Abstract
Several animal studies have shown that striatal dopamine can be released under direct control of glutamatergic corticostriatal efferents. In Parkinson’s disease (PD), abnormalities in corticostriatal interactions are believed to play an important role in the pathophysiology of the disease. Previously, we have reported that, in healthy subjects, repetitive transcranial magnetic stimulation (rTMS) of motor cortex (MC) induces focal dopamine release in the ipsilateral putamen. In the present study, using [11C]raclopride PET, we sought to investigate early PD patients with evidence of unilateral motor symptoms. We measured in the putamen changes in extracellular dopamine concentration following rTMS (intensity, 90% of the resting motor threshold; frequency, 10 Hz) of the left and right MC. The main objective was to identify potential differences in corticostriatal dopamine release between the hemisphere associated with clear contralateral motor symptoms (symptomatic hemisphere) and the presymptomatic stage of the other hemisphere (asymptomatic hemisphere). Repetitive TMS of MC caused a binding reduction in the ipsilateral putamen of both hemispheres. In the symptomatic hemisphere, while the amount of TMS-induced dopamine release was, as expected, smaller, the size of the significant cluster of change in [11C]raclopride binding was, instead, 61.4% greater than in the asymptomatic hemisphere. This finding of a spatially enlarged area of dopamine release, following cortical stimulation, may represent a possible in vivo expression of a loss of functional segregation of cortical information to the striatum and an indirect evidence of abnormal corticostriatal transmission in early PD. This has potential implications for models of basal ganglia function in PD.
Keywords: Parkinson’s disease, positron emission tomography, raclopride, motor cortex stimulation, transcranial magnetic stimulation
Introduction
The striatum receives two major projections: glutamatergic from most of the cerebral cortex, and dopaminergic from the subtantia nigra and ventral tegmental area (Bouyer et al., 1984; Sesack & Pickel, 1992). While several studies have attempted to investigate the functional abnormalities and compensatory mechanisms of the nigrostriatal dopaminergic system in Parkinson’s disease (PD) (McGeer et al., 1989; Zigmond et al., 1990; Lee et al., 2000), little attention has been paid to the role played by the corticostriatal system in the control of dopamine release in PD. The corticostriatal neurons belong to a series of recurrent parallel loops that project back to the cerebral cortex via the thalamus (Alexander et al., 1986). The mesostriatal dopamine neurons, which are also arranged somatotopically, synapse on striatal medium spiny neurons in close proximity to the corticostriatal glutamatergic synapses, upon which they exert a modulatory influence (Bouyer et al., 1984; Sesack & Pickel, 1992). Glutamate–dopamine interactions in the striatum play a major role in the normal function of the corticostriatal system, which is involved in a wide range of motor and cognitive functions that include planning of movement, procedural memory, attention, and reward processing. Moreover, abnormalities in glutamate–dopamine interactions are thought to play a role in the pathophysiology of disorders such as PD, schizophrenia and drug addiction (Carlsson & Carlsson, 1990).
Numerous animal experiments have demonstrated that the frontal cortex exerts an influence on striatal dopamine release (Murase et al., 1993; Karreman & Moghaddam, 1996; Keck et al., 2002; Kanno et al., 2004), through the modulation of dopamine neuron firing (Murase et al., 1993; Karreman & Moghddam, 1996), but possibly also through a direct effect of corticostriatal neurons on dopamine nerve terminals (Cheramy et al., 1986; Leviel et al., 1990).
In PD, abnormalities in corticostriatal interactions (Carlsson & Carlsson, 1990; Calabresi et al., 1993, 2000) are believed to play an important role in the pathophysiology of the disease. In support of this hypothesis, electrophysiological studies in animal models of PD have shown an increased activation of corticostriatal glutamatergic transmission (Lindefors & Ungerstedt, 1990; Campbell & Bjorklund, 1994; Calabresi et al., 2000) with an enhanced excitability of striatal target neurons (Calabresi et al., 1993; Florio et al., 1993; Campbell & Bjorklund, 1994; Onn & Grace, 1999; Calabresi et al., 2000). These changes are associated with a functional reorganization of the cortical inputs to the striatum and a loss of functional segregation as also shown by the increase in cellular dye coupling between striatal neurons (Calabresi et al., 1993, 2000;Florio et al., 1993; Onn & Grace, 1999; Onn et al., 2000). Together, the increased excitability of the corticostriatal pathway and the loss of functional segregation of cortical information to the striatum are believed to have important functional implications in the pathogenesis of PD motor symptoms (Calabresi et al., 1993, 2000; Florio et al., 1993; Onn & Grace, 1999).
Previous studies have shown that brain imaging, with positron emission tomography (PET) and functional magnetic resonance imaging (fMRI), and neurophysiological recordings, with electroencephalographic and single-cell recording, can be used to measure transcranial magnetic stimulation-induced changes in brain activity to study functional connectivity in the human brain (Fox et al., 1997; Paus et al., 1998, 2001; Siebner et al., 1998, 2003; Strafella & Paus, 2001; Strafella et al., 2001, 2003, 2004; Bestmann et al., 2004). Recently, we provided evidence supporting corticostriatal control of dopamine release in humans by using PET and the dopamine receptor ligand [11C]raclopride to measure dopamine release in the striatum following repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex (Strafella et al., 2001) and motor cortex (Strafella et al., 2003). Dopamine release was only detected focally in the ipsilateral caudate nucleus (Strafella et al., 2001) and putamen (Strafella et al., 2003).
In vivo binding of benzamide tracers such as [11C]raclopride has been shown to be inversely proportional to levels of extracellular dopamine (Endres et al., 1997; Laruelle, 2000). In humans, this method has also been used to measure dopamine release in response to drugs (Dewey et al., 1993; Breier et al., 1997; de la Fuente-Fernandez et al., 2001, 2004; Piccini et al., 2003; Stoessl & de la Fuente-Fernandez, 2003; Piccini, 2004) and to motor and behavioural tasks (Koepp et al., 1998; Ouchi et al., 2002; Goerendt et al., 2003; Ginovart et al., 2004).
In the present study, using [11C]raclopride PET, we sought to investigate recently diagnosed, early, PD patients with evidence of unilateral symptoms. We measured in the putamen changes in extracellular dopamine concentration following rTMS (intensity 90% of the resting motor threshold, frequency 10 Hz) of the left and right primary motor cortex (MC). The main objective was to identify potential differences in corticostriatal dopamine release between the hemisphere clearly associated with contralateral motor symptoms and the presymptomatic stage of the other hemisphere in which, conceivably, functional compensatory mechanisms from surviving dopaminergic terminals (Zigmond et al., 1990; McGeer et al., 1989; Lee et al., 2000) are still preventing the appearance of PD symptoms.
Materials and methods
Experimental design
Seven patients (Table 1), recently diagnosed with early unilateral PD (Hoehn–Yahr stages I), participated in the study after having given written informed consent. They were investigated, off medication after overnight withdrawal (12–18 h) of their antiparkinsonian medications, with [11C]raclopride PET to measure changes in striatal dopamine release. Each underwent two [11C]raclopride PET sessions (total injected dose 20 mCi), one after rTMS of the MC contralateral to the clinically affected hemibody (hereafter referred to as the symptomatic hemisphere) and one following rTMS of the MC contralateral to the asymptomatic side (hereafter referred to as the asymptomatic hemisphere). The scan order was randomized across subjects and the scans were performed at the same time on two consecutive days. Autonomic parameters and subjective ratings were collected throughout both test sessions. During the study the subjects were asked to relax and keep their eyes closed. The experiments were approved by the Research Ethics Committee of the Montreal Neurological Institute and Hospital.
Table 1.
Clinical features of PD patients
| Subject | Age (years) | Disease duration (months) | UPDRS* (III) off medication | Medication | Dose (mg/day) |
|---|---|---|---|---|---|
| 1 | 61 | 11 | 19 | Pramipexole | 1.5 |
| 2 | 66 | 8 | 13 | Levodopa | 100 |
| 3 | 58 | 12 | 17 | Pramipexole | 0.75 |
| 4 | 60 | 13 | 19 | Levodopa | 100 |
| 5 | 57 | 11 | 17 | Pramipexole | 1.5 |
| 6 | 40 | 9 | 14 | None | – |
| 7 | 53 | 10 | 17 | Pramipexole | 0.75 |
| Mean ± SD | 56.4 ± 8.26 | 10.5 ± 1.71 | 16.5 ± 2.2 |
UPDRS III, Unified Parkinson’s Disease Rating Scale, motor section.
Values are divided by 108.
Transcranial magnetic stimulation
Repetitive TMS was carried out with a Magstim high-speed magnetic stimulator (Magstim, UK). This device permits delivery of high-frequency magnetic stimuli through the same coil. TMS was delivered through a circular coil (9 cm external diameter) orientated so that the induced electric current flowed in a posterior–anterior direction over the hand area of the MC. The coil was held in the scanner in a fixed position by a mechanical arm over the area where the lowest motor threshold was obtained.
The motor threshold, which was determined for each individual prior to the PET session, was defined as the lowest stimulus intensity able to elicit, from the first dorsal interosseous (FDI), five motor evoked potentials (MEPs) of at least 50 μV amplitude in a series of 10 stimuli delivered over the MC at intervals > 5 s. MEPs were recorded with Ag-AgCl surface electrodes fixed on the skin with a belly tendon montage. Electromyogram (EMG) signal was filtered (50 Hz–50 KHz bandpass) and displayed on the EMGrapher screen (Keypoint, Medtronic, Canada).
The stimulation intensity was set at 90% of the resting motor threshold for the FDI to avoid EMG responses. Four rTMS blocks were delivered 10 min apart in the scanner prior to image acquisition. Each block consisted of 15 10-pulse trains of 1 s duration (i.e. 10 Hz) with an intertrain interval of 10 s. Subthreshold rTMS over either of the stimulation sites induced no EMG responses in the relaxed FDI or in the abductor pollicis brevis or extensor digitorum communis.
Subjective ratings and autonomic measures
Electrodermal level, respiration rate and temperature were measured for 2.5 min during a baseline period at the start of the study and during the rest periods following each block of rTMS. After the baseline period and after each rest period, subjects rated their level of comfort, fatigue, anxiety, mood, irritation and pain on a seven-point Likert scale ranging from −3 to +3. For the baseline ratings, subjects were asked to rate how they were currently feeling, while ratings following blocks of rTMS referred to how they felt during the preceding rTMS stimulation. The autonomic and behavioural measures were analysed using repeated-measures ANOVA.
PET and imaging analysis
PET scans were obtained with a CTI-Siemens HR plus tomograph operating in 3-D mode, yielding images of resolution 4.2 mm full-width at half-maximum. Within 5 min of the end of the rTMS session, 10 mCi of [11C]raclopride was injected into the left antecubital vein over 60 s and emission data were then acquired over a period of 60 min in 26 frames of progressively increasing duration. After the emission scan, a 10-min transmission scan was performed with a rotating radioactive source (68Ga) for attenuation correction.
Each hemi-parkinsonian patient underwent two [11C]raclopride PET sessions, one after rTMS of the MC of the symptomatic hemisphere and one after rTMS of the MC of the asymptomatic hemisphere. The effect of rTMS on striatal dopamine release is strictly limited to the ipsilateral hemisphere (Strafella et al., 2001, 2003); thus, the change in striatal [11C]raclopride binding potential (BP) of the stimulated hemisphere was compared with the ipsilateral striatal [11C]raclopride BP of the nonstimulated hemisphere of the other session (baseline).
A high-resolution MRI (Siemens Sonata 1.5 T; T1-weighted images, 1 mm slice thickness) of each subject’s brain was acquired and transformed into standardized stereotaxic space (Talairach & Tournoux, 1998) using automated feature-matching to the Montreal Neurological Institute template (Collins et al., 1994).
PET frames were summed, registered to the corresponding MRI (Woods et al., 1993) and transformed into standardized stereotaxic space using the transformation parameters previously determined for the MRI. Thus, coordinates listed in the results are in Talairach space and correspond to the so-called MNI305 template. Voxelwise [11C]raclopride BP was calculated using a simplified reference tissue (cerebellum) method (Lammertsma & Hume, 1996; Gunn et al., 1997) to generate statistical parametric images of change in BP (Aston et al., 2000). This method uses the residuals of the least-squares fit of the compartmental model to the data at each voxel to estimate the standard deviation of the BP estimate, thus greatly increasing degrees of freedom. Only peaks falling within the striatum were considered, because this is the only brain structure where receptor-specific [11C]raclopride binding is detected. A reduction in [11C]raclopride BP is indicative of an increase in extracellular dopamine concentration (Dewey et al., 1993; Breier et al., 1997). For the purpose of the group analysis, images were flipped along the horizontal axis so that the side of all symptomatic hemispheres was set on the left while the asymptomatic hemisphere was on the right.
A threshold level of t ≥ 4.2 was considered significant (P < 0.05, two-tailed) corrected for multiple comparisons (Worsley et al., 1996), assuming a search volume equal to the entire striatum, an effective image filter of 6 mm full-width at half-maximum and 276 degrees of freedom (Aston et al., 2000). In each subject, BP values of the putamen were extracted with spherical regions of interest (radius 5 mm) drawn on the MRI at the level of the statistical peak defined by the parametric map (asymptomatic hemisphere, x = 35, y = 4, z = 0; symptomatic hemisphere, x = −30, y = 7, z = 5).
Results
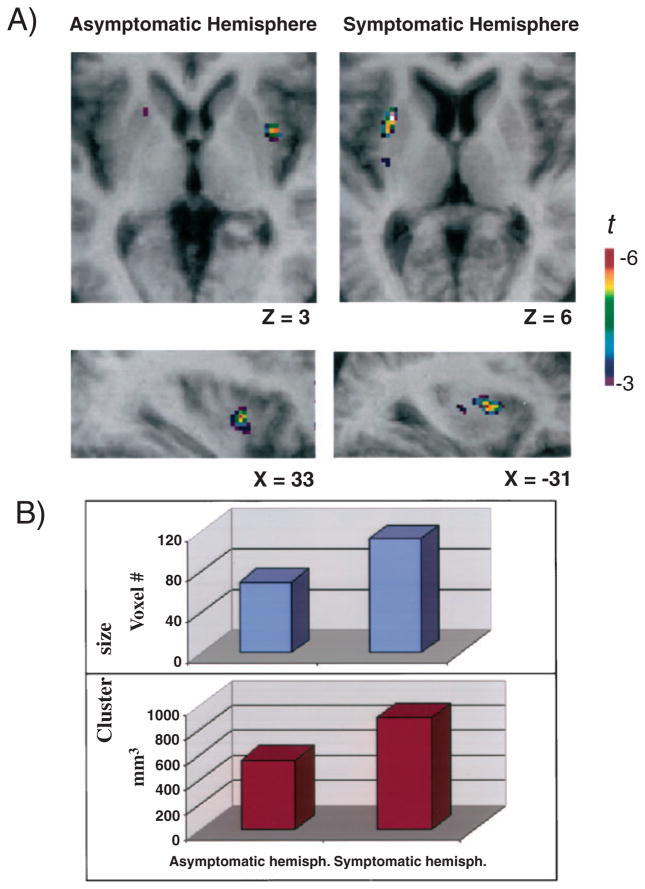
Repetitive TMS of the MC was associated with a reduction in [11C]raclopride BP in the ipsilateral putamen of both the asymptomatic and the symptomatic hemisphere (Fig. 1A). This reduction in [11C]raclopride BP is indicative of an increase in dopamine neurotransmission following cortical stimulation.
Fig. 1.
rTMS-induced dopamine release. (A) Axial and sagittal sections of the statistical parametric map of the change in [11C]raclopride BP overlaid upon the average MRI of all subjects in stereotaxic space. (B) The figure displays the size (voxel number and mm3) of the significant cluster of dopamine release in the putamen for the asymptomatic and symptomatic hemispheres.
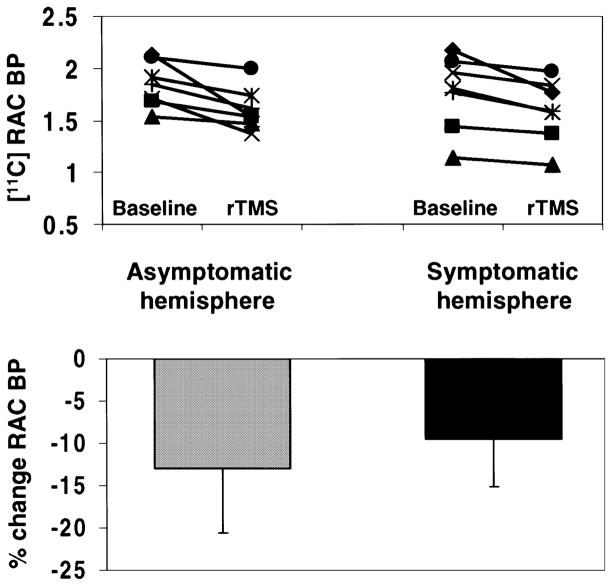
In the asymptomatic hemisphere, rTMS of the MC induced a 12.99% decrease in [11C]raclopride BP (mean ± SD: baseline, 1.847 ± 0.22; rTMS, 1.607 ± 0.20) whereas, in the symptomatic hemisphere, rTMS produced a 9.47% reduction in BP (baseline, 1.762 ± 0.36; rTMS, 1.595 ± 0.30). The magnitude of change in binding was significantly less in the putamen of the symptomatic hemisphere (ANCOVA after adjusting for baseline differences, F1,5 = 19.75, P < 0.01). Figure 2 displays percentage changes and BP values from the putamen of the asymptomatic and symptomatic hemisphere obtained from regions of interest drawn at the axial level of the statistical peaks.
Fig. 2.
Binding potentials. On the top, [11C]raclopride binding potentials (baseline and following rTMS), from the putamen of the asymptomatic (left) and symptomatic (right) hemispheres, extracted from a spherical region of interest (radius 5 mm) centred at the x, y, and z coordinates of the statistical peak revealed by the parametric map. On the bottom, the figure displays the percentage changes in [11C]raclopride BP for the asymptomatic (left) and symptomatic (right) hemispheres. The magnitude of change in binding was significantly less in the putamen of the symptomatic than the asymptomatic hemisphere (P < 0.01 by ANCOVA after adjusting for baseline differences).
The area of decrease in [11C]raclopride BP, located in the lateral part of the putamen, had its peak at coordinates x = 35, y = 4, z = 0 for the asymptomatic hemisphere and at x = −30, y = 7, z = 5 for the symptomatic hemisphere. While in the former the significant cluster, extending from y = −2 to y = 7 in the anteroposterior direction (where y = 0 mm is the level of the anterior commissure), involved 70 voxels, corresponding to a volume of 560 mm3, in the latter the significant cluster, extending from y = −6 to y = 12, counted 113 voxels corresponding to a volume of 904 mm3 (Fig. 1B). This represented an increase in cluster size for the symptomatic hemisphere of 61.4%.
These changes in [11C]raclopride BP were not related to the stimulation intensity. There were no statistically significant differences in any other striatal areas (i.e. caudate nucleus or nucleus accumbens), nor was any difference observed in motor threshold between the symptomatic (mean ± SD, 49.1 ± 0.98%) and asymptomatic (50.6% ± 2.73%) hemispheres. The statistical analysis of autonomic and behavioural measures revealed no significant main effect of stimulation site or condition (before and after rTMS), nor any significant site × condition interaction (Tables 2 and 3).
Table 2.
Mean autonomic parameters before and after rTMS of symptomatic and asymptomatic hemisphere
| Symptomatic hemisphere |
Asymptomatic hemisphere |
|||
|---|---|---|---|---|
| Before rTMS | After rTMS | Before rTMS | After rTMS | |
| Electrodermal level (μΩ) | 5.88 ± 0.47 | 5.62 ± 0.47 | 5.50 ± 0.24 | 5.45 ± 0.26 |
| Respiration rate | 9.14 ± 2.46 | 9.74 ± 2.95 | 8.70 ± 1.98 | 8.66 ± 1.26 |
| Temperature (°C) | 27.91 ± 2.78 | 28.35 ± 2.86 | 31.21 ± 2.77 | 29.86 ± 3.87 |
Mean values are shown ±SD.
Table 3.
Behavioural ratings before and after rTMS of symptomatic and asymptomatic hemisphere*
| Symptomatic hemisphere |
Asymptomatic hemisphere |
|||
|---|---|---|---|---|
| Before rTMS | After rTMS | Before rTMS | After rTMS | |
| Discomfort–comfort | 1.78 ± 1.20 | 1.98 ± 0.98 | 1.22 ± 1.92 | 1.19 ± 1.78 |
| Anxious–calm | 1.22 ± 2.11 | 1.37 ± 2.00 | 1.44 ± 1.94 | 1.37 ± 1.94 |
| Fatigued–rested | 1.33 ± 1.87 | 1.30 ± 1.76 | 1.44 ± 1.01 | 1.58 ± 1.00 |
| Sad–happy | 1.56 ± 1.24 | 1.44 ± 1.13 | 1.28 ± 1.25 | 1.35 ± 1.22 |
| Irritated–soothed | 1.11 ± 1.90 | 1.11 ± 1.90 | 1.06 ± 1.98 | 0.96 ± 2.01 |
| Feel pain–do not feel pain | 1.33 ± 1.41 | 1.47 ± 1.10 | 1.44 ± 1.24 | 1.50 ± 1.21 |
Seven-point Likert scale, ranging from −3 to +3. Values are presented as mean ± SD.
Discussion
We have shown that rTMS of the human MC in hemi-parkinsonian patients evoked release of dopamine in the ipsilateral putamen, as detected by [11C]raclopride PET. The most interesting finding was that, in the symptomatic hemisphere, while the amount of TMS-induced striatal dopamine release was, as expected, smaller, the size of the significant cluster of change in [11C]raclopride BP was, instead, 61.4% greater than in the asymptomatic side (Figs 1 and 2).
The observed changes in [11C]raclopride BP in the ipsilateral putamen of patients with PD confirms our previous experiments performed in normal subjects in which we showed that rTMS of the dorsolateral prefrontal cortex and motor cortex was associated with reduced [11C]raclopride binding in its main projection area of the striatum, namely the ipsilateral head of the caudate nucleus (Strafella et al., 2001) and putamen (Strafella et al., 2003). The putamen is the principal input nucleus for somatic motor control in the basal ganglia and receives somatotopically organized corticostriatal projections from the frontal motor areas (Kemp & Powel, 1970; Kunzle, 1975; Jones et al., 1977; Liles & Updyke, 1985; Whitworth et al., 1991; Parent & Hazrati, 1995; Inase et al., 1996; Takada et al., 1998; Tokuno et al., 1999). Anterograde tracing studies in monkeys have shown that corticostriatal fibers originating in the MC project to the lateral part of the putamen with a dorsoventral arrangement, the leg represented in the dorsal putamen, the face more ventrally and the arm lying in between these two areas (Jones et al., 1977; Kunzle, 1975; Liles & Updyke, 1985; Whitworth et al., 1991; Takada et al., 1998). Moreover, in monkeys there is distal-to-proximal somatotopy, with the distal forelimb portion of the MC projecting to the most lateral part of the putamen (Tokuno et al., 1999). This anatomical location corresponds exactly to the cluster of dopamine release found in our present study following stimulation of the hand area of the MC (Fig. 1A). This is also in accordance with our previous report of dopamine release confined to the lateral part of the ipsilateral putamen following rTMS of the finger representation in the MC (Strafella et al., 2003).
One limitation of the present study relates to the brain area stimulated by our rTMS. While the hand MC projections were certainly stimulated, we cannot exclude the possibility that adjacent cortical areas, namely premotor and somatosensory cortex, were also stimulated, which may have contributed to dopamine release. However, the premotor corticostriatal projections map to the dorsomedial putamen (Takada et al., 1998), where there was no change in tracer binding. The somatosensory corticostriatal projections target the same areas as MC in the lateral putamen (Flaherty & Graybiel, 1993).
Although a direct corticostriatal influence on striatal dopamine terminals most probably accounts for the spatial selectivity of the rTMS effect in our study, we cannot exclude involvement of other anatomical pathways. Frontal cortical neurons also project to the substantia nigra (Sesack & Pickel, 1992; Naito & Kita, 1994) where they can modulate the firing of dopamine neurons projecting to the striatum (Murase et al., 1993; Karreman & Moghddam, 1996). However, little is known about the somatotopic organization of this system.
In the present study, the amount of TMS-induced striatal dopamine release was less in the symptomatic hemisphere than in the contralateral hemisphere. This was not surprising, and two main reasons could account for this finding: the more advanced degeneration of dopaminergic terminals and the failure of the well described functional compensatory mechanisms from surviving dopaminergic terminals (McGeer et al., 1989; Zigmond et al., 1990; Lee et al., 2000), which are mainly characterized by an increase in synthesis and release of dopamine as well as by a reduced dopamine inactivation rate in the presynaptic space of nerve terminals (Zigmond et al., 1990; Lee et al., 2000).
The most intriguing and, we believe, exciting finding of this study was the larger cluster of change in [11C]raclopride BP (61.4%) involving the symptomatic side (Fig. 1A and B). There are a number of possible interpretations that could explain our observation. Indeed, Zigmond et al. (1990) have suggested that the field of influence of residual dopamine terminals increases with denervation. That is, because of the partial loss of re-uptake sites, released dopamine diffuses out to more distant regions of the receptor population in the dopamine-denervated striatum. According to this model, dopamine molecules released after rTMS could possibly interact with larger areas of the receptor population in the symptomatic putamen. However, several electrophysiological studies have also shown that in conditions of dopamine denervation there exists a hyperactivity of corticostriatal glutamatergic transmission (Lindefors & Ungerstedt, 1990; Calabresi et al., 1993, 2000; Campbell & Bjorklund, 1994) with increased numbers of striatal neurons responding to cortical stimulation (Florio et al., 1993). Therefore, as an alternative, one may propose that because of such corticostriatal hyperactivity rTMS could be responsible for an abnormal release of glutamate which by diffusing into the extrasynaptic space (Onn et al., 2000) may activate, either directly or indirectly via nitric oxide (Onn et al., 2000), larger areas of dopaminergic terminals in the symptomatic hemisphere.
While there is certainly little knowledge about the mechanisms by which rTMS exerts remote effects, either one of the proposed explanations seem to support the hypothesis that the larger cluster of change in [11C]raclopride BP in the symptomatic hemisphere (where compensatory mechanisms have presumably failed) may represent an in vivo measure of a loss of functional segregation of cortical information to the striatum, thus representing indirect evidence of abnormal corticostriatal transmission in symptomatic PD.
In contrast, the more focal release of dopamine observed in the putamen of the contralateral hemisphere in the presymptomatic stage (Fig. 1A and B), similar to what has also been described in normal subjects (Strafella et al., 2003) may well represent the result of the functional compensatory changes (McGeer et al., 1989; Zigmond et al., 1990; Lee et al., 2000) taking place in these patients and still preventing the appearance of the motor symptoms.
The finding of a spatially enlarged area of dopamine release following cortical stimulation as a possible indirect expression of loss of functional segregation of striatal neurons has implications for models of basal ganglia function in PD. One of these models proposes that, during action, there is specific enhancement of activity in corticostriatal loops involved in the current task with concomitant suppression of competing motor networks (Mink, 1996). The neuroanatomical arrangement of the corticostriatal system in a centre-surround inhibitory pattern is thought to facilitate this focusing function (Parent & Hazrati, 1993; Mink, 1996; Mink, 2003). The abnormal corticostriatal excitability and loss of neuronal functional segregation described in PD may well contribute to the de-arrangement of this selective centre-surround inhibitory pattern leading to an impaired inhibition of competing motor patterns and underlying some of the motor symptoms observed in this condition.
Our findings open new avenues for two different types of in vivo studies in PD. In the first place, the opportunity for longitudinal studies, to test whether the enlarged area of dopamine release as a consequence of the corticostriatal activation is simply a nonspecific sign of PD or could possibly be considered an index for the later development of motor fluctuations (i.e. dyskinesias). In the second place, the possibility of testing the capability of antiparkinsonian medications (i.e. L-dopa, dopamine agonists) in restoring (or worsening) spatially confined area of dopamine release, as observed in healthy subjects.
Acknowledgments
We wish to thank the staff of the McConnell Brain Imaging and Medical Cyclotron Units for their assistance in carrying out the studies and Dr Keith Worsley and Dr Chuanhong Liao for statistical assistance. This work was funded by the Canadian Institutes of Health Research, the Fonds de la Recherche en Santé du Québec and the Canada Foundation for Innovation to A.P.S.
Abbreviations
- BP
binding potential
- EMG
electromyogram
- FDI
first dorsal interosseous
- fMRI
functional magnetic resonance imaging
- MC
motor cortex
- MEP
motor evoked potential
- PD
Parkinson’s disease
- PET
positron emission tomography
- rTMS
repetitive transcranial magnetic stimulation
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Aston JA, Gunn RN, Worsley KJ, Evans AC, Dagher A. Statistical method for the analysis of positron emission tomography neuroreceptor ligand data. Neuroimage. 2000;12:245–256. doi: 10.1006/nimg.2000.0620. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci. 2004;19:1950–1962. doi: 10.1111/j.1460-9568.2004.03277.x. [DOI] [PubMed] [Google Scholar]
- Bouyer JJ, Park DH, Joh TH, Pickel VM. Chemical and structural analysis of the relation between cortical inputs and tyrosine hydroxylase-containing terminals in rat neostriatum. Brain Res. 1984;302:267–275. doi: 10.1016/0006-8993(84)90239-7. [DOI] [PubMed] [Google Scholar]
- Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Bernardi G. Electrophysiology of dopamine denervated striatal neurons. Trends Neurosci. 2000;23:S57–S63. doi: 10.1016/s1471-1931(00)00017-3. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Mercuri NB, Sancesario G, Bernardi G. Electrophysiology of dopamine denervated striatal neurons. Implications for Parkinson’s disease. Brain. 1993;116:433–452. [PubMed] [Google Scholar]
- Campbell K, Bjorklund A. Prefrontal corticostriatal afferents maintain increased enkephalin gene expression in the dopamine-denervated rat striatum. Eur J Neurosci. 1994;6:1371–1383. doi: 10.1111/j.1460-9568.1994.tb00328.x. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Carlsson A. Interactions between glutamatergic and monoaminergic systems within the basal ganglia – implications for schizophrenia and Parkinson’s disease. Trends Neurosci. 1990;13:272–276. doi: 10.1016/0166-2236(90)90108-m. [DOI] [PubMed] [Google Scholar]
- Cheramy A, Romo R, Godeheu G, Glowinski J. In vivo presynaptic control of dopamine release in the cat caudate nucleus – II. Facilitatory or inhibitory influence of 1-glutamate. Neuroscience. 1986;19:1081–1090. doi: 10.1016/0306-4522(86)90124-7. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volume tric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Dewey SL, Smith GS, Logan J, Brodie JD, Fowler JS, Wolf AP. Striatal binding of the PET ligand 11C-raclopride is altered by drugs that modify synaptic dopamine levels. Synapse. 1993;13:350–356. doi: 10.1002/syn.890130407. [DOI] [PubMed] [Google Scholar]
- Endres CJ, Kolachana BS, Saunders RC, Su T, Weinberger D, Breier A, Eckelman WC, Carson RE. Kinetic modeling of [11C]raclopride: combined PET-microdialysis studies. J Cereb Blood Flow Metab. 1997;17:932–942. doi: 10.1097/00004647-199709000-00002. [DOI] [PubMed] [Google Scholar]
- Flaherty AW, Graybiel AM. Two input systems for body representations in the primate striatal matrix: experimental evidence in the squirrel monkey. J Neurosci. 1993;13:1120–1137. doi: 10.1523/JNEUROSCI.13-03-01120.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florio T, Di Loreto S, Cerrito F, Scarnati E. Influence of prelimbic and sensorimotor cortices on striatal neurons in the rat. electrophysiological evidence for converging inputs and the effects of 6-OHDA-induced degeneration of the substantia nigra. Brain Res. 1993;619:180–188. doi: 10.1016/0006-8993(93)91610-5. [DOI] [PubMed] [Google Scholar]
- Fox P, Ingham R, George MS, Mayberg H, Ingham J, Roby J, Martin C, Jerabek P. Imaging human intra-cerebral connectivity by PET during TMS. Neuroreport. 1997;8:2787–2791. doi: 10.1097/00001756-199708180-00027. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernandez R, Lu JQ, Sossi V, Jivan S, Schulzer M, Holden JE, Lee CS, Ruth TJ, Calne DB, Stoessl AJ. Biochemical variations in the synaptic level of dopamine precede motor fluctuations in Parkinson’s disease: PET evidence of increased dopamine turnover. Ann Neurol. 2001;49:298–303. doi: 10.1002/ana.65.abs. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernandez R, Sossi V, Huang Z, Furtado S, Lu JQ, Calne DB, Ruth TJ, Stoessl AJ. Levodopa-induced changes in synaptic dopamine levels increase with progression of Parkinson’s disease: implications for dyskinesias. Brain. 2004;127:2747–2754. doi: 10.1093/brain/awh290. [DOI] [PubMed] [Google Scholar]
- Ginovart N, Wilson AA, Houle S, Kapur S. Amphetamine pretreatment induces a change in both D2-receptor density and apparent affinity: a [11C]raclopride positron emission tomography study in cats. Biol Psychiatry. 2004;55:1188–1194. doi: 10.1016/j.biopsych.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Goerendt IK, Messa C, Lawrence AD, Grasby PM, Piccini P, Brooks DJ. Dopamine release during sequential finger movements in health and Parkinson’s disease: a PET study. Brain. 2003;126:312–325. doi: 10.1093/brain/awg035. [DOI] [PubMed] [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–287. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- Inase M, Sakai ST, Tanji J. Overlapping corticostriatal projections from the supplementary motor area and the primary motor cortex in the macaque monkey: an anterograde double labeling study. J Comp Neurol. 1996;373:283–296. doi: 10.1002/(SICI)1096-9861(19960916)373:2<283::AID-CNE10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Jones EG, Coulter JD, Burton H, Porter R. Cells of origin and terminal distribution of corticostriatal fibers arising in the sensory-motor cortex of monkeys. J Comp Neurol. 1977;173:53–80. doi: 10.1002/cne.901730105. [DOI] [PubMed] [Google Scholar]
- Kanno M, Matsumoto M, Togashi H, Yoshioka M, Mano Y. Effects of acute repetitive transcranial magnetic stimulation on dopamine release in the rat dorsolateral striatum. J Neurol Sci. 2004;217:73–81. doi: 10.1016/j.jns.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Karreman M, Moghaddam B. The prefrontal cortex regulates the basal release of dopamine in the limbic striatum: an effect mediated by ventral tegmental area. J Neurochem. 1996;66:589–598. doi: 10.1046/j.1471-4159.1996.66020589.x. [DOI] [PubMed] [Google Scholar]
- Keck ME, Welt T, Muller MB, Erhardt A, Ohl F, Toschi N, Holsboer F, Sillaber I. Repetitive transcranial magnetic stimulation increases the release of dopamine in the mesolimbic and mesostriatal system. Neuropharmacology. 2002;43:101–109. doi: 10.1016/s0028-3908(02)00069-2. [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TP. The cortico-striate projection in the monkey. Brain. 1970;93:525–546. doi: 10.1093/brain/93.3.525. [DOI] [PubMed] [Google Scholar]
- Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, Brooks DJ, Bench CJ, Grasby PM. Evidence for striatal dopamine release during a video game. Nature. 1998;393:266–268. doi: 10.1038/30498. [DOI] [PubMed] [Google Scholar]
- Kunzle H. Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in Macaca fascicularis. Brain Res. 1975;88:195–209. doi: 10.1016/0006-8993(75)90384-4. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- Lee CS, Samii A, Sossi V, Ruth TJ, Schulzer M, Holden JE, Wudel J, Pal PK, de la Fuente-Fernandez R, Calne DB, Stoessl AJ. In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson’s disease. Ann Neurol. 2000;47:493–503. [PubMed] [Google Scholar]
- Leviel V, Gobert A, Guibert B. The glutamate-mediated release of dopamine in the rat striatum: further characterization of the dual excitatory-inhibitory function. Neuroscience. 1990;39:305–312. doi: 10.1016/0306-4522(90)90269-a. [DOI] [PubMed] [Google Scholar]
- Liles SL, Updyke BV. Projection of the digit and wrist area of precentral gyrus to the putamen. relation between topography and physiological properties of neurons in the putamen. Brain Res. 1985;339:245–255. doi: 10.1016/0006-8993(85)90089-7. [DOI] [PubMed] [Google Scholar]
- Lindefors N, Ungerstedt U. Bilateral regulation of glutamate tissue and extracellular levels in caudate-putamen by midbrain dopamine neurons. Neurosci Lett. 1990;115:248–252. doi: 10.1016/0304-3940(90)90463-j. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Akiyama H, McGeer EG. Comparison of neuronal loss in Parkinson’s disease and aging. In: Calne DB, Comi G, Crippa D, et al., editors. Parkinsonism and Aging. Raven Press; New York: 1989. pp. 25–34. [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch Neurol. 2003;60:1365–1368. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- Murase S, Grenhoff J, Chouvet G, Gonon FG, Svensson TH. Prefrontal cortex regulates burst firing and transmitter release in rat mesolimbic dopamine neurons studied in vivo. Neurosci Lett. 1993;157:53–56. doi: 10.1016/0304-3940(93)90641-w. [DOI] [PubMed] [Google Scholar]
- Naito A, Kita H. The cortico-nigral projection in the rat: an anterograde tracing study with biotinylated dextran amine. Brain Res. 1994;637:317–322. doi: 10.1016/0006-8993(94)91252-1. [DOI] [PubMed] [Google Scholar]
- Onn SP, Grace AA. Alterations in electrophysiological activity and dye coupling of striatal spiny and aspiny neurons in dopamine-denervated rat striatum recorded in vivo. Synapse. 1999;33:1–15. doi: 10.1002/(SICI)1098-2396(199907)33:1<1::AID-SYN1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Onn SP, West AR, Grace AA. Dopamine-mediated regulation of striatal neuronal and network interactions. Trends Neurosci. 2000;23:S48–S56. doi: 10.1016/s1471-1931(00)00020-3. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Yoshikawa E, Futatsubashi M, Okada H, Torizuka T, Sakamoto M. Effect of simple motor performance on regional dopamine release in the striatum in Parkinson disease patients and healthy subjects: a positron emission tomography study. J Cereb Blood Flow Metab. 2002;22:746–752. doi: 10.1097/00004647-200206000-00013. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Anatomical aspects of information processing in primate basal ganglia. Trends Neurosci. 1993;16:111–116. doi: 10.1016/0166-2236(93)90135-9. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC. Dose-dependent reduction of cerebral blood flow during rapid-rate transcranial magnetic stimulation of the human sensorimotor cortex. J Neurophysiol. 1998;79:1102–1107. doi: 10.1152/jn.1998.79.2.1102. [DOI] [PubMed] [Google Scholar]
- Paus T, Sipila PK, Strafella AP. Synchronization of neuronal activity in the human primary motor cortex by transcranial magnetic stimulation: an EEG study. J Neurophysiol. 2001;86:1983–1990. doi: 10.1152/jn.2001.86.4.1983. [DOI] [PubMed] [Google Scholar]
- Piccini P. Neurodegenerative movement disorders: the contribution of functional imaging. Curr Opin Neurol. 2004;17:459–466. doi: 10.1097/01.wco.0000137538.84115.3c. [DOI] [PubMed] [Google Scholar]
- Piccini P, Pavese N, Brooks DJ. An in vivo study of striatal and cortical endogenous dopamine release following pharmacological challenges in Parkinson’s disease. Ann Neurol. 2003;53:647–653. doi: 10.1002/ana.10526. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320:145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Peller M, Lee L. Applications of combined TMS-PET studies in clinical and basic research. Clin Neurophysiol. 2003;56 (Suppl):63–72. doi: 10.1016/s1567-424x(09)70210-7. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Willoch F, Peller M, Auer C, Boecker H, Conrad B, Bartenstein P. Imaging brain activation induced by long trains of repetitive transcranial magnetic stimulation. Neuroreport. 1998;9:943–948. doi: 10.1097/00001756-199803300-00033. [DOI] [PubMed] [Google Scholar]
- Stoessl AJ, de la Fuente-Fernandez R. Dopamine receptors in Parkinson’s disease: imaging studies. Adv Neurol. 2003;91:65–71. [PubMed] [Google Scholar]
- Strafella AP, Paus T. Cerebral blood-flow changes induced by paired-pulse transcranial magnetic stimulation of the primary motor cortex. J Neurophysiol. 2001;85:2624–2629. doi: 10.1152/jn.2001.85.6.2624. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21:RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Fraraccio M, Dagher A. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain. 2003;126:2609–2615. doi: 10.1093/brain/awg268. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Vanderwerf Y, Sadikot AF. Effects of transcranial magnetic stimulation of the motor cortex on subthalamic neuronal activity. Eur J Neurosci. 2004;20:2245–2249. doi: 10.1111/j.1460-9568.2004.03669.x. [DOI] [PubMed] [Google Scholar]
- Takada M, Tokuno H, Nambu A, Inase M. Corticostriatal projections from the somatic motor areas of the frontal cortex in the macaque monkey: segregation versus overlap of input zones from the primary motor cortex, the supplementary motor area, and the premotor cortex. Exp Brain Res. 1998;120:114–128. doi: 10.1007/s002210050384. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme; Stuttgart: 1998. [Google Scholar]
- Tokuno H, Inase M, Nambu A, Akazawa T, Miyachi S, Takada M. Corticostriatal projections from distal and proximal forelimb representations of the monkey primary motor cortex. Neurosci Lett. 1999;269:33–36. doi: 10.1016/s0304-3940(99)00401-2. [DOI] [PubMed] [Google Scholar]
- Whitworth RH, Jr, LeDoux MS, Gould HJ., 3rd Topographic distribution of connections from the primary motor cortex to the corpus striatum in Aotus trivirgatus. J Comp Neurol. 1991;307:177–188. doi: 10.1002/cne.903070202. [DOI] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J Comput Assist Tomogr. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Abercrombie ED, Berger TW, Grace AA, Stricker EM. Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci. 1990;13:290–296. doi: 10.1016/0166-2236(90)90112-n. [DOI] [PubMed] [Google Scholar]