshake or a tasteless solution. Order of presentation was randomized across participants. The chocolate milkshake consisted of four scoops of Häagen-Daz vanilla ice cream, 1.5 cups of 2% milk, and 2 tablespoons of Hershey's chocolate syrup. The calorie-free tasteless solution, which was designed to mimic the natural taste of saliva, consisted of 25 mm KCl and 2.5 mm NaHCO3. We used artificial saliva because water has a taste that activates the taste cortex (Zald and Pardo, 2000). Images were presented for 2 s using MATLAB (Mathworks). Taste delivery occurred 7–10 s after onset of the cue and lasted 5 s. Each event of interest lasted 5 s. Each run consisted of 20 events of milkshake intake and 20 events of tasteless solution intake. Fluids were delivered using programmable syringe pumps (BS-8000; Braintree Scientific) controlled by MATLAB to ensure consistent volume, rate, and timing of taste delivery. Sixty milliliter syringes filled with chocolate milkshake and tasteless solution were connected via tygon tubing through a wave guide to a manifold attached to the head coil in the MRI scanner. The manifold fit into the participants' mouths and delivered the taste to a consistent segment of the tongue (Fig. 2). This procedure has been successfully used in the past to deliver liquids in the scanner and has been described in detail previously (Stice et al., 2008b). Participants were instructed to swallow when they saw the “swallow” cue. Images were presented with a digital projector/reverse screen display system to a screen at the back end of the MRI scanner bore and were visible via a mirror mounted on the head coil.
Figure 1.
Example of timing and ordering of presentation of pictures and drinks during the run.
Figure 2.
The gustatory manifold was anchored to the table. New tubing and syringes were used for each subject and the mouthpiece was cleaned and sterilized between uses.
Imaging and statistical analysis
Scanning was performed by a Siemens Allegra 3 tesla head-only MRI scanner. A standard birdcage coil was used to acquire data from the entire brain. A thermo foam vacuum pillow and additional padding was used to restrict head motion. In total, 152 scans were collected during each of the functional runs. Functional scans used a T2* weighted gradient single-shot echo planar imaging sequence [echo time = 30 ms, repetition time (TR) = 2000 ms, flip angle = 80°] with an in-plane resolution of 3.0 × 3.0 mm2 [64 × 64 matrix; 192 × 192 mm2 field of view (FOV)]. To cover the whole brain, 32 slices (4 mm thick; interleaved acquisition, no skip) were acquired along the AC–PC transverse, oblique plane, as determined by the midsagittal section. Structural scans were collected using an inversion recovery T1 weighted sequence (MP-RAGE) in the same orientation as the functional sequences to provide detailed anatomic images aligned to the functional scans. High-resolution structural MRI sequences (FOV = 256 × 256 mm2, 256 × 256 matrix, thickness = 1.0 mm, n ≈ 160 slices) were acquired.
Data were preprocessed and analyzed using SPM5 (Wellcome Department of Imaging Neuroscience, London, UK) in MATLAB (Worsley and Friston, 1995). Images were time-acquisition corrected to the slice obtained at 50% of the TR. Functional images were realigned to the mean. Anatomical and functional images were normalized to the standard MNI template brain implemented in SPM5 (ICBM152, based on an average of 152 normal MRI scans). Normalization resulted in a voxel size of 3 mm3 for functional images and 1 mm3 for structural images. Functional images were smoothed with a 6 mm full-width at half-maximum isotropic Gaussian kernel.
To identify brain regions activated by consumption of palatable food, we contrasted blood oxygenation level-dependent (BOLD) response during receipt of milkshake versus receipt of tasteless solution. We considered the arrival of a taste in the mouth to be consummatory reward, rather than when the taste was swallowed, but acknowledge that postingestive effects contribute to the reward value of food (O'Doherty et al., 2002). Condition-specific effects at each voxel were estimated using general linear models. Vectors of the onsets for each event of interest were compiled and entered into the design matrix so that event-related responses could be modeled by the canonical hemodynamic response function, as implemented in SPM5, consisting of a mixture of two gamma functions that emulate the early peak at 5 s and the subsequent undershoot. To account for the variance induced by swallowing the solutions, we included the time of the swallow cue (subjects were trained to swallow at this time) as a control variable. We also included temporal derivatives of the hemodynamic function to obtain a better model of the data (Henson et al., 2002). A 128 s high-pass filter (per SPM5 convention) was used to remove low-frequency noise and slow drifts in the signal.
Individual maps were constructed to compare the activations within each participant for the contrast milkshake receipt–tasteless receipt. Between-group comparisons were then performed using random-effects models to account for interparticipant variability. Parameter estimates were entered into a second-level 2 × 2 random-effects ANOVA (milkshake receipt–tasteless receipt) by weight-gain group versus weight-stable group, weight-gain group versus weight-loss group, or weight-stable group versus weight-loss group. The significance of BOLD activation was determined by considering both the maximum intensity of a response as well as the extent of the response. We performed regions-of-interest (ROIs) searches using peaks in the dorsal striatum identified previously (Stice et al., 2008a) as centroids to define 10-mm-diameter spheres. Significance for these a priori ROIs was assessed at a statistical threshold of p < 0.005 uncorrected and cluster extent ≥3 voxels. To adjust for the fact that we conducted multiple comparisons, we report false discovery rate (FDR)-corrected p values (p < 0.05).
Validation.
Evidence suggests that this fMRI paradigm is a valid measure of individual differences in anticipatory and consummatory food reward (Stice et al., 2008b). Participants rated the milkshake as significantly (r = 0.68) more pleasant than the tasteless solution per a visual analog scale. Pleasantness ratings of the milkshake correlated with activation in the parahippocampal gyrus in response to milkshake receipt (r = 0.72), a region that is sensitive to the devaluation of food (Small et al., 2001). Activation in regions representing consummatory food reward in response to milkshake receipt in this fMRI paradigm correlated (r = 0.84–0.91) with self-reported perceived pleasantness for a variety of foods, as assessed with an adapted version of the Food Craving Inventory (White et al., 2002). Activation in response to consummatory food reward in this fMRI paradigm correlates (r = 0.82–0.95) with how hard participants work for food and how much food they work for in an operant behavioral task that assesses individual differences in food reinforcement (Saelens and Epstein, 1996). A preliminary study using the same paradigm with college women (N = 20) found that women who expect food to be rewarding, as assessed with the Eating Expectancy Inventory, show greater activation in the ventromedial prefrontal cortex, cingulate gyrus, frontal operculum, amygdala, and parahippocampal gyrus (η2 = 0.21–0.42) in response to milkshake receipt than did women who expect food to be less rewarding.
Results
We tested whether subjects who showed a >2.5% increase in BMI over the 6 month follow-up (N = 8; mean percentage BMI change = 4.41; range, 2.6–8.2) exhibited a reduction in caudate activation in response to milkshake intake relative to those who showed a <2% change in BMI (N = 12; mean percentage BMI change = 0.05; range, −0.64–1.7) to provide a direct test of the a priori hypothesis that weight gain would be associated with a reduction in striatal response to palatable food relative to weight-stable participants. Exploratory analyses also tested whether participants who showed a >2.5% decrease in BMI (N = 6; mean percentage BMI change = −4.7; range, −3.1–−6.8) exhibited differential change in striatal response to palatable food than participants who remained weight stable or gained weight. In terms of raw weight change, this translated into a mean weight change of 6.4 lbs for the weight-gain group, a mean weight change of 0.5 lbs for the weight-stable group, and a mean weight change of −6.8 lbs for the weight-loss group. Although groups did not differ on BMI at baseline, we controlled for this variable. Because there was some variation in the time of day at which the baseline and follow-up scans were conducted across subjects that might have influenced results, we also controlled for the difference in time of the two scans (in hours). Parameter estimates from milkshake–tasteless contrasts were entered into a second-level 2×2×2 random-effects ANOVA (e.g., weight gain–weight stable by milkshake receipt–tasteless receipt by 6 month follow-up–baseline.
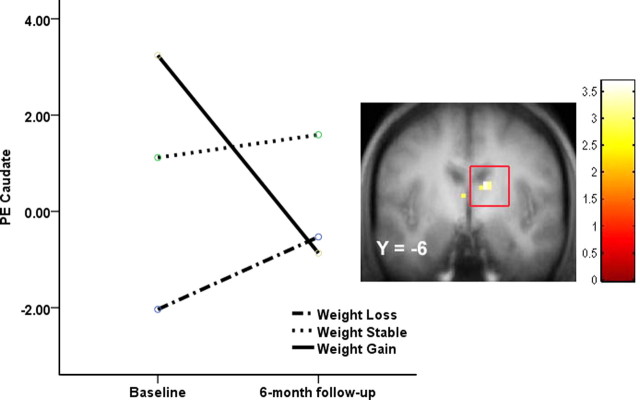
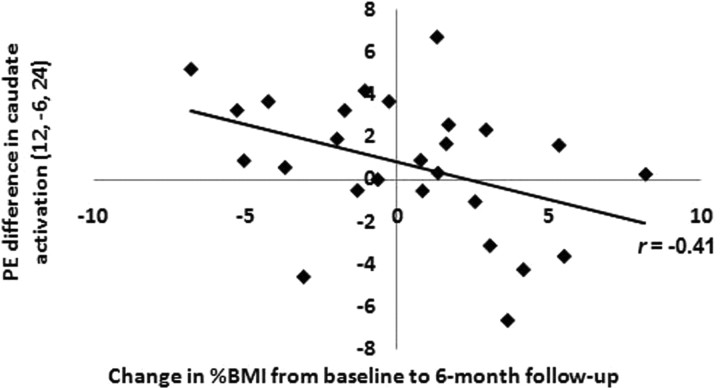
As hypothesized, the weight-gain group showed significantly less activation in right caudate in response to milkshake intake at 6 month follow-up compared with baseline relative to changes observed in weight-stable participants (12, −6, 24; Z = 3.44; FDR-corrected p = 0.03; r = −0.35; 9, 0, 15; Z = 2.96, FDR-corrected p = 0.03, r = −0.26) (Fig. 3). The weight-loss group did not show significant changes in activation in the caudate in response to milkshake intake compared with the weight-gain or weight-stable groups (Fig. 3). To illustrate the relation between the continuous measure of degree of weight gain and the magnitude of the reduction in striatal responsivity to palatable food, we regressed change in BMI against change in right caudate (12, −6, 24) activation for all participants in SPSS, controlling for baseline BMI and scan-time difference (Fig. 4). To determine whether change in the right caudate for those who gained weight compared with those who maintained weight was significantly greater than in the mirror region of the left caudate, we compared the activation in the right and left caudate using ROI analysis. We conducted an ANOVA testing the interaction between hemisphere, time, and group for the contrast between activation in response to receipt of milkshake versus tasteless solution. There was no significant interaction (F (1,18) = 0.91, p = 0.35). Thus, although our analyses revealed a significant time-by-group interaction in the right caudate, but not the left caudate, we cannot conclude that the observed effect was significantly lateralized.
Figure 3.
Coronal section showing less activation in the right caudate (12, −6, 24; Z = 3.44; FDR-corrected p = 0.03; p < 0.05) in the weight-gain group (N = 8; ≥2% BMI gain) versus the weight-stable group (N = 12; ≤2% BMI change) during milkshake receipt–tasteless receipt at 6 month follow-up compared with baseline with a scatter plot of the extracted caudate parameter estimates estimated for each group. PE, Parameter estimate.
Figure 4.
Scatter plot showing change in right caudate activation during milkshake receipt–tasteless receipt at 6 month follow-up compared with baseline as a function of change in percentage BMI. PE, Parameter estimate.
Discussion
Results indicate that weight gain was associated with a reduction in striatal activation in response to palatable food intake relative to baseline response, which is a novel contribution to the literature because, to our knowledge, this is the first prospective fMRI study to investigate change in striatal response to food consumption as a function of weight change. These findings extend results from experiments that indicate that high-fat and high-sugar diets result in a reduction in signaling capacity of DA-based reward circuitry and reward sensitivity in rodents (Colantuoni et al., 2001; Bello et al., 2002; Kelley et al., 2003; Johnson and Kenny, 2010). These findings also dovetail with evidence that treatment-induced weight loss produces increased D2 receptor availability in humans (Steele et al., 2010) and upregulation of genes that govern DA signaling capacity in mice (Yamamoto, 2006). Collectively, these data suggest that overeating contributes to a reduction in striatal response to palatable foods.
The above findings, taken in conjunction with evidence that low striatal responsivity to palatable foods increases risk for future weight gain if coupled with genotypes associated with reduced signaling capacity of DA-based reward circuitry (Stice et al., 2008a), implies that there may be a feedforward process of vulnerability, wherein low initial striatal responsivity to food may increase risk for overeating, which contributes to D2 receptor downregulation and blunted striatal responsivity to food, thereby further increasing the risk for future overeating and consequent weight gain. If this feedforward model of the relation of striatal responsivity to food and overeating is replicated in independent studies, it would suggest that future research should evaluate behavioral and pharmacological interventions that increase D2 receptors and signaling capacity in DA-based reward circuitry as a means of preventing or treating obesity. This working model would also imply that prevention programs and health policy should strive to reduce intake of high-fat and high-sugar foods during development to avoid a further blunting of striatal responsivity to food and reduce risk for future weight gain in vulnerable populations.
It is important to acknowledge, however, that the present study and the previous study that predicted weight gain (Stice et al., 2008a) involved participants who were already overweight at the baseline assessment. Thus, it is possible that overeating had already contributed to a blunted striatal response to food. It would be useful to examine responsivity of reward regions to food receipt among lean individuals at high and low risk for future weight gain to better characterize any abnormalities that exist before unhealthy weight gain. It is also important to note that hyposensitivity of reward circuitry to food intake is only one of a multitude of etiologic processes that likely increase risk for obesity and further that obesity is a heterogeneous condition that may have qualitatively distinct etiologic pathways (Davis et al., 2009).
It is also important to consider the limitations of this study. First, we did not directly assess DA functioning, so we can only speculate that changes in DA signaling contribute to the observed change in striatal responsivity. However, Hakyemez et al. (2008) confirmed that there is a positive relation between oral d-amphetamine-induced DA release in the ventral striatum assessed via positron emission tomography (PET) and BOLD activation assessed via fMRI in the same region during anticipation (motor preparation to obtain) of monetary reward (r = 0.51), paralleling results from another PET/fMRI study (Schott et al., 2008). Second, we did not conduct weight measurements at the same time of day for participants at the baseline and 6 month follow-up assessments, which might have introduced error in our modeling of weight change. However, we did standardize time since last meal by asking participants to abstain from any type of intake of food or beverages (other than water) for 3 h before being weighed. We also found that BMI showed high 1 month test–retest reliability (r = 0.99) in a previous study that likewise did not conduct weight measurements at the same time of day at baseline and the follow-up assessment (Stice et al., 2006). Third, we could not confirm that participants actually abstained from eating for 4–6 h before the fMRI scans, which may have introduced unnecessary variance.
In conclusion, the present results, taken in combination with past findings, suggest that low responsivity of DA-based reward circuitry to food intake may increase risk for overeating, and further that this overeating results in an additional attenuation in reward circuitry responsivity, thereby increasing risk for future weight gain in a feedforward manner. This working model may explain why obesity typically shows a chronic course and is resistant to treatment.
Footnotes
This study was supported by National Institutes of Health Grants R1MH64560A and DK080760.
References
- Bello NT, Lucas LR, Hajnal A. Repeated sucrose access influences dopamine D2 receptor density in the striatum. Neuroreport. 2002;13:1575–1578. doi: 10.1097/00001756-200208270-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Cull JG, Comings DE. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J R Soc Med. 1996;89:396–400. doi: 10.1177/014107689608900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, Schwartz GJ, Moran TH, Hoebel BG. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12:3549–3552. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- Davis CA, Levitan RD, Reid C, Carter JC, Kaplan AS, Patte KA, King N, Curtis C, Kennedy JL. Dopamine for “wanting” and opioids for “liking”: a comparison of obese adults with and without binge eating. Obesity. 2009;17:1220–1225. doi: 10.1038/oby.2009.52. [DOI] [PubMed] [Google Scholar]
- Dietz WH, Robinson TN. Use of the body mass index (BMI) as a measure of overweight in children and adolescents. J Pediatr. 1998;132:191–193. doi: 10.1016/s0022-3476(98)70426-3. [DOI] [PubMed] [Google Scholar]
- Fetissov SO, Meguid MM, Sato T, Zhang LH. Expression of dopaminergic receptors in the hypothalamus of lean and obese Zucker rats and food intake. Am J Physiol Regul Integr Comp Physiol. 2002;283:R905–R910. doi: 10.1152/ajpregu.00092.2002. [DOI] [PubMed] [Google Scholar]
- Hakyemez HS, Dagher A, Smith SD, Zald DH. Striatal dopamine transmission in healthy humans during a passive monetary reward task. Neuroimage. 2008;39:2058–2065. doi: 10.1016/j.neuroimage.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Henson RN, Price CJ, Rugg MD, Turner R, Friston KJ. Detecting latency differences in event-related BOLD responses: application to words versus nonwords and initial versus repeated face presentations. Neuroimage. 2002;15:83–97. doi: 10.1006/nimg.2001.0940. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Will MJ, Steininger TL, Zhang M, Haber SN. Restricted daily consumption of a highly palatable food (chocolate Ensure) alters striatal enkephalin gene expression. Eur J Neurosci. 2003;18:2592–2598. doi: 10.1046/j.1460-9568.2003.02991.x. [DOI] [PubMed] [Google Scholar]
- Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psychiatry. 1991;48:648–654. doi: 10.1001/archpsyc.1991.01810310066012. [DOI] [PubMed] [Google Scholar]
- Noble EP, Gottschalk LA, Fallon JH, Ritchie TL, Wu JC. D2 dopamine receptor polymorphism and brain regional glucose metabolism. Am J Med Genet. 1997;74:162–166. [PubMed] [Google Scholar]
- O'Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Orosco M, Rouch C, Nicolaïdis S. Rostromedial hypothalamic monoamine changes in response to intravenous infusions of insulin and glucose in freely feeding obese Zucker rats: a microdialysis study. Appetite. 1996;26:1–20. doi: 10.1006/appe.1996.0001. [DOI] [PubMed] [Google Scholar]
- Ritchie T, Noble EP. Association of seven polymorphisms of the D2 dopamine receptor gene with brain receptor-binding characteristics. Neurochem Res. 2003;28:73–82. doi: 10.1023/a:1021648128758. [DOI] [PubMed] [Google Scholar]
- Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, Klapp BF. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Saelens BE, Epstein LH. Reinforcing value of food in obese and non-obese women. Appetite. 1996;27:41–50. doi: 10.1006/appe.1996.0032. [DOI] [PubMed] [Google Scholar]
- Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, Seidenbecher CI, Coenen HH, Heinze HJ, Zilles K, Düzel E, Bauer A. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J Neurosci. 2008;28:14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- Steele KE, Prokopowicz GP, Schweitzer MA, Magunsuon TH, Lidor AO, Kuwabawa H, Kumar A, Brasic J, Wong DF. Alterations of central dopamine receptors before and after gastric bypass surgery. Obes Surg. 2010;20:369–374. doi: 10.1007/s11695-009-0015-4. [DOI] [PubMed] [Google Scholar]
- Stice E, Shaw E, Burton E, Wade E. Dissonance and healthy weight eating disorder prevention programs: a randomized efficacy trial. J Consult Clin Psychol. 2006;74:263–275. doi: 10.1037/0022-006X.74.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008a;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008b;117:924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. Neuroimage. 2010;50:1618–1625. doi: 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW, 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Szczypka MS, Kwok K, Brot MD, Marck BT, Matsumoto AM, Donahue BA, Palmiter RD. Dopamine production in the caudate putamen restores feeding in dopamine-deficient mice. Neuron. 2001;30:819–828. doi: 10.1016/s0896-6273(01)00319-1. [DOI] [PubMed] [Google Scholar]
- Tupala E, Hall H, Bergström K, Mantere T, Räsänen P, Särkioja T, Tiihonen J. Dopamine D2 receptors and transporters in type 1 and 2 alcoholics measured with human whole hemisphere autoradiography. Hum Brain Mapp. 2003;20:91–102. doi: 10.1002/hbm.10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, Alexoff D, Ding YS, Wong C, Ma Y, Pradhan K. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42:1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS. The role of dopamine in motivation for food in humans: implications for obesity. Expert Opin Ther Targets. 2002;6:601–609. doi: 10.1517/14728222.6.5.601. [DOI] [PubMed] [Google Scholar]
- White MA, Whisenhunt BL, Williamson DA, Greenway FL, Netemeyer RG. Development and validation of the food-craving inventory. Obes Res. 2002;10:107–114. doi: 10.1038/oby.2002.17. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited–again. [letter; comment] Neuroimage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Yamamoto T. Neural substrates for the processing of cognitive and affective aspects of taste in the brain. Arch Histol Cytol. 2006;69:243–255. doi: 10.1679/aohc.69.243. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. Cortical activation induced by intraoral stimulation with water in humans. Chem Senses. 2000;25:267–275. doi: 10.1093/chemse/25.3.267. [DOI] [PubMed] [Google Scholar]