Abstract
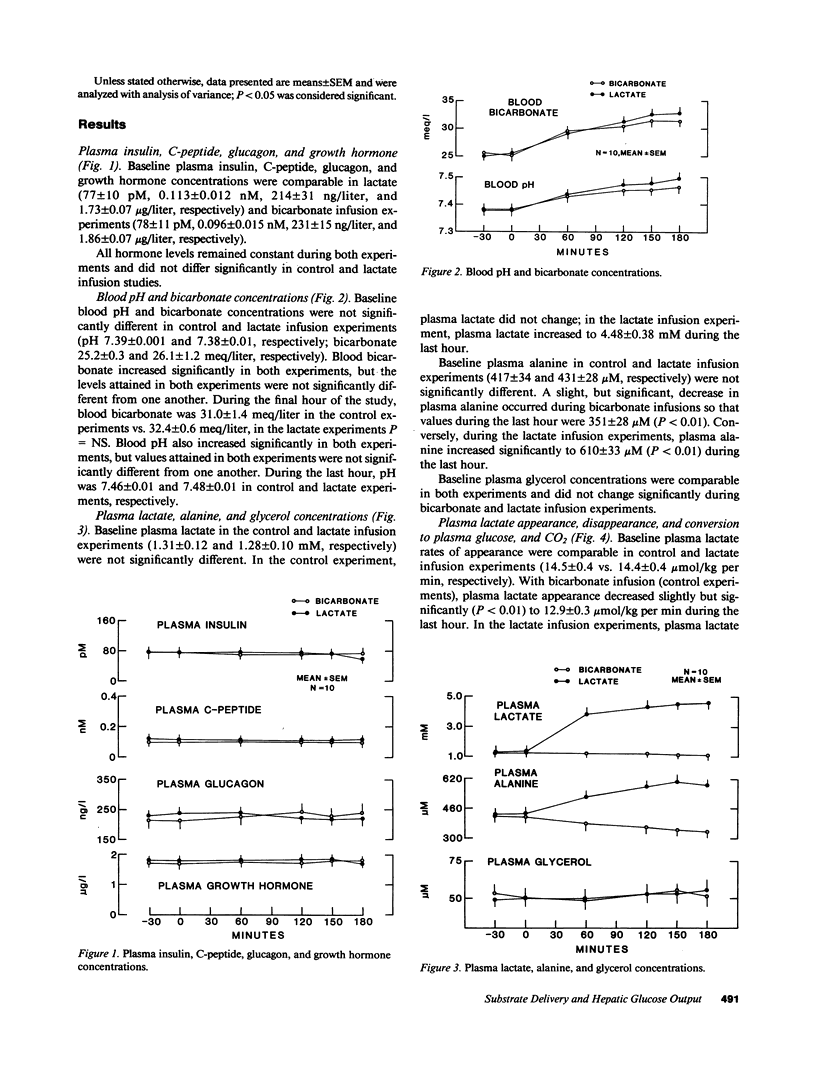
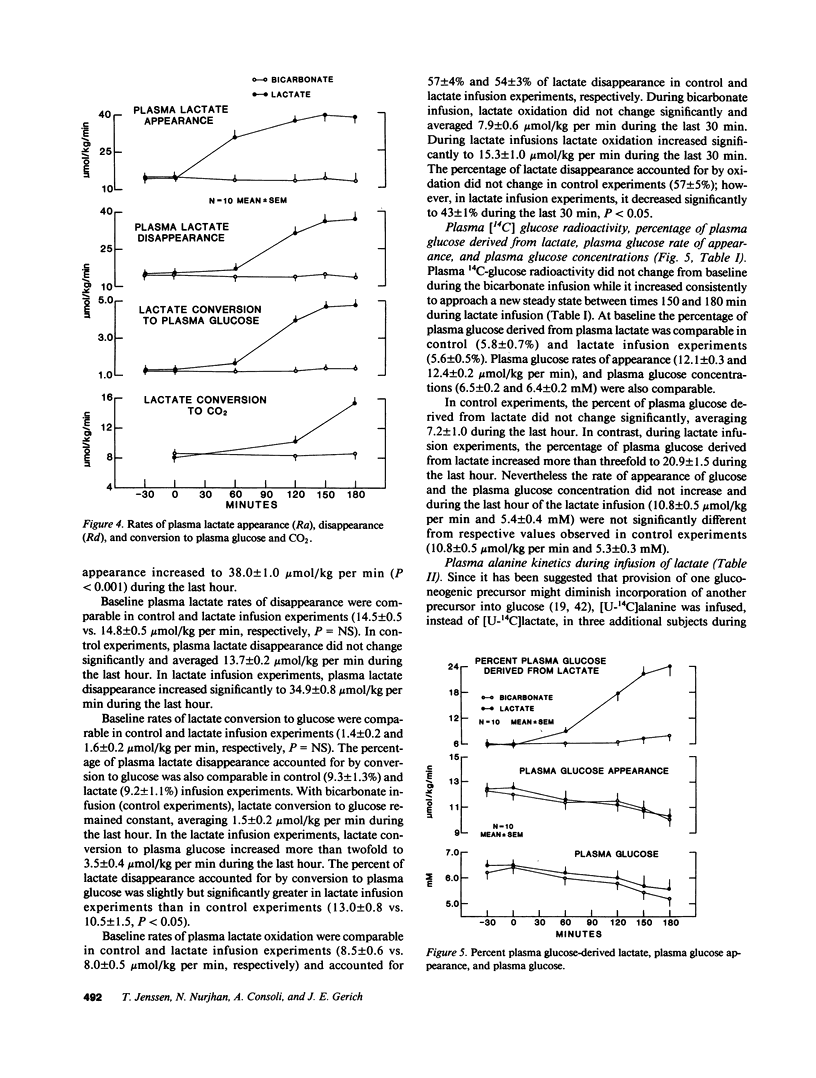
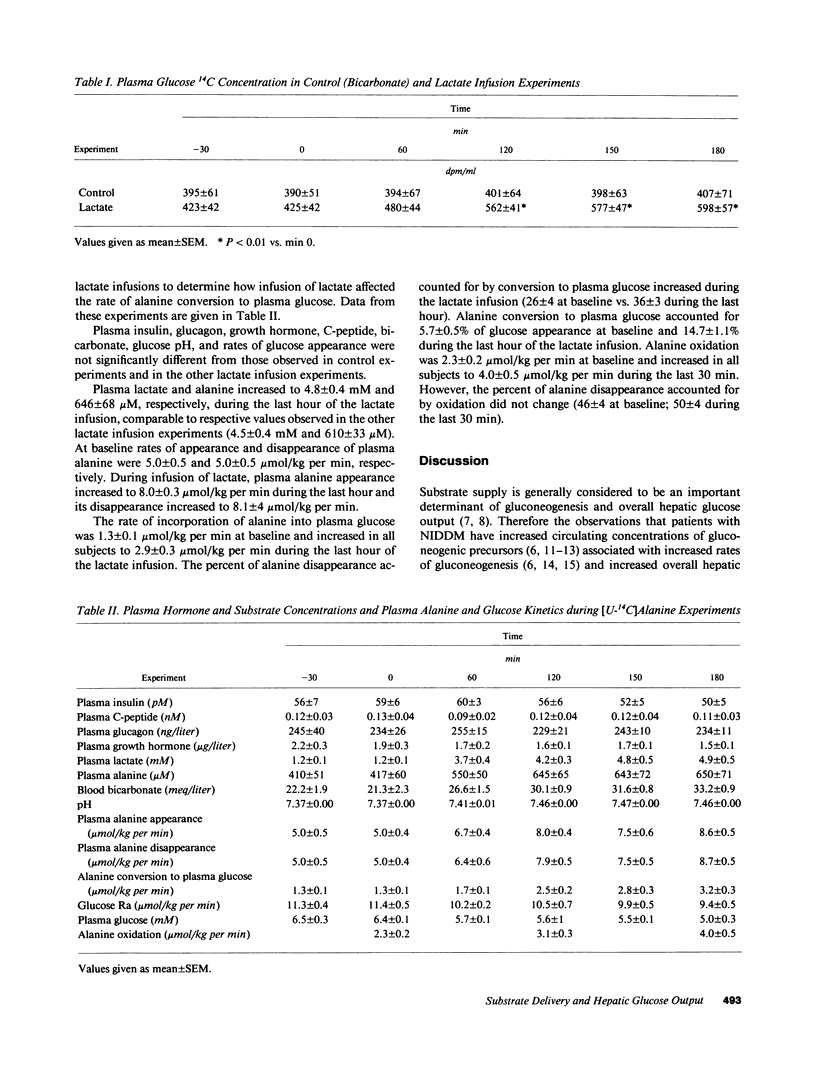
It has been proposed that increased supply of gluconeogenic precursors may be largely responsible for the increased gluconeogenesis which contributes to fasting hyperglycemia in non-insulin-dependent diabetes mellitus (NIDDM). Therefore, to test the hypothesis that an increase in gluconeogenic substrate supply per se could increase hepatic glucose output sufficiently to cause fasting hyperglycemia, we infused normal volunteers with sodium lactate at a rate approximately double the rate of appearance observed in NIDDM while clamping plasma insulin, glucagon, and growth hormone at basal levels. In control experiments, sodium bicarbonate was infused instead of sodium lactate at equimolar rates. In both experiments, [6-3H]-glucose was infused to measure glucose appearance and either [U-14C]lactate or [U-14C]alanine was infused to measure the rates of appearance and conversion of these substrates into plasma glucose. Plasma insulin, glucagon, growth hormone, C-peptide, and glycerol concentrations, and blood bicarbonate and pH in control and lactate infusion experiments were not significantly different. Infusion of lactate increased plasma lactate and alanine to 4.48 +/- 3 mM and 610 +/- 33 microM, respectively, from baseline values of 1.6 +/- 0.2 mM and 431 +/- 28 microM, both P less than 0.01; lactate and alanine rates of appearance increased to 38 +/- 1.0 and 8.0 +/- 0.3 mumol/kg per min (P less than 0.01 versus basal rates of 14.4 +/- 0.4 and 5.0 +/- 0.5 mumol/kg per min, respectively). With correction for Krebs cycle carbon exchange, lactate incorporation into plasma glucose increased nearly threefold to 10.4 mumol/kg per min and accounted for about 50% of overall glucose appearance. Alanine incorporation into plasma glucose increased more than twofold. Despite this marked increase in gluconeogenesis, neither overall hepatic glucose output nor plasma glucose increased and each was not significantly different from values observed in control experiments (10.8 +/- 0.5 vs. 10.8 +/- 0.5 mumol/kg per min and 5.4 +/- 0.4 vs. 5.3 +/- 0.3 mM, respectively). We, therefore, conclude that in normal humans there is an autoregulatory process independent of changes in plasma glucose and glucoregulatory hormone concentrations which prevents a substrate-induced increase in gluconeogenesis from increasing overall hepatic glucose output; since this process cannot be explained on the basis of inhibition of gluconeogenesis from other substrates, it probably involves diminution of glycogenolysis. A defect in this process could explain at least in part the increased hepatic glucose output found in NIDDM.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlborg G., Hagenfeldt L., Wahren J. Influence of lactate infusion on glucose and FFA metabolism in man. Scand J Clin Lab Invest. 1976 Mar;36(2):193–201. doi: 10.1080/00365517609055248. [DOI] [PubMed] [Google Scholar]
- Bier D. M., Arnold K. J., Sherman W. R., Holland W. H., Holmes W. F., Kipnis D. M. In-vivo measurement of glucose and alanine metabolism with stable isotopic tracers. Diabetes. 1977 Nov;26(11):1005–1015. doi: 10.2337/diab.26.11.1005. [DOI] [PubMed] [Google Scholar]
- Bolli G., De Feo P., Perriello G., De Cosmo S., Ventura M., Campbell P., Brunetti P., Gerich J. E. Role of hepatic autoregulation in defense against hypoglycemia in humans. J Clin Invest. 1985 May;75(5):1623–1631. doi: 10.1172/JCI111869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucolo G., David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973 May;19(5):476–482. [PubMed] [Google Scholar]
- Campbell P. J., Bolli G. B., Cryer P. E., Gerich J. E. Pathogenesis of the dawn phenomenon in patients with insulin-dependent diabetes mellitus. Accelerated glucose production and impaired glucose utilization due to nocturnal surges in growth hormone secretion. N Engl J Med. 1985 Jun 6;312(23):1473–1479. doi: 10.1056/NEJM198506063122302. [DOI] [PubMed] [Google Scholar]
- Chiasson J. L., Atkinson R. L., Cherrington A. D., Keller U., Sinclair-Smith B. C., Lacy W. W., Liljenquist J. E. Effects of fasting on gluconeogenesis from alanine in nondiabetic man. Diabetes. 1979 Jan;28(1):56–60. doi: 10.2337/diab.28.1.56. [DOI] [PubMed] [Google Scholar]
- Chiasson J. L., Liljenquist J. E., Lacy W. W., Jennings A. S., Cherrington A. D. Gluconeogenesis: methodological approaches in vivo. Fed Proc. 1977 Feb;36(2):229–235. [PubMed] [Google Scholar]
- Chiasson J. L., Liljenquist J. E., Sinclair-Smith B. C., Lacy W. W. Gluconeogenesis from alanine in normal postabsorptive man. Intrahepatic stimulatory effect of glucagon. Diabetes. 1975 Jun;24(6):574–584. doi: 10.2337/diab.24.6.574. [DOI] [PubMed] [Google Scholar]
- Chochinov R. H., Bowen H. F., Moorhouse J. A. Circulating alanine disposal in diabetes mellitus. Diabetes. 1978 Apr;27(4):420–426. doi: 10.2337/diab.27.4.420. [DOI] [PubMed] [Google Scholar]
- Connor H., Woods H. F. Quantitative aspects of L(+)-lactate metabolism in human beings. Ciba Found Symp. 1982;87:214–234. doi: 10.1002/9780470720691.ch12. [DOI] [PubMed] [Google Scholar]
- Consoli A., Kennedy F., Miles J., Gerich J. Determination of Krebs cycle metabolic carbon exchange in vivo and its use to estimate the individual contributions of gluconeogenesis and glycogenolysis to overall glucose output in man. J Clin Invest. 1987 Nov;80(5):1303–1310. doi: 10.1172/JCI113206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consoli A., Nurjhan N., Capani F., Gerich J. Predominant role of gluconeogenesis in increased hepatic glucose production in NIDDM. Diabetes. 1989 May;38(5):550–557. doi: 10.2337/diab.38.5.550. [DOI] [PubMed] [Google Scholar]
- DEBODO R. C., STEELE R., ALTSZULER N., DUNN A., BISHOP J. S. ON THE HORMONAL REGULATION OF CARBOHYDRATE METABOLISM; STUDIES WITH C14 GLUCOSE. Recent Prog Horm Res. 1963;19:445–488. [PubMed] [Google Scholar]
- Davidson M. B. Autoregulation by glucose of hepatic glucose balance: permissive effect of insulin. Metabolism. 1981 Mar;30(3):279–284. doi: 10.1016/0026-0495(81)90152-9. [DOI] [PubMed] [Google Scholar]
- De Feo P., Perriello G., Ventura M. M., Brunetti P., Santeusanio F., Gerich J. E., Bolli G. B. The pancreatic-adrenocortical-pituitary clamp technique for study of counterregulation in humans. Am J Physiol. 1987 Apr;252(4 Pt 1):E565–E570. doi: 10.1152/ajpendo.1987.252.4.E565. [DOI] [PubMed] [Google Scholar]
- Diamond M. P., Rollings R. C., Steiner K. E., Williams P. E., Lacy W. W., Cherrington A. D. Effect of alanine concentration independent of changes in insulin and glucagon on alanine and glucose homeostasis in the conscious dog. Metabolism. 1988 Jan;37(1):28–33. doi: 10.1016/0026-0495(88)90025-x. [DOI] [PubMed] [Google Scholar]
- Dietze G., Wicklmayr M., Hepp K. D., Bogner W., Mehnert H., Czempiel H., Henftling H. G. On gluconeogenesis of human liver. Accelerated hepatic glucose formation induced by increased precursor supply. Diabetologia. 1976 Dec;12(6):555–561. doi: 10.1007/BF01220631. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Mallette L. E., Jefferson L. S., Wong E. H., Friedmann N., Miller T. B., Jr, Park C. R. The hormonal control of hepatic gluconeogenesis. Recent Prog Horm Res. 1970;26:411–461. doi: 10.1016/b978-0-12-571126-5.50014-5. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Park C. R. Control of gluconeogenesis in liver. I. General features of gluconeogenesis in the perfused livers of rats. J Biol Chem. 1967 Jun 10;242(11):2622–2636. [PubMed] [Google Scholar]
- Faber O. K., Binder C. C-peptide response to glucagon. A test for the residual beta-cell function in diabetes mellitus. Diabetes. 1977 Jul;26(7):605–610. doi: 10.2337/diab.26.7.605. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J., Hendler R. Influence of maturity-onset diabetes on splanchnic glucose balance after oral glucose ingestion. Diabetes. 1978 Feb;27(2):121–126. doi: 10.2337/diab.27.2.121. [DOI] [PubMed] [Google Scholar]
- Foster D. M., Hetenyl G., Jr, Berman M. A model for carbon kinetics among plasma alanine, lactate, and glucose. Am J Physiol. 1980 Jul;239(1):E30–E38. doi: 10.1152/ajpendo.1980.239.1.E30. [DOI] [PubMed] [Google Scholar]
- Freymond D., Bogardus C., Okubo M., Stone K., Mott D. Impaired insulin-stimulated muscle glycogen synthase activation in vivo in man is related to low fasting glycogen synthase phosphatase activity. J Clin Invest. 1988 Nov;82(5):1503–1509. doi: 10.1172/JCI113758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzell R. T., Hendrick G. K., Biggers D. W., Lacy D. B., Donahue D. P., Green D. R., Carr R. K., Williams P. E., Stevenson R. W., Cherrington A. D. Role of gluconeogenesis in sustaining glucose production during hypoglycemia caused by continuous insulin infusion in conscious dogs. Diabetes. 1988 Jun;37(6):749–759. doi: 10.2337/diab.37.6.749. [DOI] [PubMed] [Google Scholar]
- Garber A. J., Bier D. M., Cryer P. E., Pagliara A. S. Hypoglycemia in compensated chronic renal insufficiency. Substrate limitation of gluconeogenesis. Diabetes. 1974 Dec;23(12):982–986. doi: 10.2337/diab.23.12.982. [DOI] [PubMed] [Google Scholar]
- Glinsmann W. H., Hern E. P., Lynch A. Intrinsic regulation of glucose output by rat liver. Am J Physiol. 1969 Apr;216(4):698–703. doi: 10.1152/ajplegacy.1969.216.4.698. [DOI] [PubMed] [Google Scholar]
- Hall S. E., Braaten J. T., McKendry J. B., Bolton T., Foster D., Berman M. Normal alanine-glucose relationships and their changes in diabetic patients before and after insulin treatment. Diabetes. 1979 Aug;28(8):737–745. [PubMed] [Google Scholar]
- Hall S. E., Saunders J., Sönksen P. H. Glucose and free fatty acid turnover in normal subjects and in diabetic patients before and after insulin treatment. Diabetologia. 1979 May;16(5):297–306. doi: 10.1007/BF01223618. [DOI] [PubMed] [Google Scholar]
- Haymond M. W., Ben-Galim E., Strobel K. E. Glucose and alanine metabolism in children with maple syrup urine disease. J Clin Invest. 1978 Aug;62(2):398–405. doi: 10.1172/JCI109141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems D. A., Whitton P. D. Control of hepatic glycogenolysis. Physiol Rev. 1980 Jan;60(1):1–50. doi: 10.1152/physrev.1980.60.1.1. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Hetenyi G., Jr, Perez G., Vranic M. Turnover and precursor-product relationships of nonlipid metabolites. Physiol Rev. 1983 Apr;63(2):606–667. doi: 10.1152/physrev.1983.63.2.606. [DOI] [PubMed] [Google Scholar]
- Huijing F. Glycogen metabolism and glycogen-storage diseases. Physiol Rev. 1975 Oct;55(4):609–658. doi: 10.1152/physrev.1975.55.4.609. [DOI] [PubMed] [Google Scholar]
- Karl I. E., Pagliara A. S., Kipnis D. M. A microfluorometric enzymatic assay for the determination of alanine and pyruvate in plasma and tissues. J Lab Clin Med. 1972 Sep;80(3):434–441. [PubMed] [Google Scholar]
- Katz J. Determination of gluconeogenesis in vivo with 14C-labeled substrates. Am J Physiol. 1985 Apr;248(4 Pt 2):R391–R399. doi: 10.1152/ajpregu.1985.248.4.R391. [DOI] [PubMed] [Google Scholar]
- Kelley D., Mitrakou A., Marsh H., Schwenk F., Benn J., Sonnenberg G., Arcangeli M., Aoki T., Sorensen J., Berger M. Skeletal muscle glycolysis, oxidation, and storage of an oral glucose load. J Clin Invest. 1988 May;81(5):1563–1571. doi: 10.1172/JCI113489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisberg R. A. Glucose-lactate inter-relations in man. N Engl J Med. 1972 Jul 20;287(3):132–137. doi: 10.1056/NEJM197207202870307. [DOI] [PubMed] [Google Scholar]
- Kreisberg R. A., Pennington L. F., Boshell B. R. Lactate turnover and gluconeogenesis in normal and obese humans. Effect of starvation. Diabetes. 1970 Jan;19(1):53–63. doi: 10.2337/diab.19.1.53. [DOI] [PubMed] [Google Scholar]
- Kreisberg R. A., Siegal A. M., Owen W. C. Glucose-lactate interrelationships: effect of ethanol. J Clin Invest. 1971 Jan;50(1):175–185. doi: 10.1172/JCI106471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljenquist J. E., Mueller G. L., Cherrington A. D., Perry J. M., Rabinowitz D. Hyperglycemia per se (insulin and glucagon withdrawn) can inhibit hepatic glucose production in man. J Clin Endocrinol Metab. 1979 Jan;48(1):171–175. doi: 10.1210/jcem-48-1-171. [DOI] [PubMed] [Google Scholar]
- Mazzeo R. S., Brooks G. A., Schoeller D. A., Budinger T. F. Disposal of blood [1-13C]lactate in humans during rest and exercise. J Appl Physiol (1985) 1986 Jan;60(1):232–241. doi: 10.1152/jappl.1986.60.1.232. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Kuwajima M., Newgard C. B., Foster D. W., Katz J. From dietary glucose to liver glycogen: the full circle round. Annu Rev Nutr. 1987;7:51–73. doi: 10.1146/annurev.nu.07.070187.000411. [DOI] [PubMed] [Google Scholar]
- McGuire E. A., Helderman J. H., Tobin J. D., Andres R., Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol. 1976 Oct;41(4):565–573. doi: 10.1152/jappl.1976.41.4.565. [DOI] [PubMed] [Google Scholar]
- Nilsson L. H., Hultman E. Liver glycogen in man--the effect of total starvation or a carbohydrate-poor diet followed by carbohydrate refeeding. Scand J Clin Lab Invest. 1973 Dec;32(4):325–330. doi: 10.3109/00365517309084355. [DOI] [PubMed] [Google Scholar]
- Prager R., Wallace P., Olefsky J. M. Direct and indirect effects of insulin to inhibit hepatic glucose output in obese subjects. Diabetes. 1987 May;36(5):607–611. doi: 10.2337/diab.36.5.607. [DOI] [PubMed] [Google Scholar]
- Reaven G. M., Hollenbeck C., Jeng C. Y., Wu M. S., Chen Y. D. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes. 1988 Aug;37(8):1020–1024. doi: 10.2337/diab.37.8.1020. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Herrera M. G. Glucose regulation of hepatic gluconeogenesis. Am J Physiol. 1968 Jun;214(6):1346–1351. doi: 10.1152/ajplegacy.1968.214.6.1346. [DOI] [PubMed] [Google Scholar]
- Sacca L., Hendler R., Sherwin R. S. Hyperglycemia inhibits glucose production in man independent of changes in glucoregulatory hormones. J Clin Endocrinol Metab. 1978 Nov;47(5):1160–1163. doi: 10.1210/jcem-47-5-1160. [DOI] [PubMed] [Google Scholar]
- Searle G. L., Cavalieri R. R. Determination of lactate kinetics in the human analysis of data from single injection vs. continuous infusion methods. Proc Soc Exp Biol Med. 1972 Mar;139(3):1002–1006. doi: 10.3181/00379727-139-36284. [DOI] [PubMed] [Google Scholar]
- Shikama H., Ui M. Glucose load diverts hepatic gluconeogenic product from glucose to glycogen in vivo. Am J Physiol. 1978 Oct;235(4):E354–E360. doi: 10.1152/ajpendo.1978.235.4.E354. [DOI] [PubMed] [Google Scholar]
- Shulman G. I., Lacy W. W., Liljenquist J. E., Keller U., Williams P. E., Cherrington A. D. Effect of glucose, independent of changes in insulin and glucagon secretion, on alanine metabolism in the conscious dog. J Clin Invest. 1980 Feb;65(2):496–505. doi: 10.1172/JCI109693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele R., Winkler B., Altszuler N. Inhibition by infused glycerol of gluconeogenesis from other precursors. Am J Physiol. 1971 Sep;221(3):883–888. doi: 10.1152/ajplegacy.1971.221.3.883. [DOI] [PubMed] [Google Scholar]
- Wright K. S., Beck-Nielsen H., Kolterman O. G., Mandarino L. J. Decreased activation of skeletal muscle glycogen synthase by mixed-meal ingestion in NIDDM. Diabetes. 1988 Apr;37(4):436–440. doi: 10.2337/diab.37.4.436. [DOI] [PubMed] [Google Scholar]
- Zawadzki J. K., Wolfe R. R., Mott D. M., Lillioja S., Howard B. V., Bogardus C. Increased rate of Cori cycle in obese subjects with NIDDM and effect of weight reduction. Diabetes. 1988 Feb;37(2):154–159. doi: 10.2337/diab.37.2.154. [DOI] [PubMed] [Google Scholar]



