Abstract
CD56bright lymphocytes become abundant in human uterus during every menstrual cycle, following the surge in pituitary-derived luteinizing hormone (LH) which initiates final oocyte maturation. While the uterus is host to some CD56bright cells prior to ovulation, the rapid increase is thought due to proliferation of the resident population, accompanied by recruitment of CD56bright lymphocytes from the circulation. Rapid increase of CD56bright cells is concurrent with onset of decidualization, the transformation uterine stromal cells into secretory decidual cells. Uterine CD56bright cells proliferate and differentiate to become the predominant lymphocytes of the post-ovulatory uterus. These distinct, tissue-specific Natural Killer (NK) cells either die prior to menses or expand in number during early pregnancy then decline towards the end of the first trimester. Since lymphocytes home to tissues from the circulation, we investigated mechanisms of NK cell traffic over the course of natural menstrual cycles by measuring functional interactions between CD56+ cells from blood and endothelial cells using the Stamper-Woodruff assay of lymphocyte adhesion to frozen tissue sections. While a baseline level of adhesion was maintained throughout the cycle, elevated L-selectin dependent adhesion of peripheral blood CD56bright cells occurred during a peri-ovulatory window. However, there were no significant menstrual cycle induced changes in transcription of L-selectin, alpha 4 integrin or LFA-1 or in expression of these proteins by NK cells, suggesting the enhanced adhesion was due to post-translational modifications of these molecules. Quantitative RT-PCR failed to amplify message for LH receptor or the alpha or beta forms of progesterone or estrogen receptors from blood NK cell subsets. Thus, we conclude that the actions of LH, E2 and P4 on NK cells that promote interactions with endothelium and potential uterine homing are indirectly mediated through the responsiveness of other cell types.
INTRODUCTION
Human and murine endometria respond to ovarian steroid priming by transforming into mature decidua (Maslar, 1988), a step essential for successful implantation (Genbacev et al., 2003). Amongst the many molecules secreted by decidua, specific cytokine and chemotactic signals and protease inhibitors are thought to orchestrate maternal contributions to successful implantation and the uterine modifications required to sustain pregnancy (Hanna et al., 2003; Kitaya et al., 2003b; Kao et al., 2002; Kao et al., 2003). Due to difficulties associated with study of the peri-implantation uterus, the maternal compartment remains more enigmatic than fetal and placental development but expression profiling studies using specific, menstrual cycle-timed endometrial biopsies from fertile and infertile women, have recently become available and will likely provide new insights into decidualization and implantation (Kao et al., 2002; Kao et al., 2003; Carson et al., 2002; Borthwick et al., 2003).
Decidualization of human and murine endometria is associated with the appearance and proliferation of two unique maternal cell types; Natural Killer (NK) cells and immature dendritic cells (DC) (King, 2000; Chaouat et al., 2003; Kammerer et al., 2003; Robertson et al., 2003). These cells emerge in precise microdomains within implantation sites, proliferate and many localize to the spiral arteries. Studies of mice genetically-deficient in lymphocytes identified three uterine (u)NK cell functions; 1) support of terminal decidual cell differentiation, 2) sensitization of spiral arteries that allows their pregnancy-associated dilation and elongation, 3) formation of a transient lymphoid aggregate at the portal for vessels and nerves servicing implant sites (Guimond et al., 1997; Croy et al., 2000). These uNK cell deficient mice retain their fertility, delivering a normal number of pups. UNK cells mediate these functions through secretion of interferon (IFN)-γ(Ashkar and Croy, 1999; Ashkar et al., 2000) which changes expression of target genes such as A2M, a major plasma cytokine binding and protease inhibiting molecule active in spiral artery modification (Croy et al., 2003; He et al., 2004).
In humans, decidua-associated (d)NK cells are present in low numbers in the pre-ovulatory uterus, but increase rapidly post-ovulation (King et al., 1996b). DNK cells are distinguished from peripheral blood NK cells by their expression of CD56 at ten-fold higher levels, lack of expression of CD16 and limited lytic ability (Moffett-King, 2002). Despite the fact that timing of the increased abundance of dNK cells is tightly regulated by hormones, the hormone effects are thought to be indirect as dNK do not express progesterone receptors (PR) (King et al., 1996a) or the predominant estrogen receptor (ERα). DNK cells are reported to express ERβ (Henderson et al., 2003), a form of ER which attenuates gene transcription (Kuiper and Gustafsson, 1997). Indeed, dNK cells do not respond to hormonal stimulation in vitro by proliferation, cytokine secretion or alterations in lytic ability (King et al., 1996b; Kitaya et al., 2003a).
Progesterone regulates decidual production of cytokines such as IL-15, necessary for NK cell proliferation and differentiation (Verma et al., 2000; Okada et al., 2000), leukemia inhibitory factor (LIF), required for implantation and IL-11 which contributes to further decidualization in an autocrine manner (Robb et al., 2002; Dimitriadis et al., 2002). Progesterone also induces stromal cell production of CXCL9 (Mig) and CXCL10 (IP-10) (Kitaya et al., 2004), ligands of CXCR3, a chemokine receptor expressed by peripheral blood NK cells (Campbell et al., 2001; Inngjerdingen et al., 2001). Chemokine receptors that are highly up-regulated in decidua during the luteal phase include CXCR1, CCR2b and CCR5 (Dominguez et al., 2003). Thus, major roles for P4 include induction of decidualization, induction of chemokines capable of recruiting NK cells and up-regulation of IL-15, the cytokine essential for differentiation and maintenance of dNK cells (Ashkar et al., 2003).
In contrast, the ovarian hormone estradiol (E2) induces vasodilation or relaxation in vessels through interactions with endothelial cells throughout the cardiovascular system (Sullivan et al., 1995; Mendelsohn, 2002). These effects are particularly profound in the pregnant uterus, where branches from the uterine arteries known as spiral arteries, elongate, dilate and become tortuous. Spiral artery modification yields high flow, low resistance vessels with reduced wall structure and proximal (i.e. towards fetus) replacement of endothelium with intravascular trophoblast. These structural changes, which occur during the 3rd and 4th months of human pregnancy, shortly after the onset in decline of dNK cells and between gestation days 9–10 in mice, when uNK cells are at their peak in number and function, are thought to be required in humans for normal rates of conceptus growth and maternal health in later gestation (Sattar et al., 2003). The cells controlling spiral artery modification in mice and women appear to differ (uNK vs trophoblast cells, respectively). However, this difference may lie in the proportion of the vessel changed by each cell type (i.e. uNK cells functioning more distally to fetal trophoblasts and the spiral arterial segment being relatively longer in mice) or trophoblast invasion in humans may be facilitated by prior dNK cell sensitization of these vessels (as occurs in mice) during the implantation period (Adamson et al., 2003). Human decidual (d)NK cells, like their murine counterparts, express major angiogenic molecules such as vascular endothelial and placental growth factors (Smith, 2001). Thus, if the roles of uNK cells in mice and dNK cells in humans are analogous, dNK support of decidualization can be linked to fertility/implantation, while dNK contributions to spiral artery modification can be associated with the development of pre-eclampsia and some small for gestational age (SGA) pregnancies (Khong et al., 1986).
Trafficking of Pre-dNK cells to the uterus
Self-renewing murine uNK cell progenitors do not reside within the uterus (Chantakru et al., 2002). Transplantable progenitors of murine uNK cells were found in all lymphoid tissues, but particularly in the spleen during pregnancy, suggesting a hormonal induction of NK cell mobility or maturation (Chantakru et al., 2002). Mobilization of blood-borne lymphocytes to tissue depends on their sequential adhesive interactions with endothelial cells under wall shear stress induced by hemodynamic flow (Springer, 1995).
Homing of naive T and B lymphocytes to secondary lymphoid tissues such as lymph nodes (LN) and Peyer’s Patches (PP) is a well described four step cascade of events that occurs in post capillary venules in secondary immune tissues or at sites of inflammation (Springer, 1994; Butcher and Picker, 1996; von Andrian and Mempel, 2003). First, a sequence of transient adhesive interactions under shear force between adhesion molecules constitutively expressed on circulating lymphocytes (L-selectin and/or α4β7 integrin) and their respective ligands, peripheral node addressins (PNAd, includes CD34, GlyCAM-1, podocalyxin, Sgp200) (Rosen, 2004) and mucosal addressin cell adhesion molecule (MadCAM), on specialized high endothelial venules (HEV) cause circulating lymphocytes to progressively slow, tether, roll and stop. Subsequent rapid activation by chemokines such as CCL19 (MIP3β), CCL21 (6Ckine) and CXCL12 (SDF1) induces lateral mobility and temporary enhanced affinity of LFA-1 for its ligand, ICAM-1, resulting in firm attachment, then transendothelial migration along a chemokine gradient into tissue. Endothelial expression of ligands and tissue-specific secretion of chemoattractants enables B cell migration at follicular sites and T cell egress at interfollicular areas in LN or PP (Warnock et al., 2000; Constantin et al., 2000; Butcher et al., 1999).
These steps in homing apply to extravasation of leukocytes to tertiary sites in response to specific inflamation-induced chemokine signals. Migrating cells include memory lymphocytes, dendritic cells and neutrophils (Picker and Butcher, 1992). Sites of inflammation are also characterized by production of cytokines which up-regulate expression of adhesion molecules such as E-selectin, VCAM-1 and ICAM-1 on vascular endothelium that facilitate egress of immune cells (Butcher et al., 1999).
Much less is known about NK cell trafficking. Circulating NK cells are subdivided based on expression of CD3, CD16 and CD56. The majority of NK cells express CD16 strongly and CD56 weakly (CD56dim), but about 10% of blood NK cells have higher expression of CD56 and lack expression of CD16 (CD56bright) (Robertson and Ritz, 1990). CD3 expressing CD56+ cells are designated NK-T cells because they also express a somatically rearranged αβT cell receptor, thus are considered of the T cell lineage rather than the NK cell lineage. The CD56bright subset is further discriminated from the CD56dim subset by expression of high affinity IL-2R, high expression of L-selectin and α4 andβ1 integrins, but low expression of LFA-1 (Frey et al., 1998). CD56 bright and dim cells also differ in their biologic functions; CD56dim cells have greater lytic ability, while CD56bright cells have immunoregulatory roles through secretion of cytokines (Cooper et al., 2001). CD56+ populations respond uniquely to chemotactic signaling; CD56dim cells migrate in response to CXCL8 (IL-8) and CX3CL1 (fractalkine) and strongly express their receptors CXCR1 and CX3CR1. CD56bright cells express the chemokine receptors CCR5 (which binds CCL3 (MIP1α), CCL4 (MIP1β) and CCL5 (RANTES)) and CCR7 (which binds CCL19 (MIP3β) and CCL21 (6Ckine)) (Campbell et al., 2001). NK-T cells share expression of all chemokine receptors found on the CD56bright subset and have in addition, CCR1, 2 and 6. While all CD56+ populations express CXCR3 (binds CXCL9 (Mig), CXCL10 (IP-10) and CXCL11(ITAC)) and CXCR4 (binds CXCL12 (SDF1)), NK cells do not readily migrate to secondary lymphoid tissues such as LN or PP, but are found in spleen (Campbell et al., 1998). Decidual NK cells have a unique expression pattern, strongly expressing, CCR1, 2 and 5, CXCR3 and 4 and CX3CR1(Red-Horse et al., 2001). The expression of both phenotypic and chemokine receptors for each CD56+ population is summarized in Table 1. CD56bright cells, through their intense expression of L-selectin (Frey et al., 1998; Kruse et al., 1999) and specific chemokine receptor array (Campbell et al., 2001; Inngjerdingen et al., 2001) and their ability to adhere in an L-selectin-dependent manner to frozen sections of LN (Frey et al., 1998), appear predisposed to tissue-selective homing. We addressed whether L-selectin-mediated interactions could contribute to the trafficking of NK cells or their precursors to the decidualizing uterus.
Table 1.
Trafficking phenotype of NK cell subsets
| Dim | Blood subsets Bright | NK-T | Decidual NK | |
|---|---|---|---|---|
| CD56 | + | ++ | + | +++ |
| CD16 | + | − | − | − |
| CD3 | − | − | + | − |
| L-selectin | + | ++ | + | − |
| α4 integrin | + | ++ | + | ++ |
| LFA-1 | + | + | + | ++ |
| CCR1 | − | − | + | ++ |
| CCR2 | − | − | + | ++ |
| CCR5 | − | ++ | + | ++ |
| CCR7 | − | ++ | − | +/− |
| CXCR1 | + | − | − | − |
| CXCR2 | + | − | + | − |
| CXCR3 | + | ++ | ++ | ++ |
| CXCR4 | ++ | ++ | ++ | ++ |
| CX3CR1 | + | ++ | ++ | ++ |
+expressed
++strongly expressed
+++very strongly expressed
− not expressed
The Stamper-Woodruff adhesion assay is a model of lymphocyte trafficking potential (Stamper and Woodruff, 1976) which evaluates specific functional adhesive interactions between viable lymphocytes and endothelium presented in frozen tissue sections. The assay is performed on a rotating table to mimic the shear forces of flowing blood necessary to engage adhesion molecules on both lymphocytes and endothelium. Using this assay, we demonstrated that lymphocytes from pregnant or ovarian steroid hormone-treated ovariectomized mice had increased interactions with endothelium over lymphocytes from non-pregnant donors (Chantakru et al., 2003). These interactions were mediated by two well-characterized lymphocyte homing receptors, L-selectin and alpha4 integrin since pre-treatment of the lymphocytes with function-blocking antibodies to either molecule, or the substrate tissue with antibodies to their counter-receptors PNAd and MAdCAM, removed the enhancement. Furthermore, independent but coordinated, pregnancy and steroid hormone-induced changes occurred in endothelium of the uterus and LN but not in endothelium of other organs. The changes in endothelia were recognized by murine splenocytes, human blood lymphocytes and indicator cell lines expressing specific receptors. (Chantakru et al., 2003). Importantly, we found the human lymphocytes adhering to microvessels of midgestation decidua were enriched for CD56bright NK cells. While CD56bright NK cells were ~3% of the blood lymphocytes applied to the sections, they constituted 75% of the adherent cells.
RECRUITMENT OF DECIDUAL NATURAL KILLER CELLS
Because stromal triggering towards decidualization and uterine recruitment of NK cells coincide in women during each menstrual cycle, we hypothesized that CD56bright cells in human blood contained precursors of dNK cells and that interactions of these cells with uterine endothelium would vary over the menstrual cycle. We further postulated that endocrine changes in the menstrual cycle induce synchronous changes in uterine endothelium that promote NK cell trafficking into human uterus and that defects in the migration pathway or its regulation could compromise blastocyst implantation and/or limit structural changes to the arteries supporting human placental development. Because the substrate tissue needed for the in vitro cell adhesion assay has a limited useful storage life of 10–15 days, uterine tissue from inbred mice was used as the substrate tissue for evaluating endocrinological modifications of the functions of human blood NK cells.
Serial blood samples from 18 normally cycling women were studied. NK cell subsets, hormone levels and functional adhesion to uterine endothelium from gd7 mice were monitored over a menstrual cycle. Adhesive function of total blood lymphocytes (PBL) as well as the CD56bright cell subset was studied on alternate days using endothelium in LN (n=14) or decidualized uterus (n=6). Figure 1 depicts the highlights of this study; on LN substrates, adhesion of PBL and CD56bright cells was highly variable between donors but consistently peaked at the luteinizing hormone (LH) surge. This rise in adhesion however, did not reach statistical significance. With uterine endothelial substrates, dramatic gains in adhesion were observed for both PBL and NK cells (p=0.004) with all adhesion localized to decidua basalis (van den Heuvel et al., 2005).
Figure 1. Adhesion of PBL and CD56bright Cells to Mouse Decidua.
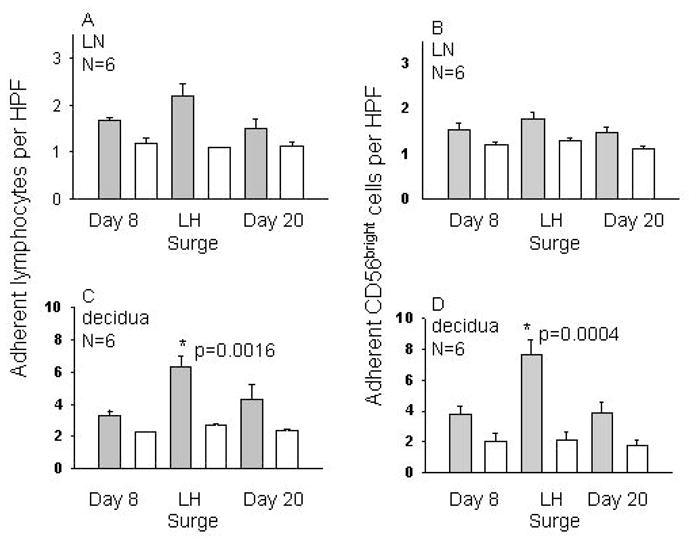
Mean adhesion of human PBL or CD56bright cells at 3 timepoints during a menstrual cycle to frozen endothelium from mice at gd7 is presented. 5 × 106 cells were overlaid onto frozen tissue under shear for 30 min, then non-adhesive cells were rinsed off. Adhesion was evaluated in 50 fields by 2 researchers. Panels A and B show mean adhesion to HEV in LN, while C and D show adhesion to decidua. Panels A and C illustrate total PBL adhesion and B and D depict adhesion of CD56bright cells only. Solid bars show untreated cells, while open bars show adhesion of identical aliquots pre-treated with a function blocking antibody to L-selectin.
The strongest statistical correlation was between estrogen concentration and number of adherent cells. During a typical 28 day menstrual cycle, E2 levels rise from cycle day 8 to cycle day 13, when a surge of LH down-regulates E2. We found that CD56bright adhesion peaked only during a narrow window from cycle day 10 to 12, just prior to ovulation (cycle day 14). Thus, it appeared that trafficking of peripheral blood NK cells might be directly regulated by rising levels of E2 and/or LH but not P4. Function blocking antibodies to L-selectin or alpha 4 integrin significantly reduced adhesion and eliminated the peri-ovulatory enhancement, suggesting that recruitment is dependent upon classic adhesion molecules used in inflammatory processes.
To further investigate the role of hormones on the adhesive interactions of NK cells with endothelium, total lymphocytes collected from male donors that would not be primed by menstrual stage, were cultured in serum-free RPMI with LH, E2 and P4. Hormone effects were titrated in ranges found in plasma during the menstrual cycle. LH ranged from 5 to 250 IU/ml, E2 from 100 to 800 pg/ml and P4 from 1 to 8 ng/ml. After 4 hr in culture, significant changes occurred in CD56 cell adhesion to endothelium from gd 7 mouse decidua basalis. These experiments are summarized in Figure 2. A positive association of adhesion was found with E2 and with LH, but not with P4 forboth PBL and CD56bright cells. The effects of E2 and LH were not additive when combined treatment was used. These data strongly suggest hormonal complicity in the peri-ovulatory change in functional adhesion of NK cells.
Figure 2.
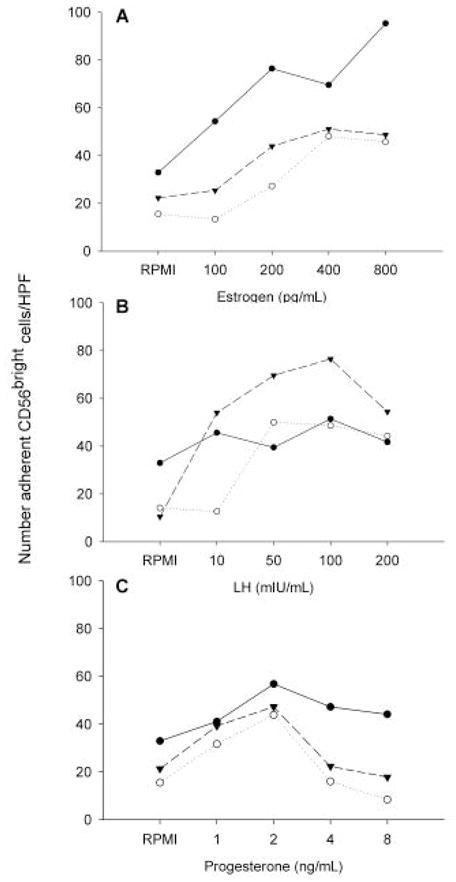
The role of hormones on adhesive interactions of NK cells with endothelium. Total lymphocytes collected from 3 male donors were cultured for 4 h in serum-free RPMI alone or with varying concentrations of E2 (panel A), LH (panel B) or P4 (panel C) as shown on the lower axis, then applied to frozen sections of gestational mouse decidua. Post-culture adhesion of CD56bright cells from individual donors to mouse decidua is shown.
In further studies, quantitative PCR was performed on NK cell subsets derived from magnetically separated PBL of women at day 5 or at day of LH surge. No transcripts for either of the ER or PR isoforms or for the LH receptor were detected in any of the subsets, although GAPDH and genes for L-selectin, alpha 4 integrin and LFA-1 were detected and the hormone receptor transcripts were amplified from control tissues (ovary or uterus) as summarized in Table 2. Thus, the hormonally effected changes in NK cell function must be mediated through another cell population in the PBL cultures. In-depth studies of direct endocrinological involvement in maternal immune regulation have often given apparently conflicting results. Effects of hormones, especially E2, on NK cell numbers, subsets and activity have been extensively reported in relation to the menstrual cycle (Hunt et al., 2000; Flynn et al., 2000; Bouman et al., 2001; Yovel et al., 2001; Sulke et al., 1985; Souza et al., 2001; Jones et al., 1997), pregnancy (Veenstra et al., 2002; Watanabe et al., 1997), reproductive pathologies (Provinciali et al., 1995; Somigliana et al., 1999; Searle et al., 1999), contraceptive use (Auerbach et al., 2002; Scanlan et al., 1995) and metatastic disease (Baral et al., 1995; Roszkowski et al., 1997) but there are no reports of an influence of sex hormones on NK cell trafficking in the human.
Table 2.
Summary of realtime RT-PCR analyses for genes transcribed in blood NK cell subsets
| Dim | Bright | NK-T | Ovary/uterus | ||||
|---|---|---|---|---|---|---|---|
| d5 | LH | d5 | LH | d5 | LH | ||
| LHR | − | − | − | − | − | − | + |
| ER | − | − | − | − | − | − | + |
| ER | − | − | − | − | − | − | + |
| PR A | − | − | − | − | − | − | + |
| PR B | − | − | − | − | − | − | + |
| L-selectin | 7 | 4 | 8 | 4 | 8 | 4 | ND |
| alpha 4 integrin | 1259 | 1251 | 871 | 1245 | 1115 | 1120 | ND |
| LFA-1 | 21 | 14 | 12 | 15 | 11 | 10 | ND |
| GAPDH | + | + | + | + | + | + | + |
Values are copy numbers relative to GAPDH expression averaged from 7 samples.
+ positive
− not detected
ND Not done
Quantitative PCR was also undertaken on the above samples to determine whether changes in transcription of adhesion molecules might account for enhanced lymphocyte interactions with endothelium at the LH surge. There were no differences in the numbers of transcripts for L-selectin, alpha4 integrin or LFA-1 between day 5 and day of LH surge in either NK cell subset. This confirmed our earlier observation by flow cytometry that there was no change in mean fluorescence intensity in expression of L-selectin, alpha4 integrin or LFA-1 over the menstrual cycle. These results suggest that hormone promoted changes in membrane receptor clustering must be investigated.
CONCLUSIONS
The changes in lymphocyte/endothelial interactions that we have described have the potential to underlie cyclic movement of progenitors of dNK cells from peripheral lymphoid tissue to the uterus. Once localized to peri-vascular and decidual sites where they are in contact with fetal trophoblast, dNK cells contribute to modifying the endometrial environment to promote implantation and alter the maternal vasculature. These inaugural studies demonstrate dynamic, coordinated indirect regulation of adhesive molecule function on CD56bright cells and endothelium by ovarian steroid hormones, as summarized in Figure 3.
Figure 3.
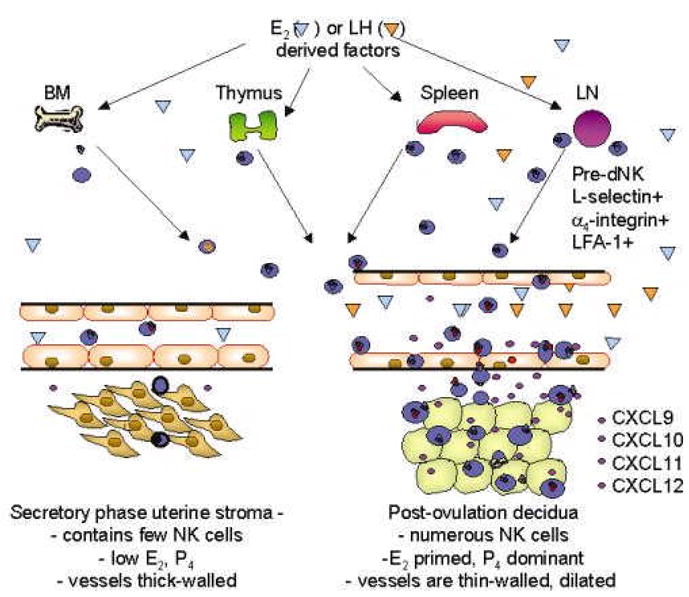
A model of pre-dNK cell trafficking from lymphoid reservoirs to decidualizing uterus. In the early secretory phase and luteal phase of the menstrual cycle, few NK cells ( )enter and leave the uterus in normal immune surveillance. Increased peri-ovulatory plasma concentrations of E2 and/or LH induce expression of unknown factors (
)enter and leave the uterus in normal immune surveillance. Increased peri-ovulatory plasma concentrations of E2 and/or LH induce expression of unknown factors ( ) which mobilize NK cell egress from reservoirs into the circulation, particularly LN. NK cells leave the blood in increased numbers and preferentially enter the uterus in response to a gradient of chemokines (CXCR3 and 4 ligands
) which mobilize NK cell egress from reservoirs into the circulation, particularly LN. NK cells leave the blood in increased numbers and preferentially enter the uterus in response to a gradient of chemokines (CXCR3 and 4 ligands  ) secreted by the E2 primed stromal cells in the post ovulatory uterus. L-selectin is shed as they traverse the decidua and localize in decidual cells proximal to blood vessels. P4 acts on E2-primed stroma to initiate decidualization as well as on vascular endothelium to promote NK cell secreted IFNγ thinning of the vessel wall and dilation of the major arteries (Kitaya et al., 2004; Red-Horse et al., 2001; Hanna et al., 2003).
) secreted by the E2 primed stromal cells in the post ovulatory uterus. L-selectin is shed as they traverse the decidua and localize in decidual cells proximal to blood vessels. P4 acts on E2-primed stroma to initiate decidualization as well as on vascular endothelium to promote NK cell secreted IFNγ thinning of the vessel wall and dilation of the major arteries (Kitaya et al., 2004; Red-Horse et al., 2001; Hanna et al., 2003).
Acknowledgments
The work described here was supported by NSERC, CIHR, the Ontario Women’s Health Scholarship Award and the Canada Research Chairs Program. We thank J.E. Lewis, A. Simpson, K. Hatta, M.E. Junkins, S. Burke and Y. Fang for technical support and Dr. C. Tayade for his most helpful discussions. We greatly appreciate the co-operation of our blood donors without whom our study would not have been possible.
Abbreviations
- A2M
alpha2macroglobulin
- dNK
decidual NK cell (human)
- DC
dendritic cell
- E2
estradiol
- ER
estradiol receptor
- HEV
high endothelial venule
- ICAM-1
intercellular adhesion molecule-1
- LN
Lymph Node
- LFA-1
leucocyte function associated molecule -1
- LH
luteinizing hormone
- LHR
luteinizing hormone receptor
- LIF
leukemia inhibitory factor
- MAdCAM
mucosal addressin cell adhesion molecule
- NK
Natural Killer cell
- PBL
peripheral blood lymphocytes
- PNAd
peripheral lymph node addressin
- PP
Peyers Patches
- P4
progesterone
- PR
progesterone receptor
- PZP
pregnancy zone protein
- SGA
small for gestational age
- uNK
uterine NK cell (murine)
Reference List
- Adamson SL, Lu Y, Whiteley KJ, Holmyard D, Hemberger M, Pfarrer C, Cross JC. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol. 2003;250:358–373. doi: 10.1016/s0012-1606(02)90773-6. [DOI] [PubMed] [Google Scholar]
- Ashkar AA, Black GP, Wei Q, He H, Liang L, Head JR, Croy BA. Assessment of requirements for IL-15 and IFN regulatory factors in uterine NK cell differentiation and function during pregnancy. J Immunol. 2003;171:2937–2944. doi: 10.4049/jimmunol.171.6.2937. [DOI] [PubMed] [Google Scholar]
- Ashkar AA, Croy BA. Interferon-gamma contributes to the normalcy of murine pregnancy. Biol Reprod. 1999;61:493–502. doi: 10.1095/biolreprod61.2.493. [DOI] [PubMed] [Google Scholar]
- Ashkar AA, di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 2000;192:259–270. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach L, Hafner T, Huber JC, Panzer S. Influence of low-dose oral contraception on peripheral blood lymphocyte subsets at particular phases of the hormonal cycle. Fertil Steril. 2002;78:83–89. doi: 10.1016/s0015-0282(02)03173-4. [DOI] [PubMed] [Google Scholar]
- Baral E, Nagy E, Berczi I. Modulation of natural killer cell-mediated cytotoxicity by tamoxifen and estradiol. Cancer. 1995;75:591–599. doi: 10.1002/1097-0142(19950115)75:2<591::aid-cncr2820750224>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Borthwick JM, Charnock-Jones DS, Tom BD, Hull ML, Teirney R, Phillips SC, Smith SK. Determination of the transcript profile of human endometrium. Mol Hum Reprod. 2003;9:19–33. doi: 10.1093/molehr/gag004. [DOI] [PubMed] [Google Scholar]
- Bouman A, Moes H, Heineman MJ, de Leij LF, Faas MM. Cytokine production by natural killer lymphocytes in follicular and luteal phase of the ovarian cycle in humans. Am J Reprod Immunol. 2001;45:130–134. doi: 10.1111/j.8755-8920.2001.450302.x. [DOI] [PubMed] [Google Scholar]
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–53. 209–253. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Bowman EP, Murphy K, Youngman KR, Siani MA, Thompson DA, Wu L, Zlotnik A, Butcher EC. 6-C-kine (SLC), a lymphocyte adhesion-triggering chemokine expressed by high endothelium, is an agonist for the MIP-3beta receptor CCR7. J Cell Biol. 1998;141:1053–1059. doi: 10.1083/jcb.141.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JJ, Qin S, Unutmaz D, Soler D, Murphy KE, Hodge MR, Wu L, Butcher EC. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol. 2001;166:6477–6482. doi: 10.4049/jimmunol.166.11.6477. [DOI] [PubMed] [Google Scholar]
- Carson DD, Lagow E, Thathiah A, Al-Shami R, Farach-Carson MC, Vernon M, Yuan L, Fritz MA, Lessey B. Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Mol Hum Reprod. 2002;8:871–879. doi: 10.1093/molehr/8.9.871. [DOI] [PubMed] [Google Scholar]
- Chantakru S, Miller C, Roach LE, Kuziel WA, Maeda N, Wang WC, Evans SS, Croy BA. Contributions from self-renewal and trafficking to the uterine NK cell population of early pregnancy. J Immunol. 2002;168:22–28. doi: 10.4049/jimmunol.168.1.22. [DOI] [PubMed] [Google Scholar]
- Chantakru S, Wang WC, van den Heuvel M, Bashar S, Simpson A, Chen Q, Croy BA, Evans SS. Coordinate Regulation of Lymphocyte-Endothelial Interactions by Pregnancy-Associated Hormones. J Immunol. 2003;171:1132–1145. doi: 10.4049/jimmunol.171.8.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouat G, Ledee-bataille N, Zourbas S, Dubanchet S, Sandra O, Martal J, Ostojojic S, Frydman R. Implantation: can immunological parameters of implantation failure be of interest for pre-eclampsia? J Reprod Immunol. 2003;59:205–217. doi: 10.1016/s0165-0378(03)00048-2. [DOI] [PubMed] [Google Scholar]
- Constantin G, Majeed M, Giagulli C, Piccio L, Kim JY, Butcher EC, Laudanna C. Chemokines trigger immediate beta2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity. 2000;13:759–769. doi: 10.1016/s1074-7613(00)00074-1. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- Croy BA, di Santo JP, Greenwood JD, Chantakru S, Ashkar AA. Transplantation into genetically alymphoid mice as an approach to dissect the roles of uterine natural killer cells during pregnancy--a review. Placenta. 2000;21(Suppl A):S77–80. 77–80. doi: 10.1053/plac.1999.0518. [DOI] [PubMed] [Google Scholar]
- Croy BA, Esadeg S, Chantakru S, van den Heuvel M, Paffaro VA, Jr, He H, Black G, Ashkar AA, Kiso Y, Zhang J. Update on pathways regulating the activation of uterine Natural Killer cells, their interactions with decidual spiral arteries and homing of their precursors to the uterus. J Reprod Immunol. 2003;59:175–191. doi: 10.1016/s0165-0378(03)00046-9. [DOI] [PubMed] [Google Scholar]
- Dimitriadis E, Robb L, Salamonsen LA. Interleukin 11 advances progesterone-induced decidualization of human endometrial stromal cells. Mol Hum Reprod. 2002;8:636–643. doi: 10.1093/molehr/8.7.636. [DOI] [PubMed] [Google Scholar]
- Dominguez F, Galan A, Martin JJ, Remohi J, Pellicer A, Simon C. Hormonal and embryonic regulation of chemokine receptors CXCR1, CXCR4, CCR5 and CCR2B in the human endometrium and the human blastocyst. Mol Hum Reprod. 2003;9:189–198. doi: 10.1093/molehr/gag024. [DOI] [PubMed] [Google Scholar]
- Flynn L, Byrne B, Carton J, Kelehan P, O’Herlihy C, O’Farrelly C. Menstrual cycle dependent fluctuations in NK and T-lymphocyte subsets from non-pregnant human endometrium. Am J Reprod Immunol. 2000;43:209–217. doi: 10.1111/j.8755-8920.2000.430405.x. [DOI] [PubMed] [Google Scholar]
- Frey M, Packianathan NB, Fehniger TA, Ross ME, Wang WC, Stewart CC, Caligiuri MA, Evans SS. Differential expression and function of L-selectin on CD56bright and CD56dim natural killer cell subsets. J Immunol. 1998;161:400–408. [PubMed] [Google Scholar]
- Genbacev OD, Prakobphol A, Foulk RA, Krt, Ilic D, Singer MS, Yang ZQ, Kiessling LL, Rosen SD, Fisher SJ. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science. 2003;299:405–408. doi: 10.1126/science.1079546. [DOI] [PubMed] [Google Scholar]
- Guimond MJ, Luross JA, Wang B, Terhorst C, Danial S, Croy BA. Absence of natural killer cells during murine pregnancy is associated with reproductive compromise in TgE26 mice. Biol Reprod. 1997;56:169–179. doi: 10.1095/biolreprod56.1.169. [DOI] [PubMed] [Google Scholar]
- Hanna J, Wald O, Goldman-Wohl D, Prus D, Markel G, Gazit R, Katz G, Haimov- Kochman R, Fujii N, Yagel S, Peled A, Mandelboim O. CXCL12 expression by invasive trophoblasts induces the specific migration of CD16- human natural killer cells. Blood. 2003;102:1569–1577. doi: 10.1182/blood-2003-02-0517. [DOI] [PubMed] [Google Scholar]
- He H, McCartney DJ, Wei Q, Esadeg SM, Zhang J, Foster RA, Hayes MA, Tayade C, Van Leuven F, Croy BA. Characterization of a Murine Alpha 2 Macroglobulin Gene Expressed in Reproductive and Cardiovascular Tissue. Biol Reprod. 2004 doi: 10.1095/biolreprod.104.029835. [DOI] [PubMed] [Google Scholar]
- Henderson TA, Saunders PT, Moffett-King A, Groome NP, Critchley HO. Steroid receptor expression in uterine natural killer cells. J Clin Endocrinol Metab. 2003;88:440–449. doi: 10.1210/jc.2002-021174. [DOI] [PubMed] [Google Scholar]
- Hunt JS, Miller L, Roby KF, King A. Female steroid hormones regulate production of pro-inflammatory molecules in uterine leukocytes. Hum Reprod Update. 2000;6:28–36. doi: 10.1016/s0165-0378(97)00060-0. [DOI] [PubMed] [Google Scholar]
- Inngjerdingen M, Damaj B, Maghazachi AA. Expression and regulation of chemokine receptors in human natural killer cells. Blood. 2001;97:367–375. doi: 10.1182/blood.v97.2.367. [DOI] [PubMed] [Google Scholar]
- Jones RK, Bulmer JN, Searle RF. Cytotoxic activity of endometrial granulated lymphocytes during the menstrual cycle in humans. Biol Reprod. 1997;57:1217–1222. doi: 10.1095/biolreprod57.5.1217. [DOI] [PubMed] [Google Scholar]
- Kammerer U, Eggert AO, Kapp M, McLellan AD, Geijtenbeek TB, Dietl J, van Kooyk Y, Kampgen E. Unique appearance of proliferating antigen-presenting cells expressing DC-SIGN (CD209) in the decidua of early human pregnancy. Am J Pathol. 2003;162:887–896. doi: 10.1016/S0002-9440(10)63884-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, Osteen K, Lessey BA, Giudice LC. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144:2870–2881. doi: 10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- Kao LC, Tulac S, Lobo S, Imani B, Yang JP, Germeyer A, Osteen K, Taylor RN, Lessey BA, Giudice LC. Global gene profiling in human endometrium during the window of implantation. Endocrinology. 2002;143:2119–2138. doi: 10.1210/endo.143.6.8885. [DOI] [PubMed] [Google Scholar]
- Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93:1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- King A. Uterine leukocytes and decidualization. Hum Reprod Update. 2000;6:28–36. doi: 10.1093/humupd/6.1.28. [DOI] [PubMed] [Google Scholar]
- King A, Gardner L, Loke YW. Evaluation of oestrogen and progesterone receptor expression in uterine mucosal lymphocytes. Hum Reprod. 1996a;11:1079–1082. doi: 10.1093/oxfordjournals.humrep.a019300. [DOI] [PubMed] [Google Scholar]
- King A, Jokhi PP, Burrows TD, Gardner L, Sharkey AM, Loke YW. Functions of human decidual NK cells. Am J Reprod Immunol. 1996b;35:258–260. doi: 10.1111/j.1600-0897.1996.tb00041.x. [DOI] [PubMed] [Google Scholar]
- Kitaya K, Nakayama T, Daikoku N, Fushiki S, Honjo H. Spatial and temporal expression of ligands for CXCR3 and CXCR4 in human endometrium. J Clin Endocrinol Metab. 2004;89:2470–2476. doi: 10.1210/jc.2003-031293. [DOI] [PubMed] [Google Scholar]
- Kitaya K, Yasuda J, Nakayama T, Fushiki S, Honjo H. Effect of female sex steroids on human endometrial CD16neg CD56bright natural killer cells. Fertil Steril. 2003a;79(Suppl 1):730–734. doi: 10.1016/s0015-0282(02)04818-5. [DOI] [PubMed] [Google Scholar]
- Kitaya K, Yasuda J, Yagi I, Tada Y, Fushiki S, Honjo H. IL-15 expression at human endometrium and decidua. Biol Reprod. 2003b;63:683–687. doi: 10.1095/biolreprod63.3.683. [DOI] [PubMed] [Google Scholar]
- Kruse A, Merchant MJ, Hallmann R, Butcher EC. Evidence of specialized leukocyte-vascular homing interactions at the maternal/fetal interface. Eur J Immunol. 1999;29:1116–1126. doi: 10.1002/(SICI)1521-4141(199904)29:04<1116::AID-IMMU1116>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Gustafsson JA. The novel estrogen receptor-beta subtype: potential role in the cell- and promoter-specific actions of estrogens and anti-estrogens. FEBS Lett. 1997;410:87–90. doi: 10.1016/s0014-5793(97)00413-4. [DOI] [PubMed] [Google Scholar]
- Maslar IA. The progestational endometrium. Sem Reprod Immunol. 1988;6:115–128. [Google Scholar]
- Mendelsohn ME. Genomic and nongenomic effects of estrogen in the vasculature. Am J Cardiol. 2002;90:3F–6F. doi: 10.1016/s0002-9149(02)02418-9. [DOI] [PubMed] [Google Scholar]
- Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- Okada H, Nakajima T, Sanezumi M, Ikuta A, Yasuda K, Kanzaki H. Progesterone enhances interleukin-15 production in human endometrial stromal cells in vitro. J Clin Endocrinol Metab. 2000;85:4765–4770. doi: 10.1210/jcem.85.12.7023. [DOI] [PubMed] [Google Scholar]
- Picker LJ, Butcher EC. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992;10:561–591. doi: 10.1146/annurev.iy.10.040192.003021. [DOI] [PubMed] [Google Scholar]
- Provinciali M, Di Stefano G, Muzzioli M, Garzetti GG, Ciavattini A, Fabris N. Relationship between 17-beta-estradiol and prolactin in the regulation of natural killer cell activity during progression of endometriosis. J Endocrinol Invest. 1995;18:645–652. doi: 10.1007/BF03349783. [DOI] [PubMed] [Google Scholar]
- Red-Horse K, Drake PM, Gunn MD, Fisher SJ. Chemokine ligand and receptor expression in the pregnant uterus:reciprocal patterns in complementary cell subsets suggest functional roles. Am J Pathol. 2001;159:2199–2213. doi: 10.1016/S0002-9440(10)63071-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb L, Dimitriadis E, Li R, Salamonsen LA. Leukemia inhibitory factor and interleukin-11: cytokines with key roles in implantation. J Reprod Immunol. 2002;57:129–141. doi: 10.1016/s0165-0378(02)00012-8. [DOI] [PubMed] [Google Scholar]
- Robertson MJ, Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990;76:2421–2438. [PubMed] [Google Scholar]
- Robertson SA, Redman CW, McCracken SA, Hunt JS, Dimitriadis E, Moffett-King A, Chamley L. Immune modulators of implantation and placental development-a workshop report. Placenta 2003. 2003;24:S16–S20. doi: 10.1053/plac.2002.0937. [DOI] [PubMed] [Google Scholar]
- Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- Roszkowski PI, Hyc A, Stopinska-Gluszak U, Malejczyk J. Natural killer cell activity and sex hormone levels in mastopathy. Gynecol Endocrinol. 1997;11:399–404. doi: 10.3109/09513599709152567. [DOI] [PubMed] [Google Scholar]
- Sattar N, Ramsay J, Crawford L, Cheyne H, Greer IA. Classic and novel risk factor parameters in women with a history of preeclampsia. Hypertension. 2003;42:39–42. doi: 10.1161/01.HYP.0000074428.11168.EE. [DOI] [PubMed] [Google Scholar]
- Scanlan JM, Werner JJ, Legg RL, Laudenslager ML. Natural killer cell activity is reduced in association with oral contraceptive use. Psychoneuroendocrinology. 1995;20:281–287. doi: 10.1016/0306-4530(94)00059-j. [DOI] [PubMed] [Google Scholar]
- Searle RF, Jones RK, Bulmer JN. Phenotypic analysis and proliferative responses of human endometrial granulated lymphocytes during the menstrual cycle. Biol Reprod. 1999;60:871–878. doi: 10.1095/biolreprod60.4.871. [DOI] [PubMed] [Google Scholar]
- Smith SK. Regulation of angiogenesis in the endometrium. Trends Endocrinol Metab. 2001;12:147–151. doi: 10.1016/s1043-2760(01)00379-4. [DOI] [PubMed] [Google Scholar]
- Somigliana E, Vigano P, Vignali M. Endometriosis and unexplained recurrent spontaneous abortion: pathological states resulting from aberrant modulation of natural killer cell function? Hum Reprod Update. 1999;5:40–51. doi: 10.1093/humupd/5.1.40. [DOI] [PubMed] [Google Scholar]
- Souza SS, Castro FA, Mendonca HC, Palma PV, Morais FR, Ferriani RA, Voltarelli JC. Influence of menstrual cycle on NK activity. J Reprod Immunol. 2001;50:151–159. doi: 10.1016/s0165-0378(00)00091-7. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–72. 827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- Stamper HBJ, Woodruff JJ. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med. 1976;144:828–833. doi: 10.1084/jem.144.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulke AN, Jones DB, Wood PJ. Variation in natural killer activity in peripheral blood during the menstrual cycle. Br Med J (Clin Res Ed ) 1985;290:884–886. doi: 10.1136/bmj.290.6472.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan TRJ, Karas RH, Aronovitz M, Faller GT, Ziar JP, Smith JJ, O’Donnell TFJ, Mendelsohn ME. Estrogen inhibits the response-to-injury in a mouse carotid artery model. J Clin Invest. 1995;96:2482–2488. doi: 10.1172/JCI118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M, Horrocks J, Bashar S, Taylor S, Burke S, Hatta K, Lewis JE, Croy BA. Menstrual Cycle Hormones Induce Changes in Functional Interactions Between Lymphocytes and Decidual Vascular Endothelial Cells. 2005 doi: 10.1210/jc.2004-1742. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra vNA, Bouman A, Moes H, Heineman MJ, de Leij LF, Santema J, Faas MM. Cytokine production in natural killer cells and lymphocytes in pregnant women compared with women in the follicular phase of the ovarian cycle. Fertil Steril. 2002;77:1032–1037. doi: 10.1016/s0015-0282(02)02976-x. [DOI] [PubMed] [Google Scholar]
- Verma S, Hiby SE, Loke YW, King A. Human decidual natural killer cells express the receptor for and respond to the cytokine interleukin 15. Biol Reprod. 2000;62:959–968. doi: 10.1095/biolreprod62.4.959. [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- Warnock RA, Campbell JJ, Dorf ME, Matsuzawa A, McEvoy LM, Butcher EC. The role of chemokines in the microenvironmental control of T versus B cell arrest in Peyer’s patch high endothelial venules. J Exp Med. 2000;191:77–88. doi: 10.1084/jem.191.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Iwatani Y, Kaneda T, Hidaka Y, Mitsuda N, Morimoto Y, Amino N. Changes in T, B, and NK lymphocyte subsets during and after normal pregnancy. Am J Reprod Immunol. 1997;37:368–377. doi: 10.1111/j.1600-0897.1997.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Yovel G, Shakhar K, Ben-Eliyahu S. The effects of sex, menstrual cycle, and oral contraceptives on the number and activity of natural killer cells. Gynecol Oncol. 2001;81:254–262. doi: 10.1006/gyno.2001.6153. [DOI] [PubMed] [Google Scholar]


