Abstract
Alcohol-dependent outpatients with persisting insomnia were treated with either gabapentin or trazodone. Patients were assessed at baseline and after 4 to 6 weeks on medication using the Sleep Problems Questionnaire (SPQ). Of 55 cases initially treated, 9% dropped out due to morning drowsiness. Of the remaining 50 cases, 34 were treated with gabapentin (mean dose ± SD = 888 ± 418 mg) at bedtime and 16 were treated with trazodone (105 ± 57 mg) at bedtime. Both groups improved significantly on the SPQ, however, the gabapentin group improved significantly more than the trazodone group. Controlled studies are warranted to replicate these findings.
Keywords: alcoholism, insomnia, drug therapy, gabapentin, trazodone
Introduction
Insomnia is common and may increase the risk of relapse in treated alcoholics, even after controlling for other clinical variables.1 Unfortunately, the abuse potential of commonly used sedative-hypnotic drugs limit their use in this population.2,3 Mood stabilizers and sedating antidepressants have recently emerged as possibly effective medications for treating patients with substance use disorders and comorbid insomnia.3,4
Trazodone is a sedating antidepressant that has been used to treat alcoholism.5 Both depressed6 and healthy adults7 have improved sleep on trazodone, presumably due to its serotonergic activity. Gabapentin, is an antiepileptic drug that has also been used in the treatment of alcohol-related disorders, because of its anticonvulsant, sedative, and anxiolytic effects.4,8 Gabapentin increases brain concentrations of gamma-amino-butyric acid (GABA) and decreases glutamatergic activity.9 For these reasons, we compared trazodone vs. gabapentin to treat insomnia associated with alcohol dependence.
Methods
Of 71 alcohol-dependent outpatients consecutively referred to an addiction psychiatrist for complaints of insomnia, 55 met study criteria and received either gabapentin or trazodone after giving written informed consent. To qualify, patients (1) met DSM-IV criteria for alcohol dependence as determined by the psychiatric evaluation; (2) had insomnia that persisted despite 4 or more weeks of abstinence as verified by breath tests and urine drug screens; (3) did not have insomnia due to substance intoxication or withdrawal, medications, or an unstable mental or medical disorder (other than alcohol dependence); and (4) had normal serum creatinine levels and liver transaminases (because gabapentin is eliminated by the kidney and trazodone is metabolized in the liver). Patients were informed that gabapentin and trazodone were alternative agents for treating insomnia without the worry of dependence associated with other sleeping pills. After discussing the potential side effects and benefits of each medication, the patient and psychiatrist made a collaborative decision about which medication to use, as ordinarily occurs in clinical practice.
Gabapentin-treated patients were started on 300 mg by mouth at bedtime, and increased as needed by 300 mg each night to a maximum of 1800 mg as a single bedtime dose. Trazodone-treated patients were started at 25 mg by mouth at bedtime, and increased as needed by 25 mg every two nights to a maximum of 300 mg as a single bedtime dose. Patients were instructed to take their medication 30–60 minutes before bedtime.
The Sleep Problems Questionnaire (SPQ),10 was used to assess the severity of insomnia at baseline (t1) and 4–6 weeks later at follow-up (t2). The SPQ is a 4-item, self-administered measure of the past 1-month. Initial insomnia, middle insomnia, terminal insomnia, and feeling tired and worn out upon awakening are each assessed by 1 item, which have scores ranging from 0 (no disturbance) to 5 (daily disturbance) for a maximum total score of 20. A previous report indicated that the SPQ was sensitive to improvement in sleep over a 4–6 week period in gabapentin-treated alcoholic outpatients.4
Results
Of the 55 patients who consented to take medication, 2 (11%) of 18 patients stopped trazodone and 3 (8%) of 37 patients stopped gabapentin after the first dose due to excessive sleepiness or drowsiness the next day. These five patients were not included in any further analyses. Of the remaining 50 patients, 16 received trazodone, titrated to a mean ± SD dose of 105 ± 57 mg at bedtime (range 50 to 300 mg), and 34 received gabapentin, titrated to a mean dose of 888 ± 418 mg at bedtime (range 300 to 1800 mg). The two medication groups differed in sample size, because the choice of medication for each patient resulted from a collaborative patient-physician discussion rather than random assignment.
The trazodone and gabapentin groups did not differ significantly in terms of age (mean ± SD = 44 ± 14 years), gender (62% female), race (84% Caucasian, 12% African-American, 4% other), and employment (66% employed), although there was a lower percentage of men in the trazodone group vs. the gabapentin group (25% vs. 44%; χ2=1.69, df=1, p=0.19). Sixty-four percent of the sample had a diagnosis of nicotine dependence and 66% had at least one other substance dependence disorder without significant differences between groups. Comorbid but stable mood or anxiety disorders were present in 14 (88%) of the trazodone-treated patients and 29 (85%) of the gabapentin-treated patients.
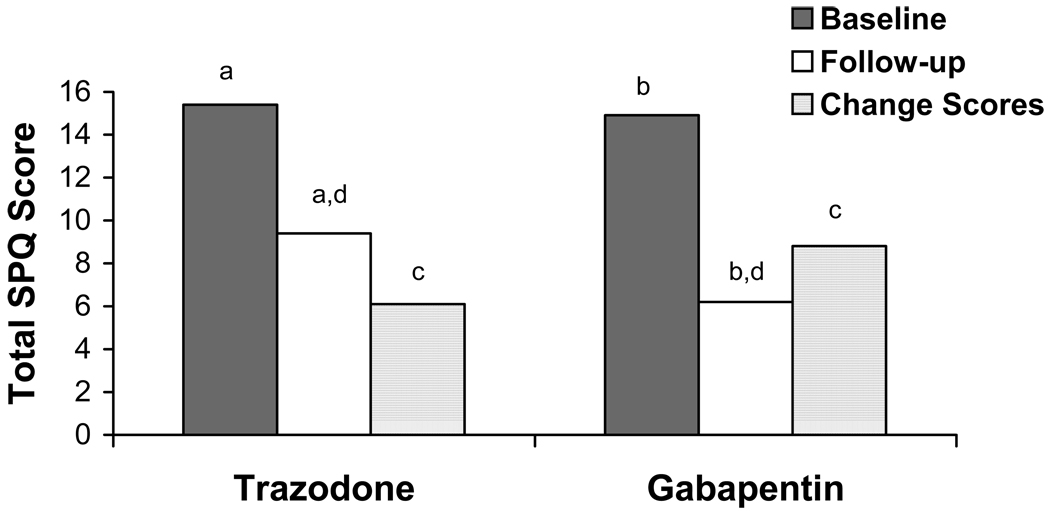
As shown in Figure 1, total SPQ scores did not differ between the trazodone and gabapentin groups at baseline (15.4 ± 3.6 and 14.9 ± 3.8, respectively). Both groups showed significant improvement in sleep from baseline to follow-up (for trazodone group: paired samples t test = 7.1, df=15, p<l0.001; for gabapentin group: paired samples t test = 12.7, df=33, p<0.001). When comparing total SPQ change scores, however, the gabapentin group (8.8 ± 4.0) improved significantly more than the trazodone group (6.1 ± 3.4; t=−2.35, df=48, p=0.023). A linear regression analysis demonstrated that medication group significantly predicted overall SPQ change scores after controlling for age, gender, and pre-treatment total sleep score.
Figure 1.
Sleep problems questionnaire (SPQ) scores before and after medication treatment with trazodone vs. gabapentin. Matched letter pairs indicate significant (p<0.05) differences between baseline and follow-up for the trazodone (a) and gabapentin (b) groups, and between medication groups for change scores (c) and follow-up scores (d).
The SPQ individual item scores were dichotomized because of skewed distributions. Patients scoring from 3 to 5 on an individual item were classified as having the symptom because it occurred more than 7 days in the past 31 days, whereas patients scoring from 0 to 2 were classified as not having the symptom. Results are shown in Table 1. Patients treated with trazodone vs. gabapentin were more likely to endorse initial insomnia at both baseline (100% vs. 76%, p=0.043) and follow-up (38% vs. 9%, p=0.022). The two groups did not differ at baseline in terms of middle insomnia (84%), terminal insomnia (70%), or feeling tired and worn out upon awakening (86%). At follow-up, however, the trazodone group was more likely than the gabapentin group to wake up “feeling tired and worn out” (69% vs. 12%; χ2=16.82, Fisher’s exact test: p<0.001).
Table 1.
Sleep characteristics at baseline (t1) and follow-up (t2) by medication group.
| Sleep Variablesa | Medication Group | |
|---|---|---|
| trazodone | gabapentin | |
| (N=16) | (N=34) | |
| Initial insomnia at t1 (%) | 100 | 76.5* |
| Initial insomnia at t2 (%) | 37.5 | 8.8* |
| Middle insomnia at t1 (%) | 81.3 | 85.3 |
| Middle insomnia at t2 (%) | 37.5 | 23.5 |
| Terminal insomnia at t1 (%) | 56.3 | 76.5 |
| Terminal insomnia at t2 (%) | 25.0 | 14.7 |
| Tired & worn out upon awakening at t1 (%) | 87.5 | 85.3 |
| Tired & worn out upon awakening at t2 (%) | 68.8 | 11.8*** |
p<0.05
p< 0.01
p=0.001
Variables were analyzed by Fisher’s exact test. All tests were two-tailed.
Two patients from each medication group admitted to drinking 1 or more drinks for at least 1 day during the study period.
Discussion
After at least 4 weeks of abstinence, alcoholic patients with persistent insomnia reported significant sleep improvement during treatment with either gabapentin or trazodone. Although the overall sleep of each medication group improved significantly over time, patients who received gabapentin improved significantly more than did patients who received trazodone. At follow-up, gabapentin-treated patients were less likely than trazodone-treated patients to have initial insomnia and to awaken in the morning feeling tired and worn out. Both gabapentin and trazodone were well tolerated as indicated by low dropout rates that did not differ significantly between the two groups. All dropouts occurred after the first dose of medication because of morning drowsiness.
These findings must be viewed as preliminary. Patients were not randomized, no placebo control group was used, and neither patients nor psychiatrist was blinded to medication. Other limitations include the small sample size, brief follow-up period, and lack of polysomnographic measures to complement subjective reports of insomnia and to rule out other sleep disorders. It is also not known whether the dosages of the two medications were equivalent. Finally, the study was not able to determine the effect of medication on relapse rates.
Despite its limitations, this is the first study to compare gabapentin and trazodone for treating insomnia in alcohol-dependent patients. Double-blind, randomized controlled trials are indicated to investigate the relative efficacy of these two agents vs. placebo.
Acknowledgments
Supported in part by Grant K24 AA 00304 from the National Institute on Alcohol Abuse and Alcoholism to Dr. Brower.
References
- 1.Brower KJ. Insomnia, alcoholism and relapse. Sleep Med. Rev. doi: 10.1016/s1087-0792(03)90005-0. in press. [DOI] [PubMed] [Google Scholar]
- 2.Madrak LN, Rosenberg M. Zolpidem abuse. Am. J. Psychiatry. 2001;158:1330–1331. doi: 10.1176/appi.ajp.158.8.1330-a. [DOI] [PubMed] [Google Scholar]
- 3.Longo LP, Johnson B. Treatment of insomnia in substance abusing patients. Psychiatr. Ann. 1998;28:154–159. [Google Scholar]
- 4.Karam-Hage M, Brower KJ. Gabapentin treatment for insomnia associated with alcohol dependence. Am. J. Psychiatry. 2000;157:151. doi: 10.1176/ajp.157.1.151. [DOI] [PubMed] [Google Scholar]
- 5.Janiri L, Hadjichristos A, Buonanno A, Rago R, Mannelli P, de Risio S. Adjuvant trazodone in the treatment of alcoholism: an open study. Alcohol Alcohol. 1998;33:362–365. doi: 10.1093/oxfordjournals.alcalc.a008405. [DOI] [PubMed] [Google Scholar]
- 6.Mashiko H, Niwa S, Kumashiro H, et al. Effect of trazodone in a single dose before bedtime for sleep disorders accompanied by a depressive state: dose-finding study with no concomitant use of hypnotic agent. Psychiatry Clin. Neurosci. 1999;53:193–194. doi: 10.1046/j.1440-1819.1999.00532.x. [DOI] [PubMed] [Google Scholar]
- 7.Yamadera H, Suzuki H, Nakamura S, Endo S. Effects of trazodone on polysomnography, blood concentration and core body temperature in healthy volunteers. Psychiatry Clin. Neurosci. 1999;53:189–191. doi: 10.1046/j.1440-1819.1999.00531.x. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet U, Banger M, Leweke FM, Maschke M, Kowalski T, Gastpar M. Treatment of alcohol withdrawal syndrome with gabapentin. Pharmacopsychiatry. 1999;32:107–109. doi: 10.1055/s-2007-979203. [DOI] [PubMed] [Google Scholar]
- 9.Taylor CP. Gabapentin: mechanisms of action. In: Levy RH, Mattson RH, Meldrum BS, Perucca E, editors. Antiepileptic Drugs. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2002. pp. 321–334. [Google Scholar]
- 10.Jenkins CD, Stanton BA, Niemcryk SJ, Rose RM. A scale for the estimation of sleep problems in clinical research. J. Clin. Epidemiol. 1988;41:313–321. doi: 10.1016/0895-4356(88)90138-2. [DOI] [PubMed] [Google Scholar]