Abstract
Hyperaldosteronism has been associated with endothelial dysfunction and impaired vascular reactivity in patients with hypertension or congestive heart failure. The mineralocorticoid receptor (MR) antagonists spironolactone and eplerenone have been shown to reduce morbidity and mortality, in part, by ameliorating the adverse effects of aldosterone on vascular function. Although spironolactone and eplerenone are increasingly utilized in patients with cardiovascular disease, widespread clinical use is limited by the development of gynecomastia with spironolactone and hyperkalemia with both agents. This suggests that the development of newer agents with favorable side effect profiles is warranted.
Keywords: aldosterone, congestive heart failure, endothelial function, eplerenone, hypertension, spironolactone
1. Introduction
Elevated levels of aldosterone are associated with endothelial dysfunction and impaired vascular reactivity in patients with congestive heart failure or hypertension that is ameliorated by treatment with a mineralocorticoid receptor (MR) antagonist [1–3]. In patients with congestive heart failure, left ventricular dysfunction decreases afferent renal arteriole blood flow resulting in a 20-fold increase in aldosterone levels compared with normal subjects. Under these circumstances, aldosterone-mediated sodium and water retention leads to inappropriate intravascular volume expansion and clinical symptoms consistent with hypervolemia [4]. Traditionally, endothelial dysfunction was believed to be a consequence of these hemodynamic changes; however, identification of MRs in blood vessels has led to the realization that aldosterone has pleiotropic effects in the vasculature that extend beyond the regulation of sodium and water balance [5].
The renin-angiotensin-aldosterone system has evolved as a primary target for goal directed therapy in patients with cardiovascular disease. Angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARB) are established pharmacologic options in patients with hypertension, left ventricular dysfunction, and diabetes mellitus-associated microvascular disease [6–8]; however, clinical studies have shown that aldosterone levels may remain elevated despite effective therapy with these agents. By some estimates, this phenomenon, referred to as aldosterone breakthrough, may occur in up to 50% of patients on ACE inhibitor or ARB therapy [9]. The cardiovascular consequences of aldosterone breakthrough are profound and include endothelial dysfunction as well as worsening left ventricular function, diminished exercise capacity, and decreased renal function [9]. At present, two MR antagonists, spironolactone and eplerenone, are utilized clinically to limit the adverse cardiovascular effects of hyperaldosteronism. This review will detail the pharmacological profiles of these drugs with focus on vascular endothelial function and detail aspects of each agent that warrant consideration for the development of future therapies.
2. Aldosterone and endothelial function
The association between aldosterone and endothelial dysfunction was originally observed by Farquharson and colleagues who examined endothelial function in 16 healthy males after aldosterone infusion (12 pmol/min/kg for 4 hr). In these subjects, endothelium-dependent vasodilation was decreased significantly and did not result from a reduction in forearm blood flow or systemic blood pressure [10]. In contrast, two other studies performed in healthy individuals reported conflicting results. In the first study, aldosterone infusion (1400 pmol/min for 30 min) increased forearm blood flow but had no effect on endothelium-dependent vasodilation while, in the second study, infusion of aldosterone (55 pmol/1000 mL/min for 10 min) improved acetylcholine-stimulated vasodilation [11,12]. In addition to the differences in dose and duration of aldosterone infusion, the disparities in these results may be explained, in part, by sodium and/or potassium shifts or nonMR-mediated effects of aldosterone. Nonetheless, the observations made by Farquharson and colleagues, that aldosterone induces endothelial dysfunction, are in agreement with clinical studies performed in patients with congestive heart failure or hypertension (vide infra).
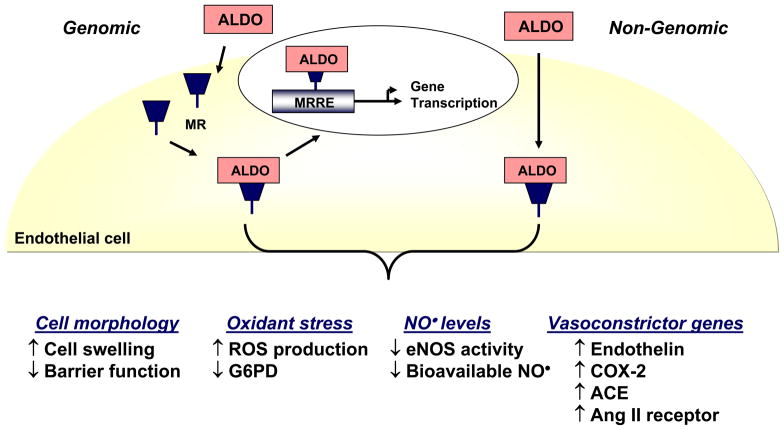
An understanding of the mechanism(s) by which aldosterone induces endothelial dysfunction has been elucidated from in vitro studies and animal models (Figure 1). Aldosterone modulates vascular endothelial cell morphology and barrier function leading to cell swelling, increased stiffness, and gap formation between cells [13]. These effects are exacerbated by exposure to pathophysiologically relevant concentrations of sodium [14]. Aldosterone increases endothelial cell oxidant stress by augmenting reactive oxygen species accumulation via NADPH oxidase activation, uncoupled eNOS, and decreased antioxidant enzyme capacity [15,16]. This, in turn, decreases levels of bioavailable nitric oxide and impairs endothelium-dependent vasodilation in rodent models, independent of changes in blood pressure [16–18]. Aldosterone may also promote endothelial dysfunction through the enhanced production of vasoconstrictor factors: aldosterone increases the expression of endothelin, cyclooxygenase-2, ACE, and angiotensin II receptors [19]. Regardless of the mechanism(s) by which aldosterone induces endothelial dysfunction many of these effects are abrogated by MR antagonism. It is, therefore, of interest to speculate that some of these mechanisms may be operative in patients and underlie the observed clinical benefits of MR blockade on endothelial function.
Figure 1.
Biological effects of aldosterone in vascular endothelial cells. Aldosterone (ALDO) binds to and activates the mineralocorticoid receptor (MR) to stimulate electrolyte flux and intracellular signaling pathways. These effects may occur through binding of the MR to a MR response element (MRRE) to initiate de novo gene transcription (genomic) or through receptor-mediated pathways in the absence of transcription (non-genomic). The net result is an increase in cell swelling and rigidity with the appearance of gaps between cells resulting in a loss of endothelial barrier function. Aldosterone increases endothelial oxidant stress by enhancing reactive oxygen species (ROS) production and decreasing expression of glucose-6-phosphate dehydrogenase (G6PD), a key antioxidant enzyme in the vascular endothelium. There is a decrease in levels of bioavailable nitric oxide (NO•) owing to diminished activity of the endothelial isoform of nitric oxide synthase (eNOS) as well as sequestration of NO• by ROS. These deleterious effects of aldosterone on endothelial vasodilator signaling pathways is augmented further by increased expression of the vasoconstrictor mediators endothelin-1, cyclooxygenase-2 (COX-2) metabolites, angiotensin converting enzyme (ACE) and angiotensin II receptors.
3. Spironolactone
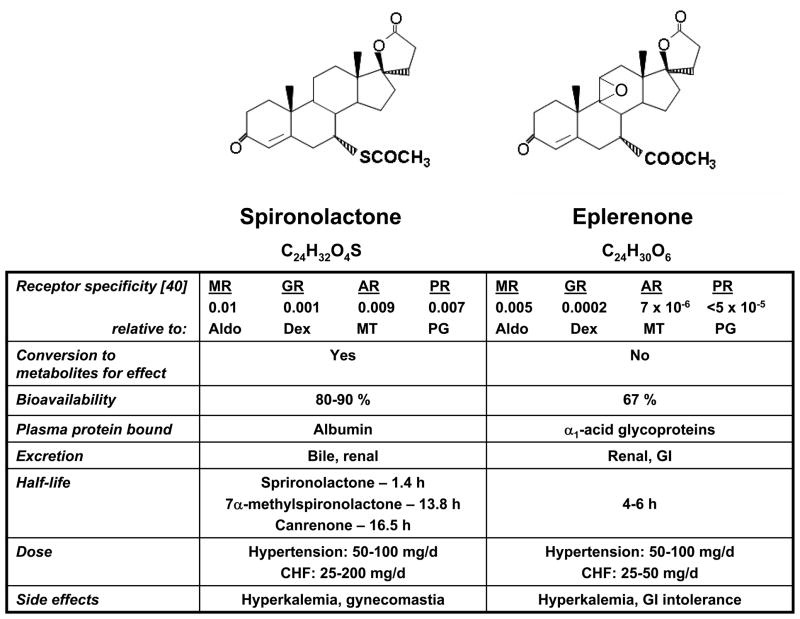
Spironolactone is a competitive MR antagonist that was synthesized initially as a diuretic to inhibit renal transport of sodium leading to salt and water excretion with potassium sparing [20]. Spironolactone is structurally similar to progesterone and as such is a nonselective MR blocker with antiprogesterone and antiandrogen properties [21] (Figure 2).
Figure 2.
Chemical structure and pharmacologic profile of spironolactone and eplerenone. The nonselective mineralocorticoid receptor antagonist spironolactone is structurally similar to progesterone and, as such, binds to the progesterone, androgen, and mineralocorticoid receptors. Eplerenone, a derivative of spironolactone with a 9,11-epoxy in the lactone ring and the substitution of a carboxymethyl group in place of the 17α-thioacetyl group, is selective for the mineralocorticoid receptor. The differences in chemical structure between these drugs accounts for their distinct pharmacological profiles. MR, mineralocorticoid receptor; GR, glucocorticoid receptor; AR, androgen receptor; PR, progesterone receptor; Aldo, aldosterone; Dex, dexamethasone; MT, methyltrienolone; PG, progesterone.
3.1 Pharmacokinetics
Spironolactone is administered orally with an estimated absorption and bioavailability of 80–90%. Although food may increase the absorption of spironolactone by 100%, clinical studies have shown that there is no therapeutic difference when given in the fed or fasted state [22]. In the liver, spironolactone is converted rapidly by deacetylation, dethiolation, and thiomethylation to the metabolites canrenone, 7α-methylspironolactone, and 6β-hydroxy-7α-methylspironolactone, which account for the majority of aldosterone inhibition [23]. Both spironolactone (88%) and canrenone (99%) are bound to plasma albumin and the active metabolites of are excreted in both bile and urine [24].
Spironolactone typically has a slow onset of action with a maximal response detected 48 hours after the first dose of drug. This delay has been attributed to the time needed to accumulate therapeutic steady state levels of the active metabolites [20]. High performance liquid chromatography analysis has identified 7α-methylspironolactone, and not canrenone, as the predominant active metabolite that accounts for the majority of the potassium-sparing effects of this drug [25]. Compared to spironolactone, the relative antimineralocorticoid effects of the metabolites canrenone and 7α-methylspironolactone is 1.1 and 1.28 [24]. The half-life of spironolactone was determined in studies of normal volunteers administered 100 mg/day for 15 days. Here, the half-life of spironolactone was found to be 1.4 hours while the half-lives of the metabolites canrenone and 7α-methylspironolactone were 16.5 and 13.8 hours, respectively. In patients with cirrhosis, similar to what may be observed in patients with congestive heart failure and hepatic congestion, the half-lives of these metabolites were increased significantly: the half-life of spironolactone was 9 hours and the half-lives of canrenone and 7α-methylspironolactone were 58 and 24 hours, respectively [26].
3.2 Spironolactone and endothelial function
The beneficial effect of spironolactone on the cardiovascular toxicity of hyperaldosteronism was demonstrated in the landmark Randomized Aldactone Evaluation Study (RALES). In patients with congestive heart failure and an ejection fraction ≤ 35%, spironolactone (25 mg/day) therapy was associated with a 30% reduction in mortality owing to a decrease in the progression of congestive heart failure and sudden death compared to placebo-treated patients [27].
Although not examined directly in the RALES trial, it has been suggested that some of the observed clinical benefits may have resulted from improved endothelial function [28]. When present, endothelial dysfunction itself is an independent predictor of major adverse cardiac events and associated with worse clinical outcomes [29]. A number of smaller studies lend credibility to this hypothesis. In patients with New York Heart Association (NYHA) class II–III heart failure treated with ACE inhibitors and diuretics, endothelium-dependent vascular reactivity increased nearly 2-fold in patients treated with spironolactone 50 mg/day as compared to placebo-treated patients [3]. These findings were confirmed in patients with mild (NYHA class I–II) as well as advanced (NYHA class III–IV) heart failure [30,31].
Improved endothelial function and vascular reactivity with spironolactone has also been demonstrated in patients with hypertension. In a study of 80 patients with resistant hypertension being treated with three or more medications, including an ACE inhibitor, brachial artery endothelium-dependent vasodilation was decreased significantly in patients with hyperaldosteronism compared to those with plasma aldosterone levels within the normal range. In a subset of patients treated with spironolactone 12.5–25 mg/day for three months, endothelial function improved in patients with hyperaldosteronism, independent of changes in blood pressure [32]. Increased endothelium-dependent vasodilation was also seen in spironolactone-treated patients with aldosterone levels in the normal range implying that currently accepted normal levels of aldosterone may, in fact, have deleterious consequences for endothelial function.
Interestingly, spironolactone does not improve vascular endothelial function in all patient subsets. In patients with type 2 diabetes mellitus, spironolactone 50 mg/day decreased endothelial function by 45% compared to placebo-treated patients, an effect that was more pronounced in patients that were being treated with ACE inhibitors [33]. Similarly, in patients with established coronary artery disease, without evidence of congestive heart failure, treatment with spironolactone 12.5–50 mg/day for three months had no effect on endothelial function compared to placebo [34]. These observations suggest that the beneficial effects of spironolactone on endothelial function may be disease-specific.
4. Eplerenone
Eplerenone is a selective MR antagonist that is chemically derived from spironolactone by the addition of a 9,11-epoxy in the lactone ring and the substitution of a carboxymethyl group in place of the 17α-thioacetyl group [23,35] (Figure 2). The addition of the 9,11-epoxy group reduced the affinity of this compound for the progesterone and androgen receptors leading to its utilization as an alternative to spironolactone.
4.1 Pharmacokinetics
Following oral administration, eplerenone is absorbed from the intestine with a bioavailability of 67% [36,37]. Food does not influence drug absorption or bioavailability and peak plasma concentrations are reached by 1.5 hours [23,37]. In plasma, eplerenone is protein bound in a concentration-dependent manner, primarily to α1-acid glycoprotein [36].
Eplerenone is metabolized to its inactive metabolites, 6β-OH eplerenone and the open lactone ring form of eplerenone, by cytochrome P-450 isoenzyme 3A4. Therefore, coadministration of drugs such as ketoconazole, verapamil, or erythromycin, which inhibit cytochrome P-450 3A4, may necessitate a reduction in the dose of eplerenone [37,38]. Eplerenone is excreted in urine (67%) and feces (37%), predominantly as metabolites; only 6.8% of the eliminated drug is the intact parent compound and its elimination half-life is 4–6 hours [36,37]. The maximum concentration for eplerenone is increased in the elderly (age ≥ 65 years), decreased in black compared with white individuals, and increased in patients with renal insufficiency indicating that patient subsets do not respond uniformly to eplerenone and select groups may require additional monitoring with dose adjustments [34,35,39].
Although the relative antialdosterone effect of eplerenone has not been determined, in studies performed with isolated rat MRs, eplerenone was found to have a 10–20 fold lower affinity for the receptor than spironolactone. In contrast, when examined in vivo, a lower dose of eplerenone (0.8 mg/kg) than spironolactone (1.7 mg/kg) was required to inhibit aldosterone-MR binding by 50% [40]. This discrepancy may be explained by differences in drug-plasma protein binding properties (spironolactone > eplerenone) that influence drug availability at the receptor level. Eplerenone has a 500-fold lower affinity for the androgen receptor than spironolactone leading to the observed decrease in adverse endocrine-related side effects [41].
4.2 Eplerenone and endothelial function
In 2003, the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival (EPHESUS) trial extended the findings of RALES by demonstrating that early administration of eplerenone (50 mg/day) to patients with acute myocardial infarction complicated by heart failure resulted in a 15% decrease in death, owing to the prevention of sudden cardiac death [42]. Similar to the RALES trial, it has been suggested that some of the observed benefits of eplerenone in this patient population may be attributed to improved endothelial function, although this was not assessed in the trial [43]. Insight into the beneficial effects of eplerenone on endothelial function, therefore, may be gained from preclinical studies and inferred from studies in diabetic patients.
In a hypertensive rat model, eplerenone has been shown to improve endothelial function and vascular reactivity by decreasing oxidant stress, enhancing bioavailable NO, and limiting vascular remodeling, independent of changes in blood pressure [44]. Similarly, in atherosclerotic rabbits or nonhuman primates, eplerenone restored endothelium-dependent vasodilation without affecting blood pressure or plasma cholesterol levels [45,46].
Additional support for the beneficial effects of eplerenone on endothelial function comes from a study that examined coronary artery function and myocardial perfusion reserve in diabetic patients. In this study, diabetic patients with albuminuria and no prior history of coronary artery disease were treated with eplerenone (50 mg/day) or hydrochlorothiazide (12.5 mg/day) for 6 weeks and examined coronary circulatory function. In these patients, eplerenone increased left ventricular myocardial perfusion reserve significantly compared to hydroclorothiazide. Interestingly, eplerenone did not increase brachial artery vascular reactivity; however, it should be recognized that prior studies of MR blockade with spironolactone in diabetic patients was associated with a marked decrease in endothelial function. As this was not observed in the present study, it may be concluded that eplerenone limited endothelial dysfunction in this patient population [47].
5. Adverse effects of mineralocorticoid receptor antagonists
5.1 Hyperkalemia
The major adverse effects associated with MR antagonists are related to electrolyte and endocrinologic disturbances. In a MR-dependent process, aldosterone increases potassium excretion in the late distal convoluted tubule and collecting ducts of principal cells in the kidney [5]. MR antagonists impair this regulatory function of aldosterone to induce clinically relevant elevations in serum potassium levels. Thus, the therapeutic efficacy of both spironolactone and eplerenone is limited by the development of secondary hyperkalemia, an effect that is dose-dependent. In the RALES trial, serum potassium levels increased by a mean of 0.3 mmol/L in spironolactone-treated patients and 2% of patients developed severe hyperkalemia (≥ 6 mmol/L) [27]; in the EPHESUS trial, severe hyperkalemia was reported in 5.5% of eplerenone-treated patients [18]. These figures, however, may underestimate the true incidence of hyperkalemia in clinical practice, since both trials excluded patients at increased risk for hyperkalemia and limited use of additional medications known to increase potassium levels [39]. Various comorbidities increase the risk of MR antagonist-induced hyperkalemia and include advanced age, renal insufficiency, diabetes mellitus, and end-stage congestive heart failure. Medications shown to further increase the risk of hyperkalemia in patients on MR antagonist therapy include ACE inhibitors, ARBs, β-blockers, potassium supplements, heparin sulfate, and nonsteroidal anti-inflammatory drugs [39]. Eplerenone has also been associated with gastrointestinal intolerance; whether or not this phenomenon is related to tissue-specific binding of the drug and electrolyte abnormalities remains unknown [37].
5.2 Endocrine abnormalities
Spironolactone has secondary effects on androgen hormone balance related to its non-selective blockade of androgen and progesterone receptors in addition to the MR. Spironolactone decreases 17α-reductase activities and lowers plasma testosterone and androstenedione resulting in gynecomastia, impotence, and menstrual irregularities [48]. In RALES, up to 10% of men reported gynecomastia with spironolactone therapy compared to 1% in the placebo group [27]. Similarly, in patients with hypertension, 6.9% of men treated with spironolactone experienced gynecomastia; this effect was found to be dose-dependent and occur at any time after treatment was started. Typically, however, gynecomastia is fully reversed by cessation of spironolactone therapy [48]. Eplerenone, in contrast to spironolactone, is a selective MR antagonist and is not associated with gynecomastia [42].
6. Conclusion
The aldosterone antagonists spironolactone and eplerenone have become a mainstay in the therapy of cardiovascular disease to limit the adverse consequences of hyperaldosteronism. There has been increasing recognition that one of clinical benefits of MR blockade is improved endothelial function, which may, in part, explain the reduction in morbidity in mortality observed in the RALES and EPHESUS trials. The mechanisms by which these agents prevent endothelial dysfunction is likely multifactorial and involves decreased vascular oxidant stress, increased bioavailable nitric oxide, and a reduction in vasoconstrictor agents. Despite these positive effects, widespread use of spironolactone and eplerenone is restricted by the side effect profile; both drugs cause hyperkalemia and spironolactone is associated with gynecomastia. As such, there is a need to develop newer agents that retain the ability to specifically block MR activation absent a rise in serum potassium.
Acknowledgments
This work was supported in part by an American Heart Association Grant-in-Aid and NIH grant HL081110 (J.A.L.).
References
- 1.Blacher J, Amah G, Girerd X, Kheder A, Ben Mais H, London GM, Safar ME. Association between increased plasma levels of aldosterone and decreased systemic arterial compliance in subjects with essential hypertension. Am J Hypertens. 1997;10(12 Pt 1):1326–1334. doi: 10.1016/s0895-7061(97)00301-4. [DOI] [PubMed] [Google Scholar]
- 2.Duprez DA, De Buyzere ML, Rietzschel ER, Taes Y, Clement DL, Morgan D, Cohn JN. Inverse relationship between aldosterone and large artery compliance in chronically treated heart failure patients. Eur Heart J. 1998;19(9):1371–1376. doi: 10.1053/euhj.1998.1099. [DOI] [PubMed] [Google Scholar]
- 3••.Farquharson CA, Struthers AD. Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failure. Circulation. 2000;101(6):594–597. doi: 10.1161/01.cir.101.6.594. In patients with congestive heart failure, this study was the first to demonstrate impaired endothelium-dependent vascular reactivity was improved by treatment with spironolactone. [DOI] [PubMed] [Google Scholar]
- 4•.Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001;345(23):1689–1697. doi: 10.1056/NEJMra000050. This comprehensive review details the cardiovascular effects of hyperaldosteronism in the clinical setting of congestive heart failure. [DOI] [PubMed] [Google Scholar]
- 5.Booth RE, Johnson JP, Stockand JD. Aldosterone. Adv Physiol Educ. 2002;26(1–4):8–20. doi: 10.1152/advan.00051.2001. [DOI] [PubMed] [Google Scholar]
- 6.Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O’Rourke M. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113(9):1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 7.Tokmakova M, Solomon SD. Inhibiting the renin-angiotensin system in myocardial infarction and heart failure: lessons from SAVE, VALIANT and CHARM, and other clinical trials. Curr Opin Cardiol. 2006;21(4):268–272. doi: 10.1097/01.hco.0000231394.79609.24. [DOI] [PubMed] [Google Scholar]
- 8.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 9•.Bomback AS, Klemmer PJ. The incidence and implications of aldosterone breakthrough. Nat Clin Pract Nephrol. 2007;3(9):486–492. doi: 10.1038/ncpneph0575. A comprehensive study of the incidence of aldosterone breakthrough and the clinical implications for patients being treated ACE inhibitors and ARBs. [DOI] [PubMed] [Google Scholar]
- 10••.Farquharson CA, Struthers AD. Aldosterone induces acute endothelial dysfunction in vivo in humans: evidence for an aldosterone-induced vasculopathy. Clin Sci (Lond) 2002;103(4):425–431. doi: 10.1042/cs1030425. This was the first study to demonstrate that aldosterone induces endothelial dysfunction by decreasing bioavailable nitric oxide when infused into healthy volunteers. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt BM, Oehmer S, Delles C, Bratke R, Schneider MP, Klingbeil A, Fleischmann EH, Schmieder RE. Rapid nongenomic effects of aldosterone on human forearm vasculature. Hypertension. 2003;42(2):156–160. doi: 10.1161/01.HYP.0000083298.23119.16. [DOI] [PubMed] [Google Scholar]
- 12.Nietlispach F, Julius B, Schindler R, Bernheim A, Binkert C, Kiowski W, Brunner-La Rocca HP. Influence of acute and chronic mineralocorticoid excess on endothelial function in healthy men. Hypertension. 2007;50(1):82–88. doi: 10.1161/HYPERTENSIONAHA.107.088955. [DOI] [PubMed] [Google Scholar]
- 13.Oberleithner H, Riethmuller C, Ludwig T, Hausberg M, Schillers H. Aldosterone remodels human endothelium. Acta Physiol (Oxf) 2006;187(1–2):305–312. doi: 10.1111/j.1748-1716.2006.01574.x. [DOI] [PubMed] [Google Scholar]
- 14.Oberleithner H, Riethmuller C, Schillers H, MacGregor GA, de Wardener HE, Hausberg M. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci U S A. 2007;104(41):16281–16286. doi: 10.1073/pnas.0707791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwashima F, Yoshimoto T, Minami I, Sakurada M, Hirono Y, Hirata Y. Aldosterone induces superoxide generation via Rac1 activation in endothelial cells. Endocrinology. 2008;149(3):1009–1014. doi: 10.1210/en.2007-0864. [DOI] [PubMed] [Google Scholar]
- 16•.Leopold JA, Dam A, Maron BA, Scribner AW, Liao R, Handy DE, Stanton RC, Pitt B, Loscalzo J. Aldosterone impairs vascular reactivity by decreasing glucose-6-phosphate dehydrogenase activity. Nat Med. 2007;13(2):189–197. doi: 10.1038/nm1545. This study demonstrated that aldosterone induced endothelial dysfunction by increasing reactive oxygen species production as well as decreasing antioxidant enzyme capacity. In vivo, this was associated with impaired endothelium-dependent vascular reactivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagata D, Takahashi M, Sawai K, Tagami T, Usui T, Shimatsu A, Hirata Y, Naruse M. Molecular mechanism of the inhibitory effect of aldosterone on endothelial NO synthase activity. Hypertension. 2006;48(1):165–171. doi: 10.1161/01.HYP.0000226054.53527.bb. [DOI] [PubMed] [Google Scholar]
- 18.Pu Q, Neves MF, Virdis A, Touyz RM, Schiffrin EL. Endothelin antagonism on aldosterone-induced oxidative stress and vascular remodeling. Hypertension. 2003;42(1):49–55. doi: 10.1161/01.HYP.0000078357.92682.EC. [DOI] [PubMed] [Google Scholar]
- 19.Cachofeiro V, Miana M, de Las Heras N, Martin-Fernandez B, Ballesteros S, Fernandez-Tresguerres J, Lahera V. Aldosterone and the vascular system. J Steroid Biochem Mol Biol. 2008 doi: 10.1016/j.jsbmb.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Sica DA. Pharmacokinetics and pharmacodynamics of mineralocorticoid blocking agents and their effects on potassium homeostasis. Heart Fail Rev. 2005;10(1):23–29. doi: 10.1007/s10741-005-2345-1. [DOI] [PubMed] [Google Scholar]
- 21.Greenblatt DJ, Koch-Weser J. Adverse reactions to spironolactone. A report from the Boston Collaborative Drug Surveillance Program. JAMA. 1973;225(1):40–43. doi: 10.1001/jama.225.1.40. [DOI] [PubMed] [Google Scholar]
- 22.Overdiek HW, Merkus FW. Influence of food on the bioavailability of spironolactone. Clin Pharmacol Ther. 1986;40(5):531–536. doi: 10.1038/clpt.1986.219. [DOI] [PubMed] [Google Scholar]
- 23.Nolan PE., Jr Integrating traditional and emerging treatment options in heart failure. Am J Health Syst Pharm. 2004;61 (Suppl 2):S14–22. doi: 10.1093/ajhp/61.suppl_2.S14. [DOI] [PubMed] [Google Scholar]
- 24.Pharmacia. Aldactone (spironolactone) package insert. 2003. [Google Scholar]
- 25.Gardiner P, Schrode K, Quinlan D, Martin BK, Boreham DR, Rogers MS, Stubbs K, Smith M, Karim A. Spironolactone metabolism: steady-state serum levels of the sulfur-containing metabolites. J Clin Pharmacol. 1989;29(4):342–347. doi: 10.1002/j.1552-4604.1989.tb03339.x. [DOI] [PubMed] [Google Scholar]
- 26.Sungaila I, Bartle WR, Walker SE, DeAngelis C, Uetrecht J, Pappas C, Vidins E. Spironolactone pharmacokinetics and pharmacodynamics in patients with cirrhotic ascites. Gastroenterology. 1992;102(5):1680–1685. doi: 10.1016/0016-5085(92)91730-r. [DOI] [PubMed] [Google Scholar]
- 27••.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709–717. doi: 10.1056/NEJM199909023411001. A landmark clinical trial that demonstrated a 30% decrease in mortality in patients with congestive heart failure and decreased left ventricular function that were treated with spironolactone. [DOI] [PubMed] [Google Scholar]
- 28.Struthers AD. Impact of aldosterone on vascular pathophysiology. Congest Heart Fail. 2002;8(1):18–22. doi: 10.1111/j.1527-5299.2002.00722.x. [DOI] [PubMed] [Google Scholar]
- 29.Hadi HA, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag. 2005;1(3):183–198. [PMC free article] [PubMed] [Google Scholar]
- 30.Macdonald JE, Kennedy N, Struthers AD. Effects of spironolactone on endothelial function, vascular angiotensin converting enzyme activity, and other prognostic markers in patients with mild heart failure already taking optimal treatment. Heart. 2004;90(7):765–770. doi: 10.1136/hrt.2003.017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abiose AK, Mansoor GA, Barry M, Soucier R, Nair CK, Hager D. Effect of spironolactone on endothelial function in patients with congestive heart failure on conventional medical therapy. Am J Cardiol. 2004;93(12):1564–1566. doi: 10.1016/j.amjcard.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 32••.Nishizaka MK, Zaman MA, Green SA, Renfroe KY, Calhoun DA. Impaired endothelium-dependent flow-mediated vasodilation in hypertensive subjects with hyperaldosteronism. Circulation. 2004;109(23):2857–2861. doi: 10.1161/01.CIR.0000129307.26791.8E. This study revealed that hyperaldosteronism associated with hypertension decreased vascular endothelial function, an effect that was abrogated by spironolactone. [DOI] [PubMed] [Google Scholar]
- 33•.Davies JI, Band M, Morris A, Struthers AD. Spironolactone impairs endothelial function and heart rate variability in patients with type 2 diabetes. Diabetologia. 2004;47(10):1687–1694. doi: 10.1007/s00125-004-1510-8. In patients with diabetes mellitus and impaired endothelial function, this study revealed that spironolactone actually worsened endothelium-dependent vasodilation providing evidence that not all patient populations benefit from MR antagonism. [DOI] [PubMed] [Google Scholar]
- 34.Shah NC, Pringle SD, Donnan PT, Struthers AD. Spironolactone has antiarrhythmic activity in ischaemic cardiac patients without cardiac failure. J Hypertens. 2007;25(11):2345–2351. doi: 10.1097/HJH.0b013e3282e9a72d. [DOI] [PubMed] [Google Scholar]
- 35.Brown NJ. Eplerenone: cardiovascular protection. Circulation. 2003;107(19):2512–2518. doi: 10.1161/01.CIR.0000071081.35693.9A. [DOI] [PubMed] [Google Scholar]
- 36.Cook CS, Berry LM, Bible RH, Hribar JD, Hajdu E, Liu NW. Pharmacokinetics and metabolism of [14C]eplerenone after oral administration to humans. Drug Metab Dispos. 2003;31(11):1448–1455. doi: 10.1124/dmd.31.11.1448. [DOI] [PubMed] [Google Scholar]
- 37.Searle GD. Inspra (eplerenone) package insert. 2003. [Google Scholar]
- 38.Cook CS, Berry LM, Kim DH, Burton EG, Hribar JD, Zhang L. Involvement of CYP3A in the metabolism of eplerenone in humans and dogs: differential metabolism by CYP3A4 and CYP3A5. Drug Metab Dispos. 2002;30(12):1344–1351. doi: 10.1124/dmd.30.12.1344. [DOI] [PubMed] [Google Scholar]
- 39.Marcy TR, Ripley TL. Aldosterone antagonists in the treatment of heart failure. Am J Health Syst Pharm. 2006;63(1):49–58. doi: 10.2146/ajhp050041. [DOI] [PubMed] [Google Scholar]
- 40.de Gasparo M, Joss U, Ramjoue HP, Whitebread SE, Haenni H, Schenkel L, Kraehenbuehl C, Biollaz M, Grob J, Schmidlin J, et al. Three new epoxy-spirolactone derivatives: characterization in vivo and in vitro. J Pharmacol Exp Ther. 1987;240(2):650–656. [PubMed] [Google Scholar]
- 41.McManus F, McInnes GT, Connell JM. Drug Insight: eplerenone, a mineralocorticoid-receptor antagonist. Nat Clin Pract Endocrinol Metab. 2008;4(1):44–52. doi: 10.1038/ncpendmet0676. [DOI] [PubMed] [Google Scholar]
- 42••.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348(14):1309–1321. doi: 10.1056/NEJMoa030207. A large scale, randomized, clinical trial that demonstrated that early administration of eplerenone to patients with acute myocardial infarction and left ventricular dysfunction decreased mortality. [DOI] [PubMed] [Google Scholar]
- 43.Struthers AD. Aldosterone blockade in cardiovascular disease. Heart. 2004;90(10):1229–1234. doi: 10.1136/hrt.2003.025312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanz-Rosa D, Oubina MP, Cediel E, De las Heras N, Aragoncillo P, Balfagon G, Cachofeiro V, Lahera V. Eplerenone reduces oxidative stress and enhances eNOS in SHR: vascular functional and structural consequences. Antioxid Redox Signal. 2005;7(9–10):1294–1301. doi: 10.1089/ars.2005.7.1294. [DOI] [PubMed] [Google Scholar]
- 45.Rajagopalan S, Duquaine D, King S, Pitt B, Patel P. Mineralocorticoid receptor antagonism in experimental atherosclerosis. Circulation. 2002;105(18):2212–2216. doi: 10.1161/01.cir.0000015854.60710.10. [DOI] [PubMed] [Google Scholar]
- 46.Takai S, Jin D, Muramatsu M, Kirimura K, Sakonjo H, Miyazaki M. Eplerenone inhibits atherosclerosis in nonhuman primates. Hypertension. 2005;46(5):1135–1139. doi: 10.1161/01.HYP.0000184640.81730.22. [DOI] [PubMed] [Google Scholar]
- 47••.Joffe HV, Kwong RY, Gerhard-Herman MD, Rice C, Feldman K, Adler GK. Beneficial effects of eplerenone versus hydrochlorothiazide on coronary circulatory function in patients with diabetes mellitus. J Clin Endocrinol Metab. 2007;92(7):2552–2558. doi: 10.1210/jc.2007-0393. In contrast to what was observed with spironolactone, this study revealed that eplerenone did not decrease endothelial function in diabetic patients and improved myocardial perfusion reserve. [DOI] [PubMed] [Google Scholar]
- 48.Goldfien A. The gonadal hormones and inhibitors. In: Katzung B, editor. Basic & clinical pharmacology. McGraw-Hill; New York: 1998. pp. 653–683. [Google Scholar]