Abstract
The relationships between peak velocity and amplitude of Edinger–Westphal (EW) stimulated accommodation and disaccommodation were investigated in anesthetized, middle-aged rhesus monkeys. Accommodative responses were recorded at 30 Hz with infrared photorefraction. Peak velocity of accommodation and disaccommodation increased linearly with stimulus amplitude. Peak velocities of accommodation continued to increase with stimulus amplitudes greater than required to produce the maximum response. The peak velocity of disaccommodation did not further increase with supramaximal stimulus amplitudes beyond that achieved with maximal stimulus amplitudes. Although maximum accommodative response amplitude is reduced in older rhesus monkeys, within the methodological constraints of this study, older monkeys appear to achieve accommodative and disaccommodative peak velocities similar to adolescent monkeys for the same response amplitudes.
Keywords: disaccommodation, presbyopia, lens, refraction, aging
1. Introduction
Accommodation is defined as a dynamic change in dioptric power of the eye that enables the eye to focus on objects at different distances (Keeney et al., 1995). The optical accommodative response of the eye occurs through a contraction of the ciliary muscle, release of zonular tension around the lens equator, a decrease in lens diameter and a resulting increase in crystalline lens curvatures (Glasser and Campbell, 1998; Glasser and Kaufman, 1999; Glasser et al., 2006; Vilupuru and Glasser, 2005). The maximum amplitude of accommodation declines with advancing age, and in humans above approximately 50 years of age, the ability to accommodate is completely lost, a condition known as presbyopia (Duane, 1912; Glasser and Campbell, 1998).
Static measurement of accommodation only allows assessment of the accommodative response amplitudes for different stimulus amplitudes. Dynamic measurement of accommodation can provide information about the stability of an accommodative response, a more accurate assessment of accommodative amplitude and important information about dynamic variables such as velocity, acceleration and time constants and how these change with accommodative amplitude and age (Bharadwaj and Schor, 2005; Kasthurirangan and Glasser, 2005, 2006; Kasthurirangan et al., 2003; Schor and Bharadwaj, 2005; Vilupuru and Glasser, 2002; Vilupuru et al., 2005). Studying the dynamics of accommodation can provide insight into the neural control of accommodation (Gamlin and Reiner, 1991; Gamlin et al., 1994; Schor and Bharadwaj, 2005), biomechanics of the accommodative anatomical structures (the accommodative plant) and possible age changes and how these contribute to the development of presbyopia. In addition, dynamic analysis allows similarities and differences between change in focus from far-to-near and change in focus from near-to-far to be determined (Kasthurirangan et al., 2003; Vilupuru and Glasser, 2002).
While it is well established that accommodative amplitude decreases with age, there are conflicting results regarding age changes in the dynamics of visual stimulus driven accommodation in humans. Several studies have found a decrease in peak velocity of accommodation for a given response amplitude with increasing age (Beers and van der Heijde, 1996; Kasthurirangan and Glasser, 2006; Schaeffel et al., 1993; Sun et al., 1988), others reported no change in the amplitude vs. peak velocity relationship with increasing age (Mordi and Ciuffreda, 2004). The peak velocity of disaccommodation shows no change with age in most of the published studies (Heron et al., 1999, 2002), although some authors have reported a decrease with aging (Beers and van der Heijde, 1996; Schaeffel et al., 1993).
The dynamics of Edinger–Westphal (EW) stimulated accommodation in anesthetized, adolescent rhesus monkeys have been studied previously (Ostrin and Glasser, 2004, 2005; Vilupuru and Glasser, 2002, 2005; Vilupuru et al., 2005). Stimulation of the EW nucleus causes a depolarization of the EW neurons which propagates to and cause a contraction of the ciliary muscle and an increase in optical power of the eye (accommodation). When the stimulus is terminated, the ciliary muscle contraction ceases and the ciliary muscle is pulled back into its unaccommodated rest state, decreasing the optical power of the eye (disaccommodation). EW-stimulated accommodation in anesthetized monkeys is open-loop without visual feedback. The dynamics of the EW-stimulated accommodative response are therefore entirely dictated by the properties of the stimulus to the EW nucleus, the neural propagation to the ciliary muscle and the biomechanical characteristics of the accommodative plant (force and speed of contraction of the ciliary muscle contraction, elasticity of the choroid and visco-elastic properties of the lens, for example) and not at all by visual feedback and optical perception of blur as would occur with accommodation in conscious subjects. These experiments in anesthetized monkeys enable rigorously controlled assessment of how age changes in the biomechanical properties of the accommodative plant may influence EW-stimulated accommodative dynamics in the absence of visual feedback.
In conscious human subjects, the dynamic characteristics of accommodation or disaccommodation may be influenced by many factors including biomechanics of the plant, response amplitude, perceptual cues and visual feedback (Kasthurirangan et al., 2003), the starting point of a response (Kasthurirangan and Glasser, 2005; Schor and Bharadwaj, 2006) or neural control (Schor and Bharadwaj, 2005, 2006). There are many age changes in the biomechanics of the accommodative apparatus including an increase in elastic stiffness of the capsule (Krag and Andreassen, 2003; Krag et al., 1997), decreased compliance of the posterior attachment of the ciliary muscle (Tamm et al., 1992, 1991) and loss of accommodative ability of the lens due to increased stiffness (Glasser and Campbell, 1998, 1999; Heys et al., 2004). Given the fine balance of forces required for accommodation, age changes in the biomechanics of the plant might alter the dynamics of accommodation (Kasthurirangan and Glasser, 2006) unless compensatory neuronal control strategies occur to maintain accommodative dynamics constant with age (Schor and Bharadwaj, 2005). EW-stimulated accommodation in anesthetized monkeys offers an ideal method to study accommodative dynamics of the plant (Vilupuru and Glasser, 2002), and the possible influence of age changes in the accommodative plant. Perceptual factors such as visual feedback that may influence visual stimulus driven accommodative dynamics in conscious subjects are eliminated and any possible age-related compensatory neuronal control strategies are avoided as the monkeys are anesthetized and the stimulus is externally controlled by a stimulator.
In a previous study in adolescent rhesus monkeys, EW-stimulated accommodation produced a linear dependency of the peak velocity of accommodation and disaccommodation on the response amplitude (Vilupuru and Glasser, 2002). The aim of the present study was to use the same methodology to investigate dynamic accommodation in older, middle-aged, pre-presbyopic rhesus monkeys to submaximal and supramaximal stimulus amplitudes. This provides an opportunity, to investigate the relationship between peak velocity and accommodative amplitude in older monkeys and to determine the effects of aging on accommodative dynamics of EW-stimulated accommodation in rhesus monkeys.
2. Material and methods
2.1. Animal preparation
All experiments conformed to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Vision Research and EC Directive 86/609/EEC and were in accordance with institutionally approved animal protocols. Appropriate analgesia and anesthesia were used in all procedures to minimize or eliminate pain and discomfort. Three eyes of three rhesus monkeys (Macaca mulatta), aged 18.6 years (#69, OS), 15.3 years (#96, OS) and 14.6 years (#34, OD) respectively, were studied. The experiment was repeated in the left eye of monkey #96 (referred to here as #96 (2)). The monkeys had previously undergone total iridectomy, assessment of maximum pharmacologically stimulated accommodative amplitude (Koretz et al., 1987; Vilupuru and Glasser, 2002) and stereotaxic surgical implantation of a stimulating electrode into the EW nucleus (Crawford et al., 1989; Vilupuru and Glasser, 2002).
The monkeys are used in multiple protocols; the justification for the iridectomies and the absence of an effect on EW-stimulated accommodation responses have been described previously (Glasser et al., 2006; Kaufman and Lütjen-Drecoll, 1975; Vilupuru and Glasser, 2002).
2.2. Pharmacologically stimulated accommodation
Maximum pharmacologically stimulated accommodative amplitude was tested at least 30 days before the electrode implantation. Carbachol 40% was applied iontophoretically to the nasal and temporal cornea for 8 s each. The cornea was irrigated with saline and a rigid gas-permeable contact lens (Metro Optics, Dallas, TX) was placed on the cornea to prevent dehydration. Refraction was measured with a Hartinger coincidence refractometer (Zeiss, Jena, Germany). The eyepiece of the Hartinger refractometer was replaced with a CCD camera which was connected to a video monitor to facilitate rapid refraction readings. Measurements were taken three times each at 2-min intervals until there was no further increase in accommodation for three consecutive 2-min intervals. The contact lens was removed and carbachol was applied again for 4 s and refraction measurements were repeated for 60 min or until no further increase in accommodation occurred for three consecutive 2-min intervals.
2.3. EW-stimulated accommodation
Monkeys were anesthetized with intramuscular ketamine (Ketaset, Fort Dodge Animal Health, Fort Dodge, IA) 10 mg/kg and acepromazine (Acepromazine, Vedco, St. Joseph, MO) 0.5 mg/kg. Surgical depth anesthesia was induced by a bolus injection of 1.5 mg/kg intravenous propofol (Propo-Flo, Abbott Laboratories, Abbott Park, IL) and anesthesia was maintained with a continuous intravenous infusion of propofol 0.5 mg/kg/min (Vilupuru and Glasser, 2005).
The monkey's head was placed in a head holder upright and facing forward. The eye was held open by a lid speculum. To reduce convergence eye movements during accommodation, 4–0 nylon sutures were placed through the medial and lateral rectus muscles and held under light tension. A rigid gas-permeable contact lens was placed on the cornea. Baseline resting refraction was measured with the Hartinger coincidence refractometer.
2.4. Static accommodation measurements
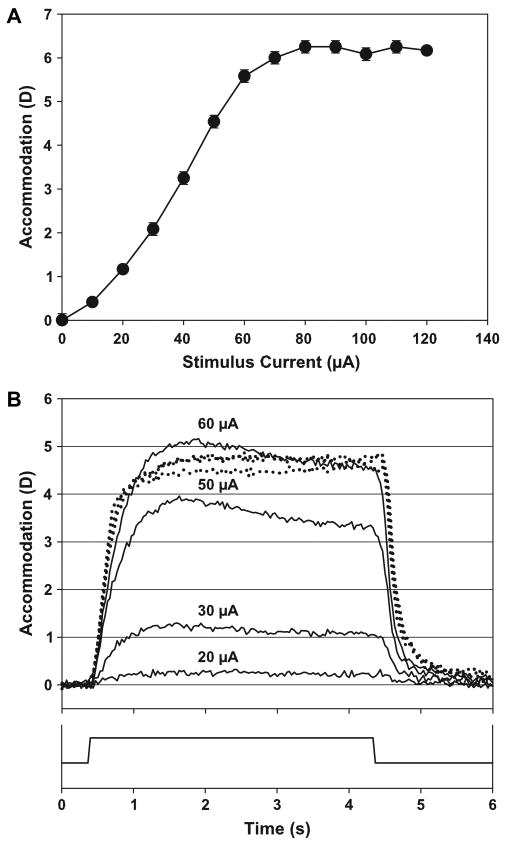
Accommodation was stimulated using an A-310 stimulator connected to an A-385 stimulus isolator (World Precision Instruments, Sarasota, FL). Four-second stimulus trains were used (frequency: 72 Hz, pulse width: 600 μs), ranging from 0 μA up to a current amplitude sufficient to produce the maximum accommodative response. In addition, three or more stimuli of current amplitudes greater than that required to produce the maximum accommodative response (supramaximal stimulus amplitudes) were delivered. For each stimulus amplitude, five consecutive 4-s duration stimulus trains with 4-s long inter-stimulus intervals were delivered. Static accommodative response amplitudes were measured towards the end of each 4-s stimulus train using the Hartinger coincidence refractometer. The mean and standard deviation of the response amplitudes achieved in the last three of the five stimulus trains were calculated. The mean values were used to obtain a static stimulus-response function (Fig. 1A). From this stimulus-response function, several submaximal and supramaximal stimulus current amplitudes, including the current amplitude required to produce the maximal accommodative response, were selected to be used for the dynamic accommodation measurements.
Fig. 1.
(A) EW-stimulated accommodative stimulus/response curve for monkey #69 OS showing accommodative response as a function of the stimulus current. The symbols represent the average of three consecutive measurements with the standard deviation shown as error bars. With supramaximal stimulus amplitudes, accommodative amplitude reached an asymptote. (B) Dynamic EW-stimulated accommodative responses for monkey #69 OS showing the time course and accommodative amplitude with different stimulus amplitudes. The three dotted lines represent responses to supramaximal stimulus amplitudes of 60 μA, 80 μA and 120 μA. The solid line at the bottom of the graph represents the stimulus onset, duration and offset.
2.5. Dynamic accommodation measurements
Procedures for dynamic photorefraction measurement of EW-stimulated accommodation have been described in detail previously (Vilupuru and Glasser, 2002, 2003). Video-based infrared photorefraction (Schaeffel et al., 1987) was used. A charge coupled device (CCD) infrared video camera (Cohu, San Diego, CA) was placed 0.3 m in front of the eye. A knife-edge aperture was attached to the front of the camera lens to cover the lower half of the lens aperture and this held a bank of 20 infrared LEDs emitting a wavelength of 890 nm. Photorefraction images were recorded to digital video tape (DSR-20, Sony, Tokyo, Japan) at a frequency of 30 Hz. The recording was subsequently analyzed off-line, frame-by-frame using image analysis software (Optimas 6.5, Media Cybernetics, Silver Springs, MD). For each stimulus amplitude, the last three of the five, 4-s stimulus trains were analyzed. Analysis of each accommodative response was started one second (30 frames) before stimulus onset and ended two seconds (60 frames) after the stimulus terminated. The software located the Purkinje image and the limbus diameter in the iridectomized eye. Vertical luminance profiles were extracted along two parallel vertical lines on either side of the Purkinje image with a length corresponding to 40% of the iridectomized pupil diameter. The luminance profiles were averaged, a linear regression line fitted to the luminance profile and a unique luminance profile slope value was obtained for each frame. A calibration function was generated to convert the measured slope values to refraction. For each of the accommodative responses analyzed, the average slope of the last 10 video frames before the stimulus terminated was calculated and plotted against the Hartinger measured refraction of the corresponding stimulus amplitude from the static stimulus-response function. The curve fitted to this slope vs. refraction data were used as the calibration curve for conversion of the photorefraction luminance slopes into refraction values. For each experiment, a unique calibration curve was obtained.
2.6. Data analysis
The resting, unaccommodated baseline refraction was determined by calculating the average refraction of the last 20 frames before stimulus onset. The refraction measurements were converted into accommodation by subtracting the refraction at each time point from the baseline. For each stimulus current amplitude, the response amplitude was calculated by averaging the maximum accommodation measurements from the last three of five stimulus trains (Table 1). In some cases, the disaccommodative phase returned to a slightly different baseline resting refraction. Therefore, disaccommodation amplitude was calculated separately with the disaccommodative baseline as the average of the last 20 measurements before stimulus termination. The accommodative responses were plotted against time. The accommodation values of the last three stimulus trains for each stimulus amplitude were collapsed into one single data set and functions consisting of an exponential function plus a polynomial were fit to the accommodative and disaccommodative phase of the dynamic responses as described previously (Vilupuru and Glasser, 2002). The fitted function was used for two reasons: first, at a sampling rate of 30 Hz, calculating the derivative from the data using a two-point difference would result in considerable noise and a clear and unequivocal peak may not be available (Kasthurirangan and Glasser, 2005); second, the intention was to compare the results to the findings of a previously published study in adolescent monkeys (Vilupuru and Glasser, 2002), therefore it was necessary to use the same methodology. The actual accommodative phase was considered to begin two video frames after stimulus onset to compensate for the response latency (Vilupuru and Glasser, 2005) and to last until termination of the stimulus. The disaccommodative phase was considered to begin two frames after stimulus termination and to continue until two seconds after stimulus termination. The goodness of fits of the exponential functions to the collapsed data was determined by examination of the residuals between the fitted functions and the collapsed data. The derivatives of the exponential functions were calculated to obtain peak velocity (Vilupuru and Glasser, 2002).
Table 1.
Maximum mean amplitudes of accommodation (±standard deviations) produced with different stimulation and measurement methods
| Monkey eye | Age (years) | Hartinger measurement of carbachol-stimulated accommodation (D) | Hartinger measurement of EW-stimulated accommodation (D) | Dynamic photorefraction measurement of EW-stimulated accommodation (D) |
|---|---|---|---|---|
| #69 OS | 18.6 | 7.58 ± 0.14 | 6.25 ± 0.14 | 5.21 ± 0.15 |
| #96 OS (1) | 15.3 | 10.58 ± 0.52 | 7.75 ± 0 | 6.38 ± 0.09 |
| #34 OD | 14.6 | 8.92 ± 0.14 | 6.08 ± 0.14 | 5.79 ± 0.11 |
| #96 OS (2) | 15.3 | – | 7.25 ± 0 | 9.60 ± 0.06 |
For each EW-stimulated accommodative response, maximum amplitude of accommodation was calculated as the average maximum refraction of the analyzed responses before stimulus termination subtracted from baseline (last 20 frames before stimulus onset). The baseline for the disaccommodative amplitude was calculated as the average of the last 20 video frames before termination of the stimulus. This baseline was subtracted from the refraction values after termination of the stimulus. The average maximum disaccommodation value of the analyzed responses was defined as the maximum amplitude of disaccommodation. Main sequence relationships (peak velocity as a function of amplitude) for accommodation and disaccommodation were determined.
3. Results
3.1. Carbachol-stimulated accommodation
Carbachol iontophoresis was used to determine the maximum accommodative amplitude of each eye before implantation of the EW electrodes. The maximum pharmacologically stimulated accommodative amplitudes achieved are shown in Table 1.
3.2. EW-stimulated static stimulus-response functions
Mean maximum amplitude of the static Hartinger measured EW-stimulated accommodative responses from all eyes was 6.79 ± 0.74 D (one eye repeated twice). The accommodative stimulus-response function of the left eye of monkey #69 is shown as an example in Fig. 1A. The EW-stimulated static accommodative amplitudes achieved are shown in Table 1.
3.3. Dynamic infrared photorefraction
3.3.1. Maximum amplitude
In each eye, increasing the stimulus amplitude ultimately resulted in a saturation of the accommodative response amplitude (Fig. 1A, B). The individual maximum EW-stimulated dynamically measured amplitudes achieved are shown in Table 1.
3.3.2. Peak velocity
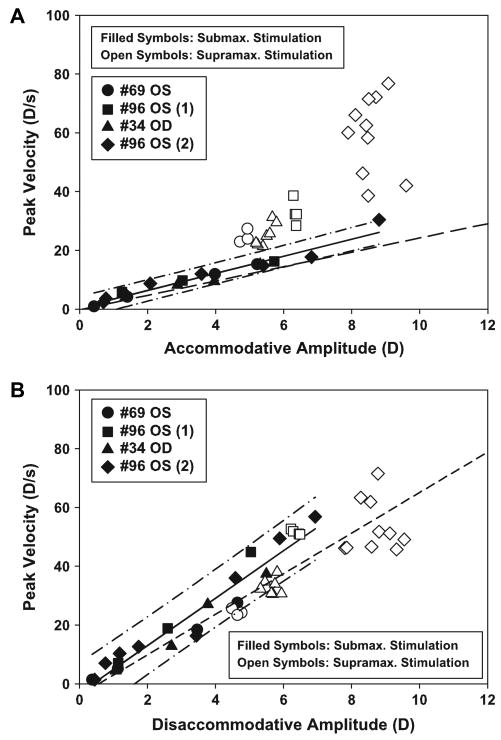
The peak velocity as a function of the submaximal to maximal stimulus amplitudes showed a linear increasing relationship for both accommodation (slope: 2.904, intercept: 0.538, r2 = 0.95, p < 0.001) and disaccommodation (slope: 8.067, intercept: −3.144, r2 = 0.93, p < 0.001). The intercepts of both regression lines were not significantly different from zero (accommodation: p = 0.438; disaccommodation: p = 0.110). The regression lines were not significantly different from the regressions from the earlier study which investigated the relationships between accommodative amplitude and peak velocity in younger, adolescent rhesus monkeys (Vilupuru and Glasser, 2002) (accommodation: slopes p = 0.207, intercepts p = 0.725; disaccommodation: slopes p = 0.153, intercepts p = 0.849) (Fig. 2A, B).
Fig. 2.
(A) Graph of the peak velocity of accommodation as a function of accommodative amplitude in four EW-stimulated monkey eyes. Solid symbols are responses from submaximal to maximum stimulations. Open symbols are responses to supramaximal stimulations. The peak velocity of the accommodative responses (excluding the supramaximal stimulations) increased linearly up to the maximum response amplitude (solid regression line; slope: 2.904, intercept: 0.538, r2: 0.95, p < 0.001). The regression line is shown with 95% confidence intervals (dash-dot). At supramaximal stimulus amplitudes, peak velocity continued to increase but without an increase in accommodative response amplitude. The dashed line is the regression line from a prior study in adolescent monkeys (Vilupuru and Glasser, 2002) (slope: 2.439, intercept: −0.199, r2: 0.89, p < 0.001). (B) Graph of the peak velocity of disaccommodation as a function of disaccommodative amplitude in EW-stimulated rhesus monkey eyes. Peak velocity increased linearly with response amplitude. Supramaximal stimuli increased neither response amplitude nor peak velocity. The solid line represents the regression of responses from sub-maximal to maximal stimuli (slope: 8.067, intercept: −3.144, r2: 0.93, p < 0.001). The 95% confidence interval is shown as dash-dots. The dashed line represents the regression from a prior study on adolescent monkeys (Vilupuru and Glasser, 2002) (slope: 6.901, intercept: −3.944, r2: 0.955, p < 0.001).
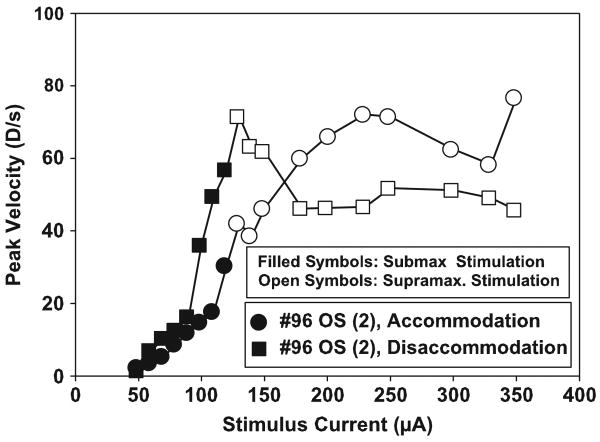
Accommodative responses to maximal stimulus amplitudes reached a mean peak velocity of 19.28 ± 7.41 D/s. The disaccommodative responses with maximal stimulus amplitudes reached a mean peak velocity of 41.66 ± 12.34 D/s. In each monkey, supramaximal stimulus amplitudes increased the peak velocity of the accommodative responses above the peak velocity achieved for the maximal stimulus amplitude, but without increasing the accommodative response amplitudes. A different range of supramaximal stimulus amplitudes was used in each monkey. Initially, only three supramaximal stimuli were used. As the subsequent experiments were performed, higher supramaximal stimuli were used. In the final experiment, 10 supramaximal stimulus amplitudes were used. There was, therefore, a wide range of peak velocities achieved to supramaximal stimulations. The mean maximum accommodative peak velocity for all eyes tested was 43.48 ± 22.64 D/s with the maximum of 76.72 D/s being achieved in monkey #96 (2), the only monkey in which high enough stimulus amplitudes were used to achieve a clear asymptote in peak velocity of accommodation. Disaccommodative responses to maximum stimulus amplitudes had a mean peak velocity of 46.98 ± 19.73 D/s. The maximum disaccommodative peak velocity was 71.50 D/s which was achieved in monkey #96 (2) (Fig. 3).
Fig. 3.
Graph of the peak velocity of accommodation and disaccommodation in an EW-stimulated monkey eye as a function of stimulus current amplitude. As a representative example, the graph of the second experiment on monkey #96 is shown. Peak velocity of accommodation continued to increase with increasing stimulus amplitude. Much higher stimulus amplitudes used resulted in an asymptote in peak velocity of accommodation. With submaximal to maximal stimulus amplitudes, peak velocity of disaccommodation was generally higher and increased faster than peak velocity of accommodation. With supramaximal stimuli, however, there was no further increase of peak velocity of disaccommodation while the peak velocity of accommodation generally continued to increase and eventually reached the peak velocity of disaccommodation.
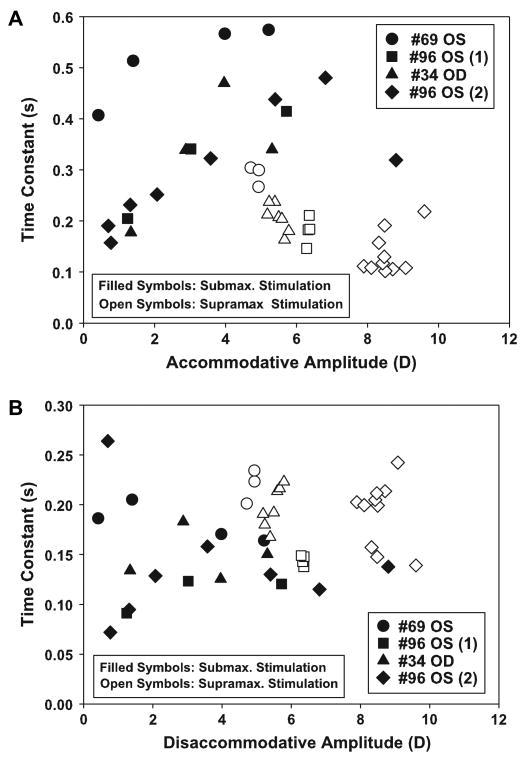
3.3.3. Time constants
Time constants of the EW-stimulated accommodative response showed, in general, an increase with accommodative response amplitude up to an amplitude of about 80% of the maximum accommodative response and then decreased with further increasing accommodative amplitudes and stimulus amplitudes (Fig. 4A).
Fig. 4.
Time constants for (A) accommodation and (B) disaccommodation as a function of the maximum accommodative amplitude in all EW-stimulated experiments. The solid symbols represent submaximal up to maximal stimulations, the open symbols represent supramaximal stimulations. For the accommodative phase, the time constant increased up to about 80% of the accommodative amplitude and then decreased again. The disaccommodative phase showed no clear trend regarding the amplitude of accommodation.
Time constants of the EW-stimulated disaccommodative response showed no clear relationship with response amplitude or stimulus amplitude and were on average smaller than the time constants of the accommodative responses. The time constants for supramaximal stimuli were on average higher than for the submaximal stimuli (Fig. 4B).
4. Discussion
The monkeys used were 18.6-, 15.3- and 14.6-years-old. At this age, the monkeys have reduced EW-stimulated accommodative amplitudes although still a large enough range to allow meaningful measurements. The mean maximum accommodation amplitudes measured in the previous study in four eyes of two younger, adolescent monkeys aged 9 and 8 years, were 13.96 ± 2.44 D for the carbachol stimulation, 13.11 ± 2.16 D for the Hartinger measurements and 14.30 ± 3.19 D for the photorefraction measurement (Vilupuru and Glasser, 2002). The monkeys in the present study have a mean accommodative amplitude of about 50% of adolescent monkeys used previously (Table 1).
In the present study, as in the prior monkey study (Vilupuru and Glasser, 2002), a single tonic step-stimulus was delivered to the EW nucleus of anesthetized monkeys to produce a step-accommodative response. Previous studies have shown that intravenously infused anesthesia (Ostrin et al., 2006; Ostrin and Glasser, 2005, 2007a,b; Vilupuru and Glasser, 2005) does not affect the neuromuscular response or accommodative dynamics relative to other anesthetics used previously (Vilupuru and Glasser, 2002). No significant differences in accommodative amplitude and dynamics were found for different anesthetic agents. (Vilupuru and Glasser, 2002; Vilupuru and Glasser, 2005). The step-stimulus is not intended to mimic the behaviorally elicited neuronal input to the EW nucleus (Gamlin et al., 1994; Judge and Cumming, 1986; Schor and Bharadwaj, 2005) or visual stimulus driven accommodation as occurs in conscious subjects. Since the monkeys are anesthetized, the accommodative response achieved is open-loop accommodation without the influence of visual stimulus driven feedback and the step-stimulus is simply used to produce an increase in response amplitude with an increase in stimulus amplitude. In anesthetized adolescent monkeys, a step-stimulus to the EW nucleus produced an increasing response amplitude with a proportionally increasing response peak velocity (Vilupuru and Glasser, 2002). This systematic linear main sequence relationship potentially provides a simple metric to understand the dynamic performance of EW-stimulated accommodation in anesthetized monkeys. The intention of the current study was to perform identical experiments in older monkeys to understand if it may be possible to use this simple metric to detect age changes in the accommodative dynamics that may be an indicator of age-related biomechanical changes in the accommodative anatomical structures (the plant) that lead to presbyopia.
Differences in the results between studies on conscious human subjects and open-loop accommodation in anesthetized EW-stimulated monkeys are expected. In conscious humans, the visual feedback from blur, disparity and contrast cues may influence accommodative dynamics. Accommodative dynamics in EW-stimulated monkeys simply shows the mechanical characteristics of the accommodative plant in response to the step-stimulus delivered to the EW nucleus in the brain.
The results from the present study suggest that the dynamics of EW-stimulated accommodative responses from submaximal to maximal stimulus amplitudes are not significantly different in adolescent and older monkeys. When compared to the prior results from adolescent monkeys (Vilupuru and Glasser, 2002), the mean regression lines of the accommodation and disaccommodation vs. peak velocity relationship in the older monkeys have a slightly steeper slope but are still within the 95% confidence interval indicating there is no significant difference between the data from the adolescent and older monkeys (Fig. 2A, B). It is of particular interest to note that the disaccommodative main sequence relationship is unchanged with age in the adolescent and older monkeys. Disaccommodation in anesthetized monkeys is a purely passive relaxation of accommodation to return the eye to the unaccommodated state when the stimulus terminates. This indicates that in the absence of any neuronal control of disaccommodation, at least as assessed by the methods use in this study, the plant is able to return the eye to the unaccommodated state as rapidly in the older eye as in the younger eye. It remains possible that with increasing age beyond 18 years, age changes in the dynamics may become evident using this methodology.
Supramaximal stimulus amplitudes used in this study in older monkeys and in another study in adolescent monkeys (Ostrin and Glasser, 2005) showed an increased peak velocity over that achieved with the maximal step-stimulus amplitudes. In the older monkeys, peak velocity of accommodation continues to increase with supramaximal stimulus amplitudes, ultimately approaching the maximum peak velocity of accommodation of about 40 D/s attained in the adolescent monkeys (Vilupuru and Glasser, 2002), while the peak velocity of disaccommodation does not. The saturation in peak velocity of disaccommodation which was in all cases substantially lower than the 120 D/s achieved in the adolescent monkeys (Vilupuru and Glasser, 2002) is anticipated, as the peak velocity of disaccommodation in anesthetized monkeys is purely dependent on the passive characteristics of the accommodative plant and the disaccommodative amplitude. Increasing supra-maximal stimulus amplitudes produce no further increase in accommodative amplitude and therefore no further increase in disaccommodative amplitude and therefore no further increase in disaccommodative peak velocity. In experiment #96 (2), 10 supramaximal EW-stimulus amplitudes were used. This showed that the peak velocity of accommodation approached the peak velocity of disaccommodation but did not reach a higher value with higher supramaximal stimulus amplitudes (Fig. 3). The same tendency was found in the other experiments. The ultimate saturation of peak velocity of the accommodative phase with supramaximal stimulus amplitudes may be related to elastic properties of the lens or the maximum attainable rate of ciliary muscle contraction.
Several possible conclusions are apparent from this study. Based on the results from the present study, the main sequence ratio for accommodation and disaccommodation was not altered by aging up to approximately 18 years of age in anesthetized EW-stimulated rhesus monkeys for submaximal stimulus amplitudes. This means that the peak velocity of accommodation and disaccommodation in anesthetized monkeys of different ages is dictated by the response amplitude per se and not by the proportion of the response amplitude relative to the maximum amplitude available. The similarity in the main sequence for disaccommodation between adolescent and older monkeys may suggest that no age changes occur in the biomechanics of the choroid and posterior zonules which pull the ciliary body, anterior zonules and lens into the unaccommodated form. The fact that the peak velocity of accommodation can increase beyond that attained with the maximum accommodative amplitude means that the lens and ciliary muscle, even in the older monkeys, is capable of higher velocities than is achieved over the linear range in a step-stimulus main sequence relationship. These results suggesting a similar main sequence relationship between adolescent and older monkeys are perhaps surprising considering the wealth of documented age changes in the accommodative plant in monkeys that would likely impact the biomechanics of accommodation (Lütjen-Drecoll et al., 1988; Tamm et al., 1991, 1992).
In the previous study in adolescent monkeys, the time constants for the EW-stimulated accommodative response increased up to a peak at about 70% of maximum accommodative amplitude and then decreased again with higher stimulus amplitudes (Vilupuru and Glasser, 2002). The time constants of accommodation in the present study show a similar pattern with a maximum at about 80% of maximum accommodative amplitude. As in the previous study in younger adolescent monkeys, the time constants for disaccommodation are smaller than time constants for accommodation and show no consistent relationship to the accommodative amplitude. However, as mentioned previously (Vilupuru and Glasser, 2002), the functions used to fit the accommodative responses were not a pure exponential, but rather an exponential combined with a polynomial. The polynomial was required to ensure the functions fit the responses since the responses were not always of a pure exponential nature. These time constants are, therefore, not “pure” time constants, but are diluted by the influence of the polynomial and so the conclusions that can be reached from these time constants are limited. They are nevertheless presented here for completeness and for comparison with prior studies.
Earlier studies of dynamic accommodation in humans suggested a linear increase in peak velocity of accommodation with accommodative amplitude (Campbell and Westheimer, 1960; Ciuffreda and Kruger, 1988; Hung and Ciuffreda, 1988). In these studies, only comparatively low amplitudes up to 3 D were measured. In a more recent study on young human subjects, accommodative amplitude and peak velocity were found to saturate at response amplitudes greater than about 3 D while for disaccommodation, a linear increase in peak velocity was found (Kasthurirangan et al., 2003). The time constants showed a linear increase for accommodation and saturation at response amplitudes over 2 D for disaccommodation. A step-stimulus model of human accommodation incorporating age changes in the biomechanics of the accommodative plant predicts a decreasing slope of the main sequence relationship with increasing age. According to several studies, the first order characteristics of accommodation over the linear range remain constant with age in conscious human subjects (Heron et al., 2001, 2002; Mordi and Ciuffreda, 2004). As an explanation, a pulse-step model of neuronal control of accommodation has been suggested (Schor and Bharadwaj, 2005). A recent study on age-related changes in accommodative dynamics in humans (Kasthurirangan and Glasser, 2006) however, found a slower increase of peak velocity of accommodation and saturation at lower accommodative response levels in older subjects compared to young subjects. No such change was found for the peak velocities of disaccommodation. This is consistent with a single time constant model for disaccommodation, where the time constant does not change with stimulus amplitude. On the other hand, the results of the present study and the previously published findings in adolescent monkeys (Vilupuru and Glasser, 2002) show no apparent age-related changes in the dynamics of accommodation and disaccommodation in response to a step-stimulus. The differences in the results between conscious humans and anesthetized monkeys could be related to the absence of neural regulative mechanisms and to the nature of the step-stimulus used in the anesthetized monkey experiments as opposed to a pulse-step signal which may occur in conscious humans. It is, however, even more likely that the fitting of an exponential function may be inadequate to fully characterize the dynamics of the accommodative response in anesthetized monkeys. The peak velocities of the accommodative and disaccommodative responses in EW-stimulated anesthetized monkeys are higher than peak velocities measured in conscious humans and the fitting of an exponential function may well obscure subtle differences between younger and older monkeys. In fact, preliminary results from more recent experiments (Baumeister, unpublished data) indicate that with a higher sampling frequency and peak velocities derived from the actual data there may be age-related differences in the main sequence in anesthetized monkeys.
There are several possible limitations of the current study to detect age dependent changes in accommodative dynamics in anesthetized monkeys. Here, in anesthetized monkeys, a step-stimulus was used rather than a pulse-step or modulating stimulus frequency (Gamlin, 1999; Schor and Bharadwaj, 2005). A step-stimulus drives the velocity in proportion to the amplitude of the step and is appropriate to simply understand the relationship between peak velocity and amplitude and how this may change with age. However, it is possible that the use of a pulse-step-stimulus on both adolescent and older monkeys may be a more sensitive or appropriate stimulus to use to detect age dependent changes. Experiments in alert behaving primates have identified that accommodative response amplitude is dependent on the frequency of firing of the EW neurons (Gamlin, 1999; Gamlin et al., 1994; Judge and Cumming, 1986). It may also be that varying the accommodative response amplitude by modulating stimulus pulse-train frequency rather than step-stimulus amplitude (Crawford et al., 1989) is a more appropriate method to characterize accommodative dynamics to probe the accommodative plant for age changes in dynamics. However, stimulus frequencies below 60 Hz delivered to the EW nucleus result in oscillations of the ciliary muscle and the lens corresponding to the stimulus frequency and these could affect the dynamic measurements (Glasser, unpublished observation). These responses may therefore, be unsuitable for characterizing accommodative dynamics. As mentioned above, it is also possible that determining main sequence relationships by fitting functions to the dynamic accommodative responses, as done here, misses subtle changes in the dynamics that may be available if velocity and acceleration information can be calculated directly from the responses using a three-point central difference algorithm. Here, for comparison with the results of the previous study in adolescent monkeys (Vilupuru and Glasser, 2002) the same methodology was used. Higher frequency recording methods and more sophisticated analysis techniques will allow velocity and acceleration information to be obtained without the need for function fitting. These approaches may be more sensitive for detecting subtle age dependent changes in accommodative dynamics.
Acknowledgments
This study was funded by NEI grant #1 RO1 EY014651 to AG and DFG grant BA 3443/1-1 to MB.
References
- Beers AP, van der Heijde GL. Age-related changes in the accommodation mechanism. Optom Vis Sci. 1996;73:235–242. doi: 10.1097/00006324-199604000-00004. [DOI] [PubMed] [Google Scholar]
- Bharadwaj SR, Schor CM. Acceleration characteristics of human ocular accommodation. Vision Res. 2005;45:17–28. doi: 10.1016/j.visres.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Campbell FW, Westheimer G. Dynamics of accommodation responses of the human eye. J Physiol. 1960;151:285–295. doi: 10.1113/jphysiol.1960.sp006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffreda KJ, Kruger PB. Dynamics of human voluntary accommodation. Am J Optom Physiol Opt. 1988;65:365–370. doi: 10.1097/00006324-198805000-00010. [DOI] [PubMed] [Google Scholar]
- Crawford K, et al. Reproducible stimulation of ciliary muscle contraction in the cynomolgus monkey via a permanent indwelling midbrain electrode. Brain Res. 1989;503:265–272. doi: 10.1016/0006-8993(89)91673-9. [DOI] [PubMed] [Google Scholar]
- Duane A. Normal values of the accommodation at all ages. J Am Med Assoc. 1912;59:1010–1012. [Google Scholar]
- Gamlin PD, Reiner A. The Edinger–Westphal nucleus: sources of input influencing accommodation, pupilloconstriction, and choroidal blood flow. J Comp Neurol. 1991;306:425. doi: 10.1002/cne.903060307. [DOI] [PubMed] [Google Scholar]
- Gamlin PD, et al. Behavior of identified Edinger–Westphal neurons during ocular accommodation. J Neurophysiol. 1994;72:2368. doi: 10.1152/jn.1994.72.5.2368. [DOI] [PubMed] [Google Scholar]
- Gamlin PD. Subcortical neural circuits for ocular accommodation and vergence in primates. Ophthalmic Physiol Opt. 1999;19:81. doi: 10.1046/j.1475-1313.1999.00434.x. [DOI] [PubMed] [Google Scholar]
- Glasser A, Campbell MC. Presbyopia and the optical changes in the human crystalline lens with age. Vision Res. 1998;38:209–229. doi: 10.1016/s0042-6989(97)00102-8. [DOI] [PubMed] [Google Scholar]
- Glasser A, Campbell MC. Biometric, optical and physical changes in the isolated human crystalline lens with age in relation to presbyopia. Vision Res. 1999;39:1991–2015. doi: 10.1016/s0042-6989(98)00283-1. [DOI] [PubMed] [Google Scholar]
- Glasser A, Kaufman PL. The mechanism of accommodation in primates. Ophthalmology. 1999;106:863. doi: 10.1016/S0161-6420(99)00502-3. [DOI] [PubMed] [Google Scholar]
- Glasser A, et al. Accommodative changes in lens diameter in rhesus monkeys. Investig Ophthalmol Vis Sci. 2006;47:278. doi: 10.1167/iovs.05-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron G, et al. Accommodation responses and ageing. Investig Ophthalmol Vis Sci. 1999;40:2872. [PubMed] [Google Scholar]
- Heron G, et al. Dynamics of the accommodation response to abrupt changes in target vergence as a function of age. Vision Res. 2001;41:507. doi: 10.1016/s0042-6989(00)00282-0. [DOI] [PubMed] [Google Scholar]
- Heron G, et al. Accommodation dynamics as a function of age. Ophthalmic Physiol Opt. 2002;22:389. doi: 10.1046/j.1475-1313.2002.00070.x. [DOI] [PubMed] [Google Scholar]
- Heys KR, et al. Massive increase in the stiffness of the human lens nucleus with age: the basis for presbyopia? Mol Vis. 2004;10:956. [PubMed] [Google Scholar]
- Hung GK, Ciuffreda KJ. Dual-mode behaviour in the human accommodation system. Ophthalmic Physiol Opt. 1988;8:327. [PubMed] [Google Scholar]
- Judge SJ, Cumming BG. Neurons in the monkey midbrain with activity related to vergence eye movement and accommodation. J Neurophysiol. 1986;55:915. doi: 10.1152/jn.1986.55.5.915. [DOI] [PubMed] [Google Scholar]
- Kasthurirangan S, et al. Amplitude dependent accommodative dynamics in humans. Vision Res. 2003;43:2945. doi: 10.1016/j.visres.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Kasthurirangan S, Glasser A. Influence of amplitude and starting point on accommodative dynamics in humans. Investig Ophthalmol Vis Sci. 2005;46:3463. doi: 10.1167/iovs.04-1408. [DOI] [PubMed] [Google Scholar]
- Kasthurirangan S, Glasser A. Age related changes in accommodative dynamics in humans. Vision Res. 2006;46:1507. doi: 10.1016/j.visres.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Kaufman PL, Lütjen-Drecoll E. Total iridectomy in the primate in vivo: surgical technique and postoperative anatomy. Invest Ophthalmol. 1975;14:766–771. [PubMed] [Google Scholar]
- Keeney AH, et al. Dictionary of Ophthalmic Optics. Butterworth-Heinemann; Newton, MA: 1995. p. 1. [Google Scholar]
- Koretz JF, et al. Slit-lamp studies of the rhesus monkey eye: II. Changes in crystalline lens shape, thickness and position during accommodation and aging. Exp Eye Res. 1987;45:317–326. doi: 10.1016/s0014-4835(87)80153-7. [DOI] [PubMed] [Google Scholar]
- Krag S, et al. Biomechanical characteristics of the human anterior lens capsule in relation to age. Investig Ophthalmol Vis Sci. 1997;38:357. [PubMed] [Google Scholar]
- Krag S, Andreassen TT. Mechanical properties of the human lens capsule. Prog Retin Eye Res. 2003;22:749. doi: 10.1016/s1350-9462(03)00063-6. [DOI] [PubMed] [Google Scholar]
- Lütjen-Drecoll E, et al. Age-related loss of morphologic responses to pilocarpine in rhesus monkey ciliary muscle. Arch Ophthalmol. 1988;106:1591–1598. doi: 10.1001/archopht.1988.01060140759051. [DOI] [PubMed] [Google Scholar]
- Mordi JA, Ciuffreda KJ. Dynamic aspects of accommodation: age and presbyopia. Vision Res. 2004;44:591. doi: 10.1016/j.visres.2003.07.014. [DOI] [PubMed] [Google Scholar]
- Ostrin L, et al. Simultaneous measurements of refraction and A-scan biometry during accommodation in humans. Optom Vis Sci. 2006;83:657. doi: 10.1097/01.opx.0000232810.61191.02. [DOI] [PubMed] [Google Scholar]
- Ostrin LA, Glasser A. The effects of phenylephrine on pupil diameter and accommodation in rhesus monkeys. Investig Ophthalmol Vis Sci. 2004;45:215. doi: 10.1167/iovs.03-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrin LA, Glasser A. Comparisons between pharmacologically and Edinger–Westphal-stimulated accommodation in rhesus monkeys. Investig Ophthalmol Vis Sci. 2005;46:609. doi: 10.1167/iovs.04-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrin LA, Glasser A. Effects of pharmacologically manipulated amplitude and starting point on Edinger–Westphal-stimulated accommodative dynamics in rhesus monkeys. Investig Ophthalmol Vis Sci. 2007a;48:313–320. doi: 10.1167/iovs.06-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrin LA, Glasser A. Edinger–Westphal and pharmacologically stimulated accommodative refractive changes and lens and ciliary process movements in rhesus monkeys. Exp Eye Res. 2007b;84:302–313. doi: 10.1016/j.exer.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffel F, et al. Infrared photoretinoscope. Appl Opt. 1987;26:1505. doi: 10.1364/AO.26.001505. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, et al. Inter-individual variability in the dynamics of natural accommodation in humans: relation to age and refractive errors. J Physiol. 1993;461:301. doi: 10.1113/jphysiol.1993.sp019515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor CM, Bharadwaj SR. A pulse-step model of accommodation dynamics in the aging eye. Vision Res. 2005;45:1237. doi: 10.1016/j.visres.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Schor CM, Bharadwaj SR. Pulse-stepmodels of control strategies for dynamic ocular accommodation and disaccommodation. Vision Res. 2006;46:242. doi: 10.1016/j.visres.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Sun FC, et al. Changes in accommodation with age: static and dynamic. Am J Optom Physiol Opt. 1988;65:492. doi: 10.1097/00006324-198806000-00009. [DOI] [PubMed] [Google Scholar]
- Tamm E, et al. Posterior attachment of ciliary muscle in young, accommodating old, presbyopic monkeys. Investig Ophthalmol Vis Sci. 1991;32:1678–1692. [PubMed] [Google Scholar]
- Tamm E, et al. Age-related loss of ciliary muscle mobility in the rhesus monkey. Role of the choroid. Arch Ophthalmol. 1992;110:871–876. doi: 10.1001/archopht.1992.01080180143043. [DOI] [PubMed] [Google Scholar]
- Vilupuru AS, Glasser A. Dynamic accommodation in rhesus monkeys. Vision Res. 2002;42:125. doi: 10.1016/s0042-6989(01)00260-7. [DOI] [PubMed] [Google Scholar]
- Vilupuru AS, Glasser A. Dynamic accommodative changes in rhesus monkey eyes assessed with A-scan ultrasound biometry. Optom Vis Sci. 2003;80:383. doi: 10.1097/00006324-200305000-00013. [DOI] [PubMed] [Google Scholar]
- Vilupuru AS, Glasser A. The relationship between refractive and biometric changes during Edinger–Westphal stimulated accommodation in rhesus monkeys. Exp Eye Res. 2005;80:349. doi: 10.1016/j.exer.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilupuru AS, et al. Dynamics of accommodative fatigue in rhesus monkeys and humans. Vision Res. 2005;45:181. doi: 10.1016/j.visres.2004.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]