Abstract
Survivors of massive inhalation of combustion smoke endure critical injuries, including lasting neurological complications. We have previously reported that acute inhalation of combustion smoke disrupts the nitric oxide homeostasis in the rat brain. In this study, we extend our findings and report that exposure to smoke induces protein nitration in the rat hippocampus and that mitochondrial proteins are a sensitive nitration target in this setting. Mitochondria have central roles in energy metabolism and cellular signaling and are critical to proper cell function. Here, analyses of the mitochondrial proteome showed elevated protein nitration in the course of a 24-h recovery following exposure to smoke. Mass spectrometry identification of several significantly nitrated mitochondrial proteins revealed diverse functions and involvement in central aspects of mitochondrial physiology. The nitrated proteins include the ubiquitous mitochondrial creatine kinase, F1-ATP synthase α subunit, dihydrolipoamide dehydrogenase (E3), succinate dehydrogenase Fp subunit, and voltage-dependent anion channel (VDAC1) protein. Furthermore, acute exposure to combustion smoke significantly compromised the respiratory capacity of hippocampal mitochondria. Interestingly, elevated protein nitration and reduced mitochondrial respiration in the hippocampus persisted beyond the time required for restoration of normal oxygen and carboxyhemoglobin blood levels after the cessation of exposure to smoke. Thus, the data indicate that timing of the different smoke inhalation-induced effects varies among tissues. Taken together, our findings suggest that nitration of essential mitochondrial proteins may contribute to the reduction in mitochondrial respiratory capacity and underlie, in part, the brain pathophysiology after acute inhalation of combustion smoke.
Keywords: combustion smoke inhalation, brain, protein nitration, mitochondrial oxygen consumption, mitochondrial proteome
INTRODUCTION
Massive smoke inhalation causes mortality and morbidity in victims of accidental fires, acts of terrorism and in combat, with severe immediate and delayed neurological impairments. The recognized neurotoxic factors in combustion-smoke are carbon monoxide, hydrogen cyanide and toxicants, which in the brain tissue may combine and synergize with free radical-generating factors as well as hypoxia and acidosis, to perturb cellular homeostasis and precipitate brain injury (Hartzell, 1996; Rossi et al., 1996; Smith et al., 1996; Roohi et al., 2001; Alarie, 2002; Raub and Benignus, 2002; Stuhmiller et al., 2006).
To obtain insights into the progression of molecular events contributing to brain pathophysiology after acute exposure to combustion smoke, we have developed a rat model of smoke inhalation injury. Using this model, we show that blood parameters as well as cellular and molecular targets in the rat brain are significantly affected by massive inhalation of smoke (Lee et al., 2005). The key hemodynamic changes include striking elevation of carboxyhemoglobin levels, reduction in oxygen saturation and blood pH, while in the brain tissue the changes include modulation of gene expression patterns, lipid peroxidation, oxidative DNA damage as well as significant modulations of the nitric oxide system (Lee et al., 2005; Chen et al., 2007). Interestingly, the timing for onset and diminution of the various manifestations differed markedly, underscoring the complex nature of smoke inhalation pathophysiology.
It is noteworthy, that in humans after carbon monoxide (CO) insult, only limited predictions can be made based on blood carboxyhemoglobin (COHb) with respect to the potential for development of neuropathologies. This is likely due to a failure of blood COHb levels to reflect the tissue specific rates of CO clearance. In fact, it appears that during recovery tissue, CO levels do not necessarily trail declining blood levels, and that tissue hypoxia slows down CO clearance during the later resolution stages, particularly in the brain and heart (Cronje et al., 2004). Since hypoxia is a major component in the setting of acute smoke inhalation, elevated brain tissue CO may persist, replacing oxygen bound to neuronal heme proteins and thus further impact oxygen availability and brain homeostasis. In our rat model, the very high COHb (72%) blood levels measured immediately after exposure, decline to near normal levels within the first 2 h of recovery. In contrast, manifestations of smoke exposure in the brain tissue tend to peak at the later recovery times. For example, the respiratory capacity of brain mitochondria is most severely compromised at 2 and 6 h post smoke when the blood COHb, oxygen saturation and pH have returned to normal (Lee et al., 2005). This finding suggests that CO clearance from brain tissue lags behind its clearance from blood, and that the severely reduced oxygenation, high CO, toxic gases and reduced pH may continue to harm the brain mitochondria.
Interestingly, gene microarray analyses revealed that genes encoding components of the nitric oxide system including the endothelial nitric oxide synthase (NOS) and capon, a NOS ligand (Wiggins et al., 2003; Thom et al., 2004) were upregulated after smoke (Lee et al., 2005). Previously, investigation of the nitric oxide (NO) system in the brain in the context of CO poisoning revealed elevation of NO, nitration of tyrosine residues (Ischiropoulos et al., 1996), increases in perivascular nitric oxide synthesis (Thom et al., 2003) with perivascular nitrotyrosine immunoreactivity in endothelial lining, and elevated synthesis of NO in the brain after CO poisoning (Thom et al., 2001) as well as neuronal NOS mediated CO-initiated NMDA activation and excitotoxicity (Wiggins et al., 2003; Thom et al., 2004).
Since modulations of the nitric oxide system have been linked with reduced mitochondrial respiration (Brookes et al., 2003; Ischiropoulos and Beckman, 2003; Radi, 2004; Franco et al., 2006), in this study, we examine the effects of acute exposure to combustion smoke on mitochondrial protein nitration and respiratory capacity of the rat hippocampal mitochondria. We show post-smoke increases in 3-nitrotyrosine immunoreactivity in the hippocampal region and demonstrate that mitochondrial proteins are a significant target for smoke-induced protein nitration. Identification of the post-smoke mitochondrial nitration targets revealed that the nitrated proteins are involved in a broad spectrum of mitochondrial functions. In addition, a substantial decline in respiratory capacity of hippocampal mitochondria was observed following exposure to smoke.
METHODS
Smoke inhalation rat model
Sprague Dawley male rats (250–300 g) were exposed to combustion smoke generated by burning aspen wood shavings for three successive 10-min periods separated by 30 sec as we described previously (Lee et al., 2005). Sham controls were given similar treatment without smoke. Rats were allowed to recover for 0, 2, 6 or 24 h. Brains were harvested and fixed for immunohistochemistry or dissected for mitochondrial isolation and gradient purification. All experiments were conducted in accordance with mandated standards of humane care and were approved by the UTMB Institutional Animal Care and Use Committee.
Immunohistochemistry
Brains were immersion fixed in 4% paraformaldehyde and coronal, paraffin embedded sections (Bregma -3.14) were prepared from control, and smoke exposed rats harvested at indicated times (n=3). Sections were dewaxed in xylene and rehydrated through graded ethanol series. Blocking was for 1 h with 1% BSA followed by 30 min with 3% goat serum. Sections were incubated with anti 3-nitrotyrosine antibody (#06-284, Upstate, Charlottesville, VA) at 1:150 for 1 h at room temperature, followed by three 5-min washes and incubation with biotinylated goat anti-rabbit antibody. The avidin:biotinylated enzyme complex (ABC reagent) was used and sections were counterstained with hematoxylin. Sections processed identically with omission of the primary antibody served as a negative control. Sections were analyzed with a Nikon Eclipse 600 microscope using Plan Fluor and 40X Plan Apo objectives. Images were captured by Nikon DXM1200 digital camera. Biotinylated goat anti-rabbit IgG (BA-1000) and Vectastain ABC kit were from Vector Lab (Burlingame, CA). Hematoxylin Gill #2 (CS401-1D) and Permount (SP15-100) were from Fisher Scientific (Hampton, NH).
Subcellular fractionation
Dissected hippocampal tissues were homogenized in hypotonic buffer (10 mm Hepes, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.5 mM PMSF, 2 μg/ml Pepstatin, and Protease Inhibitor Cocktail). Homogenates were spun at 800 g for 5′ to remove nuclei. Supernatants were combined for separation of crude mitochondria by centrifugation at 12,500 g for 8 min. Prior to proteomic analyses the crude mitochondrial pellets were subjected to further purification through a Percoll density gradient adapted from Sims et al. (Sims, 1990; Anderson and Sims, 2000). Briefly, crude mitochondrial pellets were suspended in 14% Percoll in 1X MSHE (10 mM Hepes-KOH pH 7.6, 210 mM mannitol, 70 mM sucrose, 1 mM EDTA, 1 mM EGTA, 0.15 mM spermine, 0.75 mM spermidine), layered over preformed Percoll gradient (19% on 40%) and spun at 30,700 g 10 min. Fractions in the layers interface were collected and spun at 16,700 g to obtain mitochondrial pellets.
Mitochondrial lysis and Western blotting
Gradient purified mitochondrial pellets were incubated with lysis buffer recommended by Amersham (7 M urea, 2 M thiourea, 4% CHAPS, 40 mM Tris-HCl pH 7.5, 1 mM EDTA, 1 mM EGTA, 60 mM DTT, 1 mM PMSF), followed by 2x centrifugations at 20,000 g to remove remaining particulates. Equal amounts (30 μg/well) of extracted mitochondrial proteins were resolved in 10% SDS PAGE, electrotransferred to a PVDF membrane, blocked overnight at 4°C (20 mM Tris pH 7.5, 150 mM NaCl, 0.2% Tween-20, 2% BSA), and incubated with anti 3-nitrotyrosine monoclonal antibody (#05-233, Upstate, Lake Placid, NY) at 4°C for 3 h followed by 5 washes and incubation with goat antimouse horseradish peroxidase conjugate for 3 h at RT and 5 vigorous washes prior to ECL mediated detection. Specificity of nitrotyrosine detection was confirmed by ‘in membrane’ reduction of nitrotyrosine to amino tyrosine, which does not react with the anti nitrotyrosine antibody. Reduction was accomplished by incubating the membrane with a solution of 100 mM sodium hydrosulfite (dithionite) in bicarbonate buffer for 3 h at RT followed by washing with distilled water and equilibration with wash solution (20 mM Tris pH 7.5, 150 mM NaCl, 0.2% Tween 20) prior to ON blocking as described above. Reduced and non-reduced PVDF membranes were processed and visualized simultaneously. Equal loading was confirmed by re-probing with anti VDAC polyclonal antibody (# PC548T, Calbiochem).
Two-dimensional electrophoresis (2DE) and Western blotting analyses
Gradient purified mitochondrial pellet proteins extracted as described above were separated using the IPGphor isoelectric focusing (IEF) system from Amersham. Briefly, solubilized gradient purified mitochondrial proteins in 200 μl lysis buffer, at 30 or 100 μg for SYPRO Ruby staining or for immunodetection, respectively, were separated on isoelectric pH gradient (IPG) strips (Immobiline Drystrip, 3–10 NL pH range). Rehydration of strips was at 50v/14 h, followed by isoelectric focusing (IEF) at 500v/1 h, 1000v/1.5 h and 8000v/1.5 h. Subsequently, strips were equilibrated with SDS buffer (50 mM Tris-HCl pH 8.8, 6 M urea, 30% glycerol, 2% SDS, 1% DTT, and bromophenol blue followed by 15 min in 50 mM Tris-HCl pH 8.8, 6 M urea, 30% glycerol, 2% SDS, 2.5% iodoacetamide, bromophenol blue. Strips were embedded in 0.5% agarose in the slot of Criterion 8–16% gradient gels with protein standards loaded in parallel. Electrophoresis was at 150V for 150 min at 4°C. Prior to electrotransfer to PVDF membranes, gels were soaked in transfer buffer (20 mM Tris-HCl, 96 mM glycine, and 20% methanol) and processed for immunodetection as described above. Parallel two dimensional profiles of 30 μg protein samples were visualized with SYPRO Ruby protein stain (Molecular Probes) after fixation in 10% methanol/7.5% acetic acid solution and following destaining imaged with a 480/620 nm excitation/emission filter. For Western blotting analyses, 100 μg samples were subjected to simultaneous separation procedure and run in parallel in the first and second dimension. Anti 3-nitrotyrosine monoclonal antibodies (#05-233, Upstate) were used for immunoblotting.
Mass spectrometry
Spots detected by Western analyses with anti 3-nitrotyrosine antibodies were aligned with spots visualized with SYPRO Ruby. Preparations from three rats per group were analyzed. Three sets of two 2D gels were analyzed per rat (n=3). Spots matched with confidence (n=3) were excised, in gel digested with trypsin (Opii et al., 2007) and subjected to matrix-assisted laser desorption ionization time of flight/time of flight mass spectrometry (MALDI-TOF/TOF-MS) analysis using the Applied Biosystems model 4700 Proteomics Analyzer for protein fingerprinting at the Proteomics Core Facility at UTMB. Applied Biosystems software in conjunction with MASCOT was employed to search databases for peptide identification. Protein matches were based on probabilities determined by expectation values, MASCOT protein scores and peptide coverage. Probability-based scores were estimated by comparison of search results against random population and were reported as 10×log10(p) where p is the absolute probability. Only spots whose identity was confirmed by three independent analyses are reported. Mass spectrometry and sequence matching analyses were carried out at the Proteomics Core facility at UTMB.
Mitochondrial respiration assay
The rate of oxygen consumption by isolated hippocampal mitochondria was measured using a Clark-type oxygen electrode in a continuously stirred, sealed/closed chamber (Yellow Springs Instrument 5300, Yellow Springs, OH) with Omega software. Oxygen consumption by hippocampal mitochondria was measured in a total volume of 0.6 ml medium containing 230 mM manitol and 70 mM sucrose, 10 mM potassium phosphate pH 7.4, 10 mM Tris HCl, pH 7.4, 5 mM MgCl2 and isolated mitochondria at concentration of 400 μg protein/ml. Glutamate (10 mM) or succinate (10 mM in the presence of the Complex l inhibitor, rotenone) were used to drive Complex l and Complex II respiration rates, respectively. Reactions were initiated by the addition of mitochondria and following rate stabilization, by subsequent additions of ADP to100 μM final concentration to induce state-3 respiration. After a constant state-4 respiration was reached, ADP addition/depletion was repeated twice. Oxygen consumption in the presence of ADP (state 3) or depletion of ADP (state 4) was calculated for sham-controls and mitochondria isolated at the indicated recovery times after exposure to smoke (n=6). Statistical analyses were carried out with SigmaStat and p values were obtained using one-way ANOVA and multiple comparisons versus control group by Holm-Sidak method. * Indicates different from control; P<0.05 was considered significant.
RESULTS
Smoke inhalation-induced 3-nitrotyrosine immunoreactivity in hippocampal mitochondria
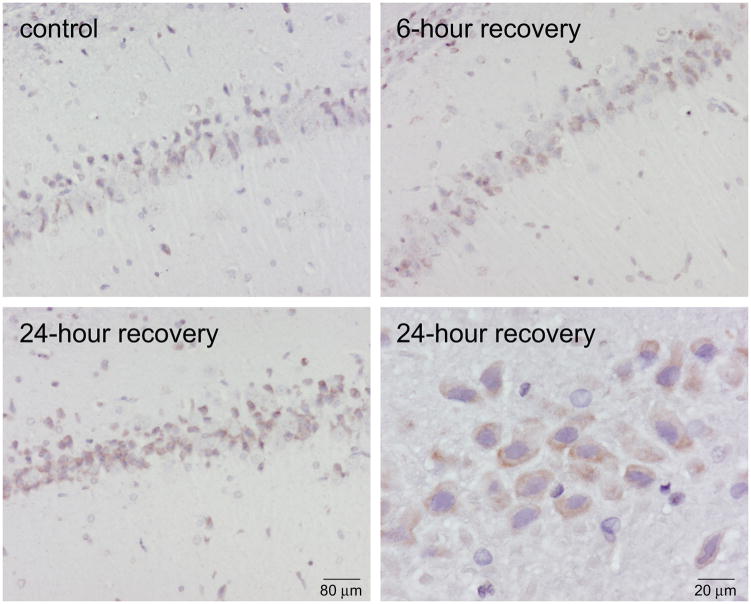
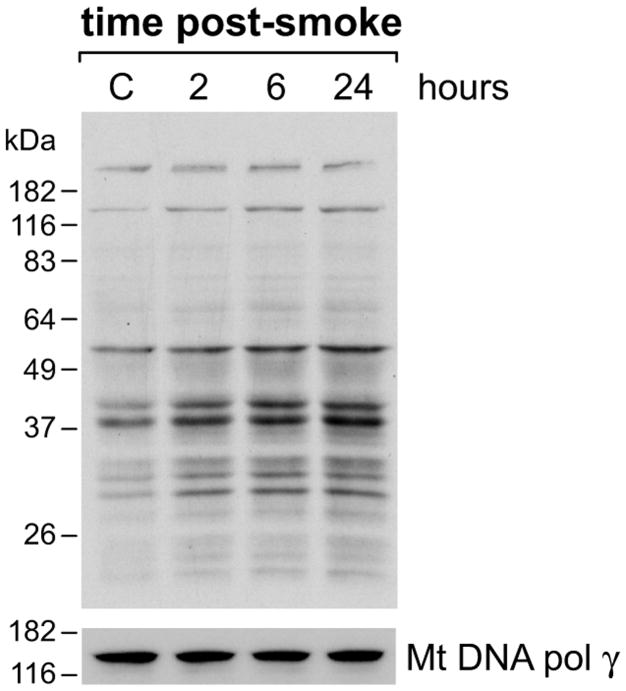
Nitrotyrosine immunoreactivity, which reflects nitric oxide or peroxynitrite-mediated protein modifications, increased in the hippocampal region after acute exposure to combustion smoke. Elevated 3-nitrotyrosine (3-NT) immunoreactivity was seen in the course of 24-h recovery after exposure to smoke (Fig. 1). High magnification revealed predominantly cytoplasmic staining (bottom, right). To determine whether the mitochondrial proteins were a significant target for nitration, hippocampal mitochondria were isolated, Percoll gradient purified, lysed and their nitration status was examined by Western blotting analyses (Fig. 2). An intensification of 3-NT immunoreactivity in the course of post-smoke recovery was seen for mitochondrial proteins resolved in SDS-PAGE. Re-probing for the mitochondrial voltage dependent anion channel (VDAC) protein confirmed equal loading and demonstrated that the levels of VDAC remain unchanged in the course of recovery after exposure to smoke. To confirm 3-NT signal specificity, identical pairs of membranes were prepared and one of each set was reduced with Na2S2O4 prior to immunoblotting for 3-NT; the resulting absence of signal confirmed the specificity of 3-NT detection (not shown).
Figure 1.
Smoke inhalation induced 3-nitrotyrosine (3-NT) immunoreactivity in the rat hippocampus. Photomicrographs show intensified 3-NT staining in hippocampus of rats after exposure to smoke compared to controls (n=3). Higher magnification (40x) shows primarily cytoplasmic localization of immunoreactivity in hippocampal neurons. 3-NT staining is significantly intensified at 24 h after exposure to smoke. Cell nuclei were counterstained with hematoxylin.
Figure 2.
Immunodetection of nitrated proteins in mitochondria purified from hippocampi of rats at different recovery points after exposure to combustion smoke. Western blotting analyses shows intensification of 3-NT positive bands across a broad range of molecular weights of proteins resolved in 10% SDS-PAGE. Equal loading was confirmed by re-probing for the mitochondrial channel protein VDAC.
Proteomic analyses of hippocampal mitochondria proteins following acute exposure to combustion smoke
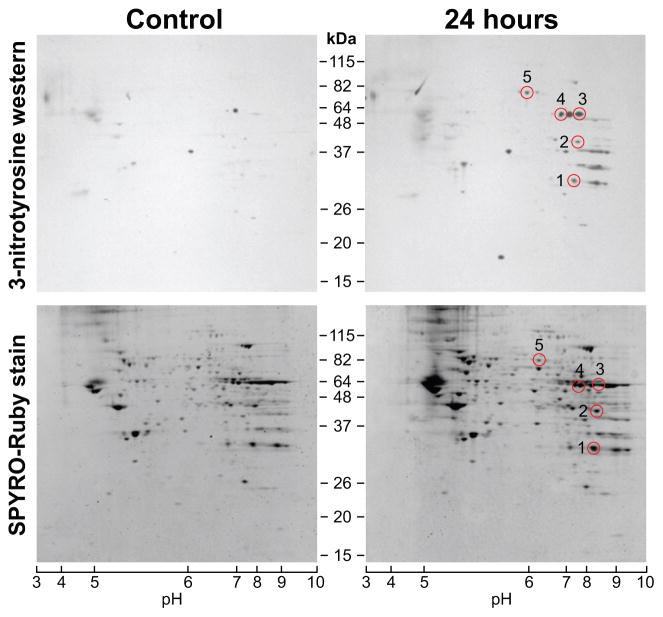
Protein nitration after exposure to smoke was postulated in view of changes in NO flux revealed by the alterations in expression of genes associated with the NO system and by the corresponding immunohistochemical analyses (Lee et al., 2005). In this study, the smoke inhalation-induced changes in hippocampal mitochondrial proteome were assessed by two dimensional electrophoresis (2DE) followed by electrotransfer to PVDF membranes and immunodetection of nitrated proteins. Mitochondrial proteins from hippocampi of controls and 24-h recovery rats were resolved by 2DE, over an IEF range of pH 3–10 followed by separation in an 8–16% gradient SDS-PAGE (Fig. 3, bottom).
Figure 3.
Two-dimensional analyses of mitochondrial proteins from hippocampi of rats after acute exposure to combustion smoke. 2DE profiles of mitochondrial proteins and corresponding detection of 3-nitrotyrosine (3-NT) immunoreactive mitochondrial proteins. Mitochondrial proteins from controls and rats at 24 h after smoke were resolved in the first dimension by IEF (pH range 3–10) followed by separation in a gradient 8–16% SDS-PAGE. The bottom panels show protein profiles visualized by SYPRO-Ruby protein stain for sham-controls and rats 24 h post smoke. Corresponding Western blots of parallel gels electrotransferred to PVDF membranes and probed for 3-NT are given in top panels. Western blotting reveals a distinct pattern of nitrated proteins when compared to controls. SYPRO-Ruby spots were aligned with Western blot signals (circles), excised, in gel digested with trypsin and analyzed by MS. Nitrated protein spots identified with high confidence are listed and described in Table 1.
The mitochondrial proteins resolved by 2DE (Fig. 3, bottom) were probed for presence of 3-nitrotyrosine modified proteins (Fig. 3, top). A significant intensification of 3-NT immunoreactivity was observed for mitochondrial proteins purified after exposure to smoke when compared to mitochondria from sham-control rats. One of each pair of identical simultaneously-processed 2DE gels was electro-transferred to a PVDF membrane, which was then subjected to immunoblotting with the anti 3-NT antibody. Positive signals detected by Western analyses were aligned with SYPRO Ruby visualized protein spots on simultaneously run gels. SYPRO Ruby spots which, were matched with the 3-NT signals in triplicate pairs were excised and subjected to in gel trypsin digestion and subsequent analysis by mass spectrometry (MS). 3-NT positive proteins, which were conclusively identified by MS in triplicate analyses of mitochondria purified from the 24-h recovery rats, are demarcated by circles on Western blots (Fig. 3, top). The list of the MS identified proteins (numbered circles), which became nitrated is given in Table 1.
Table 1.
Smoke-induced Nitration of Mitochondrial Proteins
| Spot no. | Protein name | Accession no. | Peptides identified | Sequence coverage (%) | P value | Function | Localization |
|---|---|---|---|---|---|---|---|
| 1 | Voltage dependent anion channel 1 (VDAC1) | gi|48734887 | 9 | 39 | <10−8 | Ion transport | Mitochondrial membrane |
| 2 | Ubiquitous Mitochondrial creatine kinase | gi|57539 | 9 | 16 | <10−5 | Energy metabolism | Mitochondria |
| 3 | Mitochondrial F1-ATP synthase α subunit | gi|40538742 | 21 | 44 | <10−8 | ATP production | Mitochondrial membrane |
| 4 | Dihydrolipoamide dehydrogenase (E3) | gi|40786469 | 16 | 32 | <10−8 | Pyruvate dehydrogenas e complex TCA Cycle | Mitochondrial matrix |
| 5 | Succinate Dehydrogenase Fp subunit | gi|52782765 | 13 | 23 | <10−8 | Electron transport | Mitochondrial inner membrane |
Hippocampal mitochondria respiration is compromised by acute exposure to combustion smoke
To determine to what extent the observed changes in protein nitration are associated with a compromise in mitochondrial function, we compared rates of oxygen consumption by hippocampal mitochondria isolated from sham-controls and from rats recovering after acute exposure to combustion smoke. The rates of oxygen consumption by hippocampal mitochondria were measured using the Clark-type electrode. State 3 and 4 respiration rates were determined for mitochondria driven either by glutamate or by succinate in the presence of rotenone, under conditions of depletion and repletion of ADP, respectively. Hippocampal mitochondria showed substantial reduction in oxygen consumption immediately after exposure to smoke (0-h) and at the 2, 6 and 24-h recovery times. The most significant reduction was measured at 2 h after exposure to smoke, with partial restoration of the capacity for oxygen consumption in the course of recovery (Fig. 4). A significant compromise in respiratory capacity was measured with either the glutamate or succinate substrate.
Figure 4.
Oxygen consumption rates by hippocampal mitochondria measured in the course of recovery following acute exposure to combustion smoke. Oxygen consumption was measured using the Clark-type electrode system. Respiration was driven by glutamate or by succinate in the presence of rotenone. Oxygen consumption for the indicated time points after exposure to smoke was measured for state 3 (black) and state 4 (open) respirations. The values are expressed as mean ± SEM and presented as bar graphs (n=6). * Indicates different from control (P< 0.05).
DISCUSSION
Inhalation of combustion smoke produces a complex insult with complicated dynamics, including dramatic increases in blood carboxyhemoglobin as well as decreases in oxygen saturation and blood pH. While upon cessation of smoke inhalation, hemodynamic parameters return fairly quickly to normal, the brain tissue recovery clearly lags behind (Lee et al., 2005; Chen et al., 2007). Since hypoxia is a major component in the setting of acute smoke inhalation, elevated brain tissue carbon monoxide may persist, replacing oxygen bound to neuronal heme proteins (Vallone et al., 2004) and thus further impact oxygen availability and brain homeostasis to more acutely perturb neuronal homeostasis, exacerbate oxidative, and nitrative stress and precipitate neural injury (Moncada and Bolanos, 2006). Our earlier studies revealed smoke inhalation-induced modulations of the nitric oxide (NO) system in the rat brain. Because changes in the NO system have been linked with altered mitochondrial energy metabolism, in the current study, we investigated the brain mitochondria in the context of smoke-induced perturbations of NO homeostasis. To assess how mitochondria are affected in this setting, protein nitration and oxygen consumption by hippocampal mitochondria were examined. We found that mitochondrial proteins in the rat hippocampus become significantly nitrated after exposure to smoke. Identity of nitrated proteins revealed involvement in diverse aspects of mitochondrial physiology, and invoked the question as to whether protein nitration and compromised bioenergetics may contribute the development of smoke-initiated delayed neuropathologies.
Multiple mitochondrial mutations and modifications of mitochondrial proteins have been implicated in etiology of human diseases, including neurodegeneration and neuromuscular disorders (Schon and Manfredi, 2003; Leshinsky-Silver et al., 2005; Wallace, 2005; Calvo et al., 2006; Edvardson et al., 2007; Schapira, 2008). Recent studies show that the mitochondrial proteome is a major target of oxidative and nitrative stress that can be induced by chronic age-associated events, as well as acute insults, such as traumatic brain injury (Opii et al., 2007), ischemia/reoxygenation (Koeck et al., 2004), manganese toxicity (Zhang et al., 2005), restraint (Liu et al., 2004), alcohol, inflammation and diverse disease states, with the mitochondrial proteome presenting either altered protein abundance or posttranslational modifications, or both (Bailey et al., 2005). Consistently with these findings, our study demonstrates that a significant nitration of mitochondrial proteins occurs after acute exposure to smoke. Interestingly, some of the proteins, which are nitrated in our setting, have been previously recognized as nitration targets under other pathological conditions. Biological relevance of protein nitration needs also to be considered in the context of differences in the turnover of non-modified versus nitrated proteins. Thus, transient mitochondrial dysfunction may be envisioned as a result of accumulation of nitrated proteins because of impaired degradation process (Elfering et al., 2004; Tatsuta and Langer, 2008). Notwithstanding, while nitration of mitochondrial proteins is considered a mechanism by which oxidative/nitrative stress contributes to mitochondrial dysfunction, protein de-nitration attributed to a putative denitrase has also been proposed (Koeck et al., 2004) suggesting that when strictly controlled, protein nitration/denitration may also serve as a regulatory mechanism.
Using immunodetection in conjunction with proteomic analyses we identified several proteins, which become significantly nitrated after exposure to smoke. The nitrated proteins include the ubiquitous mitochondrial creatine kinase (CK), F1-ATP synthase α subunit, dihydrolipoamide dehydrogenase (E3), succinate dehydrogenase Fp subunit and voltage dependent anion channel 1 (VDAC1) protein. Below, we discuss the significance of these findings in the context of the specific roles of affected proteins, as to how the smoke inhalation-induced nitration may modulate their activities and subsequently contribute to mitochondrial dysfunction.
Creatine kinase catalyzes phosphorylation of creatine by ATP at sites of ATP production, thus playing a key role in energy homeostasis with different isozymes localized at sites of energy production (mitochondria) and utilization (cytosol) (Wallimann et al., 1992). Reduced activity of CK can reduce ADP supply to the mitochondrial ATP synthase and thereby compromise energy generation (Wallimann et al., 1998). The particular importance of CK in the brain, where energy demands may increase rapidly in health and pathology, has been recently described (Delwing et al., 2007; Andres et al., 2008). Interestingly, our earlier studies revealed elevation in the mitochondrial creatine kinase mRNA at 24 h after exposure to smoke (Lee et al., 2005), while here, we report that the protein becomes nitrated about the same time. Since in vitro catalytic activity of creatine kinase is reduced by nitration (Wendt et al., 2003; Kanski et al., 2005b), it is plausible that its observed upregulated expression after smoke serves to compensate for reduced activity.
The mitochondrial F1-ATP synthase α subunit is a component of the ATP generating complex V. F1-ATP synthase is central to energy generation and able to respond to varying tissue energy demands (Das, 2003). The enzyme can be inhibited under different pathological conditions, including anoxia and trigger a slow onset of excitotoxicity in the brain. Interestingly, F1-ATP synthase α subunit deficiency has been linked with oxidative stress (Castegna et al., 2004), identified as a nitration target in the heart mitochondria in aging (Kanski et al., 2005a) and more recently as an oxidation target in traumatic brain injury (Opii et al., 2007). It is likely therefore, that also after exposure to smoke F1-ATP synthase nitration will adversely affect mitochondrial energy production.
Dihydrolipoamide dehydrogenase (E3) is a component of the pyruvate dehydrogenase complex and thus central to aerobic carbohydrate metabolism and mitochondrial energy generation. In vitro studies showed that the enzyme is adversely affected by nitrosylation (Yan et al., 2007) suggesting that this might be the case also in vivo. While no decline in dihydrolipoamide dehydrogenase protein levels is observed in normal aging of the brain, a compromised activity was seen in neurodegeneration including Alzheimer’s disease (Gibson et al., 2000).
Succinate dehydrogenase Fp-flavin (FAD) containing subunit is a major component of the inner membrane anchored Complex II that catalyzes oxidation of succinate. The importance of Complex II activity in brain mitochondria is underscored by the fact that administration of Complex II inhibitor, 3-nitroproprionic acid, generates Huntington Disease (HD)-like phenotype. In addition, Complex II HD-like defects can be induced in vitro in striatal neurons (Benchoua et al., 2006). We observed that the succinate dehydrogenase Fp subunit becomes significantly nitrated after acute inhalation of combustion smoke. Furthermore, we found that after smoke when the succinate dehydrogenase Fp subunit becomes nitrated, mitochondrial respiration driven by succinate via Complex ll, is significantly reduced.
The voltage dependent anion channel (VDAC) 1 is a pore forming protein located in the outer membrane where it controls adenine nucleotides exchange between cytosol and mitochondria, with a role also in cytochorome c release. VDAC has been recognized as a nitration target in multiple studies (Turko et al., 2003; Aulak et al., 2004; Kanski et al., 2005a), as well as an oxidation target in aging and other pathological conditions (Opii et al., 2007). Generally, posttranslational modifications of VDAC, including nitration are thought to perturb ADP-ATP exchange and adversely impact cellular energetics.
Because all the proteins identified here are central to the different aspects of mitochondrial energy metabolism and their modifications, whether genetic or exogenous, have been implicated in mitochondrial dysfunction, it is plausible that their modifications by nitration contribute to the overall compromise in respiratory capacity of hippocampal mitochondria detected in this study. Remarkably, new proteomic analyses revealed tissue-specific heterogeneities of the mitochondrial proteome (Johnson et al., 2007; Pagliarini et al., 2008) suggesting that brain-specific mitochondrial susceptibilities may also exist. Because neurons are particularly susceptible to insults involving disrupted oxygenation and prone to escalating generation of free radicals, the impact of smoke inhalation on the status and activities of mitochondrial proteins and consequently on mitochondrial function is likely to be exacerbated in the brain compared to tissues with lower oxidative metabolic rates. Hence, a further investigation of smoke initiated molecular events that impact brain mitochondria is critical to help understand the roles of mitochondria in the development of smoke inhalation-induced neuropathologies.
Acknowledgments
Funding/Support: This work was supported by Shriners Hospitals for Children grant SHG8670 and National Institutes of Health grants ES014613 and NS039449 to EWE.
Role of the Sponsor: The funding organizations played no role in the design and conduct of the study, in the collection, management, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest Statement: Authors have no conflicts of interest to declare.
References
- Alarie Y. Toxicity of fire smoke. Crit Rev Toxicol. 2002;32:259–289. doi: 10.1080/20024091064246. [DOI] [PubMed] [Google Scholar]
- Anderson MF, Sims NR. Improved recovery of highly enriched mitochondrial fractions from small brain tissue samples. Brain Res Brain Res Protoc. 2000;5:95–101. doi: 10.1016/s1385-299x(99)00060-4. [DOI] [PubMed] [Google Scholar]
- Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR. Functions and effects of creatine in the central nervous system. Brain Res Bull. 2008;76:329–343. doi: 10.1016/j.brainresbull.2008.02.035. [DOI] [PubMed] [Google Scholar]
- Aulak KS, Koeck T, Crabb JW, Stuehr DJ. Dynamics of protein nitration in cells and mitochondria. Am J Physiol Heart Circ Physiol. 2004;286:H30–38. doi: 10.1152/ajpheart.00743.2003. [DOI] [PubMed] [Google Scholar]
- Bailey SM, Landar A, Darley-Usmar V. Mitochondrial proteomics in free radical research. Free Radic Biol Med. 2005;38:175–188. doi: 10.1016/j.freeradbiomed.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Benchoua A, Trioulier Y, Zala D, Gaillard MC, Lefort N, Dufour N, Saudou F, Elalouf JM, Hirsch E, Hantraye P, Deglon N, Brouillet E. Involvement of mitochondrial complex II defects in neuronal death produced by N-terminus fragment of mutated huntingtin. Mol Biol Cell. 2006;17:1652–1663. doi: 10.1091/mbc.E05-07-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes PS, Kraus DW, Shiva S, Doeller JE, Barone MC, Patel RP, Lancaster JR, Jr, Darley-Usmar V. Control of mitochondrial respiration by NO*, effects of low oxygen and respiratory state. J Biol Chem. 2003;278:31603–31609. doi: 10.1074/jbc.M211784200. [DOI] [PubMed] [Google Scholar]
- Calvo S, Jain M, Xie X, Sheth SA, Chang B, Goldberger OA, Spinazzola A, Zeviani M, Carr SA, Mootha VK. Systematic identification of human mitochondrial disease genes through integrative genomics. Nat Genet. 2006;38:576–582. doi: 10.1038/ng1776. [DOI] [PubMed] [Google Scholar]
- Castegna A, Thongboonkerd V, Klein J, Lynn BC, Wang YL, Osaka H, Wada K, Butterfield DA. Proteomic analysis of brain proteins in the gracile axonal dystrophy (gad) mouse, a syndrome that emanates from dysfunctional ubiquitin carboxyl-terminal hydrolase L-1, reveals oxidation of key proteins. J Neurochem. 2004;88:1540–1546. doi: 10.1046/j.1471-4159.2003.02288.x. [DOI] [PubMed] [Google Scholar]
- Chen L, Lee HM, Greeley GH, Jr, Englander EW. Accumulation of oxidatively generated DNA damage in the brain: a mechanism of neurotoxicity. Free Radic Biol Med. 2007;42:385–393. doi: 10.1016/j.freeradbiomed.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronje FJ, Carraway MS, Freiberger JJ, Suliman HB, Piantadosi CA. Carbon monoxide actuates O(2)-limited heme degradation in the rat brain. Free Radic Biol Med. 2004;37:1802–1812. doi: 10.1016/j.freeradbiomed.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Das AM. Regulation of the mitochondrial ATP-synthase in health and disease. Mol Genet Metab. 2003;79:71–82. doi: 10.1016/s1096-7192(03)00069-6. [DOI] [PubMed] [Google Scholar]
- Delwing D, Cornelio AR, Wajner M, Wannmacher CM, Wyse AT. Arginine administration reduces creatine kinase activity in rat cerebellum. Metab Brain Dis. 2007;22:13–23. doi: 10.1007/s11011-006-9028-z. [DOI] [PubMed] [Google Scholar]
- Edvardson S, Shaag A, Kolesnikova O, Gomori JM, Tarassov I, Einbinder T, Saada A, Elpeleg O. Deleterious mutation in the mitochondrial arginyl-transfer RNA synthetase gene is associated with pontocerebellar hypoplasia. Am J Hum Genet. 2007;81:857–862. doi: 10.1086/521227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfering SL, Haynes VL, Traaseth NJ, Ettl A, Giulivi C. Aspects, mechanism, and biological relevance of mitochondrial protein nitration sustained by mitochondrial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2004;286:H22–29. doi: 10.1152/ajpheart.00766.2003. [DOI] [PubMed] [Google Scholar]
- Franco MC, Arciuch VG, Peralta JG, Galli S, Levisman D, Lopez LM, Romorini L, Poderoso JJ, Carreras MC. Hypothyroid phenotype is contributed by mitochondrial complex I inactivation due to translocated neuronal nitric-oxide synthase. J Biol Chem. 2006;281:4779–4786. doi: 10.1074/jbc.M512080200. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Park LC, Sheu KF, Blass JP, Calingasan NY. The alpha-ketoglutarate dehydrogenase complex in neurodegeneration. Neurochem Int. 2000;36:97–112. doi: 10.1016/s0197-0186(99)00114-x. [DOI] [PubMed] [Google Scholar]
- Hartzell GE. Overview of combustion toxicology. Toxicology. 1996;115:7–23. doi: 10.1016/s0300-483x(96)03492-0. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H, Beckman JS. Oxidative stress and nitration in neurodegeneration: cause, effect, or association? J Clin Invest. 2003;111:163–169. doi: 10.1172/JCI17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischiropoulos H, Beers MF, Ohnishi ST, Fisher D, Garner SE, Thom SR. Nitric oxide production and perivascular nitration in brain after carbon monoxide poisoning in the rat. J Clin Invest. 1996;97:2260–2267. doi: 10.1172/JCI118667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DT, Harris RA, French S, Blair PV, You J, Bemis KG, Wang M, Balaban RS. Tissue heterogeneity of the mammalian mitochondrial proteome. Am J Physiol Cell Physiol. 2007;292:C689–697. doi: 10.1152/ajpcell.00108.2006. [DOI] [PubMed] [Google Scholar]
- Kanski J, Behring A, Pelling J, Schoneich C. Proteomic identification of 3-nitrotyrosine-containing rat cardiac proteins: effects of biological aging. Am J Physiol Heart Circ Physiol. 2005a;288:H371–381. doi: 10.1152/ajpheart.01030.2003. [DOI] [PubMed] [Google Scholar]
- Kanski J, Hong SJ, Schoneich C. Proteomic analysis of protein nitration in aging skeletal muscle and identification of nitrotyrosine-containing sequences in vivo by nanoelectrospray ionization tandem mass spectrometry. J Biol Chem. 2005b;280:24261–24266. doi: 10.1074/jbc.M501773200. [DOI] [PubMed] [Google Scholar]
- Koeck T, Fu X, Hazen SL, Crabb JW, Stuehr DJ, Aulak KS. Rapid and selective oxygen-regulated protein tyrosine denitration and nitration in mitochondria. J Biol Chem. 2004;279:27257–27262. doi: 10.1074/jbc.M401586200. [DOI] [PubMed] [Google Scholar]
- Lee HM, Greeley GH, Herndon DN, Sinha M, Luxon BA, Englander EW. A rat model of smoke inhalation injury: influence of combustion smoke on gene expression in the brain. Toxicol Appl Pharmacol. 2005;208:255–265. doi: 10.1016/j.taap.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Leshinsky-Silver E, Lev D, Tzofi-Berman Z, Cohen S, Saada A, Yanoov-Sharav M, Gilad E, Lerman-Sagie T. Fulminant neurological deterioration in a neonate with Leigh syndrome due to a maternally transmitted missense mutation in the mitochondrial ND3 gene. Biochem Biophys Res Commun. 2005;334:582–587. doi: 10.1016/j.bbrc.2005.06.134. [DOI] [PubMed] [Google Scholar]
- Liu XH, Qian LJ, Gong JB, Shen J, Zhang XM, Qian XH. Proteomic analysis of mitochondrial proteins in cardiomyocytes from chronic stressed rat. Proteomics. 2004;4:3167–3176. doi: 10.1002/pmic.200300845. [DOI] [PubMed] [Google Scholar]
- Moncada S, Bolanos JP. Nitric oxide, cell bioenergetics and neurodegeneration. J Neurochem. 2006;97:1676–1689. doi: 10.1111/j.1471-4159.2006.03988.x. [DOI] [PubMed] [Google Scholar]
- Opii WO, Nukala VN, Sultana R, Pandya JD, Day KM, Merchant ML, Klein JB, Sullivan PG, Butterfield DA. Proteomic identification of oxidized mitochondrial proteins following experimental traumatic brain injury. J Neurotrauma. 2007;24:772–789. doi: 10.1089/neu.2006.0229. [DOI] [PubMed] [Google Scholar]
- Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, Mootha VK. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raub JA, Benignus VA. Carbon monoxide and the nervous system. Neurosci Biobehav Rev. 2002;26:925–940. doi: 10.1016/s0149-7634(03)00002-2. [DOI] [PubMed] [Google Scholar]
- Roohi F, Kula RW, Mehta N. Twenty-nine years after carbon monoxide intoxication. Clin Neurol Neurosurg. 2001;103:92–95. doi: 10.1016/s0303-8467(01)00119-6. [DOI] [PubMed] [Google Scholar]
- Rossi J, 3rd, Ritchie GD, Macys DA, Still KR. An overview of the development, validation, and application of neurobehavioral and neuromolecular toxicity assessment batteries: potential applications to combustion toxicology. Toxicology. 1996;115:107–117. doi: 10.1016/s0300-483x(96)03498-1. [DOI] [PubMed] [Google Scholar]
- Schapira AH. Mitochondria in the etiology and pathogenesis of Parkinson’s disease. Lancet Neurol. 2008;7:97–109. doi: 10.1016/S1474-4422(07)70327-7. [DOI] [PubMed] [Google Scholar]
- Schon EA, Manfredi G. Neuronal degeneration and mitochondrial dysfunction. J Clin Invest. 2003;111:303–312. doi: 10.1172/JCI17741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims NR. Rapid isolation of metabolically active mitochondria from rat brain and subregions using Percoll density gradient centrifugation. J Neurochem. 1990;55:698–707. doi: 10.1111/j.1471-4159.1990.tb04189.x. [DOI] [PubMed] [Google Scholar]
- Smith SM, Stuhmiller JH, Januszkiewicz AJ. Evaluation of lethality estimates for combustion gases in military scenarios. Toxicology. 1996;115:157–165. doi: 10.1016/s0300-483x(96)03504-4. [DOI] [PubMed] [Google Scholar]
- Stuhmiller JH, Long DW, Stuhmiller LM. An internal dose model of incapacitation and lethality risk from inhalation of fire gases. Inhal Toxicol. 2006;18:347–364. doi: 10.1080/08958370500516010. [DOI] [PubMed] [Google Scholar]
- Tatsuta T, Langer T. Quality control of mitochondria: protection against neurodegeneration and ageing. EMBO J. 2008;27:306–314. doi: 10.1038/sj.emboj.7601972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom SR, Fisher D, Manevich Y. Roles for platelet-activating factor and *NO-derived oxidants causing neutrophil adherence after CO poisoning. Am J Physiol Heart Circ Physiol. 2001;281:H923–930. doi: 10.1152/ajpheart.2001.281.2.H923. [DOI] [PubMed] [Google Scholar]
- Thom SR, Fisher D, Zhang J, Bhopale VM, Cameron B, Buerk DG. Neuronal nitric oxide synthase and N-methyl-D-aspartate neurons in experimental carbon monoxide poisoning. Toxicol Appl Pharmacol. 2004;194:280–295. doi: 10.1016/j.taap.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Thom SR, Fisher D, Zhang J, Bhopale VM, Ohnishi ST, Kotake Y, Ohnishi T, Buerk DG. Stimulation of perivascular nitric oxide synthesis by oxygen. Am J Physiol Heart Circ Physiol. 2003;284:H1230–1239. doi: 10.1152/ajpheart.01043.2002. [DOI] [PubMed] [Google Scholar]
- Turko IV, Li L, Aulak KS, Stuehr DJ, Chang JY, Murad F. Protein tyrosine nitration in the mitochondria from diabetic mouse heart. Implications to dysfunctional mitochondria in diabetes. J Biol Chem. 2003;278:33972–33977. doi: 10.1074/jbc.M303734200. [DOI] [PubMed] [Google Scholar]
- Vallone B, Nienhaus K, Matthes A, Brunori M, Nienhaus GU. The structure of carbonmonoxy neuroglobin reveals a heme-sliding mechanism for control of ligand affinity. Proc Natl Acad Sci U S A. 2004;101:17351–17356. doi: 10.1073/pnas.0407633101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallimann T, Dolder M, Schlattner U, Eder M, Hornemann T, Kraft T, Stolz M. Creatine kinase: an enzyme with a central role in cellular energy metabolism. MAGMA. 1998;6:116–119. doi: 10.1007/BF02660927. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J. 1992;281(Pt 1):21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt S, Schlattner U, Wallimann T. Differential effects of peroxynitrite on human mitochondrial creatine kinase isoenzymes. Inactivation, octamer destabilization, and identification of involved residues. J Biol Chem. 2003;278:1125–1130. doi: 10.1074/jbc.M208572200. [DOI] [PubMed] [Google Scholar]
- Wiggins AK, Shen PJ, Gundlach AL. Neuronal-NOS adaptor protein expression after spreading depression: implications for NO production and ischemic tolerance. J Neurochem. 2003;87:1368–1380. doi: 10.1046/j.1471-4159.2003.02099.x. [DOI] [PubMed] [Google Scholar]
- Yan LJ, Yang SH, Shu H, Prokai L, Forster MJ. Histochemical staining and quantification of dihydrolipoamide dehydrogenase diaphorase activity using blue native PAGE. Electrophoresis. 2007;28:1036–1045. doi: 10.1002/elps.200600574. [DOI] [PubMed] [Google Scholar]
- Zhang S, Fu J, Zhou Z. Changes in the brain mitochondrial proteome of male Sprague-Dawley rats treated with manganese chloride. Toxicol Appl Pharmacol. 2005;202:13–17. doi: 10.1016/j.taap.2004.06.001. [DOI] [PubMed] [Google Scholar]