Summary
In primates, including women, and in rodents, Natural Killer lymphocytes (NK cells) have a unique relationship with the decidualizing uterus. Implantation sites from genetically-modified and transplanted mice have proven useful models for understanding potential mechanisms involved in recruitment, activation and functions of human CD56bright uterine (u)NK cells. Key findings are reviewed. In mice, uNK precursor cells are recruited from secondary lymphoid tissues and are activated coincident with their uterine arrival. UNK cells proliferate, produce cytokines (interferon gamma (IFN-γ) and interleukins (IL)-18 and IL-27), and terminally differentiate into granulated lymphocytes. Many uNK cells proliferate within the myometrium at each implantation site forming a structure, the mesometrial lymphoid aggregate of pregnancy (MLAp) that surrounds blood vessels servicing each placenta. Post-mitotic uNK cells are abundant within decidua basalis; frequently (>25%) associating with spiral arteries, intramurally and intralumenally. From midgestation, uNK cell numbers decline. Studies of implantation sites in mice lacking uNK cells, IFN-γ, components of IFN-γ-induction and -signalling pathways or IFN-γ-regulated genes, indicate that uNK cell-derived IFN-γ is essential in triggering pregnancy-induced spiral artery modification. Decidual maintenance and uNK cell death are additional effects of uNK-cell-derived IFN-γ. Thus, during the first half of gestation, UNK cells contribute to and sustain important changes in the maternal placental bed.
Keywords: decidua basalis, lymphocyte differentiation, lymphocyte activation, interferon gamma, spiral artery modification
Introduction
During uterine decidualization in women, non-human primates and rodents, lymphocytes of the Natural Killer (NK) lineage appear and become abundant as large, granulated cells (Fig 1; King-Moffett, 2002; Parr et al., 1987; Peel, 1989). NK cells differ from T- and B-lymphocytes because they lack somatically rearranged antigen-sensing receptors (Natarajan et al., 2002). NK cells contribute to innate immunity, participating in early immune protection, before clonal expansion of B and T lymphocytes. NK cell functions are lysis and cytokine production with individual cells having single or dual capacity. Lysis is directed against virally-infected cells and tumors cells. Interferon-gamma (IFN-γ), which restricts viral infection, is a major cytokine product (Trinchieri, 1995). Uterine (u)NK cells are predominantly activated, cytokine producing NK cells (Moffett-King, 2002). Fifteen years lapsed between NK cell identification in marrow, blood, and spleen and recognition of endometrial granulocytes (human designation) and granulated metrial gland cells (murine designation) as NK cells.
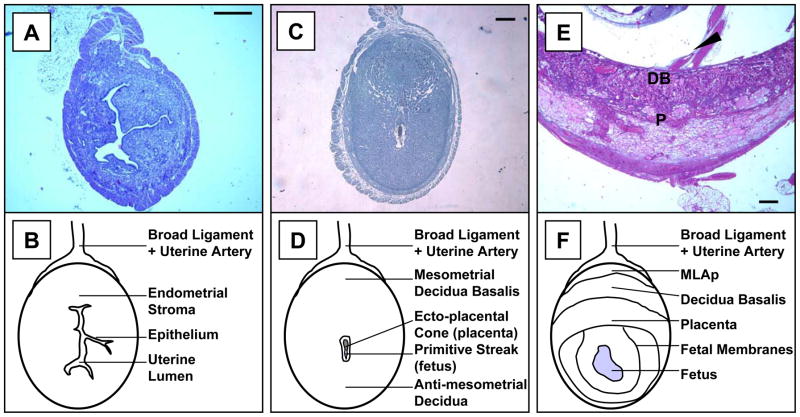
Figure 1.
Photomicrographs (A, C, E) and line drawings (B, D, F) of the virgin (A, B), gd 6 (C, D) and gd 10 (E, F) mouse uteri. In all images, the uterine artery access through the broad ligament (mesometrium) is placed to the top. Implantation triggers transformation of endometrial stroma into decidua. This process begins anti-mesometrially and progresses mesometrially (compare A to C) resulting in formation of the decidua basalis (DB) and loss of the uterine lumen. UNK cells are first found in DB. As additional structures differentiate, uNK cells also localize between the layers of the uterine wall, in a transient structure called the mesometrial lymphoid aggregate of pregnancy (MLAp), marked with arrowhead in (E), and are found at lower frequency within the placenta (P). Bar in (A) represents 400 μm and 200 μm in (C and E). (A and E) are stained with H&E; (C) is stained with DBA lectin and hematoxylin. UNK cell granules are distinctive in sections stained with Periodic Acid Schiff’s (PAS) reagent or DBA lectin (Peel, 1989; Paffaro et al., 2003). DBA lectin also stains uNK cell plasma membranes but PAS does not (Paffaro et al., 2003). UNK cells are somewhat difficult to recognize by H&E staining as their granules, which are histochemically eosinophilic, are relatively indistinct. Virgin uterus is DBA lectin negative.
UNK cells share many, but not all, of their features with peripheral NK cells. For example in humans, most blood NK cells analysed by flow cytometry express the surface marker CD16, an immunoglobulin domain receptor, and have dim expression of CD56, an adhesion molecule. About 1% of circulating lymphocytes are CD16-, CD56bright NK cells and these co-express high levels of the vascular addressin L-selectin (Campbell et al., 2001). In contrast, most human uNK cells express CD56 brightly but lack CD16 and L-selectin (Searle et al., 1999). Lymphotoxin β Receptor (LTβR) is a murine example. LTβR essential for peripheral NK cell differentiation and secondary lymphoid tissue formation (Fu and Chaplin, 1999). However uNK cells differentiate and an architecturally correct MLAp forms in pregnant LTβRo/o mice (Kather et al., 2003). Such findings limit the usefulness of studying NK cells not isolated from or functioning within the uterus and make the unique features of uNK cell recruitment, activation and differentiation key questions. It is of critical importance to recognize that these processes occur without pregnancy or fetal trophoblast tissue. In women, uNK cells differentiate in every menstrual cycle, 3–5 days after the LH surge (Bulmer et al., 1987; King, 2000). In rodents, induction of artificial deciduomata induces fully mature uNK cells (Peel, 1989).
The question of UNK cell function during pregnancy is also of tremendous interest. The cells are highly mobile and their high content of lytic molecules plus partnering antigen receptor display makes them potentially dangerous to trophoblast cells in implanting primate blastocysts and in developing placentae (primates and rodents). UNK cells could play essential physiological roles limiting normal trophoblast invasion or could destroy trophoblast, leading to pregnancy loss. Full definition of uNK cell antigen recognition on trophoblast and the intracellular signaling involved in uNK cell activation, differentiation and senescence would resolve such functional debates and provide clinically-relevant information. Mice have proven excellent models for characterization of uNK cell biology by allowing manipulative studies impossible in pregnant women (Table 1). Strains of genetically mutant mice are available to address uNK cell regulation and studies are relatively short in duration (mouse pregnancy is 19–20 days). Further, murine pregnancies can be interrupted and entire implantation sites studied. Inbred strains permit investigations of replicate pregnancies in time-course analyses that precisely define gestation’s changing cell and tissue relationships (Fig. 1). Murine uNK cell analyses are usually histological or molecular because no successful culture conditions or long-term cell lines are known. For our studies, at least 6 implantation sites derived from 2 or 3 pregnant females on at least 2 different days of gestational (gd) are serially sectioned for study. From each implant site 11 central sections are selected for computer-based image analysis in a manner ensuring no duplicate counting of individual cells occurs. Control pregnancies are matched by genetic background as closely as possible, being gd-matched sibling matings or matings of a congenic inbred partner strain. Human uNK cell study occurs at more restricted times, largely those associated with biopsy or elective termination and is genetically heterogeneous. In addition to histopathology, flow cytometry is widely used to study lymphocytes dissociated from human specimens and primary cloning has been achieved (Christmas et al., 1990). Application of expression microarrays and laser capture microdissection combined with quantitative analyses will provide information unique to human uNK cells and their environment, an environment distinct from that of the rodent anatomically, endocrinologically and in gestational length. The mouse will remain a key tool in critical assessment of new data from women because it can move studies from correlative findings to genetically-defined in vivo gestational data with potential for experimental interventions. UNK cells are infrequent or absent at term in both mice and women (Delgado et al., 1996; King, 2000), making term tissue irrelevant for defining uNK cell functions.
Table 1.
Summary of histological findings in uNK cell deficient mice ± bone marrow transplantation
| Strain | Manipulations | Histological Findings | ||||
|---|---|---|---|---|---|---|
| Phenotype | Name | UNK | DB | SA | MLAp | |
| Normal | C57Bl/6J 129J Balb/cJ CD1 |
- | + | Cellular | Modified | Present |
| T ± B deficient | Nude SCID |
- | + | Cellular | Modified | Present |
| NK/uNK, T ± B deficient | IL-2/15Rβ0/0 IL- 2/15Rγo/o IL-15o/o Tgε26 RAG-2o/o/γo/o |
- | - | Hypocellular | Unmodified | Absent |
| Bone Marrow graft into uNK deficient | RAG-2o/o/γo/o | + SCID BM + C57Bl/6J BM + IL-15o/o BM + βERKO BM + αERKO BM |
+ | Cellular | Modified | Present |
NK Cell Differentiation and Establishing the uNK Cell Lineage
i) Origins
NK cells are generated from pluripotent bone marrow (BM) stem cells. Their differentiation in mice is highly dependent upon stromal factors including stem cell factor, interleukin (IL)-7 and IL-15 (Kennedy et al., 2000; Rosmaraki et al., 2001). Lineage-committed NK-precursor cells have limited self renewal ability and are present in secondary lymphoid tissues (blood, spleen, lymph nodes (LN)) but not uterus (Chantakru et al., 2002 and references therein). Transplants from NK cell sufficient mice to NK cell deficient mice established that uNK precursors are present in fetal liver; fetal, neonatal and adult thymus; marrow, LN and spleen (Chantakru et al., 2002). Only spleen showed enhanced ability to generate uNK cells if harvested from pregnant donors (gd 3-7) while LN draining pregnant uteri were devoid of uNK precursors (Fig. 2). These observations strongly suggest that, prior to implantation, endocrine signals coordinately mobilize uNK precursors from spleen and induce mechanisms for trapping and retaining these circulating cells within the uterus (Chantakru et al., 2002).
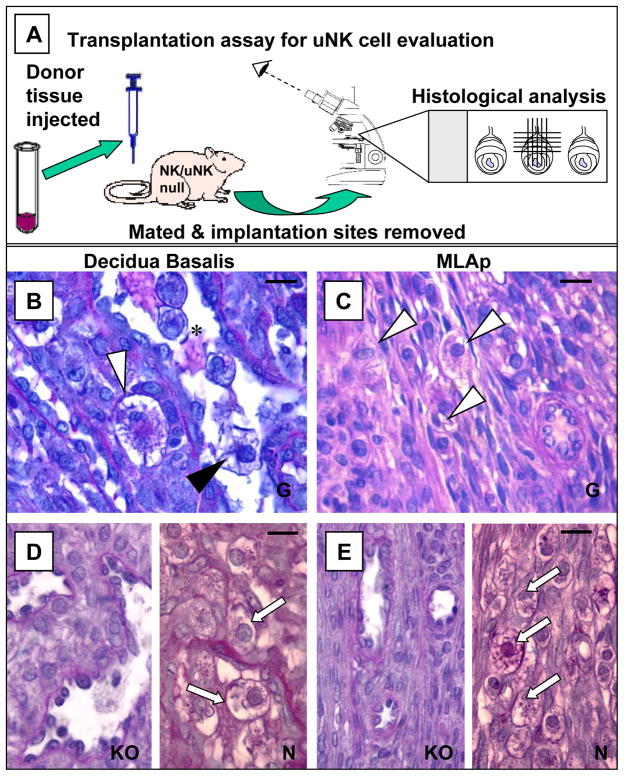
Figure 2.
The in vivo assay system, used to assess uNK cell precursor differentiation, homing and regulation, and to define uNK cell functions, depends on pregnancy in mice genetically deficient in the NK/uNK cell lineages. At present, the most reliable mouse used for these studies and manipulations is the RAG-2o/o/γco/o double knockout. The RAG-2o/o/γco/o is alymphoid but fertile under appropriate housing. Cell suspensions or tissues are grafted into virgin or mated uNK cell deficient recipients. Donors can be normal or altered in the gene(s) of interest. At different times after transplantation, implantation sites are analysed for graft-derived uNK cells. Any and all uNK cells will be graft derived in RAG-2o/o/γco/o recipients. Independent microdomains (usually decidua basalis (DB) and the mural, mesometrial lymphoid aggregate of pregnancy (MLAp)) are assessed within each implantation site. The procedure is summarized in (A). (B) and (C) show graft-derived uNK cells in the DB and MLAp respectively (white and black arrowheads), 10 days after inoculation of peripheral lymph node cell suspensions from gd 5 C57Bl/6J mice to mated (gd 0) RAG-2o/o/co/o recipients. UNK cells in the MLAp are smaller, have fewer cytoplasmic granules and more mitotic figures than those in DB (compare C to B), findings replicating implantation sites in pregnant, genetically normal mice. (B) also illustrates that the common intravascular (*) position of uNK cells (black arrowhead) in DB is replicated in transplant recipients. (D) illustrates the DB in gd 10 unmanipulated RAG-2o/o/γco/o (KO) and C57Bl/6J (N) females. (E) illustrates the MLAp in gd 10 unmanipulated RAG-2o/o/γco/o (KO) and C57Bl/6J females. No uNK cells are found in unmanipulated RAG-2o/o/γco/o but numerous uNK cells (arrows) are present in these tissues in normal mice. Bars represent 20 μm; all sections PAS stained.
ii) Ovarian Hormone Effects
We functionally addressed the postulated steroid hormone-regulated interactions between lymphocytes and endothelium by assaying human blood lymphocyte adhesion to high endothelial venules in cryostat sections of mouse subcutaneous LN pools (PLN). Lymphocytes from anonymous, blood-bank donors were evaluated using PLN from virgin and pregnant mice. Endothelium from pregnant donors attracted more cells (Chantakru et al., 2002). Endothelium in PLN from ovariectomized mice given estradiol or progesterone or both, also gained adhesive function equaling pregnancy-induced gains. Antibody blocking studies established that L-selectin and α4-integrin ligands mediated the functional changes. Adhesion assays were extended using mouse uterine tissues as substrate (virgin, pregnant or steroid-hormone-treated) instead of PLN. Binding again occurred via L-selectin and α4-integrin dependent mechanisms and numbers of cells adhering/mm2 of uterus were elevated if tissue came from pregnant or steroid hormone-treated (E2, P4 or both), ovariectomized mice. Prelabelling the human lymphocytes with anti-CD56 revealed that gains in functional adhesion to DB occurred in both CD56+ and CD56- cells but greatest enrichment occurred in CD56+ cells (>70 fold compared to the starting blood cell suspension; Chantakru et al., 2002). Gain in ability of endothelium to attract lymphocytes was not systemic since no pregnancy- or steroid hormone-induced changes were observed in pancreatic endothelium (unpublished/submitted).
To determine if hormones regulate lymphocytes independently but coordinately with endothelium, two studies were performed. First, splenic lymphocyte suspensions from virgin, pregnant or ovariectomized mice treated with placebo, E2 and/or P4, were tested for adhesion to PLN from a single donor. Adhesion rates for splenocytes from virgin and ovariectomized, placebo-treated mice were similar and statistically lower than adhesion of splenocytes from pregnant or steroid hormone treated donors indicating the pregnancy, E2 and P4 enhance the ability of murine lymphocytes to interact with endothelium (unpublished/submitted). For the second study, women were bled thrice weekly over their menstrual cycle and adhesion to constant mouse tissue was assessed. A dynamic, predictable pattern was found with significant gains in cells adhering to PLN and to decidualized uterus at the LH surge (unpublished/submitted). These data are consistent with an hypothesis of periovulatory mobilization of human uNK cell precursors to blood and their uterine appearance at LH+3-5 (King, 2000). Limited duration and importance of the luteal phase in mice may explain why different hormones appear to mediate the pregnancy-associated gains in lymphocyte-endothelial interactions in women and mice. Whether these dynamic changes reflect important in vivo steps in recruitment of uNK precursors to human uteri remains to be addressed experimentally. In an in vivo model of fever-range hyperthermia, doubling in vitro adhesion correlated with quadrupled lymphocyte trafficking to the targeted site (Evans et al., 2001).
iii) IL-15
IL-15, a stromal cell and macrophage-derived cytokine is critical for NK cell differentiation in human and murine lymphoid tissue (Carson et al., 1994; Kennedy et al., 2000). IL-15 acts on precursor and immature NK cells but not during NK progenitor cell differentiation. Human and murine endometria express IL-15 (Ye et al., 1996; Kitaya et al., 2000; Okada et al., 2000; Dunn et al., 2002). In mice, IL-15 mRNA is transcribed between gd 6-11 (Ye et al., 1996, unpublished). In humans, IL-15 transcription is more abundant during the secretory than proliferative cycle phase and is sustained in early pregnancy with localization to endothelium and perivascular stromal cells of decidual spiral arteries (SA; Kitaya et al., 2000). These correlative time course data strongly suggest that IL-15 participates in uNK cell differentiation while the localization data suggest IL-15 may contribute to chemotactic localization of uNK cells within the lumens and walls of the spiral arteries.
IL-15 shares two of its’ three receptor chains (β and γ) with IL-2, a cytokine not normally found in gestational uteri. Mice genetically ablated for either shared receptor or for IL-15 do not differentiate uNK cells (Guimond et al., 1998; Croy et al., 2003). To establish that IL-15 contributes to terminal uNK cell differentiation, reconstitution and blocking BM transplantation experiments were conducted. The reconstitution experiment involved transplants between two strains that do not differentiate uNK cells. Alymphoid mice (RAG-2o/o/common cytokine receptor chain γ (γc)o/o, a double knockout strain that makes IL-15), were grafted with BM from IL-15o/o mice, then mated and studied histologically. UNK cell differentiation occurred at frequencies identical to those observed in normal congenic C57Bl/6J. For the blocking experiment, C57Bl/6J BM was given to IL-15o/o mice. This BM produces normal uNK cells when grafted into alymphoid mice (Ashkar et al., 2000), but was totally blocked from differentiation in uteri of IL-15o/o recipients (Croy et al., 2003 and unpublished/submitted). These experiments indicate that IL-15 is the crucial factor regulating uNK cell differentiation and provokes questions concerning regulation of uterine IL-15 and whether its deficiency or overabundance has clinical consequences in women, as suggested for recurrent spontaneous aborters by Chegini et al., (2002). Interferon regulatory factor (IRF)-1, a key regulator of IL-15 in marrow, is expressed in human and mouse uterus (Jabbour et al., 1999; Kitaya et al., 2001; unpublished/submitted) but does not appear to regulate murine uterine IL-15 (Ashkar et al., 1999 and submitted). In humans, uterine IRF-1 appears regulated by prolactin (Jabbour et al., 1999) while uterine IL-15 expression appears regulated by progesterone (Okada et al., 2000; Kitaya et al., 2000) and prostaglandins (Dunn et al., 2002). Additional information on uterine specific regulation of IL-15 is needed.
Clearly, ovarian steroid hormones act on uterine stroma, including endothelium, as discussed above, in ways that promote differentiation of uNK cells but whether the hormones act directly on the lymphocytes or their precursors is less clear. Availability of mice ablated for the estrogen receptor (ER) α or β (αERKO and βERKO) (Couse and Korach, 1999) permitted functional assessment of ER utilization by uNK cells. Marrow transplanted from αERKO and βERKO to alymphoid mice fully reconstituted uNK cells, indicating no direct functional action in mouse uNK cells through estrogen receptors. This conclusion was supported by RT-PCR analysis of highly purified (99%) gd12 and 13 uNK cells, isolated rapidly from perfused, normal (C57Bl/6J) mice using a newly-reported protocol involving magnetic beads coated with the lectin Dolichos biflorus agglutinin (DBA) (Paffaro et al., 2003, Borzychowski et al., 2003). mRNA for ERα and ERβ were absent while mRNA for other genes was detected (Borzychowski et al., 2003). Human uNK cells are recently reported ERα negative, as in earlier studies, but ERβ expressing (Henderson et al., 2003), suggesting species difference or transcripts from rare contaminating cells. In both human and mouse, studies of the complex interactions between the endocrine and stromal environments on lymphocyte differentiation within the uterus will be profitable and of clinical importance for endometrial as well as reproductive health.
NK Cell and uNK Cell Activation
Lymphocyte activation requires signals additional to those involved in lymphocyte differentiation. IL-12 is a cytokine important for induction of IFN-γ synthesis in NK and T cells (Trinchieri, 1995). IL-12’s action is enhanced by IL-18 (Nakanishi et al., 2001). IL-12 and IL-18 are found in normal human and mouse implantation sites (Devergne et al., 2001; Yoshino et al., 2001; Zourbas et al., 2001; Chaouat et al., 2002; Zhang et al., 2003). IL-18 shows a dynamic protein expression pattern by immunohistology, appearing first in gd 4-6 decidual stromal cells, then exclusively from gd 8-14 in uNK cells (Chaouat et al., 2002; Zhang et al., 2003). Pregnant mice dually deficient in IL-12 and IL-18 (Takeda et al., 1998) have been compared to littermates deficient in only one or neither cytokine (Zhang et al., 2003). Females and males matched in genotype were mated. UNK cell differentiation was morphologically and numerically similar in the four strains. In the three cytokine deficient strains, mid-gestation IFN-γ (after gd 8) was somewhat reduced and SA dilation was impaired but not as severely as in IFN-γo/o implantation sites. This suggested additional cytokines contribute to uNK cell activation. Two recently described cytokines, IL-23 and IL-27, are related to IL-12 and contribute to IFN-γ induction (Parham et al., 2002; Pflanz et al., 2002 and references therein). IL-23 and IL–27 expression is absent from virgin and gd 3 mesometrial uterus but present in mesometrial decidua from gd 4 (Zhang et al., 2003). Analyses of mRNA, from DBA lectin-purified uNK cells, suggest stromal cells produce IL-23 source while uNK cells produce IL-27. The time-course patterns for induction of these cytokines, match well with putative roles for these cytokines in uterine IFN-γ regulation in normal mice because IFN-γ protein is absent mesometrially from virgin and pre-implantation gd 3 uteri but is detected from gd 6, by ELISA. Peak mesometrial IFN-γ occurs at gd 10 and then declines (Ashkar et al., 2000).
NK cells interact with other cells via surface receptors that are classified as activating or inhibitory, depending on whether lytic activity is triggered when the receptor is engaged in in vitro assays. Individual cells display multiple receptors to give target recognition specificity. The ligands initially defined for NK cell receptors were transplantation antigens. Because uNK cells become activated in primates and rodents in the absence of conception, the role of transplantation antigen recognition, especially non-self antigen recognition, in uNK cell activation is unclear. Trophoblast has restricted transplantation antigen expression that, in outbred matings, will include paternally-derived, non-self antigens (Moffett-King, 2002). The human uNK cell population expresses a full repertoire of immunoglobulin-like NK cell receptors, although in different proportions than expressed by blood NK cells (Hiby et al., 1997). The major NK cells receptors in mice are lectin-like LY49 gene family members. UNK cells from C57Bl/6 mice express all of the LY49 NK receptors typical of the strain (unpublished). This sharing of receptors between blood NK and uNK cells suggests that uNK cell activation will display many features in common with NK cell activation in other tissues and predicts that the unique properties of uNK cells are not defined by these receptors.
A second type of NK cell activation receptor in mice and humans is NKG2D. The Rae-1 family of five retinoic acid-induced oncodevelopmental antigens was recently found to engage murine NKG2D, while the homologous human ligands are products of the HLA-associated genes MICA and MICB (Cerwenka and Lanier, 2001). Interactions between murine NK cells and Rae-1 genes are normally studied in vitro using embryonic cell line targets. In vivo expression of Rae-1 is only reported in midgestation developing mouse brain (Nomura et al., 1996). We addressed transcription of Rae 1-α-β-γ-δ-ε in uteri from C57Bl/6 and 129/J strains by RT-PCR. Non-pregnant uteri expressed no Rae-1 signal but strain appropriate Rae-1 expression was induced by gd 6 in DB and sustained at gd 10 in DB, MLAp and placenta.
Immunohistochemistry using a pan Rae-1α polyclonal antibody, showed extra-fetal expression was most strongly localized to trophoblast (unpublished). These and other developmental antigens expressed by trophoblast may be of major importance in sustaining uNK cell activation once trophoblasts and NK cells begin to intermingle (in mice from about gd 8). Roles for oncodevelopmental antigens are not yet explored in humans.
Functions of uNK cells
i) uNK Deficient and IFN-γ Signal Disrupted Mice
Several mouse strains have major (>99%) or absolute deficits in NK cells. Five, each having different gene deletions, are uNK cell deficient and have common histopathology (Table 1; Guimond et al., 1998; Ashkar et al., 2000). Three anomalies accompany absence of uNK cells; absence of myometrial MLAp development (i.e. a decidual bed deficit), hypocellularity and edema of DB and persistence of vascular smooth muscle in the SA with limited lumen dilation and vessel lengthening at midgestation. There is no overgrowth of trophoblast and no consistent quantifiable impairment in fetal or postnatal health (Greenwood et al., 2000). Issues such as fetal hypoxia, ventricular hypertrophy or maturity onset diabetes have not been experimentally addressed in the offspring. These findings imply that the normal functions of uNK cells during pregnancy are to congregate lymphocytes myometrially at the portals of uterine arteries and veins, to interact with and provide growth support for stromal cells committed to decidual differentiation and to trigger events that culminate in normal, pregnancy-induced SA modification. These interpretations were confirmed by normalizing implantation sites in NK/uNK cell deficient mice by BM transplants from SCID (T-B-) mice (Guimond et al., 1998). To address effector mechanisms, NK/uNK cell deficient females were transplanted with BM deleted for IFN-γ, IFN-γ Rα or their downstream signaling molecule Stat-1. Implantation sites in the recipients revealed that IFN-γ is not required to initiate uNK cell differentiation but low concentrations are required for full maturation and senescence of uNK cells. Terminal UNK cell maturation required ~1 IU/implantation site, which is adequately provided by other cell types (Ashkar et al., 2000). UNK cell-derived IFN-γ provides higher tissue concentrations (>6 IU/implantation site) and these levels support decidual integrity and SA modification. Daily infusions of mrIFN-γ (100 IU to 3000 IU/treatment) into gd 6-11 alymphoid females were effective in induction of normal SA modification and decidual maintenance in the absence of uNK cells (Ashkar et al., 2000). Thus, pro-inflammatory cytokines act physiologically in normal pregnancy.
ii) The α2-Macroglobulin Gene Family, Possible Targets of uNK-cell Derived IFN-γ
Because our work suggested IFN-γ acts on gene expression in a complex tissue that includes lymphocytes, stromal, vascular smooth muscle, endothelial and decidual cells, a cDNA microarray (GEMc, Incyte, St. Louis, MO) was undertaken. We compared maternal mesometrium from C57Bl/6J mice at gd 6 to gd 10. Forty-three genes, documented amongst the hundreds regulated by IFN-γ, were present on the array and differentially expressed (unpublished). Among these IFN-γ-regulated genes were mouse α2-macroglobulin (MAM) and its receptor. A related EST777415, defined as α2-macroglobulin (α2M) precursor-like, was among the most differentially up regulated genes. The array results were extended by Northern analyses. Non decidualized mouse uterus (virgin and gd 3) did not express EST777415 but expression was induced by gd6, peaked at gd 10 and 12 and then declined by gd 14 (unpublished). α2M family members are abundant plasma proteins that regulate the bioavailability of proteases and cytokines in tissue (Borth, 1994). The genes map closely in mice to the NK cell LY49 receptor gene complex on chromosome 6 (Ensembl, Mouse Genome Browser BLASTView).
To address potential roles of MAM and its related, expressed family member Murinoglobulin-1 (MUG-1) in implantation sites, histological analyses were undertaken using MAMo/o/MUG-1o/o mice (Umans et al., 1999). Placental development was highly unusual; the labyrinth was reduced while giant cells were in excess (Fig. 3). In addition, intramural trophoblast invasion of the SA was aggressive and by midgestation reached the myometrial circular smooth muscle layer, a position normally achieved in very late gestation. UNK cells were numerous and SA, although cuffed by trophoblast, were dilated. This suggested that α2M gene family members regulate the rate at which trophoblast invades and led us to characterize EST777415, which remains expressed in decidua of MAMo/o/MUG-1o/o (unpublished).
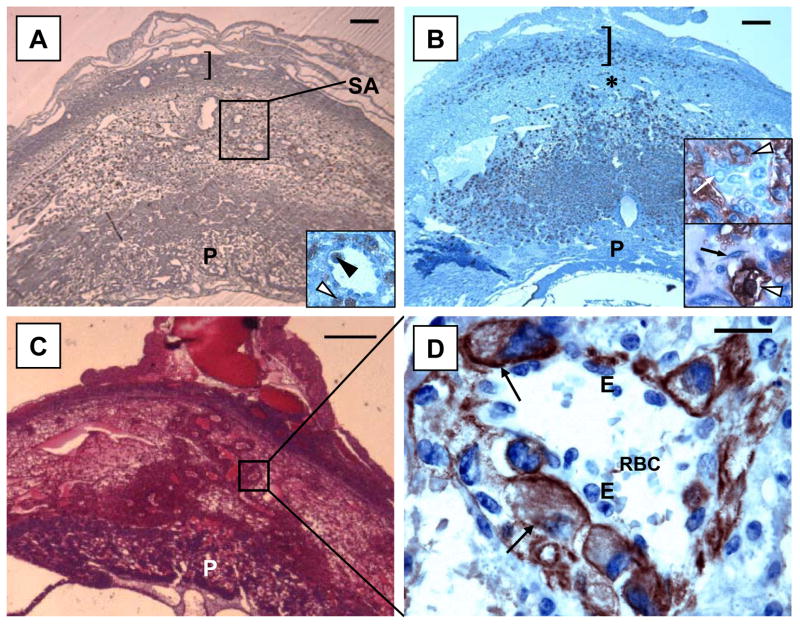
Figure 3.
Photomicrographs of gd 10 implantation sites in mice with different genetic mutations. (A) illustrates an implantation site in IL-12o/o/IL-18o/o. These mice have normal uNK cell differentiation and MLAp (]) and placental (P) development but lower than normal levels of IFN-γ at midgestation and only limited modification of the spiral arteries (SA). Intravascular (black arrowhead) and perivascular (white arrowhead) localization of uNK cells is seen in the decidua basalis of these mice (insert). (B) shows an implantation site in IFN-γo/o with over development of uNK cells and the MLAp (]). UNK cells are unusually localized in decidua basalis close to the placenta (P), leaving a band of decidua relatively void of uNK cells (*). Upper insert shows this region at gd 10 with healthy decidual cells (white arrow) clustered with darker, DBA-lectin stained uNK cells (arrowhead). Lower insert is of the same region at gd 12, when decidua basalis has become necrotic (Ashkar et al., 2000). Decidual cells have been destroyed and amorphous material fills the spaces between cells. Small mononuclear cells and fibroblasts (black arrow) replace the decidual cell population. UNK cells (arrowhead) appear less granulated at this time point (gd 12) than in normal mice. No spiral artery modification occurs in IFN-γo/o pregnancy. (C and D) illustrate an implantation site in MAMo/o/MUG-1o/o with relative overgrowth of trophoblast particularly along the spiral artery. (D) is a higher magnification (x1000) of the boxed area in (C) on a serial section stained with anti-cytokeratin antibody. It illustrates intramural trophoblast cells. Erythrocytes (RBC) are present in the spiral artery lumen and endothelial cells (E) were always present between trophoblasts (arrow) and circulating cells in this segment of the vessel. Bars represent 200 μm in (A and B), 400 μm in (C) and 20 μm in (D). (A and B) are stained with DBA lectin and hematoxylin; (C) is stained with H&E; (D) is counter- stained with Haematoxylin.
A full-length cDNA, now designated α2M of mouse pregnancy (A2Mp) was obtained from EST777415 (Genbank Accession # AY185125). The predicted amino acid sequence is typical of α2M family members with bait, thiol ester and receptor-binding domains. The bait region is most distinctive. The predicted A2Mp amino acid sequence is closest to rat α2M, an acute phase protein (90%) and 71% and 63% matched to the non-acute phase reactants human α2M and Pregnancy Zone Protein, a molecule of undefined function, that is highly up regulated during human gestation. Homology to the four other α2M mouse family members is at 50% (unpublished). Unlike other members of the mouse gene family, A2Mp is not detected in liver by RT-PCR or by in situ hybridization. Rather, testes and ovaries constitutively transcribe A2Mp; uterus transcribes A2Mp only with decidualization and the gene is transcribed in lactating mammary gland. In situ hybridizations (Fig. 4) localized A2Mp to primary spermatocytes and Sertoli cells in testesand to granulosa cells of secondary and pre-ovulatory ovarian follicles, in patterns distinct to those reported for MAM (Zhu et al., 1994; Dajee et al., 1998). Expression in 24 hr postpartum, lactating mammary tissue was localized to alveolar epithelium (unpublished). Uterine localization was dynamic. At gd6, A2Mp was expressed by anti-mesometrial decidua. Signal then migrated mesometrially and by gd 8–14 was in vascular smooth muscle of SA and unmodified fibroblasts interfaced between mesometrial myometrium and decidua (full description of these experiments will be published elsewhere). One interpretation of these findings is that A2Mp binds molecules needed for cells in reproductive tissues to transition from quiescence to active differentiation.
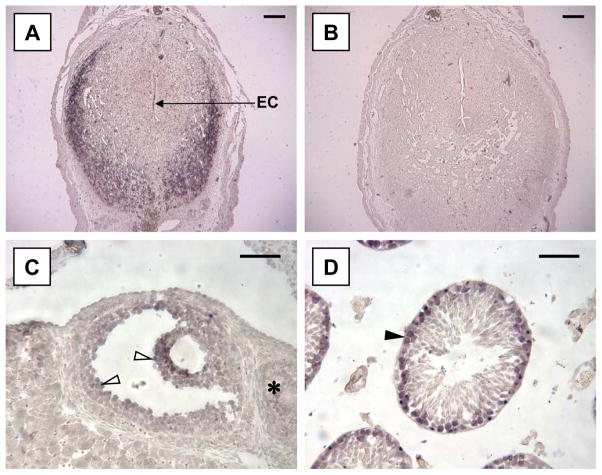
Figure 4.
In situ hybridization for A2Mp in reproductive tissues. (A) shows strong hybridization of an anti-sense DIG-labelled probe to anti-mesometrial decidual cells at gd 6. The embryonic crypt is labeled EC. (B) illustrates the sense strand hybridization negative control. (C) illustrates a secondary follicle in the ovary, showing hybridization to granulosa cells (arrowheads). Primary follicles (*) and corpora lutea (not shown) were unlabelled. (D) shows localized expression of A2Mp in a seminiferous tubule from a mature C57Bl/6J mouse (arrowhead). Hybridization appeared restricted to spermatogonia, primary spermatocytes and some Leydig cells. Magnification bar in (A and B) is 400 μm and, in (C and D) 50 μm.
We reasoned that if the A2Mp gene-derived product was the molecule of key importance induced by uNK cell-derived IFN-γ, administration of A2Mp to pregnant IFN-γo/o mice, would induce SA modification. Because no gene product is yet available but homology is significant to human α2M, human plasma-derived α2M or PBS was infused from gd 6-11 into pregnant IFN-γo/o or alymphoid mice. In both stains, native human α2M induced full SA modification while PBS induced no modification (unpublished). Thus, human α2M must have regulated proteases, cytokines or other molecules that signal vascular dilation and elongation during gestation. When human α2M, activated by methylamine to destroy its protease binding sites, was infused into pregnant alymphoid mice, SA dilation was again achieved. This experiment suggests that cytokines rather than proteases are the key molecules involved in SA destabilization. If A2Mp is the key molecule induced by uNK cell-derived IFN-γ and if A2Mp, thorough its bound molecules, effects SA dilation and elongation, it is not necessary to postulate additional vasoactive uNK cell products. However, these are known. In mice, inducible nitric oxide synthase, the enzyme generating the powerful vasodilator NO, is predominantly found in uNK cells of normal implantation sites (Hunt et al., 1996). In contrast, implantation sites in uNK cell-deficient mice display expression of this enzyme in trophoblast (Hunt et al., 1996). Vascular endothelial cell growth factor (VEGF), a molecule bound by α2M, is produced by murine and human uNK cells (Wang et al., 2000; Li et al., 2001). In humans, placental growth factor, a molecule upregulating the bioavailability of VEGF, is reported expressed exclusively in uNK cells (Li et al., 2001). Collectively these studies suggest that murine and human uNK cells contribute in various ways to the maternal vascular changes that occur in support of pregnancy.
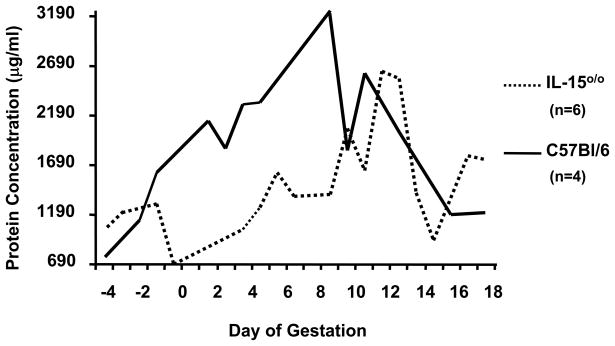
Conclusions
The rodent studies we pursue strongly suggest that uNK cells are key contributors to maternal uterine changes induced by and in support of pregnancy. They indicate that many regulatory steps are involved, each of which could have a clinical correlate culminating in syndromes such as pre-eclampsia or intrauterine growth retardation. While appealing to suggest that uNK cell deficient mice may model pre-eclampsia, our current opinion is that they model only vascular aspects. Serial urinalysis of mated NK/uNK cell deficient IL-15o/o and C57Bl/6 mice for protein (Lowry technique) failed to show proteinurea in uNK cell deficient mice above that seen in pregnant normal mice (Fig. 5). Other strains, such as BMP5 that shows pregnancy-induced glomerulosclerosis, proteinurea and hypertension (Davisson et al., 2002), or Thrombomodulin0/0, that displays trophoblast death and fibrinoid deposition (Isermann et al., 2003), are important and investigations of pre-eclampsia maybe best served by combining these or other strains as murine study models. While there are many differences between implantation sites in rodents and women, this review illustrates the dynamic and detailed information that can be collected from serial time-course studies of murine pregnancy to provide valuable concepts in understanding of the biology of human CD56bright uterine cells. The resolving power of mouse genetics, combined with transplantation, in addressing in vivo questions of regulatory mechanisms in mammalian pregnancy are illustrated. We hope others will develop and explore further variations of these approaches. (4164 Words)
Figure 5.
Analysis of mean urinary protein (Lowry assay) collected daily from individual uNK cell deficient IL-15 o/o mice (dashed line) and C57Bl/6J mice (solid line) from one estrous cycle (-4 to 0) and pregnancy (0-18). Every day, individual mice were placed in clean cages having a base composed of sterile 96 well plates. The mice were allowed to roam freely and void. Wells containing urine but no feces were collected and pooled when urine volumes of 50–200 μl appeared to be available. The uNK cell deficient mice were less proteinuric than their gestation-day matched normal congenic controls.
Acknowledgments
We thank the many collaborators who contributed genetically-manipulated mice, advice and other assistance to our studies. Immunohistology for Rae-1 was conducted by Drs. T. Seya and Y. Nishizawa, University of Osaka Prefecture. We also thank the staff of the OMAFRA animal housing facility, Guelph, for their concerns for the health of our immune deficient mice, their dedicated care of these animals and for teaching barrier husbandry procedures to all of the trainees who have contributed to this work.
Abbreviations
- α2M
alpha 2 Macroglobulin
- B
bone marrow derived
- BM
bone marrow
- γc
common cytokine receptor chain gamma
- DB
decidua basalis
- DBA
the lectin Dolichos biflorus agglutinin
- E2
estradiol
- ER
estrogen receptor
- ERKO
estrogen receptor knock out mouse
- gd
gestation day
- IFN-γ
interferon gamma
- IL
interleukin
- IRF-1
the transcription factor interferon regulatory factor-1
- LN
lymph node
- LTβR
lymphotoxin β receptor
- MAM
mouse alpha 2 Macroglobulin
- MLAp
mesometrial lymphoid aggregate of mouse pregnancy
- MUG
murinoglobulins, proteins of the mouse alpha 2 Macroglobulin gene family
- P4
progesterone
- PCR
polymerase chain reaction
- PLN
peripheral (not abdominal or thoracic) lymph nodes
- PAS
Periodic Acid Schiff’s reagent
- RAG
recombinase activating gene
- SCID
severe combined immunodeficient
- SA
spiral artery of the decidua basalis
- T
thymus-derived
- uNK cell
uterine Natural Killer lymphocyte
- VEGF
vascular endothelial growth factor
Footnotes
Supported by Awards from the Natural Sciences and Engineering Council, Canada and the Ontario Ministry of Agriculture, Food and Rural Affairs.
References
- 1.Ashkar AA, Di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. Journal of Experimental Medicine. 2000;192:259–270. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashkar AA, Croy BA. Interferon regulatory factor-1 has important functions in placental growth and modification of decidual spiral arteries. Placenta. 1999;20:A17. (and manuscript submitted) [Google Scholar]
- 3.Borth W, editor. Biology of alpha 2 Macroglobulin, its Receptor, and Related Proteins. Annals of the New York Academy of Sciences. 1994;737:1–521. [PubMed] [Google Scholar]
- 4.Borzychowski AM, Chantakru S, Minhas K, Paffaro VA, Jr, Yamada AT, He H, Korach KS, Croy BA. Functional Analysis of Murine Uterine Natural Killer Cells Genetically Devoid of Estrogen Receptors. Placenta. 2003;24:403–411. doi: 10.1053/plac.2002.0924. [DOI] [PubMed] [Google Scholar]
- 5.Bulmer JN, Hollings D, Ritson A. Immunocytochemical evidence that endometrial stromal granulocytes are granulated lymphocytes. Journal of Pathology. 1987;153:281–288. doi: 10.1002/path.1711530313. [DOI] [PubMed] [Google Scholar]
- 6.Campbell JJ, Qin S, Unutmaz D, Soler D, Murphy KE, Hodge MR, Wu L, Butcher EC. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. Journal of Immunology. 2001;166:6477–6482. doi: 10.4049/jimmunol.166.11.6477. [DOI] [PubMed] [Google Scholar]
- 7.Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri MA. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. Journal of Experimental Medicine. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerwenka A, Lanier L. Ligands for natural killer cell receptors: redundancy or specificity. Immunology Reviews. 2001;181:158–169. doi: 10.1034/j.1600-065x.2001.1810113.x. [DOI] [PubMed] [Google Scholar]
- 9.Chantakru S, Miller C, Roach LE, Kuziel WA, Maeda N, Wang WC, Evans SS, Croy BA. Contributions from self-renewal and trafficking to the uterine NK cell population of early pregnancy. Journal of Immunology. 2002;168:22–28. doi: 10.4049/jimmunol.168.1.22. [DOI] [PubMed] [Google Scholar]
- 10.Chaouat G, Zourbas S, Ostojic S, Lappree-Delage G, Dubanchet S, Ledee N, Martal J. A brief review of recent data on some cytokine expressions at the materno-foetal interface which might challenge the classical Th1/Th2 dichotomy. Journal of Reproductive Immunology. 2002;53:241–256. doi: 10.1016/s0165-0378(01)00119-x. [DOI] [PubMed] [Google Scholar]
- 11.Chegini N, Ma C, Roberts M, Williams RS, Ripps BA. Differential expression of interleukins (IL) IL-13 and IL-15 throughout the menstrual cycle in endometrium of normal fertile women and women with recurrent spontaneous abortion. Journal of Reproductive Immunology. 2002;56:93–110. doi: 10.1016/s0165-0378(02)00043-8. [DOI] [PubMed] [Google Scholar]
- 12.Christmas SE, Bulmer JN, Meager A, Johnson PM. Phenotypic and functional analysis of human CD3- decidual leucocyte clones. Immunology. 1990;71:182–189. [PMC free article] [PubMed] [Google Scholar]
- 13.Croy BA, Esadeg S, Chantakru S, van den Heuvel M, Paffaro VA, Jr, He H, Black GP, Ashkar AA, Kiso Y, Zhang J. Update on pathways regulating the activation of uterine Natural Killer cells, their interactions with decidual spiral arteries and homing of their precursors to the uterus. Journal of Reproductive Immunology. 2003 doi: 10.1016/s0165-0378(03)00046-9. in press. [DOI] [PubMed] [Google Scholar]
- 14.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocrinology Reviews. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 15.Dajee M, Georg HF, Richards JS. Stat 5b and the orphan nuclear receptors regulate expression of the α2 -macroglobulin gene in rat ovarian granulosa cells. Molecular Endocrinology. 1998;12:1393–1409. doi: 10.1210/mend.12.9.0161. [DOI] [PubMed] [Google Scholar]
- 16.Davisson RL, Hoffmann DS, Butz GM, Aldape G, Schlager G, Merrill DC, Sethi S, Weiss RM, Bates JN. Discovery of a spontaneous genetic mouse model of preeclampsia. Hypertension. 2002;39:337–342. doi: 10.1161/hy02t2.102904. [DOI] [PubMed] [Google Scholar]
- 17.Delgado SR, McBey BA, Yamashiro S, Fujita J, Kiso Y, Croy BA. Accounting for the peripartum loss of granulated metrial gland cells, a natural killer cell population, from the pregnant mouse uterus. Journal of Leukocyte Biology. 1996;59:262–269. [PubMed] [Google Scholar]
- 18.Devergne O, Coulomb-L'Hermine A, Capel F, Moussa M, Capron F. Expression of Epstein-Barr virus-induced gene 3, an interleukin-12 p40-related molecule, throughout human pregnancy: involvement of syncytiotrophoblasts and extravillous trophoblasts. American Journal of Pathology. 2001;159:1763–1776. doi: 10.1016/S0002-9440(10)63023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunn CL, Critchley HO, Kelly RW. IL-15 regulation in human endometrial stromal cells. Journal of Clinical Endocrinology and Metabolism. 2002;87:1898–1901. doi: 10.1210/jcem.87.4.8539. [DOI] [PubMed] [Google Scholar]
- 20.Evans SS, Wang WC, Bain MD, Burd R, Ostberg JR, Repasky EA. Fever-range hyperthermia dynamically regulates lymphocyte delivery to high endothelial venules. Blood. 2001;97:2727–2733. doi: 10.1182/blood.v97.9.2727. [DOI] [PubMed] [Google Scholar]
- 21.Fu YX, Chaplin DD. Development and maturation of secondary lymphoid tissues. Annual Reviews of Immunology. 1999;17:399–433. doi: 10.1146/annurev.immunol.17.1.399. [DOI] [PubMed] [Google Scholar]
- 22.Greenwood JD, Minhas K, Di Santo JP, Makita M, Kiso Y, Croy BA. Ultrastructural studies of implantation sites from mice deficient in uterine natural killer cells. Placenta. 2000;21:693–702. doi: 10.1053/plac.2000.0556. [DOI] [PubMed] [Google Scholar]
- 23.Guimond MJ, Wang B, Croy BA. Engraftment of bone marrow from severe combined immunodeficient (SCID) mice reverses the reproductive deficits in natural killer cell-deficient tg epsilon 26 mice. Journal of Experimental Medicine. 1998;187:217–223. doi: 10.1084/jem.187.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henderson TA, Saunders PT, Moffett-King A, Groome NP, Critchley HO. Steroid receptor expression in uterine natural killer cells. Journal of Clinical Endocrinology and Metabolism. 2003;88:440–449. doi: 10.1210/jc.2002-021174. [DOI] [PubMed] [Google Scholar]
- 25.Hiby SE, King A, Sharkey AM, Loke YW. Human uterine NK cells have a similar repertoire of killer inhibitory and activatory receptors to those found in blood, as demonstrated by RT-PCR and sequencing. Molecular Immunology. 1997;34:419–43. doi: 10.1016/s0161-5890(97)00032-1. [DOI] [PubMed] [Google Scholar]
- 26.Hunt JS, Miller L, Vassmer D, Croy BA. Expression of the inducible nitric oxide synthase gene in mouse uterine leukocytes and potential relationships with uterine function during pregnancy. Biology of Reproduction. 1997;57:827–836. doi: 10.1095/biolreprod57.4.827. [DOI] [PubMed] [Google Scholar]
- 27.Isermann B, Sood R, Pawlinski R, Zogg M, Kalloway S, Degen JL, Mackman N, Weiler H. The thrombomodulin-protein C system is essential for the maintenance of pregnancy. Nature Medicine. 2003;9:331–337. doi: 10.1038/nm825. [DOI] [PubMed] [Google Scholar]
- 28.Jabbour HN, Critchley HO, Yu-Lee LY, Boddy SC. Localization of interferon regulatory factor-1 (IRF-1) in nonpregnant human endometrium: expression of IRF-1 is up-regulated by prolactin during the secretory phase of the menstrual cycle. Journal of Clinical Endocrinology and Metabolism. 1999;84:4260–4265. doi: 10.1210/jcem.84.11.6142. [DOI] [PubMed] [Google Scholar]
- 29.Kather A, Chantakru S, He H, Minhas K, Foster R, Markert UR, Pfeffer K, Croy BA. Neither lymphotoxin alpha nor lymphotoxin beta receptor expression is required for biogenesis of lymphoid aggregates or differentiation of natural killer cells in the pregnant mouse uterus. Immunology. 2003;108:1–8. doi: 10.1046/j.1365-2567.2003.01586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrie K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JC, Joyce S, Peschon JJ. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. Journal of Experimental Medicine. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King A. Uterine leukocytes and decidualization. Human Reproduction Update. 2000;6:28–36. doi: 10.1093/humupd/6.1.28. [DOI] [PubMed] [Google Scholar]
- 32.Kitaya K, Yasuda J, Yagi I, Tada Y, Fushiki S, Honjo H. IL-15 expression at human endometrium and decidua. Biology of Reproduction. 2000;63:683–687. doi: 10.1095/biolreprod63.3.683. [DOI] [PubMed] [Google Scholar]
- 33.Kitaya K, Yasuda J, Fushiki S, Honjo H. Localization of interferon regulatory factor-1 in human endometrium throughout the menstrual cycle. Fertility and Sterility. 2001;75:992–996. doi: 10.1016/s0015-0282(01)01692-2. [DOI] [PubMed] [Google Scholar]
- 34.Li XF, Charnock-Jones DS, Zhang E, Hiby S, Malik S, Day K, Licence D, Bowen JM, Gardner L, King A, Loke YW, Smith SK. Angiogenic growth factor messenger ribonucleic acids in uterine natural killer cells. Journal of Clinical Endocrinology and Metabolism. 2001;86:1823–1834. doi: 10.1210/jcem.86.4.7418. [DOI] [PubMed] [Google Scholar]
- 35.Moffett-King A. Natural killer cells and pregnancy. Nature Reviews Immunology. 2002;2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 36.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annual Reviews of Immunology. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 37.Natarajan K, Dimasi N, Wang J, Mariuzza RA, Margulies DH. Structure and function of natural killer cell receptors: multiple molecular solutions to self, non-self discrimination. Annual Review of Immunology. 2002;20:853–885. doi: 10.1146/annurev.immunol.20.100301.064812. [DOI] [PubMed] [Google Scholar]
- 38.Nomura M, Zou Z, Joh T, Takihara Y, Matsuda Y, Shimada K. Genomic structures and characterization of Rae1 family members encoding GPI-anchored cell surface proteins and expressed predominantly in embryonic mouse brain. Journal of Biochemistry (Tokyo) 1996;120:987–995. doi: 10.1093/oxfordjournals.jbchem.a021517. [DOI] [PubMed] [Google Scholar]
- 39.Okada S, Okada H, Sanezumi M, Nakajima T, Yasuda K, Kanzaki H. Expression of interleukin-15 in human endometrium and decidua. Molecular Human Reproduction. 2000;6:75–80. doi: 10.1093/molehr/6.1.75. [DOI] [PubMed] [Google Scholar]
- 40.Paffaro VA, Jr, Bizinotto MC, Joazeiro PP, Yamada AT. Subset classification of mouse uterine Natural Killer cells by DBA lectin reactivity. Placenta. 2003 doi: 10.1053/plac.2002.0919. in press. [DOI] [PubMed] [Google Scholar]
- 41.Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, Pflanz S, Zhang R, Singh KP, Vega F, To W, Wagner J, O'Farrell AM, McClanahan T, Zurawski S, Hannum C, Gorman D, Rennick DM, Kastelein RA, de Waal Malefyt R, Moore KW. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. Journal of Immunology. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 42.Parr EL, Parr MB, Young JD. Localization of a pore-forming protein (perforin) in granulated metrial gland cells. Biology of Reproduction. 1987;37:1327–1335. doi: 10.1095/biolreprod37.5.1327. [DOI] [PubMed] [Google Scholar]
- 43.Peel S. Granulated metrial gland cells. Advances in Anatomy Embryology and Cell Biology. 1989;115:1–112. doi: 10.1007/978-3-642-74170-8. [DOI] [PubMed] [Google Scholar]
- 44.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 45.Rosmaraki EE, Douagi I, Roth C, Colucci F, Cumano A, Di Santo JP. Identification of committed NK cell progenitors in adult murine bone marrow. European Journal of Immunology. 2001;31:1900–1909. doi: 10.1002/1521-4141(200106)31:6<1900::aid-immu1900>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 46.Searle RF, Jones RK, Bulmer JN. Phenotypic analysis and proliferative responses of human endometrial granulated lymphocytes during the menstrual cycle. Biology Reproduction. 1999;60:871–878. doi: 10.1095/biolreprod60.4.871. [DOI] [PubMed] [Google Scholar]
- 47.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18–deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 48.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immuno-regulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annual Reviews of Immunology. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 49.Umans L, Serneels L, Overbergh L, Stas L, Van Leuven F. alpha2-macroglobulin-and murinoglobulin-1- deficient mice. A mouse model for acute pancreatitis. American Journal of Pathology. 1999;155:983–993. doi: 10.1016/s0002-9440(10)65198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang C, Umesaki N, Nakamura H, Tanaka T, Nakatani K, Sakaguchi I, Ogita S, Kaneda K. Expression of vascular endothelial growth factor by granulated metrial gland cells in pregnant murine uteri. Cell and Tissue Research. 2000;300:285–293. doi: 10.1007/s004410000198. [DOI] [PubMed] [Google Scholar]
- 51.Ye W, Zheng LM, Young JD, Liu CC. The involvement of interleukin (IL)-15 in regulating the differentiation of granulated metrial gland cells in mouse pregnant uterus. Journal of Experimental Medicine. 1996;184:2405–2410. doi: 10.1084/jem.184.6.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshino O, Osuga Y, Koga K, Tsutsumi O, Yano T, Fujii T, Kugu K, Momoeda M, Fujiwara T, Tomita K, Taketani Y. Evidence for the expression of interleukin (IL)-18, IL-18 receptor and IL-18 binding protein in the human endometrium. Molecular Human Reproduction. 2001;7:649–654. doi: 10.1093/molehr/7.7.649. [DOI] [PubMed] [Google Scholar]
- 53.Zhang JH, He H, Borzychowski AM, Takeda K, Akira S, Croy BA. Analysis of cytokine regulators inducing interferon-gamma production by mouse uterine Natural Killer cells. Biology of Reproduction. 2003 doi: 10.1095/biolreprod.103.015529. in press. [DOI] [PubMed] [Google Scholar]
- 54.Zhu LJ, Cheng CY, Phillips DM, Bardin CW. The immunohistochemical localization of alpha 2-macroglobulin in rat testes is consistent with its role in germ cell movement and spermiation. Journal of Andrology. 1994;15:575–582. [PubMed] [Google Scholar]
- 55.Zourbas S, Dubanchet S, Martal J, Chaouat G. Localization of pro-inflammatory (IL-12, IL-15) and anti-inflammatory (IL-11, IL-13) cytokines at the foeto-maternal interface during murine pregnancy. Clinical and Experimental Immunology. 2001;126:519–528. doi: 10.1046/j.1365-2249.2001.01607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]