Abstract
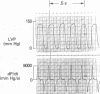
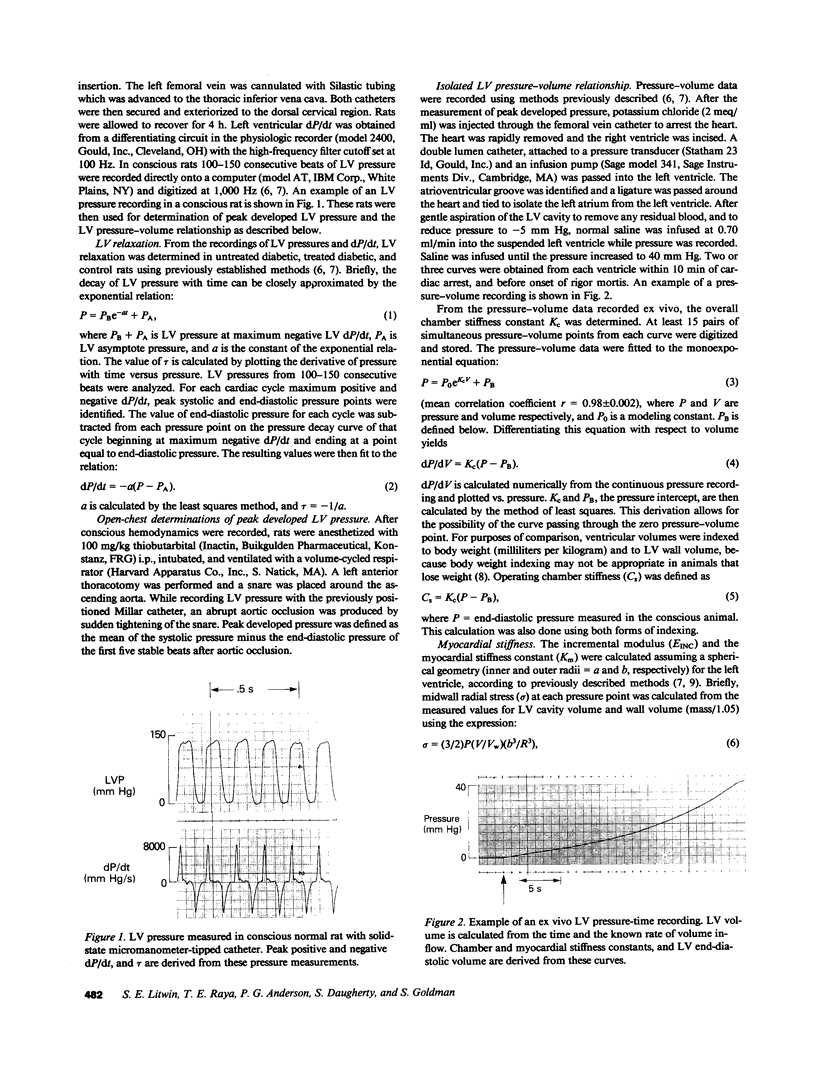
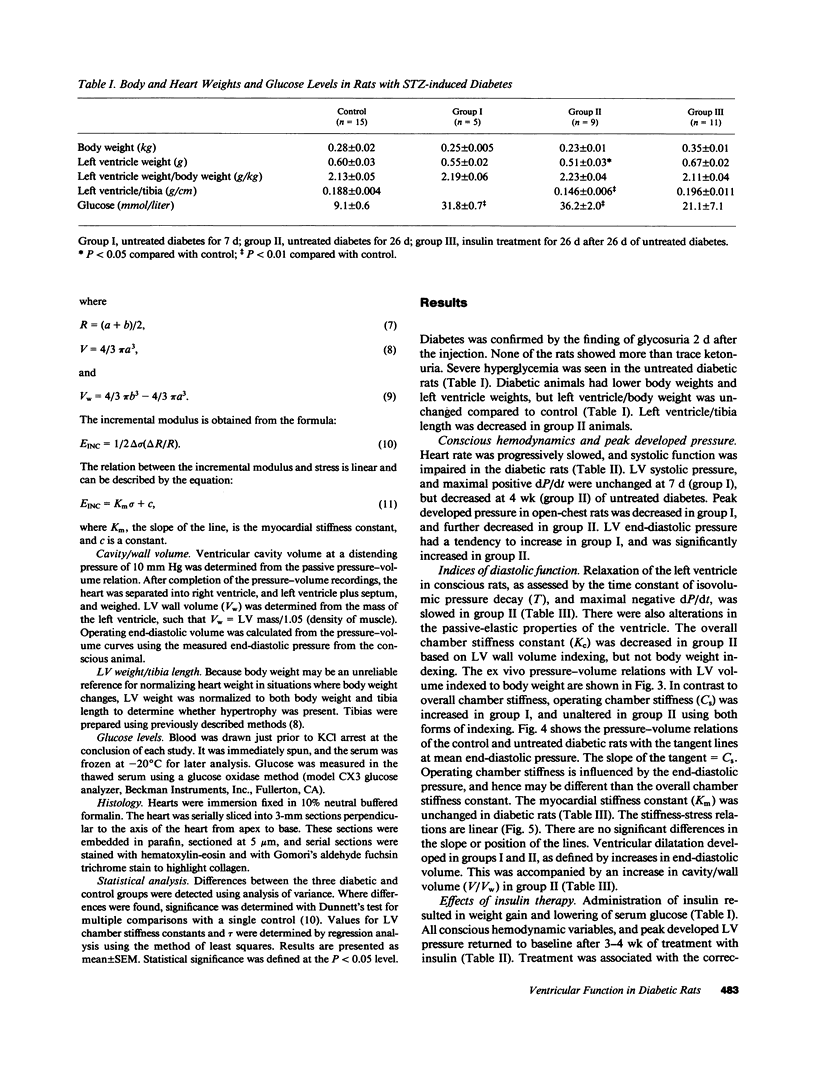
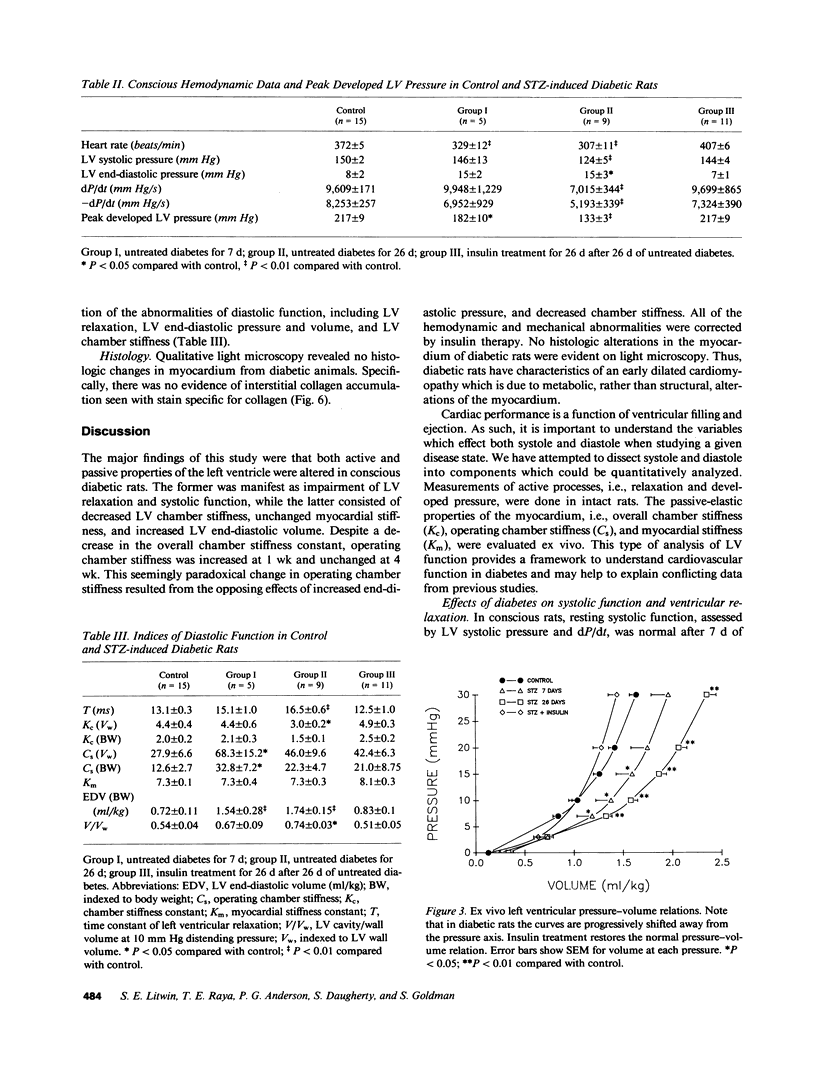
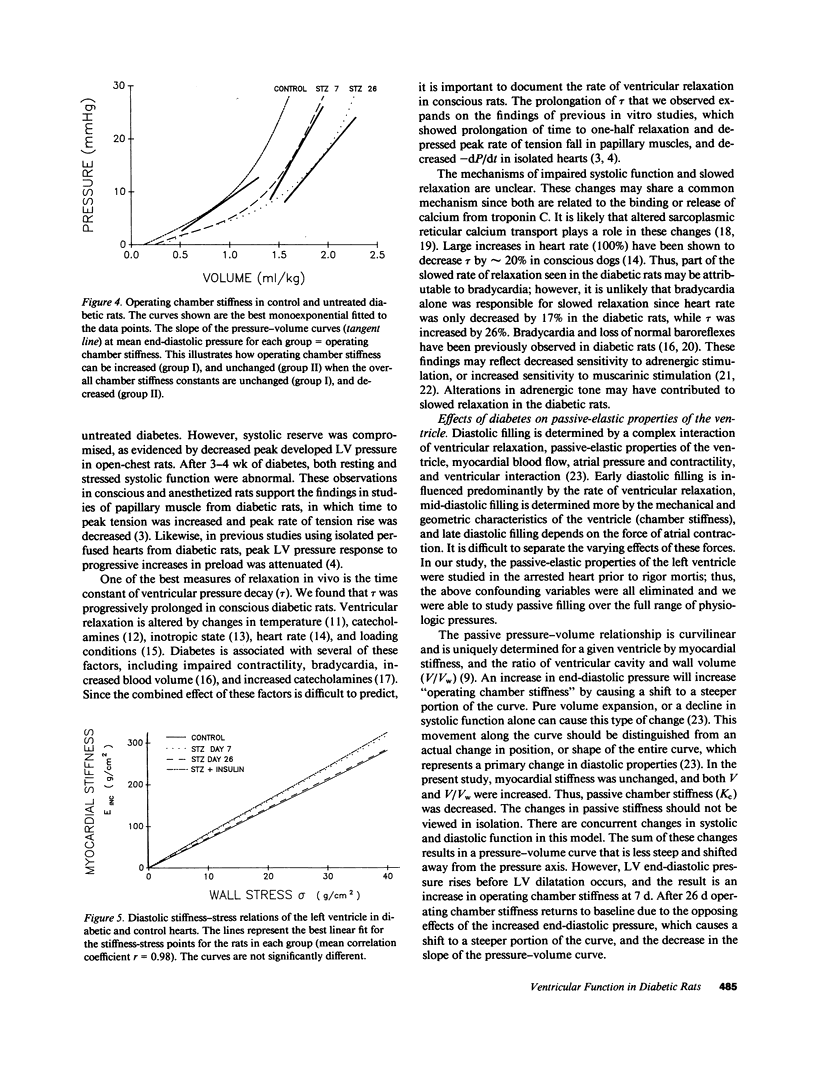
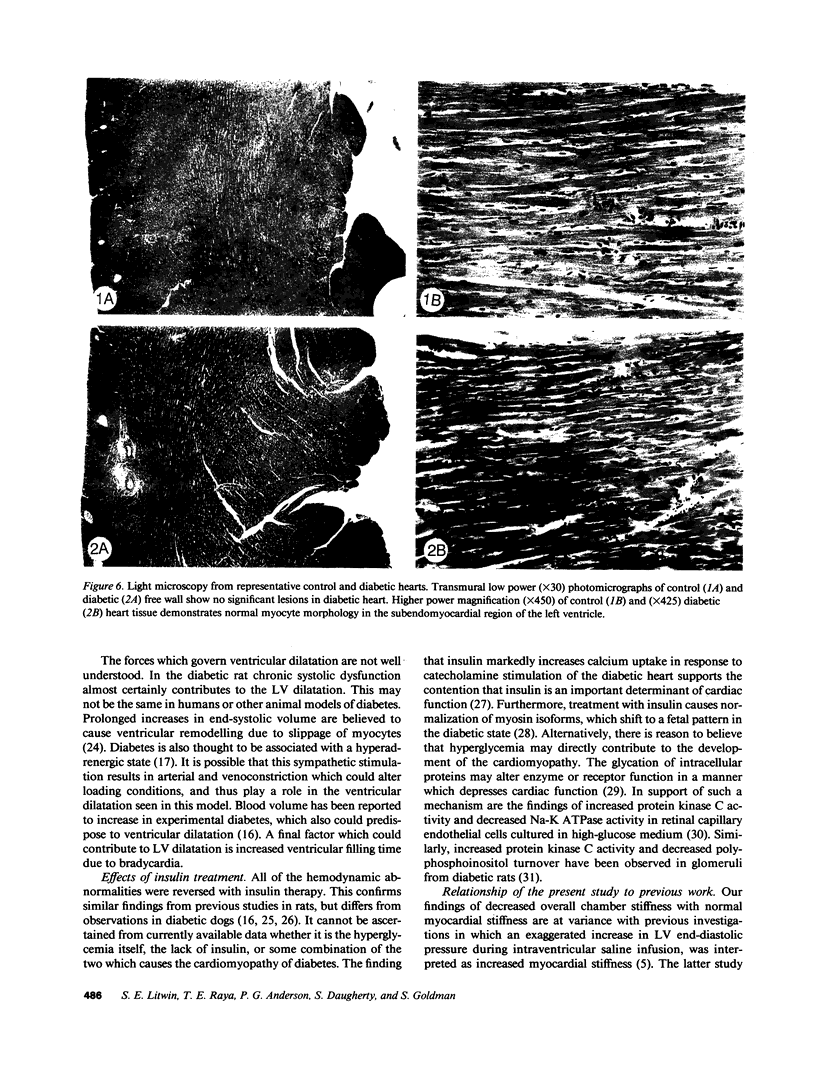
To provide an integrated assessment of changes in systolic and diastolic function in diabetic rats, we measured conscious hemodynamics and performed ex vivo analysis of left ventricular passive-elastic properties. Rats given streptozotocin (STZ) 65 mg/kg i.v. (n = 14) were compared with untreated age-matched controls (n = 15) and rats treated with insulin after administration of STZ (n = 11). After 7 d, diabetic rats exhibited decreases in heart rate and peak developed left ventricular (LV) pressure during aortic occlusion. After 26 d of diabetes there were significant decreases in resting LV systolic pressure, developed pressure, and maximal +dP/dt, whereas LV end-diastolic pressure increased and the time constant of LV relaxation was prolonged. The passive LV pressure-volume relationship was progressively shifted away from the pressure axis, and the overall chamber stiffness constant was decreased. However, "operating chamber stiffness" calculated at end-diastolic pressure was increased at 7 d, and unchanged at 26 d. LV cavity/wall volume and end-diastolic volume were increased after 26 d of diabetes. Myocardial stiffness was unchanged at both time intervals. All of the above abnormalities were reversed by the administration of insulin. We conclude that the hemodynamic and passive-elastic changes that occur in diabetic rats represent an early dilated cardiomyopathy which is reversible with insulin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronstam R. S., Carrier G. O. Insulin prevention of altered muscarinic receptor-G protein coupling in diabetic rat atria. Diabetes. 1989 Dec;38(12):1611–1616. doi: 10.2337/diab.38.12.1611. [DOI] [PubMed] [Google Scholar]
- Atkins F. L., Dowell R. T., Love S. beta-Adrenergic receptors, adenylate cyclase activity, and cardiac dysfunction in the diabetic rat. J Cardiovasc Pharmacol. 1985 Jan-Feb;7(1):66–70. doi: 10.1097/00005344-198501000-00011. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Vlassara H., Cerami A. Nonenzymatic glycosylation and the pathogenesis of diabetic complications. Ann Intern Med. 1984 Oct;101(4):527–537. doi: 10.7326/0003-4819-101-4-527. [DOI] [PubMed] [Google Scholar]
- Carbonell L. F., Salom M. G., Garcia-Estañ J., Salazar F. J., Ubeda M., Quesada T. Hemodynamic alterations in chronically conscious unrestrained diabetic rats. Am J Physiol. 1987 May;252(5 Pt 2):H900–H905. doi: 10.1152/ajpheart.1987.252.5.H900. [DOI] [PubMed] [Google Scholar]
- Chang K. S., Lund D. D. Alterations in the baroreceptor reflex control of heart rate in streptozotocin diabetic rats. J Mol Cell Cardiol. 1986 Jun;18(6):617–624. doi: 10.1016/s0022-2828(86)80969-5. [DOI] [PubMed] [Google Scholar]
- Christensen N. J. Plasma norepinephrine and epinephrine in untreated diabetics, during fasting and after insulin administration. Diabetes. 1974 Jan;23(1):1–8. doi: 10.2337/diab.23.1.1. [DOI] [PubMed] [Google Scholar]
- Courtois M. R., Kurnik P. B., Ludbrook P. A. Sensitivity of isovolumic relaxation to hypothermia during myocardial infarction. J Am Coll Cardiol. 1988 Jan;11(1):201–206. doi: 10.1016/0735-1097(88)90190-8. [DOI] [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Protein kinase C is activated in glomeruli from streptozotocin diabetic rats. Possible mediation by glucose. J Clin Invest. 1989 May;83(5):1667–1675. doi: 10.1172/JCI114066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillmann W. H. Diabetes mellitus induces changes in cardiac myosin of the rat. Diabetes. 1980 Jul;29(7):579–582. doi: 10.2337/diab.29.7.579. [DOI] [PubMed] [Google Scholar]
- Fein F. S., Kornstein L. B., Strobeck J. E., Capasso J. M., Sonnenblick E. H. Altered myocardial mechanics in diabetic rats. Circ Res. 1980 Dec;47(6):922–933. doi: 10.1161/01.res.47.6.922. [DOI] [PubMed] [Google Scholar]
- Fein F. S., Strobeck J. E., Malhotra A., Scheuer J., Sonnenblick E. H. Reversibility of diabetic cardiomyopathy with insulin in rats. Circ Res. 1981 Dec;49(6):1251–1261. doi: 10.1161/01.res.49.6.1251. [DOI] [PubMed] [Google Scholar]
- Freeman G. L., Little W. C., O'Rourke R. A. Influence of heart rate on left ventricular performance in conscious dogs. Circ Res. 1987 Sep;61(3):455–464. doi: 10.1161/01.res.61.3.455. [DOI] [PubMed] [Google Scholar]
- Ganguly P. K., Pierce G. N., Dhalla K. S., Dhalla N. S. Defective sarcoplasmic reticular calcium transport in diabetic cardiomyopathy. Am J Physiol. 1983 Jun;244(6):E528–E535. doi: 10.1152/ajpendo.1983.244.6.E528. [DOI] [PubMed] [Google Scholar]
- Gay R. G., Raya T. E., Lancaster L. D., Lee R. W., Morkin E., Goldman S. Effects of thyroid state on venous compliance and left ventricular performance in rats. Am J Physiol. 1988 Jan;254(1 Pt 2):H81–H88. doi: 10.1152/ajpheart.1988.254.1.H81. [DOI] [PubMed] [Google Scholar]
- Gilbert J. C., Glantz S. A. Determinants of left ventricular filling and of the diastolic pressure-volume relation. Circ Res. 1989 May;64(5):827–852. doi: 10.1161/01.res.64.5.827. [DOI] [PubMed] [Google Scholar]
- Gøtzsche O. Abnormal myocardial calcium uptake in streptozocin-diabetic rats. Evidence for a direct insulin effect on catecholamine sensitivity. Diabetes. 1985 Mar;34(3):287–290. doi: 10.2337/diab.34.3.287. [DOI] [PubMed] [Google Scholar]
- Gøtzsche O. Lack of cardiotoxic effect of isoproterenol in streptozotocin diabetic rats. A morphometric study of isoproterenol induced fibrosis. Virchows Arch A Pathol Anat Histol. 1982;397(1):83–91. doi: 10.1007/BF00430895. [DOI] [PubMed] [Google Scholar]
- Kannel W. B., Hjortland M., Castelli W. P. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974 Jul;34(1):29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- Lee T. S., MacGregor L. C., Fluharty S. J., King G. L. Differential regulation of protein kinase C and (Na,K)-adenosine triphosphatase activities by elevated glucose levels in retinal capillary endothelial cells. J Clin Invest. 1989 Jan;83(1):90–94. doi: 10.1172/JCI113889. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lew W. Y. Evaluation of left ventricular diastolic function. Circulation. 1989 Jun;79(6):1393–1397. doi: 10.1161/01.cir.79.6.1393. [DOI] [PubMed] [Google Scholar]
- Mirsky I. Assessment of diastolic function: suggested methods and future considerations. Circulation. 1984 Apr;69(4):836–841. doi: 10.1161/01.cir.69.4.836. [DOI] [PubMed] [Google Scholar]
- Mirsky I., Pfeffer J. M., Pfeffer M. A., Braunwald E. The contractile state as the major determinant in the evolution of left ventricular dysfunction in the spontaneously hypertensive rat. Circ Res. 1983 Dec;53(6):767–778. doi: 10.1161/01.res.53.6.767. [DOI] [PubMed] [Google Scholar]
- Modrak J. Collagen metabolism in the myocardium from streptozotocin-diabetic rats. Diabetes. 1980 Jul;29(7):547–550. doi: 10.2337/diab.29.7.547. [DOI] [PubMed] [Google Scholar]
- Parmley W. W., Sonnenblick E. H. Relation between mechanics of contraction and relaxation in mammalian cardiac muscle. Am J Physiol. 1969 May;216(5):1084–1091. doi: 10.1152/ajplegacy.1969.216.5.1084. [DOI] [PubMed] [Google Scholar]
- Penpargkul S., Fein F., Sonnenblick E. H., Scheuer J. Depressed cardiac sarcoplasmic reticular function from diabetic rats. J Mol Cell Cardiol. 1981 Mar;13(3):303–309. doi: 10.1016/0022-2828(81)90318-7. [DOI] [PubMed] [Google Scholar]
- Penpargkul S., Schaible T., Yipintsoi T., Scheuer J. The effect of diabetes on performance and metabolism of rat hearts. Circ Res. 1980 Dec;47(6):911–921. doi: 10.1161/01.res.47.6.911. [DOI] [PubMed] [Google Scholar]
- Raff G. L., Glantz S. A. Volume loading slows left ventricular isovolumic relaxation rate. Evidence of load-dependent relaxation in the intact dog heart. Circ Res. 1981 Jun;48(6 Pt 1):813–824. doi: 10.1161/01.res.48.6.813. [DOI] [PubMed] [Google Scholar]
- Raya T. E., Gay R. G., Lancaster L., Aguirre M., Moffett C., Goldman S. Serial changes in left ventricular relaxation and chamber stiffness after large myocardial infarction in rats. Circulation. 1988 Jun;77(6):1424–1431. doi: 10.1161/01.cir.77.6.1424. [DOI] [PubMed] [Google Scholar]
- Regan T. J., Lyons M. M., Ahmed S. S., Levinson G. E., Oldewurtel H. A., Ahmad M. R., Haider B. Evidence for cardiomyopathy in familial diabetes mellitus. J Clin Invest. 1977 Oct;60(4):884–899. doi: 10.1172/JCI108843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan T. J., Wu C. F., Yeh C. K., Oldewurtel H. A., Haider B. Myocardial composition and function in diabetes. The effects of chronic insulin use. Circ Res. 1981 Dec;49(6):1268–1277. doi: 10.1161/01.res.49.6.1268. [DOI] [PubMed] [Google Scholar]
- Stone P. H., Muller J. E., Hartwell T., York B. J., Rutherford J. D., Parker C. B., Turi Z. G., Strauss H. W., Willerson J. T., Robertson T. The effect of diabetes mellitus on prognosis and serial left ventricular function after acute myocardial infarction: contribution of both coronary disease and diastolic left ventricular dysfunction to the adverse prognosis. The MILIS Study Group. J Am Coll Cardiol. 1989 Jul;14(1):49–57. doi: 10.1016/0735-1097(89)90053-3. [DOI] [PubMed] [Google Scholar]
- Weisman H. F., Bush D. E., Mannisi J. A., Weisfeldt M. L., Healy B. Cellular mechanisms of myocardial infarct expansion. Circulation. 1988 Jul;78(1):186–201. doi: 10.1161/01.cir.78.1.186. [DOI] [PubMed] [Google Scholar]
- Weiss J. L., Frederiksen J. W., Weisfeldt M. L. Hemodynamic determinants of the time-course of fall in canine left ventricular pressure. J Clin Invest. 1976 Sep;58(3):751–760. doi: 10.1172/JCI108522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F. C., Spurgeon H. A., Rakusan K., Weisfeldt M. L., Lakatta E. G. Use of tibial length to quantify cardiac hypertrophy: application in the aging rat. Am J Physiol. 1982 Dec;243(6):H941–H947. doi: 10.1152/ajpheart.1982.243.6.H941. [DOI] [PubMed] [Google Scholar]
- Zarich S. W., Arbuckle B. E., Cohen L. R., Roberts M., Nesto R. W. Diastolic abnormalities in young asymptomatic diabetic patients assessed by pulsed Doppler echocardiography. J Am Coll Cardiol. 1988 Jul;12(1):114–120. doi: 10.1016/0735-1097(88)90364-6. [DOI] [PubMed] [Google Scholar]