Abstract
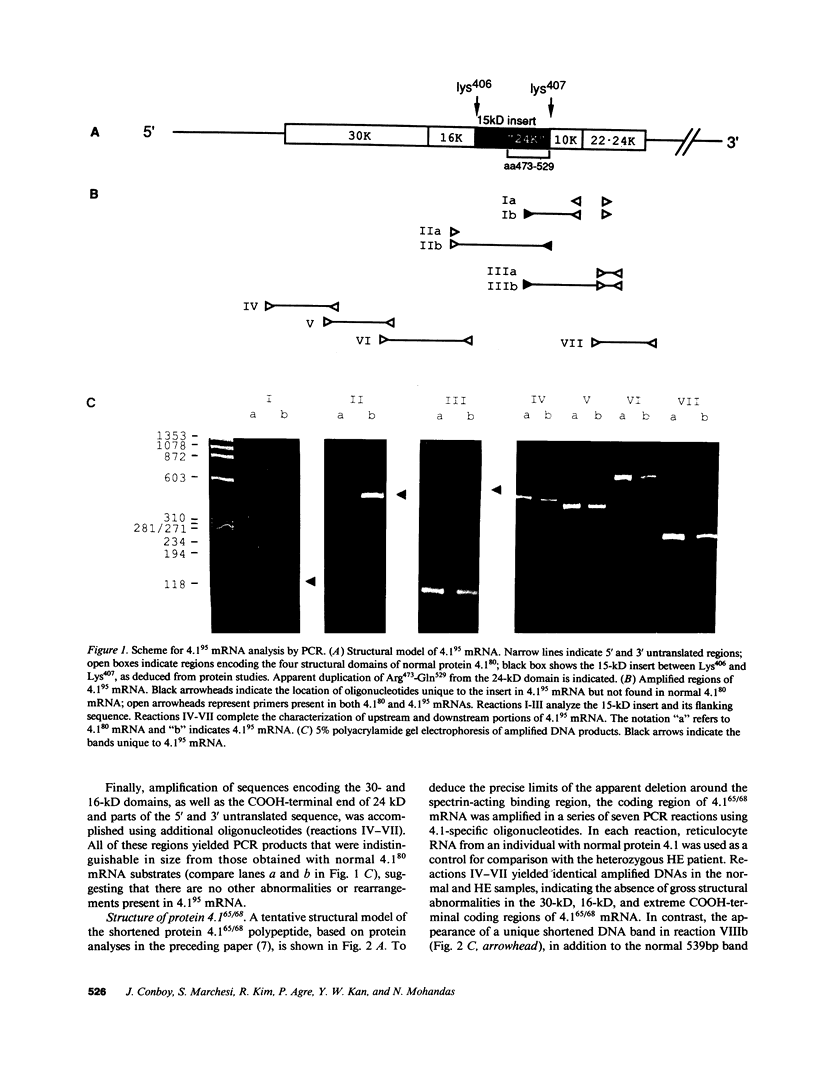
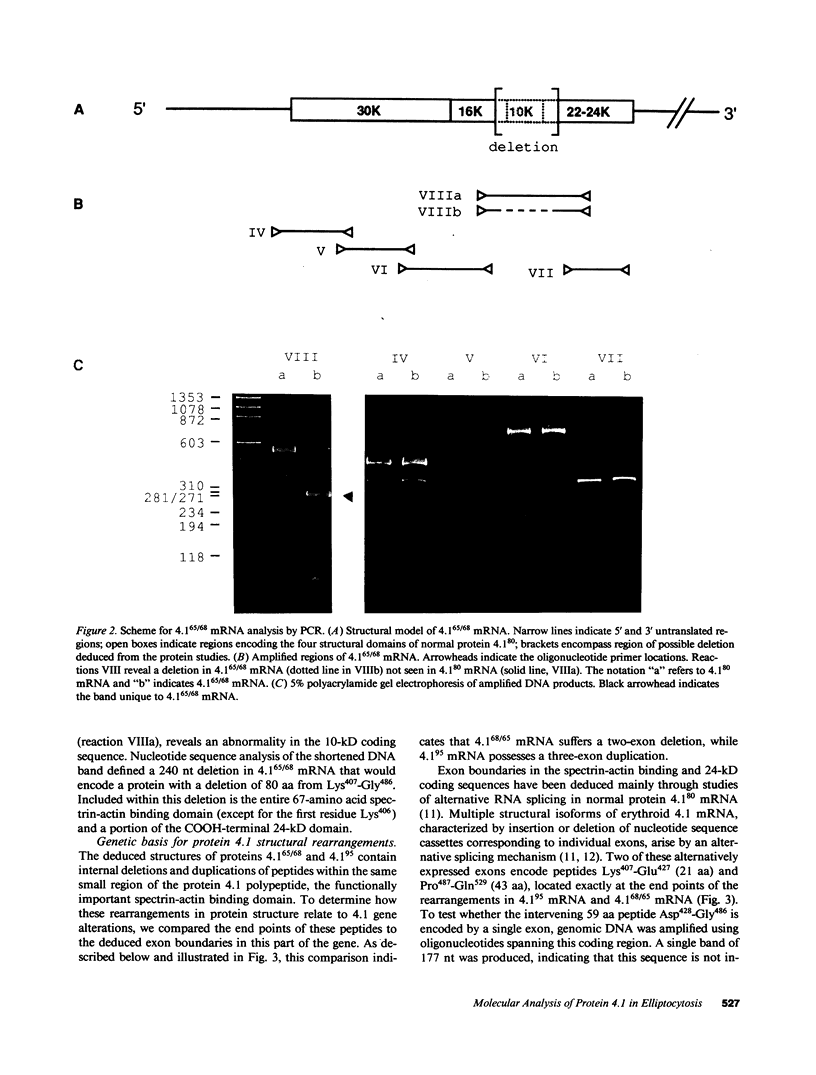
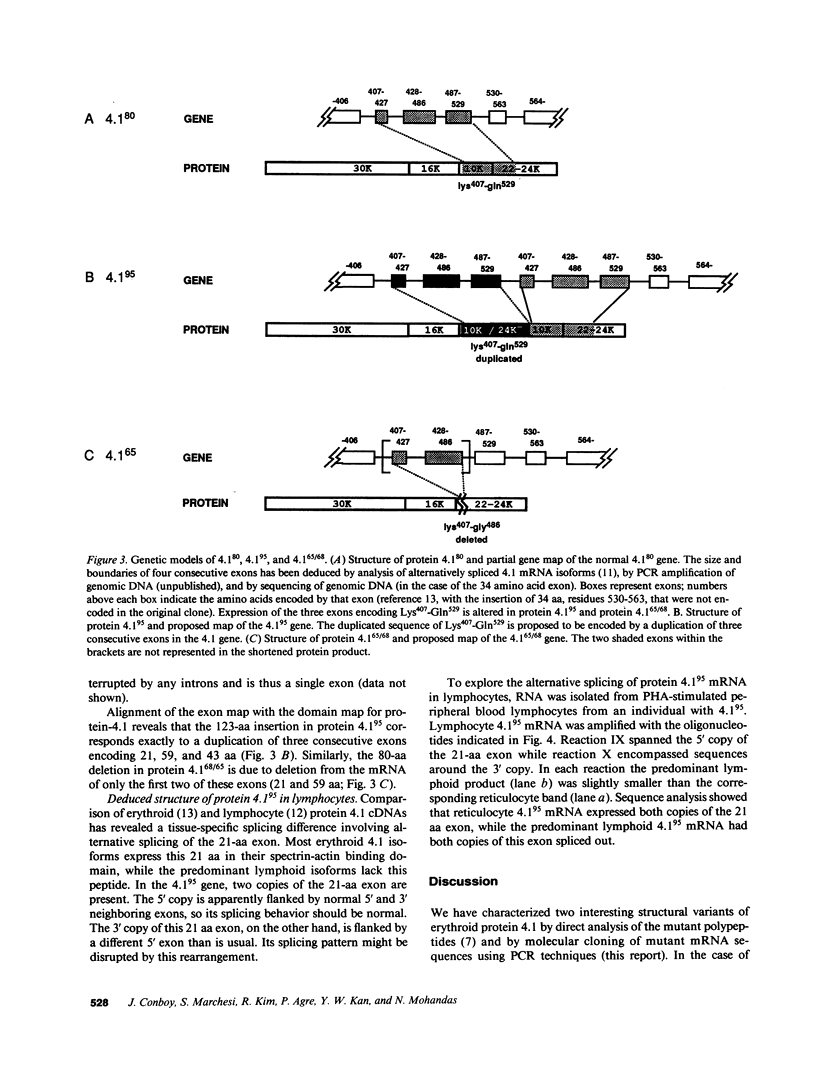
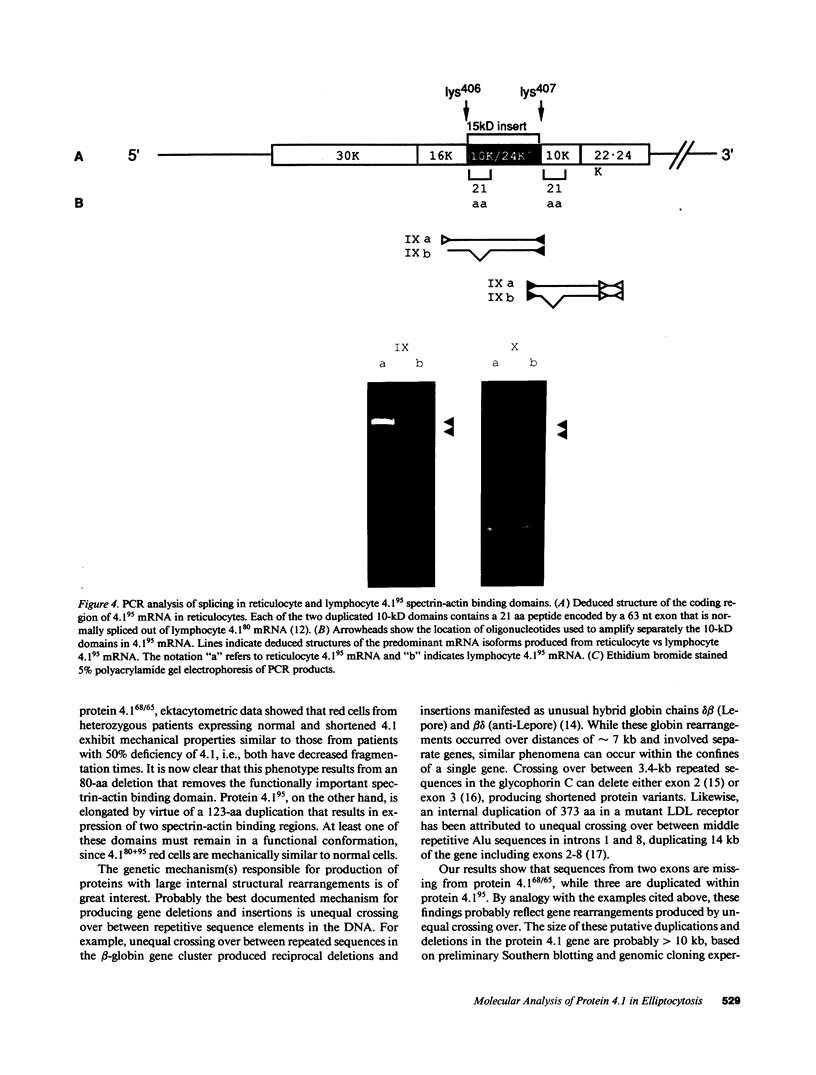
Protein 4.1 is an approximately 80-kD structural protein in the membrane skeleton which underlies and supports the erythrocyte plasma membrane. The preceding companion paper presents a biochemical study of two abnormal protein 4.1 species from individuals with the red blood cell disorder, hereditary elliptocytosis. These variants, "protein 4.1(68/65)" and "protein 4.1(95)," have altered molecular weights due to internal deletions and duplications apparently localized around the spectrin-actin binding domain. Here we use polymerase chain reaction (PCR) techniques to clone and sequence the corresponding mutant reticulocyte mRNAs, and correlate the deletion/duplication end points with exon boundaries of the gene. Protein 4.1(68/65) mRNA lacks sequences encoding the functionally important spectrin-actin binding domain due to a 240 nucleotide (nt) deletion spanning the codons for Lys407-Gly486. Protein 4.1(95) mRNA encodes a protein with two spectrin-actin binding domains by virtue of a 369 nt duplication of codons for Lys407-Gln529. These deletions and duplications correspond to gene rearrangements involving three exons encoding 21, 59, and 43 amino acids, respectively. The duplicated 21 amino acid exon in the 4.1(95) gene retains its proper tissue-specific expression pattern, being spliced into reticulocyte 4.1 mRNA and out of lymphocyte 4.1 mRNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alloisio N., Morlé L., Dorléac E., Gentilhomme O., Bachir D., Guetarni D., Colonna P., Bost M., Zouaoui Z., Roda L. The heterozygous form of 4.1(-) hereditary elliptocytosis [the 4.1(-) trait]. Blood. 1985 Jan;65(1):46–51. [PubMed] [Google Scholar]
- Colin Y., Le Van Kim C., Tsapis A., Clerget M., d'Auriol L., London J., Galibert F., Cartron J. P. Human erythrocyte glycophorin C. Gene structure and rearrangement in genetic variants. J Biol Chem. 1989 Mar 5;264(7):3773–3780. [PubMed] [Google Scholar]
- Conboy J. G., Chan J., Mohandas N., Kan Y. W. Multiple protein 4.1 isoforms produced by alternative splicing in human erythroid cells. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9062–9065. doi: 10.1073/pnas.85.23.9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy J., Kan Y. W., Shohet S. B., Mohandas N. Molecular cloning of protein 4.1, a major structural element of the human erythrocyte membrane skeleton. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9512–9516. doi: 10.1073/pnas.83.24.9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy J., Mohandas N., Tchernia G., Kan Y. W. Molecular basis of hereditary elliptocytosis due to protein 4.1 deficiency. N Engl J Med. 1986 Sep 11;315(11):680–685. doi: 10.1056/NEJM198609113151105. [DOI] [PubMed] [Google Scholar]
- Forrest S. M., Cross G. S., Flint T., Speer A., Robson K. J., Davies K. E. Further studies of gene deletions that cause Duchenne and Becker muscular dystrophies. Genomics. 1988 Feb;2(2):109–114. doi: 10.1016/0888-7543(88)90091-2. [DOI] [PubMed] [Google Scholar]
- Grimm T., Müller B., Dreier M., Kind E., Bettecken T., Meng G., Müller C. R. Hot spot of recombination within DXS164 in the Duchenne muscular dystrophy gene. Am J Hum Genet. 1989 Sep;45(3):368–372. [PMC free article] [PubMed] [Google Scholar]
- LOVRIC V. A., WALSH R. J., BRADLEY M. A. HEREDITARY ELLIPTOCYTOSIS: GENETIC LINKAGE WITH THE RH CHROMOSOME. Australas Ann Med. 1965 May;14:162–166. doi: 10.1111/imj.1965.14.2.162. [DOI] [PubMed] [Google Scholar]
- Lambert S., Conboy J., Zail S. A molecular study of heterozygous protein 4.1 deficiency in hereditary elliptocytosis. Blood. 1988 Dec;72(6):1926–1929. [PubMed] [Google Scholar]
- Lehrman M. A., Goldstein J. L., Russell D. W., Brown M. S. Duplication of seven exons in LDL receptor gene caused by Alu-Alu recombination in a subject with familial hypercholesterolemia. Cell. 1987 Mar 13;48(5):827–835. doi: 10.1016/0092-8674(87)90079-1. [DOI] [PubMed] [Google Scholar]
- Leto T. L., Marchesi V. T. A structural model of human erythrocyte protein 4.1. J Biol Chem. 1984 Apr 10;259(7):4603–4608. [PubMed] [Google Scholar]
- Marchesi S. L., Conboy J., Agre P., Letsinger J. T., Marchesi V. T., Speicher D. W., Mohandas N. Molecular analysis of insertion/deletion mutations in protein 4.1 in elliptocytosis. I. Biochemical identification of rearrangements in the spectrin/actin binding domain and functional characterizations. J Clin Invest. 1990 Aug;86(2):516–523. doi: 10.1172/JCI114738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire M., Smith B. L., Agre P. Distinct variants of erythrocyte protein 4.1 inherited in linkage with elliptocytosis and Rh type in three white families. Blood. 1988 Jul;72(1):287–293. [PubMed] [Google Scholar]
- Scharf S. J., Horn G. T., Erlich H. A. Direct cloning and sequence analysis of enzymatically amplified genomic sequences. Science. 1986 Sep 5;233(4768):1076–1078. doi: 10.1126/science.3461561. [DOI] [PubMed] [Google Scholar]
- Speicher D. W., Marchesi V. T. Erythrocyte spectrin is comprised of many homologous triple helical segments. Nature. 1984 Sep 13;311(5982):177–180. doi: 10.1038/311177a0. [DOI] [PubMed] [Google Scholar]
- Tang T. K., Leto T. L., Correas I., Alonso M. A., Marchesi V. T., Benz E. J., Jr Selective expression of an erythroid-specific isoform of protein 4.1. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3713–3717. doi: 10.1073/pnas.85.11.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernia G., Mohandas N., Shohet S. B. Deficiency of skeletal membrane protein band 4.1 in homozygous hereditary elliptocytosis. Implications for erythrocyte membrane stability. J Clin Invest. 1981 Aug;68(2):454–460. doi: 10.1172/JCI110275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple G. F., Chang J. C., Kan Y. W. Authentic beta-globin mRNA sequences in homozygous betaO-thalassemia. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3047–3051. doi: 10.1073/pnas.74.7.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wapenaar M. C., Kievits T., Hart K. A., Abbs S., Blonden L. A., den Dunnen J. T., Grootscholten P. M., Bakker E., Verellen-Dumoulin C., Bobrow M. A deletion hot spot in the Duchenne muscular dystrophy gene. Genomics. 1988 Feb;2(2):101–108. doi: 10.1016/0888-7543(88)90090-0. [DOI] [PubMed] [Google Scholar]