Abstract
PROBLEM
Enrichment of uterine Natural Killer (uNK) cells occurs during pregnancy in many species. However, functions of uNK cells and regulation of their uterine homing are not fully defined. In mice and women, uNK cells contribute to angiogenesis, a role reviewed here and now addressed in a mammal with an alternative placental type.
METHODS OF STUDY
To address lymphocyte functions, RNA from murine or porcine endometrium and lymphocytes purified from endometrium were analyzed using realtime or reverse transcription PCR. To address homing potential, human blood CD56+ lymphocytes were evaluated using both RNA and functional adhesion to endothelium presented under shear force in frozen sections of gestation day 7 C57Bl/6J implantation sites. Women were serially sampled over a menstrual cycle or a clinical preparatory cycle for embryo transfer.
RESULTS
Activation of murine uNK cells is associated with much greater increases in transcription for Eomes than for T-bet (Tbx21). Lymphocytes from normal porcine implantation sites transcribe VEGF, PlGF, IFN-γ and HIF-1α. In fertile women, increases in L-selectin- and α4-integrin-mediated interactions between CD56+ cells and endothelium occur at LH surge (cycling women) through to oocyte pick up or embryo transfer, then return to pre-LH levels.
CONCLUSIONS
Uterine lymphocytes may universally promote pregnancy-associated endometrial angiogenesis. Recruitment of uNK precursor cells from blood appears to occur in a window promoted by rising plasma estrogen and luteinizing hormone and limited by rising progesterone.
Keywords: Angiogenesis, cell homing, decidua, estrogen, luteinizing hormone, lymphocytes, pregnancy, uterus, spiral artery
INTRODUCTION
In mice, pregnancy induces the differentiation of two transient endometrial tissues; the mesometrial lymphoid aggregate of pregnancy (MLAp) and the decidua basalis (DB). These are remarkable tissues because they are traversed by large, tortuous, pregnancy-dilated maternal arteries which supply the placentae and because they contain disproportionately high numbers of lymphocytes of the Natural Killer (NK) lineage. In comparison with structures like lymph nodes (LN) and intestinal cryptopatches, the process of development of these progesterone (P4)-dependent tissues that have immune functions is poorly understood 1–3.
Uterine (u)NK cells appear during decidualization, peak in number and tissue density at gestation day (gd)10–11, then begin to die 4;5. Cells of the NK lineage are not only rare in the non-decidualized uterus but they appear to be in an immunologically naive state as evidenced by their lack of cytoplasmic granules 6–8. The self-renewing progenitors of uNK cells (pro-uNK) are derived from trafficking precursors (pre-uNK) found in peripheral lymphoid sites, in particular spleen and lymph nodes 9. To enter uterine tissues, these cells are thought to migrate through vessels in the central DB and localize to peri-vascular locations in DB and MLAp in response to specific arrays of adhesion molecules expressed on endothelium 10. Since uNK cells are highly proliferative, as evidenced by the presence of mitotic figures 7;11, progenitor and precursor cell recruitment to the uterus is likely to be of reduced significance after gd8. Once localized, pre-uNK cells differentiate and gain in size and function (acquisition of cytoplasmic granules, production of interferon (IFN)-γ etc.) as pregnancy progresses to mid gestation. Conceptus-derived signals cannot be responsible for triggering homing or the initial activation of pre- uNK cells because activated uNK cells are found in conceptus-free deciduomata 7. However, conceptus-derived signals must sustain the normal lifespan of uNK cells out to midgestation because they decline quickly in deciduomata. At gd12 of normal pregenancy, when uNK cells have become large and heavily granulated, cell division ceases and the proportion of uNK cells with features of senescent cells becomes dominant, then numbers decline 5. UNK cells have low cytotoxic function 12;13, but are potent producers of immunomodulatory cytokines such as IFN-γ, colony stimulating factors (CSF, GM-CSF), and angiogenic molecules such as vascular endothelial growth factor (VEGF), placental growth factor (PlGF), and angiopoetin (Ang2) 14–16.
Mice deficient in uNK cells retain fertility, showing that uNK cells are not essential for successful rodent pregnancy. Such mice lack development of the MLAp, have poor decidualization and the spiral arteries servicing each implantation site are more constricted, thicker walled and shorter than in appropriate strain and gd-matched control animals 17–19. Through secretion of molecules including IFN-γ 20, the potent vascular relaxant NO 21 and vascular endothelial growth factor (VEGF) 15, uNK cells promote dilation, elongation and loss of smooth muscle in spiral arteries. This increases blood vessel support capacity for developing fetuses. Production of IFN-γ is likely the major effector function of uNK cells since administration of recombinant mouse or human IFN-γ alone is sufficient to promote complete vascular modification in pregnant alymphoid mice 20;22;23. The ability to quantify changes in the histological architecture of the major vessels in implantation sites in immune deficient mice from vessels having pre-eclampsia-like features towards normal, pregnancy-modified vessels provides an excellent in vivo assay system to functionally assess molecules contributing to uNK cell homing, activation and actions.
Early, pregnancy-associated endometrial enrichment in uNK cells is also reported in pigs 24. Porcine conceptuses are non invasive and endometrial angiogenesis supporting conceptus attachment sites involves development of a subepithelial capillary plexus rather than spiral arterial modification. We wondered if the 3 fold enrichment in endometrial lymphocytes at porcine conceptus attachment sites was functionally analogous to the more abundant recruitment of uNK cells of mice and humans and promoted angiogenesis 15;25. Laser capture microdissection of endometrial lymphocytes was used to obtain RNA reflecting in utero conditions, cDNA was synthesized and used as a template for quantitative realtime PCR. Transcription profiles for VEGF, HIF-1α and IFN-γ, key regulators of angiogenesis during early pregnancy, were evaluated by relative quantification using β-actin as a housekeeping gene. Lymphocytes transcribed these molecules. Indeed, when levels of transcripts for VEGF, HIF-1α and IFN-γ in endometrial lymphocytes were compared to those from trophoblast gd15-23, lymphocytes were found to transcribe much higher levels. This suggests that, at least in pigs, lymphocytes are the major cells sensing implantation site levels of oxygen and responding by promotion of angiogenesis 16.
The human uNK cell, which expresses surface CD56 at 10–100 fold higher levels than blood bright and dim subsets respectively, increases rapidly in number in the post-ovulatory phase of the menstrual cycle to become the dominant lymphocyte of the decidualized uterus 26;27. In human pregnancy, uNK cells associate closely with extravillous trophoblast. Extensive spiral artery modification during the first trimester of human pregnancy is accomplished by highly invasive trophoblast, which replaces the vascular endothelium in maternal arteries28;29. The role of uNK cells in the human uterus/pregnancy is not yet clear, but is speculated to involve initiation of menstruation, maintenance of decidua, implantation and endometrial angiogenesis 25;27.
MOVING PRE-uNK CELLS TO THE MOUSE UTERUS
Movement of lymphocytes from lymphoid organs into the circulation and then into tissues is orchestrated by an elegant series of interactions between specific tissue-secreted chemokines and their receptors expressed on lymphocyte subsets 30;31. Engagement of the chemokine receptor (one of the seven transmembrane G-protein coupled receptors) on leukocytes results in activation of adhesion molecules that are constitutively expressed on leukocyte plasma membranes. Once activated, adhesion molecules, such as L-selectin and α4 integrin, are able to form attachments to their ligands which are expressed by endothelium (Peripheral Node Addressin (PNAd), Mucosal Addressin Cell Adhesion Molecule (MAdCAM) or Vascular Cell Adhesion Molecule, V-CAM-1) 32;33. Firm adhesion of the leukocyte to endothelium must occur prior to the leukocyte’s egress from the circulation and into a tissue 34.
Like other leucocyte subsets, NK cells and NK cell subsets display unique arrays of both chemokine receptors and adhesion molecules. These include the chemokine receptors CCR2, CCR5, CXCR3, CXCR4 and CX3CR1 35–38 and the adhesion molecules L-selectin and α4β7 integrin 39. Our recent studies, involving transplantation of pre-uNK cells from knockout mice to mated, alymphoid females, have demonstrated significant but overlapping roles for these molecules in effecting pre-uNK cell trafficking to the gestational uterus 9;40–42.
The Stamper-Woodruff adhesion assay monitors leukocyte homing potential in vitro 43. This assay applies living leukocytes or leukocyte subsets to frozen tissue sections as substrates for the tissue homing target of interest. The assay was developed to study movement of lymphocytes from the circulation into secondary lymphoid organs (i.e.LN) but has been adapted and widely applied to address questions as diverse as lymphocyte recruitment to pancreatic islets in the pre-insulitis phase of diabetes 44 to human cytotrophoblast adhesion to luminal epithelium of human luteal phase endometrial biopsies 45. For leukocyte adhesion, a constant number (usually 5×106) of cells (either mouse or human in origin; isolated from blood or organs or established cell lines with or without selected enrichment or transgenic display of the molecule of interest) is applied to 12μm sections of snap frozen murine tissue under a constant shear force at 4°C. Interactions between the viable leukocytes and the endothelium in the substrate tissue are stopped after 30 min, non-adherent cells are rinsed away and the specimens are fixed, stained and enumerated for adherent cells. We adapted the assay to assess pre-uNK cell homing to decidua. Our initial studies showed that human lymphocyte interactions with endothelium were elevated if the endothelium was presented in LN, Peyers Patches (PP) or decidua from pregnant donor mice. Not all tissues from the pregnant donors had endothelium that revealed this transient change. Further, independent but co-ordinated changes occurred in lymphocytes themselves. These independent but co-ordinated effects on specific organ endothelia and on lymphocytes were reproduced in ovariectomized animals treated with either estradiol (E2) or progesterone (P4) 46.
To further understand the physiology involved in uNK cell egress from the circulation into the uterus, we are adopting another technique from the field of leukocyte trafficking for use in gestational studies, intravital microscopy. In this technique, a vessel in an anaesthetized, living animal is visualized at high magnification. Cells passing through the vessel can be measured for speed, marginalization and tethering and the endothelium in the vessel can be assessed functionally and/or changed by drug administration. The cremaster muscle is a striated skeletal muscle associated with the spermatic cord, which is widely used for this animal preparation (Fig 1A) due to its easy exteriorization without flow impedance, the optical clarity of the preparation due to its thinness and its freedom from vibration due to cardiac or respiratory cycles. However, the cremaster is not suitable for addressing our research questions involving vascular changes which are confined to the uterus. We have been successful in imaging the spiral arteries in gd 10–12 pregnant living mice (Fig 1B) and are undertaking functional studies of these vessels with the longterm goal of assessing physiological uNK cell interactions with implantation site endothelia in vivo in real time.
Fig. 1.
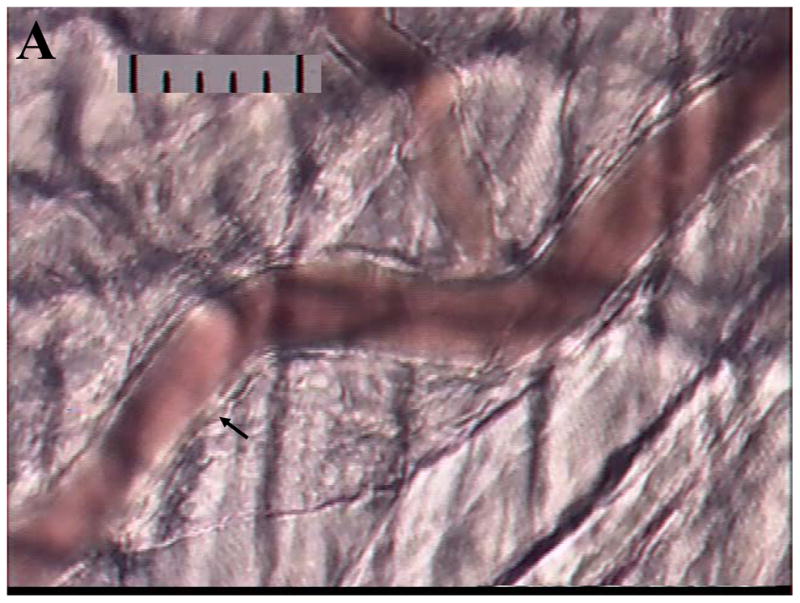
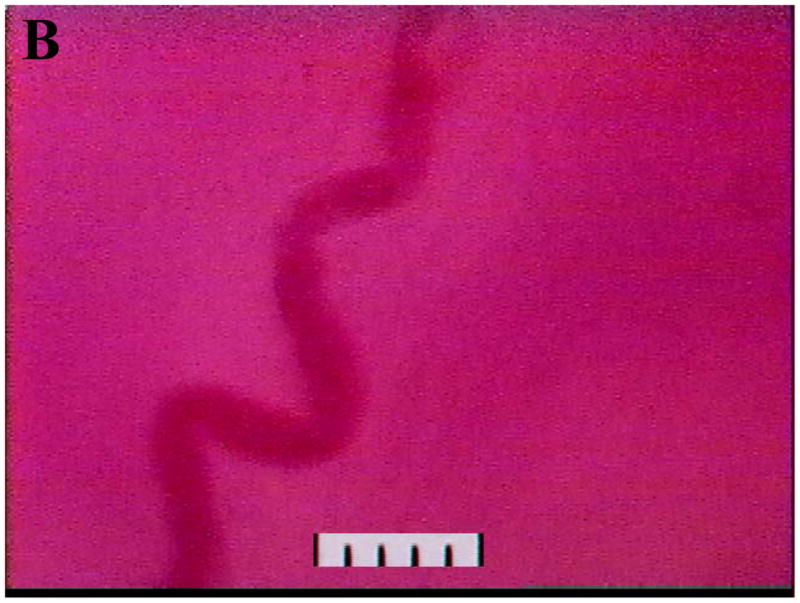
Digitized videoimages of transilluminated (A) cremaster arterioles passing through striated muscle fibres in an adult male CD1 mouse and (B) decidual spiral artery at gd 12 in an adult female CD1 mouse. Videorecordings were made using a Sony DXC-390 video camera and a BXWI Olympus microscope and a 20X objective: final magnification approximately 2000X. In (A) the cremaster muscle consists of approximately 2 layers of skeletal muscles (striated due to the actin-myosin banding pattern) running vertically and diagonally through the image. The arterioles consist of a single layer of vascular smooth muscle cells (a smooth muscle cell indicated by the arrow) wrapped circumferentially around a single layer of endothelial cells interfacing with the blood column (red). Scale: 10μm per division.
DIFFERENTIATION OF MOUSE UNK CELLS
In adult mice, NK cells differentiate from bone marrow hemopoietic progenitor cells in response to cytokines, including those that signal via receptors that contain the common cytokine receptor gamma-chain (γc). In addition to γc, pre-NK cells express CD122 (beta chain of the receptors for IL-2/IL-15), but lack NK1.1, CD49b and Ly49 gene family members which characterize mature NK cells;. Normal numbers of pre-NK cells are found in γc-deficient mice, showing that NK cell commitment is not dependent on the cytokines whose receptors incorporate γc such as IL-2, 4, 7, 9, 15, or 21. However, IL-15 plays a dominant role in early NK cell differentiation by maintaining normal numbers of immature and mature NK cells in bone marrow and spleen 47.
IL-15 has been shown to be the critical uterine stromal cytokine promoting uNK cell differentiation. Mouse uterine IL-15 production peaks at gd10 48. Mice genetically deleted in IL-15 differentiate no uNK cells during their pregnancies and their spiral arteries resemble those that cannot be gestationally modified in γc-deficient mice 49. Transplantation of bone marrow from normal mice to these strains has different implantation site outcomes. In IL-15 deficient females, there is no implantation site change and no uNK cell differentiation. In γc-deficient females, there is uNK cell differentiation and pregnancy-associated structural change in the spiral arteries 49. These data suggest that the cyclic, progesterone-regulated expression of IL-15 reported in human endometrium 50 is key to human NK cell differentiation within the uterus.
ACTIVATION OF MOUSE UNK CELLS
Differentiated uNK cells must become activated to produce IFN-γ. While many pathways involved in NK cell activation by MHC antigens, viruses, cytokines and immune-cell interactions have been defined, it cannot be assumed that uNK cells will be identical. Direct proof is necessary. For example, systemic NK cells are deficient in mice lacking IRF-1 but uNK cells are normal in number and function in IRF-1 null mice where molecules other than IRF-1 appear to regulate expression of uterine IL-15 49. Similarly, recent studies of the T-bet null mouse showed these animals were severely NK cell deficient and concluded that T-bet, a factor regulating IFN-γ gene transcription, was essential for differentiation of the NK cell lineage 51. Further study revealed that uNK cells differentiated abundantly in T-bet null mice and adequately modified implantation site spiral arteries. This led us to assess the levels of transcription of a less well studied factor associated with IFN-γ gene transcription, Eomes, in mouse uNK cells. Eomes is transcribed 1000 times more abundantly than T-bet (now formally Tbx21) between gd 8–12 and is probably the key transcription factor used by uNK cells to upregulate IFN-γ 52. Because Eomes null mice are not viable, this conclusion has not been confirmed in vivo.
IL-12, 18, 23 and 27 are cytokines known to promote and enhance IFN-γ production. They are produced by the very early decidua 53. Interestingly, about gd6, the production of uterine IL-18 moves from stromal cells to uNK cells themselves which is thought to sustain and provide autoregulation of the pro-inflammatory conditions needed for IFN-γ production 53.
The LY49 gene family encodes murine NK cell surface receptors. These are type II lectin receptors engaged by MHC molecules. Most LY49 gene products are function inhibiting receptors but several are activating receptors. Engagement of activating receptors initiates a biochemical cascade ultimately triggering cytokine production and/or cytotoxicity. We examined transcription of the LY49 gene family in C57Bl/6J mice using endometrium and isolated uNK cells. In virgin uterus the two activating receptors LY49D and H were transcribed as were several inhibitory receptors. Pregnancy induced expression of the remaining inhibitory receptors (Paffaro et al, manuscript in preparation). Thus, pregnancy appeared to upregulate signals that would dampen uNK cell function. This conclusion is the opposite to that made from the genomic study of Hiby et al addressing relationships between genes for the human NK cell receptor family known as Killer Immunoglobulin-like receptors (KIR) and pre-eclampsia. Pre-eclampsia was associated with a specific fetal genotype when the mother had a haplotype restricting NK cell activation 54. Further studies in humans at the RNA level and in both species at the protein level will be needed to determine if these are truly conflicting data. A non variant activating receptor NKG2D is shared by all human and mouse NK cells and is activated by stress antigens including the antigen family known as retinoic acid induced embryonic antigens (RAE-1) 55.
Upon NK receptor ligation, activation complexes are formed that signal through polypeptides which contain immunoreceptor tyrosine-based activation motifs (ITAMs) 56. These include KARAP/DAP12, FcRγ and CD3ζ, which interact variously to transmit signals from CD16, KIR-S, CD94 or Ly49 57. KARAP/DAP12 is an essential component for NK cell anti-viral and anti-tumor functions 58 and stimulation via this receptor results in either cytokine production (i.e.IFN-γ) or cytotoxicity. Stimulation through DAP10, which associates with NKG2D-L and is independent from ITAM signaling, results in cytotoxicity but not cytokine production 59. Murine NK cells also express 2B4, a member of the CD2 subset of IgG family of receptors which, through homophilic or heterophilic interactions, function as a co-receptors. Murine NK cells also express CD48, the ligand for 2B4, whose interaction is essential for IL-2-induced expansion, activation and secretion of IFN-γ 60. Inhibitory receptors are monomeric and express one or more intracytoplasmic immunoreceptor tyrosine-based inhibitory motifs (ITIMs) which transduce signals via the major histocompatibility complex (MHC) I ligands 59. The inhibitory effects of ITIMs are in dynamic equilibrium with ITAM activating signals, thus NK activation is in constant flux.
Gestational endometrium expresses KARAP/DAP12, FcRγ, CD3ζ and DAP10. NKG2D and RAE-1 are also expressed in normal mouse implantation sites, suggesting all the elements necessary for uNK cell activation are present during normal pregnancy 42. Studies of pregnancy in mice genetically deleted or mutated for KARAP/DAP12, FcRγ, CD3ζ and DAP10 revealed that only DAP10−/− mice had morphologically normal implantation sites and produced normal endometrial concentrations of IFN-γ. Mice without functional KARAP/DAP12 had limited spiral artery modification but the restriction was more severe in mice lacking FcRγ and CD3ζ. This suggests an unexpected role for IgG in mouse implantation sites that may reduce the stringency of the analogies between mouse and human uNK cells since human uNK cells lack CD16 and therefore would not be influenced by IgG. Mice dually deleted for DAP12 and FcRγ produced no decidual IFN-γ and did not show spiral arterial modification 42. This result is of particular interest because uNK cells are not the only source of decidual IFN-γ 20;61;62. It has been estimated in mice that ~10% of decidual IFN-γ is not uNK cell-derived. The total absence of IFN-γ suggests the non lymphocyte producing cells also use DAP12/FcRγ signal transduction.
An alternative mechanism for uNK cell activation that has not been widely explored is nerve stimulation 63. The uterus is innervated by the pelvic nerves which are mixed parasympathetic with sympathetic fibres. Grossly, nerves accompany the arterial supply that enters the uterus mesometrially. Murine uNK cells express asialo GM1 8, a brain ganglioside while human uNK cells express N-CAM1, a neural cell adhesion molecule capable of homotypic binding. To determine whether products of sympathetic nerves might participate in localization or activation of uNK cells, we mapped the sympathetic fibres in implantation sites using the sympathetic nerve restricted enzyme tyrosine hydroxylase (Fig. 2A) and compared the positions to the distribution of uNK cells (Fig 2B). Sympathetic fibres were found in the walls of the uterine arteries until they crossed the myometrium in both non pregnant and pregnant mice. No fibres were found in decidua or associated with the spiral arterial branches from the uterine artery. Thus, it seems unlikely that sympathetic innervation contributes to uNK cell localization or activation.
Fig. 2.
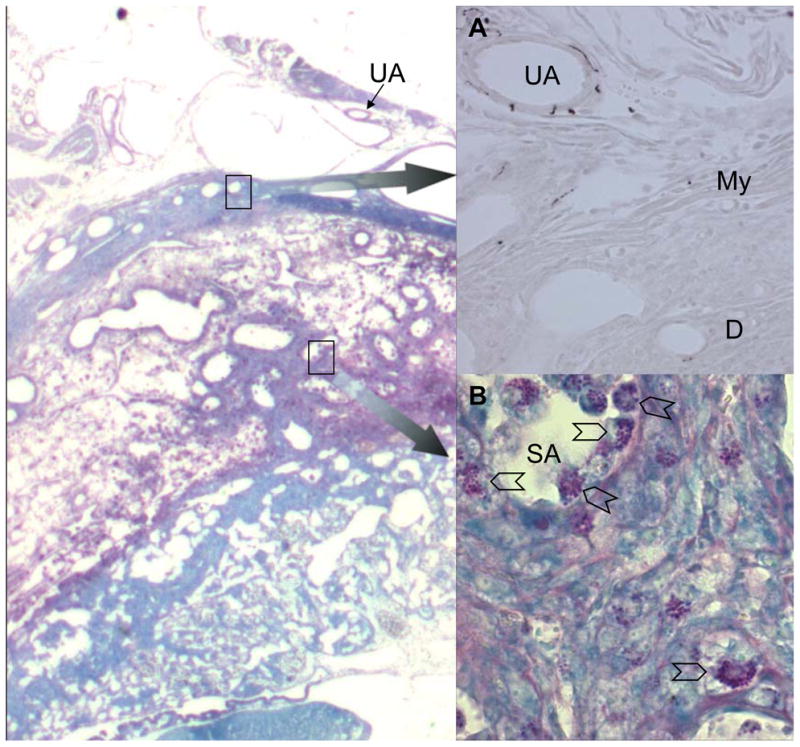
Photomicrographs of serial cryostat sections of gd10 C57Bl/6J implantation sites. Left panel shows Periodic Acid Schiff’s stained section with methylene blue counterstain, imaged at 40x. Insert (A) shows enlargement (imaged at 200x) of the upper boxed region with immunolocalization of sympathetic nerves using a primary goat antibody to tyrsoine hydroxylase (Chemicon, Th42), a rabbit anti-goat secondary reagent and DAB as the chromogen. Strong staining of sympathetic nerve fibres is seen in the walls of branches from the uterine artery (UA) as they pass in the mesometrium towards the uterus. Immunoreactivity is lost in the myometrium (My) and is absent from the decidua (D) and the walls of the spiral arterioles (SA) in the decidua x 400. Insert (B) shows enlargement (imaged at 400x) of the lower boxed region. UNK cells (chevrons) are abundant in the decidua and surrounding and within the walls and lumens of the spiral arteries. No evidence for co-localization of nerves and uNK cells was seen between gd6-12.
HUMAN UNK CELL INTERACTIONS WITH ENDOTHELIUM, AN ANALYSIS OF POTENTIAL FOR TRAFFICKING
NK cells in adult humans, like those in mice, are derived from hematopoietic stem cells found in marrow and in the bloodstream. Human NK cells become activated in the circulation and extravasate into tissues in response to inflammation. Molecules contributing to these egress-promoting interactions with endothelium include αLβ2, αMβ2, αXβ2, and α4β1 integrins, ICAM-1, PSGL-1, and L-selectin. The associated cytokines and chemokines that promote NK cell chemotaxis into tissues include IL-12, IFN-(α/β), CCL2, 3, 4, 5, 7, 8, CXCL8, and CX3CL1. NK cells are able to move into secondary and tertiary lymphoid sites without stimulation 64. These data suggest that an initial phase of uNK cell activation occurs before pre-uNK cells reach the uterus. It is unclear whether lineage differentiation of pro-, pre- and uNK cells is fully distinct from that defined for NK cells in lymphoid tissue, as suggested by microarray data 65 or if decidua creates an environment that promotes differentiation of a unique NK cell subset 66.
Using our assay of human blood lymphocyte adhesion to frozen mouse decidual tissue sections under shear force, we serially analyzed CD56+ lymphocyte adhesive function for 17 fertile women over a complete, hormonally-monitored menstrual cycle. This work established that periovulatory changes, associated with the surge in luteinizing hormone (LH), promote a 48–72 hour gain in lymphocyte adhesive function 67. This gain was L-selectin and α4 integrin dependent and could be obliterated if cells were pre-treated with antibodies blocking the functions of these molecules. Similar gains in adhesive function were induced in cells from male donors after 4 h culture in medium containing physiological levels of E2 or LH. Culture in medium containing pregnancy-associated levels of P4 did not alter adhesion in comparison to culture in medium alone.
Serial analyses of CD56+ blood lymphocyte adhesive function were also undertaken for 55 infertile women preparing clinically for embryo transfer. These patients were compared as two groups, those having natural cycles for frozen embryo transfer and those having hormonally-medicated cycles for oocyte collection, IVF and immediate embryo transfer. This study 68 found that pregnancy did not induce greater levels of adhesion than the menstrual cycle and did not sustain the elevated levels found between the LH surge and embryo transfer. In addition, administration of exogenous hormones did not alter NK cell adhesiveness to endothelium in decidual vessels. In women successfully achieving pregnancy after embryo transfer, adhesion had dropped to baseline levels at LH+18, the day of blood sampling for pregnancy diagnosis by β-hCG detection. Elevated adhesion was again found at luteal day 40 in pregnant subjects, the last time point in our analyses that coincided with the patient’s presentation for ultrasound confirmation of pregnancy. These data suggest that cyclic ovarian events promote a narrow window for pre-uNK cell homing to the human uterus. Other factors may influence the day 40 rise and new studies would be needed to determine the width of the time interval associated with this later, post implantation, gain in lymphocyte interactions with endothelium. Most interestingly, women who failed to carry a viable pregnancy to luteal day 40 did not show enhanced NK cell adhesiveness resembling that in fertile women. This suggests that in humans, uNK cells may have a role not seen in mice; of secreting molecules conducive to implantation. Alternatively, these data may suggest that blood NK cells serve as indicators for systemic adhesion molecule activation (including L-selectin) and mirror events occurring at the blastocyst surface as it interacts with endometrium45.
The functional changes in CD56+ blood lymphocyte functions are clearly hormonally synchronized. However, the influence of hormones appears to be indirect since highly purified populations of CD56+ cells do not display transcripts for the receptors for ER, PR or LHR (our unpublished data). The synchronous changes in endometrial stroma and endothelium are likely the primary, endocrinologically-targetted events and products of these changes are likely to alter the trafficking patterns of some NK cell lineage cells in the circulation and induce their functional activation. Exodus of pro- or pre-uNK cells from the circulation into decidua brings an encounter with IL-15, which drives terminal uNK cell differentiation. Encounters with IL-12, -18 and other molecules enhance the activation and maturation of the cells, sustaining them until they encounter and interact with trophoblast and engage additional activation receptors. It remains to be established if a higher level of functional activity is possible in these physiologically recruited cells that would promote fetal compromise and loss or whether these pathological events should be attributed to a different subset of specifically recruited immune cells.
Acknowledgments
Supported by NSERC, OMAFRA, Ontario Pork, CIHR, the Ontario Women’s Health Scholar Award and the Canada Research Chairs Program
References
- 1.Cupedo T, Mebius RE. Cellular interactions in lymph node development. J Immunol. 2005;174:21–25. doi: 10.4049/jimmunol.174.1.21. [DOI] [PubMed] [Google Scholar]
- 2.Kather A, Chantakru S, He H, Minhas K, Foster R, Markert UR, Pfeffer K, Croy BA. Neither lymphotoxin alpha nor lymphotoxin beta receptor expression is required for biogenesis of lymphoid aggregates or differentiation of natural killer cells in the pregnant mouse uterus. Immunology. 2003;108:338–345. doi: 10.1046/j.1365-2567.2003.01586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor RT, Lugering A, Newell KA, Williams IR. Intestinal cryptopatch formation in mice requires lymphotoxin alpha and the lymphotoxin beta receptor. J Immunol. 2004;173:7183–7189. doi: 10.4049/jimmunol.173.12.7183. [DOI] [PubMed] [Google Scholar]
- 4.Delgado SR, McBey BA, Yamashiro S, Fujita J, Kiso Y, Croy BA. Accounting for the peripartum loss of granulated metrial gland cells, a natural killer cell population, from the pregnant mouse uterus. J Leukoc Biol. 1996;59:262–269. [PubMed] [Google Scholar]
- 5.Paffaro VA, Jr, Bizinotto MC, Joazeiro PP, Yamada AT. Subset classification of mouse uterine natural killer cells by DBA lectin reactivity. Placenta. 2003;24:479–488. doi: 10.1053/plac.2002.0919. [DOI] [PubMed] [Google Scholar]
- 6.Kiso Y, McBey BA, Mason L, Croy BA. Histological assessment of the mouse uterus from birth to puberty for the appearance of LGL-1+ natural killer cells. Biol Reprod. 1992;47:227–232. doi: 10.1095/biolreprod47.2.227. [DOI] [PubMed] [Google Scholar]
- 7.Peel S. Granulated metrial gland cells. Adv Anat Embryol Cell Biol. 1989;115:1–112. doi: 10.1007/978-3-642-74170-8. [DOI] [PubMed] [Google Scholar]
- 8.Parr EL, Parr MB, Young JD. Localization of a pore-forming protein (perforin) in granulated metrial gland cells. Biol Reprod. 1987;37:1327–1335. doi: 10.1095/biolreprod37.5.1327. [DOI] [PubMed] [Google Scholar]
- 9.Chantakru S, Miller C, Roach LE, Kuziel WA, Maeda N, Wang WC, Evans SS, Croy BA. Contributions from self-renewal and trafficking to the uterine NK cell population of early pregnancy. J Immunol. 2002;168:22–28. doi: 10.4049/jimmunol.168.1.22. [DOI] [PubMed] [Google Scholar]
- 10.Kruse A, Martens N, Fernekorn U, Hallmann R, Butcher EC. Alterations in the expression of homing-associated molecules at the maternal/fetal interface during the course of pregnancy. Biol Reprod. 2002;66:333–345. doi: 10.1095/biolreprod66.2.333. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Tanaka T, Nakamura H, Umesaki N, Hirai K, Ishiko O, Ogita S, Kaneda K. Granulated metrial gland cells in the murine uterus: localization, kinetics, and the functional role in angiogenesis during pregnancy. Microsc Res Tech. 2003;60:420–429. doi: 10.1002/jemt.10280. [DOI] [PubMed] [Google Scholar]
- 12.Gambel P, Croy BA, Moore WD, Hunziker RD, Wegmann TG, Rossant J. Characterization of immune effector cells present in early murine decidua. Cell Immunol. 1985;93:303–314. doi: 10.1016/0008-8749(85)90136-4. [DOI] [PubMed] [Google Scholar]
- 13.Peel S, Adam E. The killing of rat placental cells by rat and mouse granulated metrial gland cells in vitro. Placenta. 1991;12:161–171. doi: 10.1016/0143-4004(91)90020-g. [DOI] [PubMed] [Google Scholar]
- 14.Croy BA, Guilbert LJ, Browne MA, Gough NM, Stinchcomb DT, Reed N, Wegmann TG. Characterization of cytokine production by the metrial gland and granulated metrial gland cells. J Reprod Immunol. 1991;19:149–166. doi: 10.1016/0165-0378(91)90014-h. [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Umesaki N, Nakamura H, Tanaka T, Nakatani K, Sakaguchi I, Ogita S, Kaneda K. Expression of vascular endothelial growth factor by granulated metrial gland cells in pregnant murine uteri. Cell Tissue Res. 2000;300:285–293. doi: 10.1007/s004410000198. [DOI] [PubMed] [Google Scholar]
- 16.Tayade C, Feng Y, Croy BA. An analysis of maternal and fetal gene expression during a peri-implantation life or death decision. 2005 Submitted. [Google Scholar]
- 17.Guimond MJ, Wang B, Croy BA. Engraftment of bone marrow from severe combined immunodeficient (SCID) mice reverses the reproductive deficits in natural killer cell-deficient tg epsilon 26 mice. J Exp Med. 1998;187:217–223. doi: 10.1084/jem.187.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Croy BA, di Santo JP, Greenwood JD, Chantakru S, Ashkar AA. Transplantation into genetically alymphoid mice as an approach to dissect the roles of uterine natural killer cells during pregnancy--a review. Placenta. 2000;21(Suppl A):S77–80. 77–80. doi: 10.1053/plac.1999.0518. [DOI] [PubMed] [Google Scholar]
- 19.Croy BA, Esadeg S, Chantakru S, van den Heuvel M, Paffaro VA, Jr, He H, Black G, Ashkar AA, Kiso Y, Zhang J. Update on pathways regulating the activation of uterine Natural Killer cells, their interactions with decidual spiral arteries and homing of their precursors to the uterus. J Reprod Immunol. 2003;59:175–191. doi: 10.1016/s0165-0378(03)00046-9. [DOI] [PubMed] [Google Scholar]
- 20.Ashkar AA, di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 2000;192:259–270. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunt JS, Miller L, Vassmer D, Croy BA. Expression of the inducible nitric oxide synthase gene in mouse uterine leukocytes and potential relationships with uterine function during pregnancy. Biol Reprod. 1997;57:827–836. doi: 10.1095/biolreprod57.4.827. [DOI] [PubMed] [Google Scholar]
- 22.Ashkar AA, Croy BA. Functions of uterine natural killer cells are mediated by interferon gamma production during murine pregnancy. Semin Immunol. 2001;13:235–241. doi: 10.1006/smim.2000.0319. [DOI] [PubMed] [Google Scholar]
- 23.Monk J, Leonard S, McBey B, Croy B. Induction of murine spiral artery modification by recombinant human interferon-gamma. Placenta. 2005 doi: 10.1016/j.placenta.2004.10.016. in press. [DOI] [PubMed] [Google Scholar]
- 24.Engelhardt H, Croy BA, King GJ. Evaluation of natural killer cell recruitment to embryonic attachment sites during early porcine pregnancy. Biol Reprod. 2002;66:1185–1192. doi: 10.1095/biolreprod66.4.1185. [DOI] [PubMed] [Google Scholar]
- 25.Li XF, Charnock-Jones DS, Zhang E, Hiby S, Malik S, Day K, Licence D, Bowen JM, Gardner L, King A, Loke YW, Smith SK. Angiogenic growth factor messenger ribonucleic acids in uterine natural killer cells. J Clin Endocrinol Metab. 2001;86:1823–1834. doi: 10.1210/jcem.86.4.7418. [DOI] [PubMed] [Google Scholar]
- 26.Bulmer JN, Lash GE. Human uterine natural killer cells: a reappraisal. Mol Immunol. 2005;42:511–521. doi: 10.1016/j.molimm.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 27.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 28.Burrows TD, King A, Loke YW. Expression of adhesion molecules by endovascular trophoblast and decidual endothelial cells: implications for vascular invasion during implantation. Placenta. 1994;15:21–33. doi: 10.1016/s0143-4004(05)80233-4. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A J Clin Invest. 1997;99:2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 31.Campbell JJ, Butcher EC. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol. 2000;12:336–341. doi: 10.1016/s0952-7915(00)00096-0. [DOI] [PubMed] [Google Scholar]
- 32.Berg EL, Goldstein LA, Jutila MA, Nakache M, Picker LJ, Streeter PR, Wu NW, Zhou D, Butcher EC. Homing receptors and vascular addressins: cell adhesion molecules that direct lymphocyte traffic. Immunol Rev. 1989;108:5–18. doi: 10.1111/j.1600-065x.1989.tb00010.x. [DOI] [PubMed] [Google Scholar]
- 33.von Andrian UH, Chambers JD, Berg EL, Michie SA, Brown DA, Karolak D, Ramezani L, Berger EM, Arfors KE, Butcher EC. L-selectin mediates neutrophil rolling in inflamed venules through sialyl LewisX-dependent and -independent recognition pathways. Blood. 1993;82:182–191. [PubMed] [Google Scholar]
- 34.Kunkel EJ, Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 2002;16:1–4. doi: 10.1016/s1074-7613(01)00261-8. [DOI] [PubMed] [Google Scholar]
- 35.Barlic J, Sechler JM, Murphy PM. IL-15 and IL-2 oppositely regulate expression of the chemokine receptor CX3CR1. Blood. 2003;102:3494–3503. doi: 10.1182/blood-2003-03-0946. [DOI] [PubMed] [Google Scholar]
- 36.Mack M, Cihak J, Simonis C, Luckow B, Proudfoot AE, Plachy J, Bruhl H, Frink M, Anders HJ, Vielhauer V, Pfirstinger J, Stangassinger M, Schlondorff D. Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J Immunol. 2001;166:4697–4704. doi: 10.4049/jimmunol.166.7.4697. [DOI] [PubMed] [Google Scholar]
- 37.Campbell JJ, Qin S, Unutmaz D, Soler D, Murphy KE, Hodge MR, Wu L, Butcher EC. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol. 2001;166:6477–6482. doi: 10.4049/jimmunol.166.11.6477. [DOI] [PubMed] [Google Scholar]
- 38.Hanna J, Wald O, Goldman-Wohl D, Prus D, Markel G, Gazit R, Katz G, Haimov-Kochman R, Fujii N, Yagel S, Peled A, Mandelboim O. CXCL12 expression by invasive trophoblasts induces the specific migration of CD16- human natural killer cells. Blood. 2003;102:1569–1577. doi: 10.1182/blood-2003-02-0517. [DOI] [PubMed] [Google Scholar]
- 39.Frey M, Packianathan NB, Fehniger TA, Ross ME, Wang WC, Stewart CC, Caligiuri MA, Evans SS. Differential expression and function of L-selectin on CD56bright and CD56dim natural killer cell subsets. J Immunol. 1998;161:400–408. [PubMed] [Google Scholar]
- 40.Chantakru S, Kuziel WA, Maeda N, Croy BA. A study on the density and distribution of uterine Natural Killer cells at mid pregnancy in mice genetically-ablated for CCR2, CCR 5 and the CCR5 receptor ligand, MIP-1 alpha. J Reprod Immunol. 2001;49:33–47. doi: 10.1016/s0165-0378(00)00076-0. [DOI] [PubMed] [Google Scholar]
- 41.Xie X, Kang J, Anderson LN, He H, Lu X, Croy BA. Analysis of the Contributions of L-Selectin and CXCR3 in Mediating Leukocyte Homing to Pregnant Mouse Uterus. Am J Reprod Immunol. 2005;53:1–12. doi: 10.1111/j.1600-0897.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 42.Xie X, He H, Colonna M, Seya T, Takai T, Croy BA. Pathways Participating in Activation of Mouse Uterine Natural Killer Cells During Pregnancy. Biol Reprod. 2005 doi: 10.1095/biolreprod.104.033951. [DOI] [PubMed] [Google Scholar]
- 43.Stamper HBJ, Woodruff JJ. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med. 1976;144:828–833. doi: 10.1084/jem.144.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanninen A, Taylor C, Streeter PR, Stark LS, Sarte JM, Shizuru JA, Simell O, Michie SA. Vascular addressins are induced on islet vessels during insulitis in nonobese diabetic mice and are involved in lymphoid cell binding to islet endothelium. J Clin Invest. 1993;92:2509–2515. doi: 10.1172/JCI116859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Genbacev OD, Prakobphol A, Foulk RA, Krtolica AR, Ilic D, Singer MS, Yang ZQ, Kiessling LL, Rosen SD, Fisher SJ. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science. 2003;299:405–408. doi: 10.1126/science.1079546. [DOI] [PubMed] [Google Scholar]
- 46.Chantakru S, Wang WC, van den Heuvel M, Bashar S, Simpson A, Chen Q, Croy BA, Evans SS. Coordinate Regulation of Lymphocyte-Endothelial Interactions by Pregnancy-Associated Hormones. J Immunol. 2003;171:1132–1145. doi: 10.4049/jimmunol.171.8.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vosshenrich CA, Ranson T, Samson SI, Corcuff E, Colucci F, Rosmaraki EE, di Santo JP. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J Immunol. 2005;174:1213–1221. doi: 10.4049/jimmunol.174.3.1213. [DOI] [PubMed] [Google Scholar]
- 48.Ye W, Zheng LM, Young JD, Liu CC. The involvement of interleukin (IL)-15 in regulating the differentiation of granulated metrial gland cells in mouse pregnant uterus. J Exp Med. 1996;184:2405–2410. doi: 10.1084/jem.184.6.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ashkar AA, Black GP, Wei Q, He H, Liang L, Head JR, Croy BA. Assessment of requirements for IL-15 and IFN regulatory factors in uterine NK cell differentiation and function during pregnancy. J Immunol. 2003;171:2937–2944. doi: 10.4049/jimmunol.171.6.2937. [DOI] [PubMed] [Google Scholar]
- 50.Kitaya K, Yasuda J, Yagi I, Tada Y, Fushiki S, Honjo H. IL-15 expression at human endometrium and decidua. Biol Reprod. 2003;63:683–687. doi: 10.1095/biolreprod63.3.683. [DOI] [PubMed] [Google Scholar]
- 51.Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, Gapin L, Glimcher LH. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 52.Tayade C, Fang Y, Black GP, Paffaro VA, Jr, Croy BA. Transcription of Eomes and T-bet during differentiation and effector function development of mouse uterine natural killer cells. 2005 doi: 10.1189/jlb.0305142. submitted. [DOI] [PubMed] [Google Scholar]
- 53.Zhang JH, He H, Borzychowski AM, Takeda K, Akira S, Croy BA. Analysis of cytokine regulators inducing interferon production by mouse uterine natural killer cells. Biol Reprod. 2003;69:404–411. doi: 10.1095/biolreprod.103.015529. [DOI] [PubMed] [Google Scholar]
- 54.Hiby SE, Walker JJ, O'shaughnessy KM, Redman CW, Carrington M, Trowsdale J, Moffett A. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200:957–965. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cerwenka A, Bakker AB, McClanahan T, Wagner J, Wu J, Phillips JH, Lanier LL. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity. 2000;12:721–727. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- 56.Feng J, Garrity D, Call ME, Moffett H, Wucherpfennig KW. Convergence on a distinctive assembly mechanism by unrelated families of activating immune receptors. Immunity. 2005;22:427–438. doi: 10.1016/j.immuni.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moretta L, Moretta A. Killer immunoglobulin-like receptors. Curr Opin Immunol. 2004;16:626–633. doi: 10.1016/j.coi.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 58.Tomasello E, Vivier E. KARAP/DAP12/TYROBP: three names and a multiplicity of biological functions. Eur J Immunol. 2005 doi: 10.1002/eji.200425932. [DOI] [PubMed] [Google Scholar]
- 59.Diefenbach A, Tomasello E, Lucas M, Jamieson AM, Hsia JK, Vivier E, Raulet DH. Selective associations with signaling proteins determine stimulatory versus costimulatory activity of NKG2D. Nat Immunol. 2002;3:1142–1149. doi: 10.1038/ni858. [DOI] [PubMed] [Google Scholar]
- 60.Lee KM, Forman JP, McNerney ME, Stepp S, Kuppireddi S, Guzior D, Latchman YE, Sayegh MH, Yagita H, Park CK, Oh SB, Wulfing C, Schatzle J, Mathew PA, Sharpe AH, Kumar V. Requirement of homotypic NK cell interactions through 2B4(CD244)/CD48 in the generation of NK effector functions. Blood. 2005 doi: 10.1182/blood-2005-01-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ashkar AA, Croy BA. Interferon-gamma contributes to the normalcy of murine pregnancy. Biol Reprod. 1999;61:493–502. doi: 10.1095/biolreprod61.2.493. [DOI] [PubMed] [Google Scholar]
- 62.Yeaman GR, Collins JE, Currie JK, Guyre PM, Wira CR, Fanger MW. IFN-gamma is produced by polymorphonuclear neutrophils in human uterine endometrium and by cultured peripheral blood polymorphonuclear neutrophils. J Immunol. 1998;160:5145–5153. [PubMed] [Google Scholar]
- 63.Madden KS, ThyagaRajan S, Felten DL. Alterations in sympathetic noradrenergic innervation in lymphoid organs with age. Ann NY Acad Sci. 1998;840:262–268. doi: 10.1111/j.1749-6632.1998.tb09566.x. [DOI] [PubMed] [Google Scholar]
- 64.Morris MA, Ley K. Trafficking of natural killer cells. Curr Mol Med. 2004;4:431–438. doi: 10.2174/1566524043360609. [DOI] [PubMed] [Google Scholar]
- 65.Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, Masch R, Lockwood CJ, Schachter AD, Park PJ, Strominger JL. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parham P. NK cells and trophoblasts: partners in pregnancy. J Exp Med. 2004;200:951–955. doi: 10.1084/jem.20041783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van den Heuvel MJ, Horrocks J, Bashar S, Taylor S, Burke S, Hatta K, Lewis JE, Croy BA. Menstrual cycle hormones induce changes in functional interactions between lymphocytes and decidual vascular endothelial cells. J Clin Endocrinol Metab. 2005;90:2835–2842. doi: 10.1210/jc.2004-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van den Heuvel MJ, Horrocks J, Bashar S, Hatta K, Burke S, Evans SS, Croy BA, Tekpetey FR. Periovulatory Increases in Tissue Homing Potential of Circulating CD56bright Cells Are Associated with Fertile Menstrual Cycles. J Clin Endocrinol Metab. 2005;90:3606–3613. doi: 10.1210/jc.2004-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]


