Abstract
A growing and interdisciplinary translational neuroscience research effort for neurodevelopmental disorders (NDDs) is investigating the mechanisms of dysfunction and testing effective treatment strategies in animal models and, when possible, in the clinic. NDDs with a genetic basis have received particular attention. Transgenic animals that mimic genetic insults responsible for disease in man have provided insight about mechanisms of dysfunction, and, surprisingly, have shown that cognitive deficits can be addressed in adult animals. This review will present recent translational research based on animal models of genetic NDDs, as well as pharmacotherapeutic strategies under development to address deficits of brain function for Down syndrome, fragile X syndrome, Rett syndrome, neurofibromatosis-1, tuberous sclerosis, and autism. Although these disorders vary in underlying causes and clinical presentation, common pathways and mechanisms for dysfunction have been observed. These include abnormal gene dosage, imbalance among neurotransmitter systems, and deficits in the development, maintenance and plasticity of neuronal circuits. NDDs affect multiple brain systems and behaviors that may be amenable to drug therapies that target distinct deficits. A primary goal of translational research is to replace symptomatic and supportive drug therapies with pharmacotherapies based on a principled understanding of the causes of dysfunction. Based on this principle, several recently developed therapeutic strategies offer clear promise for clinical development in man.
Key terms: neurodevelopmental disorders, pharmacotherapy, Down syndrome, fragile X syndrome, autism, neurofibromatosis-1, tuberous sclerosis, Rett syndrome
INTRODUCTION
Individuals affected with neurodevelopmental disorders (NDDs) exhibit mild to severe intellectual disabilities and may express maladaptive behaviors consistent with attention deficit hyperactivity disorder, obsessive-compulsive disorder, and autism spectrum disorders. Improved medical care, integrated educational opportunities, and symptomatic drug treatment have significantly increased the lifespan and improved daily living skills for individuals with NDDs. Drug therapies designed to address the disease state have been less successful. However, recent translational research in animal models for a number of NDDs show great promise for pharmacotherapy that targets pathology and cognitive deficits specific to these disorders.
The creation and characterization of animal models of NDDs have grown in the last decade, driving a number of promising translational research programs. Manipulating gene expression through transgenic, knockout, and knockin approaches in mice, flies, and worms permits study of the underlying anatomy, pathology, and physiology of disease. Mechanistic insights support the development of drug therapies to mitigate cognitive and behavioral deficits that could be life-changing. For example, pharmacotherapy that improves a child’s capacity to learn should provide nonlinear improvements in cognitive abilities, social function, and independence. Despite recent progress, successful therapies for NDDs require significant additional basic and clinical research.
NDDs can be distinguished by genetic and environmental causes, the nature and site of dysfunction, and the time course of cognitive and behavioral deficits during development. In this review, we focus on NDDs with a known genetic basis. These disorders are easier to characterize with animal models, better defined in terms of mechanism, and most promising for the development of principled therapeutic targets. We will also restrict our discussion to NDDs for which abnormalities in the CNS are localized to individual cells, local circuits of neurons, or specific brain regions. Translational research in animal models has been fruitful for disorders that fit this profile such as Down syndrome, fragile X syndrome, Rett syndrome, neurofibromatosis type 1, tuberous sclerosis, and autism.
The extent of genetic insults and brain pathology underlying NDDs determine the potential for pharmacotherapy. For example, phenylketonuria is a disorder with straightforward genetics and dysfunction in a specific, well-understood molecular pathway 1. Early intervention with dietary modification reduces or eliminates intellectual disability. Similar improvements in cognitive function are unlikely for other NDDs associated with severely underdeveloped brain regions or abnormal long-range projections such as fetal alcohol syndrome, microcephaly, and lissencephaly 2. However, some improvements in cognitive function may be possible by addressing dysfunction in even severely malformed regions 3. The prognosis of pharmacotherapy is better for NDDs caused by subtler changes that affect the function of local neural circuits. Translational research has identified pharmacological interventions that restore inhibitory-excitatory balance in neural circuits, compensate for dysfunctional molecular pathways, or address abnormal neurophysiology or synaptic plasticity. Therapies that address dysfunction in long-term plasticity, including both synapse strengthening via long-term potentiation (LTP) and weakening by long-term depression (LTD), are significant. These processes are generally recognized as the key substrate of learning and memory 4. Individuals with different NDDs exhibit overlapping sets of deficits due to dysfunction in common brain regions. Pharmacotherapeutic strategies that address shared forms of dysfunction have the potential to mitigate symptoms in different NDDs.
Advances in translational research offer hope to both children and adults. Remarkably, recent findings have shown improvements in learning and memory in adult animals 5,6. Mutations, deletions, or duplications of genes in NDDs may cause only modest changes in protein expression that shift the equilibrium of chemical reactions and signaling pathways. Thus, therapies that normalize function by either enhancing the activity of remaining proteins, disrupting mutant proteins, or modulating parallel and convergent pathways may improve abilities in individuals with NDDs.
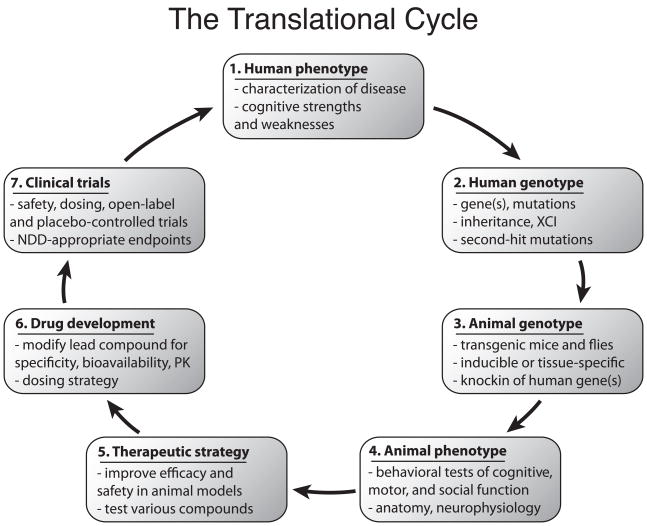
The Translational Cycle
A primary goal of translational research in the field of neurodevelopmental disorders is to replace symptomatic and supportive drug therapies with pharmacotherapies based on a principled understanding of the causes of dysfunction. We define a model of this process as the Translational Cycle. Translational research starts with diagnostic, behavioral and genetic studies in man, moves to animal models and other reduced preparations for biological and neuroscientific study then progresses to drug development and clinical studies in man based on increased knowledge and therapeutic strategies.
We describe this Translational Cycle with seven multidisciplinary steps(Fig. 1):
Figure 1. The Translational Cycle describes key events in the development of drug therapies for neurodevelopmental disorders (NDDs).
Characterization of the phenotype (1) and genotype (2) of an NDD in humans forms the basis for neuroscientific study. Creation of animal models based on the genetic causes of disease in humans (3) permits investigation of molecular, cellular, neurophysiological, and behavioral pathophysiology in mice, flies, or other organisms (4). Therapeutic strategies to address dysfunction in animal models (5) identify promising directions for drug development (6) and clinical trials (7) that quantify efficacy with endpoints that probe cognition, behavior, and quality-of-life. The primary goal of the Translational Cycle is to address the human phenotype (1). Abbreviations: X chromosome inactivation (XCI); pharmacokinetics (PK); neurodevelopmental disorder (NDD).
Human phenotype – Characterization of the disease
Human genotype – Discovery of the underlying genetics
Animal genotype – Development of animal models that mimic the genetic etiology of human disease
Animal phenotype – Behavioral testing to probe cognitive, motor, and social behaviors; studies of underlying genetics, molecular biology, neurophysiology, and anatomy in animal models
Therapeutic strategy – Development of therapeutic strategies based on biological findings in animal models and optimization for safety and efficacy
Drug development – Optimization of lead compounds to improve drug-target specificity, bioavailability, or pharmacokinetics, as well as determination of appropriate dose, dosing strategy, and route of administration.
Clinical trials – Design and execution of clinical trials in man to address cognitive deficits
In the first step of the Translational Cycle, human disorders are identified as distinct from each other and the phenotypes are characterized (Fig. 1, step 1). The first NDDs described in this manner were common disorders with external traits including skin lesions and benign tumors in neurocutaneous syndromes such as tuberous sclerosis 7–9 and craniofacial abnormalities in Down syndrome 10. Today, NDD diagnosis relies on detailed genetic and cognitive testing, behavioral phenotyping and, in some cases, neuroimaging. Improved characterization of NDDs to identify relative cognitive strengths and weaknesses (Fig. 1, step 1) has focused animal behavioral studies and determined brain regions of interest. For instance, human deficits in executive control and long-term memory implicate the frontal cortex and hippocampus, respectively.
Description of genetic causes of NDDs is a necessary step to enable the study of disease in animal models (Fig. 1, step 2). Down syndrome was the first disorder described on the basis of genetics due to the triplication of some or all of chromosome 21 11. Due to a revolution in human genetics, the genes, alleles, expression patterns, epigenetic factors, and patterns of inheritance that underlie various NDDs have been described.
Animal models form the foundation for detailed studies of the biology responsible for cognitive dysfunction in various NDDs (Fig. 1, step 3). Tools for genetically modifying mice and flies include addition or removal of genes, inducible expression of genes at particular developmental time points, and specificity of expression in cell types or tissues 12. Studies in animal models reveal mechanisms of dysfunction and suggest therapies that target these pathways and systems. However, the value of transgenic animal models is limited by the correspondence of molecular, anatomical, physiological, and behavioral pathology in animals to that in man. Careful study of brain pathology and mouse behavior establishes how well an animal model represents human disease (Fig. 1, step 4). Therapeutic strategies are evaluated in animal models by measuring markers of dysfunction and performance in behavioral tasks (Fig. 1, step 5). A particular therapeutic strategy may address only a subset of cognitive functions, so multiple therapeutic strategies that target distinct deficits and brain areas are desirable.
Significant efforts are required to translate a viable therapeutic strategy into an approved drug. Drug development optimizes a therapeutic compound to improve drug-target specificity, reduce or eliminate dangerous side effects, and determine dose and route of administration (Fig. 1, step 6). Next, a lead compound enters clinical trials in man to test safety and efficacy of therapeutic strategies discovered in animal models (Fig. 1, step7). For drugs intended to address intellectual deficits, trial design can be difficult due to relatively insensitive outcome measures such as cognitive tests. Moreover, outcome measures based on caretaker questionnaires are susceptible to bias, and the correspondence of improved outcome measures with higher functioning in daily living skills may not be straightforward. An approved pharmacotherapy for an NDD would reduce one or more cognitive deficits or maladaptive behaviors. Such a therapy completes the Translational Cycle by addressing the disease phenotype.
Important translational research challenges remain despite significant advances in the development of potential therapeutic strategies for NDDs. Animal models are often an imperfect representation of human disease or developmental disorders, and the differences between species may carry special significance for disease pathology. Moreover, higher cognitive functions in man such as language do not exist in mice or flies. Thus, improved characterization of animal models of NDDs requires better behavioral assays and physiological measurements. New animal models may improve the correspondence with human conditions. More specific and efficacious second-generation therapies require improved description of the mechanisms underlying successful pharmacotherapeutic intervention. A second set of challenges concern clinical development (Fig. 1, steps 5–7). Clinical development programs are expensive and low yield. Raising funds through government, philanthropic, and industry sources is challenging and slow.
In the following sections, we review translational research progress for several well-studied genetically-based childhood NDDs with an emphasis on research in animal models.
NEURODEVELOPMENTAL DISORDERS
Down syndrome
Down syndrome (DS) is caused by total or partial triplication of chromosome 21 (Hsa21) and occurs in approximately 1/700–1000 live births 13,14. Most individuals with DS exhibit mild to severe intellectual disability. Medical conditions such as congenital heart disease, Alzheimer’s disease, and epilepsy are common 15. Individuals with DS have particular deficits in verbal skills 16 and cognitive tasks that depend on prefrontal, hippocampal, or cerebellar function 17,18. Compared with previous decades, individuals with DS live longer and integrate more fully in social, family, and educational environments 19.
There are approximately 300–400 genes on Hsa21 20–22, but not all genes are expressed at the expected 1.5-fold level, underscoring the complexity of epigenetic interactions 23 (Fig. 2C). Segmentally trisomic mouse models of DS enable studies of the combined effect of trisomy for many genes 20,24,25. The well-studied Ts65Dn mouse is trisomic for about 100 genes on mouse chromosome 16 (Mmu16) that are homologous to those on Hsa21 26. The so-called ‘Down syndrome critical region’ (DSCR) on Mmu16 appears to be necessary for learning and plasticity deficits in mice 22, but there is conflicting evidence concerning whether the DSCR is sufficient to cause these phenotypes 27,28.
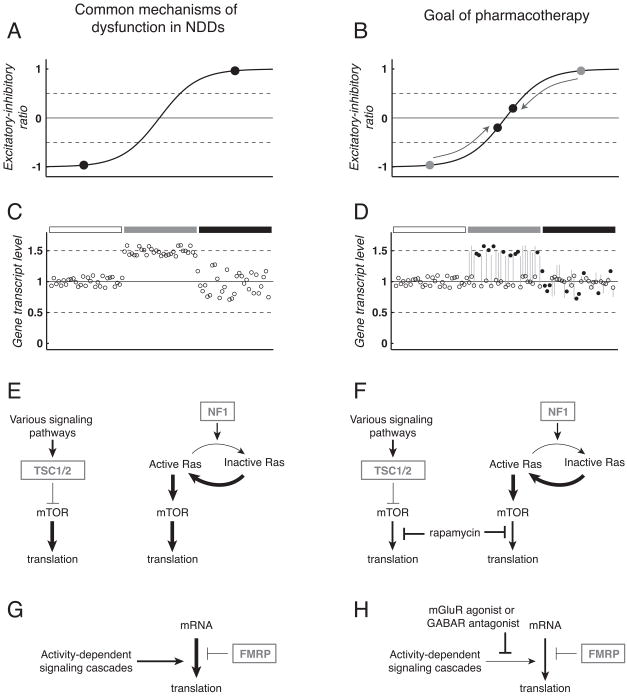
Figure 2. Neurodevelopmental disorders (NDDs) share common mechanisms of dysfunction amenable to similar therapeutic strategies.
A: Imbalanced excitation and inhibition within neuronal circuits occurs in a number of NDDs. Schematic representation of excitatory-inhibitory ratio shows pathophysiologically high (black circle, upper right) or low (black circle, bottom left) levels that exceed a theoretical window of balanced activity (between horizontal dashed lines). Note near balanced ratios are essential for normal circuit and cognitive function. B: Pharmacotherapy to address excitatory-inhibitory imbalance modifies neural circuits to achieve a level of activity that supports normal brain function (black circles, center). C: Abnormal gene dosage underlies dysfunction in many NDDs. Schematic representation of expression for several dozen genes under conditions of normal expression (left, under open rectangle), increased gene dosage for a number of genes as expected to occur in Down syndrome (center, under grey rectangle), and pseudo-realistic variability caused by gene-gene interactions and other factors (right, under black rectangle). D: Pharmacotherapy to address abnormal gene dosage may achieve normalization of altered transcript levels (center and right, under grey and black rectangles, open circles with grey lines to indicate pre-treatment expression). Improved cognitive function may occur without modifying expression of some genes (black circles). E: Dysregulated control of protein translation at synapses occurs in several NDDs. In tuberous sclerosis (TS), loss-of-function of the TSC1/2complex reduces inhibition of mTOR and leads to high levels of protein translation (left). Similarly, loss-of-function of NF1 drives increased translation via reduced inactivation of Ras in neurofibromatosis type 1 (NF-1) (right). F: In both TS and NF-1, drugs that inhibit mTOR-mediated inhibition such as rapamycin (Rapamune™ or sirolimus, Wyeth, Madison, NJ) are used to reduce translation to normal levels. G: In fragile X syndrome (FXS) activity-dependent signaling cascades drive translation at the synapse that is normally controlled by negative feedback from fragile X mental retardation protein (FMRP). H: Potential therapeutic strategies for FXS may suppress activity-dependent signals to restore normal control of translation in the absence of FMRP. In panels E–H, arrows and T-bars indicate activation and inhibition of signaling pathways, respectively, arrow and T-bar thickness represent the strength of activation or inhibition, and boxed grey text corresponds to genes absent or mutated in specific NDDs. For brevity, many components of signaling cascades have been excluded. Moreover, it should be noted that Ras, mTOR, FMRP and mGluR signaling are not independent from each other, further supporting the concept that seemingly distinct genetic lesions in NDDs converge on critical regulatory pathways to alter synaptic, circuit and cognitive function. Abbreviations: mammalian target of rapamycin (mTOR); tuberous sclerosis gene products tuberin and hamartin (TSC1/2); neurofibromatosis type 1 gene product neurofibromin (NF1); group 1 metabotropic glutamate receptor (mGluR); GABA receptor (GABAR).
Despite trisomy for only a subset of Hsa21 genes, mouse models of DS exhibit learning and memory deficits and corresponding anatomical and physiological abnormalities 24. Electron microscopy studies in Ts65Dnmice identified an excess of inhibitory synapses in the temporal cortex 29 and hippocampus 30 and enlarged dendritic spines in several brain regions 31. Ts65Dn mice have enhanced long-term depression (LTD) and reduced NMDA receptor-dependent long-term plasticity (LTP) of synapses in the CA1 region of the hippocampus 27,32–38. Signaling molecules involved in the induction of LTP are also disrupted in the hippocampus of Ts65Dn mice 39. Based on these anatomical and physiological studies, therapeutic strategies have been described that rescue LTP and deficits in hippocampal-dependent behavior.
Excessive activity of inhibitory neurons causes hippocampal LTP deficits in Ts65Dn mice, and drugs that reduce inhibition improve cognitive function in DS mice 3 (Fig. A–B). In slice studies, bath application of a GABAA receptor antagonist rescues LTP induction 34,36,37. GABAA receptor antagonists have been used to enhance LTP since the first slice studies of plasticity 40, so the viability of this strategy in vivo required direct testing. In adult Ts65Dn mice, low daily doses of a GABAA antagonist such as pentylenetetrazole (PTZ) generated improvements in the induction of hippocampal LTP and learning that lasted for months after a two-week treatment regimen had ended 35,37. GABAA antagonists can induce seizures at high doses and reduce the threshold for seizures via ‘kindling’ at moderate doses 41 but neither effect was observed at efficacious doses in mice (Garner et al., in preparation). Young DS children have increased susceptibility to seizures, so clinical development of GABAA drugs requires careful design and safety controls 3. Beginning in the 1930s, PTZ was used clinically for 50 years for a variety of indications, including schizophrenia 42, senility 43, and some forms of intellectual disability 44, but the FDA revoked PTZ approval in 1982 due to absence of efficacy data. This long history of safe use in man makes PTZ a promising candidate for clinical development. Drugs that target specific GABAA receptor subtypes, such as those containing the α5 subunit, could provide a larger therapeutic window for treatment 45. However, more work is required to develop safe α5-specific compounds 46.
Another mechanism that causes excessive inhibition in Ts65Dn mice is overexpression of G-protein-coupled inward rectifying potassium 2 (GIRK2) channels that are activated by GABAB receptors 47. As a result, GABAB activation of GIRK2 channels enhanced inhibitory currents 48, and the GABAB antagonist CGP53432 improved LTP in Ts65Dn hippocampal slices 49. Thus, drugs that target GABAB receptors offer an additional therapeutic target.
Neuromodulatory nuclei in the brainstem required for normal memory function degenerate in mouse models of DS, and drug therapies to enhance cholinergic and norepinephrinergic activity rescue behavioral deficits in DS mice. A third copy of the amyloid precursor protein (APP) gene disrupts retrograde transport of nerve growth factor (NGF), causes degeneration of basal forebrain cholinergic neurons 50,51 and may be linked to memory deficits 52. In Ts65Dn mice, elevated levels of oxidative stress contribute to basal forebrain degeneration and memory impairment 53. The cholinergic system also degrades in Alzheimer’s disease (AD), and individuals with DS commonly show early onset of AD pathology and progressive cognitive impairment 54,55. Approved AD drugs may help in DS. However, despite promising small, open-label trials, larger blinded studies have failed to find benefit of acetylcholinesterase inhibitor drugs approved for AD. The acetylcholinesterase inhibitor donepezil (Aricept™, Pfizer, New York, NY) is not efficacious in Ts65Dn mice 37 and its efficacy in man is inconclusive 56. In contrast, acute injection of the noncompetitive NMDA receptor antagonist memantine (Namenda™, Forest Laboratories, New York, NY), an approved AD drug, improved performance in contextual fear conditioning in Ts65Dn mice 33,57 and a clinical trial is underway. Rivastigmine (Exelon™, Novartis, Basel, Switzerland), an approved drug for treatment of dementia in Alzheimer’s disease and Parkinson’s disease, is also being assessed in the clinic.
Norepinephrinergic cells in another brainstem nucleus important for memory, the locus coeruleus (LC), also degenerate in Ts65Dn mice 58. Treatment with either a norepinephrine pro-drug approved to address neurogenic orthostatic hypotension, L-DOPS (Droxidopa™, Sumitomo Pharmaceuticals, Tokyo, Japan and Chelsea Therapeutics, Charlotte, NC), or xamoterol, a β-adrenergic partial agonist, improved performance in some behaviors 58. Though promising, this therapeutic strategy requires more work due to the high doses of pro-drug used.
Several additional therapeutic strategies for DS have been proposed. Ts65Dn mice have reduced neurogenesis in the hippocampus, but this phenotype can be rescued with the approved selective serotonin reuptake inhibitor (SSRI) fluoxetine (Prozac™, Eli Lilly, Indianapolis, IN) 59. Similar effects of fluoxetine on neurogenesis have been observed in rodent models of depression 60. Ts65Dn mice exhibit reduced responses to the signaling molecule sonic hedgehog (SHH) during development, causing reduced cerebellar size and cell counts 61,62, as well as neural crest deficiencies that may underlie craniofacial abnormalities 63. Delivery of SHH agonists to Ts65Dn mice during development rescued these deficits 62,63. In man these developmental stages occur in utero and during infancy, so this therapeutic strategy will be difficult to translate.
There is a long history of nutraceutical trials for DS, but a meta-study of trials using dietary supplements for DS concluded these strategies are not effective for improving cognitive function 64. Consistent with this finding, chronic administration of the nootropic piracetam, which reduces oxidative damage, did not improve cognitive function in Ts65Dn mice 65.
Fragile X syndrome
Fragile X syndrome (FXS) is the most common inherited form of intellectual disability with an incidence of ~1/3600 in males and ~1/8000 in females 66,67. Individuals with FXS are generally anxious and hypersensitive to stimuli. They exhibit facial and ear abnormalities and enlarged testes. Common diagnoses include ASD, ADHD, sleeping disorders, and seizures 68,69. Males with FXS have more severe cognitive deficits than females 70.
FXS is caused by expansion of a trinucleotide CGG repeat upstream of the fragile X mental retardation 1 gene (FMR1) 71. If the number of repeats exceeds ~200, aberrant hypermethylation represses FMR1 transcription 72,73. FMR1 encodes fragile X mental retardation protein (FMRP), an RNA binding protein that inhibits cytosolic translation 74 of various targets including those involved in the neuronal cytoskeleton such as MAP1B 75 and synapse structure e.g. PSD-95 76,77 (Fig. 2G). Consistent with these mRNA targets, FXS is associated with a high density of dendritic spines and long, thin, tortuous spines in individuals with FXS 78 and Fmr1−/− knockout mice 79–84. Zebrafish and Drosophila models of FXS confirm an evolutionarily conserved role in neuron structure and behavior 85–87.
Fmr1−/− knockout mice permit studies of brain dysfunction and tests of potential therapies to resolve these deficits 88. The behavioral phenotype of Fmr1−/− mice is less severe relative to deficits in man, including mild and strain-dependent hippocampal deficits 89–92. In contrast, anxiety and hypersensitivity phenotypes are robust in these mice 93–96. Despite mixed findings in behavioral studies, introduction of functional Fmr1 into KO animals has confirmed the role of FMRP in disease psychopathology 96,97.
Based on animal studies, the primary therapeutic strategies for FXS target excessive excitation with group 1 metabotropic glutamate receptor (mGluR5) antagonists 98 or GABAB receptor agonists 99. Clinical development is underway for both strategies. FMRP normally inhibits translation near synapses, and some forms of plasticity are enhanced in Fmr1−/− mice 82,100 (Fig. 2G). The mGluR5 pathway drives activity-dependent translation of proteins that mediate LTD, and FMRP provides negative feedback on such translation 100,101. Without this feedback, Fmr1−/− mice have enhanced mGluR5-dependent hippocampal 100,101 and cerebellar LTD 82. Over-activation of mGluR5 receptors also increases seizure susceptibility 102,103. Together these results support the mGluR theory of FXS and provide a framework for the development of new therapies 98 (Fig. 2G–H).
Antagonists of mGluR5’s are a promising category of pharmacotherapies due to genetic and pharmacological rescue of disease phenotype in animal models. Fmr1−/− mice crossed with Grm5 heterozygotes express 50% fewer mGluR5 receptors 104. Reduced mGluR5 expression rescued increased spine density, reduced ocular dominance plasticity, enhanced inhibitory avoidance extinction, and sensitivity for seizures normally observed in Fmr1−/− mice 104. Similarly, treatment with the mGluR5 antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP) rescued behavioral deficits in flies 105 and neurite branching in zebrafish 87. In Fmr1−/− mice, MPEP rescued defective prepulse inhibition 106, audiogenic seizure, and anxiety phenotypes 107.
Clinical trials in FXS are underway with several compounds that target the mGluR pathway. No clinically adverse events occurred in a small open label, single dose study of fenobam (Neuropharm Group PLC, Surrey, UK), an mGluR5 antagonist, in adults with FXS 108. Early stage trials are also underway for several other mGluR5 antagonists: STX107 (Seaside Therapeutics, Boston, MA), AFQ056(Novartis, Basel, Switzerland), and RO4917523(Roche, Basel, Switzerland). The mood stabilizer lithium carbonate (generic) is another therapeutic strategy to target overactive mGluR pathways that has shown some efficacy for improving cognition and irritability in an open-label trial in individuals with FXS (sponsored by the NIH and the FRAXA Research Foundation) 109. However, an alternative strategy for increasing excitation via the AMPA receptoragonistCX516 (Cortex Pharmaceuticals, Irvine, CA) did not show benefit in a phase II trial 110.
Anatomical and neurophysiological aspects of the GABA system suggest it may be an alternative therapeutic target for FXS 99. Expression of various GABAA receptor subunits is reduced in Fmr1−/− mice and dfmr1 knockout flies 111,112, Fmr1−/− mice have reduced cortical density of GABAergic interneurons 113, and tonic GABA transmission is reduced in Fmr1−/− mice 114. Based on these findings, a phase II trial in individuals with FXS using the GABAB agonist arbaclofen is underway (Seaside Therapeutics, Boston, MA). Ganaxolone(Marinus Pharmaceuticals, Branford, CT), a neuroactive steroid with positive allosteric activity at GABAA receptors, is in a phase II trial for epilepsy and migraine and may benefit FXS individuals with seizures 69,115.
Additional strategies have been evaluated in FXS animal models and may lead to clinically viable therapies in the future. For example, after treatment with the approved drug minocycline (generic), an antibiotic that also inhibits the metallopeptidase MMP-9 and reduces CNS inflammation, Fmr1−/− mice exhibited improved spine maturation and reduced anxiety 116. Both open-label and placebo-controlled trials with minocycline in FXS are underway (trials sponsored by the Fragile X Research Foundation of Canada and The National Fragile X Foundation). In another study, brain derived neurotrophic factor (BDNF) rescued deficits in early forms of LTP in the hippocampus 117 indicating that modulating neurotrophin signaling could also be used to normalize synaptic plasticity mechanisms and circuit function.
Rett syndrome
Rett syndrome (RTT) is an X-linked disorder with an incidence of ~1/10,000 that predominantly affects females 118. Individuals with RTT show progressive deficits beginning at ~6–18 months that include atrophy of verbal and skilled motor abilities, social withdrawal, hand wringing, respiratory difficulties, seizures, and autism spectrum behavior 119–122. De novo mutations in the Methyl-CpG binding protein 2 (MECP2) gene 123 are generally responsible for RTT 124. RTT symptoms are variable and depend on the pattern of X-chromosome inactivation (XCI) of the mutant allele 121,125–127, the nature of the MECP2 mutation, and epigenetic factors 128. MECP2 inhibits transcription by binding DNA methylated at CpG dinucleotides 129 and translation via direct interaction with RNA 130.
Several RTT mouse models have been created, including Mecp2-null mice(Mecp2y/− 131) and mouse lines with truncated versions of Mecp2 (Mecp2308/y 132 and Mecp2R168X/y 133). Constitutive and brain specific Mecp2-null mice exhibit neurological symptoms including motor impairments and respiratory issues 131, a delayed onset reduction in brain and neuron size 134, and deficits in hippocampal- and amygdalar-dependent tasks 135. In contrast, Mecp2308/y mice that express a truncated version of the gene have a more mild and mixed phenotype. These mice exhibit a progressive decline in motor function, abnormal social behavior, and altered circadian activity patterns but perform normally in conditioned fear and Morris water maze tasks 132,136.
Studies in mouse models of RTT have revealed abnormalities that may be addressable with pharmacotherapy. RTT mouse models exhibit imbalances of inhibitory and excitatory activity, deficits in long-term plasticity, and abnormal spine anatomy 130. Mecp2-null mice have reduced excitatory synaptic activity but no change in inhibitory synapses relative to wild-type mice in cultured hippocampal neurons 137 and layer V pyramidal cells in slice 138. In contrast to studies in hippocampus and cortex, young Mecp2y/− mice have reduced GABAergic inhibition and increased excitation in the ventrolateral medulla 139. Overexpression of Mecp2 increases excitatory activity and synapse number 140.
MeCP2 couples neural activity to gene regulation, a process required for synaptic plasticity. Neural activity causes Ca2+ influx that drives phosphorylation of MeCP2 and releases transcriptional repression 141. Consistent with this role, deficits have been observed in LTD and LTP in the hippocampus 142,143 and cortex 143. In contrast, overexpression of Mecp2 enhances plasticity in the hippocampus 144.
Deficits in Mecp2 mutant mice are not purely neurodevelopmental, suggesting that drug therapy is possible in adults with RTT. The phenotype of Mecp2 mutant mice can be rescued by introducing a wild-type copy of Mecp2 in neurons 145,146 (but see 147 in which introduction of Mecp2 into neurons failed to rescue the RTT phenotype) or inducing expression of functional Mecp2 in young 148 or adult mice 149. Unfortunately, such genetic manipulations are not transferable to humans. Moreover, therapies in humans will need to target downstream targets of MECP2, because MECP2 gene dosage must be carefully regulated for proper brain function 131,134,144,146. One such strategy increased lifespan and improved locomotion, breathing, and heart rate in Mecp2y/− mice with an active peptide fragment of the neurotrophic factor insulin-like growth factor 1 (IGF-1) 150. IGF-1 is approved for the treatment of severe IGF-1 deficiency (mecasermin or Increlex™, Tercica, Brisbane, CA).
To date, therapeutic strategies for RTT have focused on mitigating specific symptoms. One strategy addresses heightened anxiety and stress in individuals with RTT 151. Mecp2y/− mice over-express genes involved in glucocorticoid-mediated stress responses 152, and Mecp2308/y mice have high levels of corticotropin-releasing hormone (Crh) expression, enhanced stress responses, and elevated anxiety 153. Crh receptor 1antagonists may improve these symptoms 130.
A second therapeutic strategy is focused on respiratory deficits in RTT. Cell-autonomous reductions in aminergic neurotransmitter levels due to Mecp2 dysfunction occur in RTT individuals 154 and Mecp2−/y mice 155. Moreover, reduced norepinephrine levels cause abnormal respiratory rhythms that are a common cause of mortality 155. Desipramine (Norpramine™ or Pertofane™, Sanofi-Aventis, Paris, France), a norepinephrine reuptake inhibitor, improves respiration and extends lifespan in Mecp2-deficient mice 156,157. A clinical trial using desipramine for RTT is underway. Another strategy improved respiratory function in Mecp2-null mice with an ampakine drug that increases excitatory activity via glutamatergic AMPA receptors and enhances BDNF secretion 158. A third category of clinical development is targeting EEG abnormalities with dextromethorphan, an antagonist of NMDA receptors available as a component of over-the-counter cough suppressants.
Neurofibromatosis type 1
Neurofibromatosis type 1 (NF-1) is an autosomal dominant neurocutaneous disorder with a prevalence of approximately 1/2500–5000 159. NF-1 is caused by a mutation that inactivates the gene NF1 160, and de novo germline mutations are common 161. In addition to a number of cutaneous abnormalities, individuals with NF-1 have IQs across a broad range with mean IQs in the low-average range 162. Individuals with NF-1 express relative deficits in visual-spatial and visual-motor tasks, language, and executive function, and exhibit ADHD behavior and poor socialization 163,164.
NF1 encodes a protein, neurofibromin, that is highly expressed in the brain 165 and skin 166. Neurofibromin is a Ras GTPase Activating Protein (RasGAP) that suppresses tumor formation and inhibits protein translation via the mammalian target of rapamycin (mTOR) pathway 167 (Fig. 2E). In the absence of neurofibromin, Ras is overactive and drives abnormal cell proliferation 168. Neurofibromin also enhances the adenylyl-cyclase/cyclic AMP (AC/cAMP)pathway that couples neural activity to memory formation 169. Both Ras and cAMP pathways are promising therapeutic targets for NF-1 based on studies in transgenic mouse and fly models.
In the absence of NF1, cAMP activity is reduced, causing reduced growth and memory impairment 169–171. The small size phenotype of Nf1−/− flies can be rescued by cAMP activity 170, cAMP analogs 169, or the human NF1 transgene 171. Size rescue occurs in adult flies with inducible knockout of Nf1, confirming that the effect is not purely developmental 169,170. Moreover, learning and memory processes mediated by the Nf1-dependent component of the AC/cAMP pathway are required for olfactory learning in Drosophila 172.
The RasGAP activity of neurofibromin and its role in inhibiting mTOR activation is an alternative pathway for drug therapy. Nf1+/− heterozygous mice have mild learning deficits in some tasks 168,173 and enhanced astrocyte proliferation but do not develop neurofibromas 174,175. Homozygous deletion of Nf1 exon 23a, which is responsible for RasGAP activity, caused learning deficits 176. Moreover, spatial learning deficits in Nf1+/− mice can be rescued by crossing with K-ras+/− mice to reduce Ras pathway activity 177. Enhanced Ras activity in Nf1+/− mice causes excess inhibition and LTP deficits in the hippocampus 177 that can be resolved by systemic application of picrotoxin, a broad-acting GABAA antagonist 178. Thus, GABAA receptor antagonism is a potential therapeutic strategy for NF-1 based on reducing excessive inhibitory tone. Studies of neurofibromin RasGAP function suggest an additional therapeutic pathway but contrast with fly studies that showed learning deficits due to reduced AC/cAMP activity.
Drug therapies designed to reduce aberrant increased Ras/ERK signaling are in clinical development to address cognitive deficits in NF-1 6. The farnesyl-transferase inhibitor BMS 191563 (Bristol-Myers Squibb, New York, NY), rescues memory deficits in Nf1+/− mice by blocking post-translational modification of Ras 177. Similarly, lovastatin (Altoprev™, Shionogi Pharma, Atlanta, GA and Mevacor™, Merck, Whitehouse Station, NJ), approved to treat hypercholesterolemia, reduces Ras pathway activation by inhibiting three-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase function and rescued plasticity and learning deficits in Nf1+/− mice 179. A clinical trial with lovastatin (Altoprev™, Shionogi Pharma, Atlanta, GA and Mevacor™, Merck, Whitehouse Station, NJ), is being conducted in individuals with NF-1. Of note, there were no significant differences between treatment groups in a placebo-controlled three month trial in children with NF-1 using the lipophilic HMG-CoA reductase inhibitor simvastatin (Zocor™, Merck, Whitehouse Station, NJ) 180. More work is required to determine if inhibitors of Ras activity will be effective in humans with NF-1. Rapamycin (Rapamune™ or sirolimus, Wyeth, Madison, NJ), an inhibitor of mTOR, a downstream component of the Ras-mediated translation activation pathway, has shown promise in mouse models 181 and is approved as an immune suppressant for organ transplant and as a component in coronary stents(Fig. 2F).
Tuberous sclerosis
Tuberous sclerosis (TS) is an autosomal dominant disorder caused by a loss of function mutation in either TSC1 182 or TSC2 183. The TS birth rate is ~1/6000 184, and approximately two-thirds of cases result from a de novo mutation 185. Individuals with TS develop benign growths called hamartomas in various organs 186 with CNS pathology of tubers, giant-cell tumors, and abnormal white matter 187. Individuals with TS have deficits in memory, attention, and executive control 188,189 with frequent diagnoses of autism, attention deficit hyperactivity disorder, and sleep disorders 190. Some individuals with TS are severely disabled (30–40 IQ) while others maintain a sub-average normal IQ 191. Variability of cognitive function depends on the intensity of seizures during infancy 186,191 and the location of cortical tubers 192,193.
The proteins encoded by TSC1 and TSC2, hamartin and tuberin, form a heterodimer that interacts with dozens of gene products 194,195 to affect protein translation, cell proliferation, and synaptic plasticity 195,196. The complex functions as a GTPase activating protein (GAP) that reduces stimulation of the mammalian target of rapamycin (mTOR) pathway 197–199 (Fig. 2E). Inactivation of the Tsc1/2 complex in Drosophila drives abnormal cell growth and proliferation via the mTOR pathway 200,201 or insulin signaling 202.
Mice with conditional knockout (CKO) of Tsc1 mimic the loss-of-heterozygosity observed in hamartomas in some tissues in humans with TS, but these animals tend to have severe behavioral abnormalities 194. Neuron-, forebrain-, and glia-specific CKO mice exhibit hyperexcitability, impaired hippocampal LTP, and memory deficits 203–207. The severity of dysfunction in cells with no copies of Tsc1 relative to heterozygous animals underscores the importance of gene dosage and loss-of-heterozygosity due to second hit mutations in TS.
Some cognitive deficits in TS mice are independent of developmental abnormalities, localized pathology, or seizures. Thus, the hamartin-tuberin complex plays a role in neural circuit dysfunction that may be addressable with pharmacotherapy 194. Both Tsc1+/− and Tsc2+/− mice exhibit hippocampal-dependent learning deficits in the absence of pathology or seizures 203,208. Effects of Tsc1/2 inactivation on synaptic plasticity appear more complex. Tsc2+/− mice have a reduced threshold for the late-phase of LTP in the hippocampus that requires protein translation 203. Heterozygous “Eker rat” spontaneous Tsc2 mutants have reduced LTP and LTD relative to wild type rats 209. Young adult Tsc2+/− Eker rats are free of hamartomas and epilepsy yet exhibit a phenotype of enhanced long-term spatial memory, increased susceptibility to chemical kindling, and stronger cyclic AMP signaling 210.
Several drug therapies for TS are available or in clinical trials. Control of infantile spasms with currently approved drugs is an effective therapeutic strategy to reduce the likelihood of severe intellectual disability in individuals with TS. Vigabatrin (Sabril™, Lundbeck, Copenhagen, Denmark) is an approved inhibitor of GABA catabolism that resolves 80–100% of spasms in individuals with TS 211,212. The approved anti-epileptic drug, levetiracetam (Keppra™, UCB Pharmaceuticals, Brussels, Belgium), is an effective secondary therapy for seizures in children and adolescents with TS 213.
The mTOR inhibitor, rapamycin (Rapamune™ or sirolimus, Wyeth, Madison, NJ), is a promising candidate for TS therapy that is approved in the US for organ rejection and coronary stents 214 (Fig. 2F). Studies in rodents support the efficacy of rapamycin for improving brain function and reducing tumor size in TS. Rapamycin rescues hippocampal learning deficits, abnormal LTP threshold, development abnormalities, and seizures 203,215. Topical rapamycin improves survival 216 and reduces skin cancer growth in mice 217, and rapamycin therapy prolongs survival by inhibiting tumor progression in the Eker rat 218. Clinical trials are underway to test rapamycin in individuals with TS. Other mTOR inhibitors under development offer promise for TS, including the approved drugs CCI-779 (Torisel™ or temsirolimus, Wyeth, Madison, NJ), and RAD001 (Certican™, Afinitor™, or everolimus, Novartis, Basel, Switzerland) 219. Exogenous interferon-gamma (IFN-γ) is an additional potential therapy based on promising mouse studies 219,220. Intriguingly, in humans, a high-expressing IFN-γ allele corresponds to reduced disease severity 221.
Autism spectrum disorders
Autism spectrum disorders (ASD) are heterogeneous and include autistic disorder, Asperger syndrome, and pervasive developmental disorder-not otherwise specified (PDD-nos). ASD are diagnosed behaviorally on the basis of socialization deficits, impaired language and communication, and repetitive or restricted behaviors that generally present by age 3 (Diagnostic and Statistical Manual of Mental Disorders. 4th edition). A subset of children with autism have larger head circumference and total brain volume 222,223, and additional anatomical abnormalities are common, including an excess of local connectivity in the cerebral cortex and reduced long-distance cortico-cortical projections 224. The prevalence of ASD has increased markedly in the last two decades with an estimated prevalence in the US of ~1/150 and higher rates of diagnosis in boys 225.
Currently, most cases of ASD are idiopathic, although a significant minority of cases is genetic in origin 226. Twin and sibling studies observed high concordance rates indicating that ASD are highly heritable 227. Monogenic causes–including those discussed above for Rett syndrome, fragile X, neurofibromatosis type 1, and tuberous sclerosis–are rare. Most ASD cases have polygenic causes with complex gene-gene 228–230 and gene-environment 231,232 relationships. Linkage analysis studies have identified autism susceptibility loci on most chromosomes 228,233. Microdeletions and microduplications cause copy number variation of genes in these regions that predispose to autism 226. A comprehensive review of genetic associations with autism and the dozens of relevant mouse models is beyond the scope of this review 230,234–237. Rather, we will discuss animal models for genes and pathways that have received recent attention in translational research.
Consistent with heterogeneous genetic and behavioral factors in ASD, several mechanisms of dysfunction have been identified: imbalanced excitation and inhibition, enhanced local neuronal connectivity, and abnormal levels of modulatory neurotransmitters such as serotonin 238. The dozens of genes with potential links to ASD play roles in neurodevelopment and synaptic function. Putative autism genes include protein complexes that interact with the actin cytoskeleton at postsynaptic densities and mediate translation at synapses, including the genes NF1 and TSC1/2 that cause NF-1 and TS 229,235.
Postsynaptic neuroligins and presynaptic neurexins are cell-adhesion molecules that form a trans-synaptic signaling complex required for synapse formation and stability in vitro 239 and normal synaptic maturation and function in vivo 240,241. Linkage studies in man 228,242–244 and neurobiological studies in mice 237,240,241 confirmed the role of this complex in some cases of autism. Neuroligins and neurexins interact with synaptic scaffolding proteins 241. The neuroligin-neurexin complex promotes the formation of synapses that are subsequently pruned in an activity-dependent manner 240. This function is consistent with abnormal brain growth in autism, and mutations in neurexin-1, neuroligin-3, and neuroligin-4 are associated with ASD 241. Moreover, studies in mice with knockout of one or more neuroligin or neurexin support a role of these proteins in synapse maturation and function 237. Neuroligin-1 and neuroligin-2 enhance post-synaptic currents at excitatory 245 and inhibitory synapses 246, respectively, and set the balance of excitation and inhibition in neural circuits 247–250. Knockout mice for various mouse orthologs of neuroligin have deficits in synaptic plasticity 251,252, spatial learning 251, vocalization, grooming, and some social behaviors 253.
ASD linkage studies identified additional genes that interact with neuroligins. SHANK3 (also known as ProSAP2) binds to the cytoskeleton and proteins, including neuroligins, at synapses 254. As a key organizer of the postsynaptic density, it is not surprising that SHANK3/ProSAP2 mutations have been identified in a small number of ASD individuals 255–257. Moreover, Shank1 knockout mice exhibit altered dendritic spine and synapse structure, weak synaptic transmission, anxiety, and impaired contextual fear memory 258. The neurexin family gene Contactin Associated Protein-Like 2 (CNTNAP2) also associates with autism in linkage studies 259. In summary, neuroligins and neurexins appear to play a central role in dysfunction associated with autism and drugs to target this complex and related pathways represent a potential therapeutic strategy.
Another therapeutic strategy targets the serotonergic system 260. The serotonin transporter (5-HTT, SLC6A4) is associated with rigid-compulsive variants of autism 261,262, and circulating levels of serotonin are high in some individuals with ASD 263. The melatonin production pathway begins with serotonin, and individuals with ASD exhibit both abnormal melatonin levels and sleep disturbances 264. Drugs that target the serotonergic system may help children with ASD. However, clinical trials with SSRI antidepressants have had mixed results 265.
Various psychotropic drugs are prescribed to mitigate symptoms of ASD. Prescribed drugs include stimulants, antidepressants, adrenergic agonists, antipsychotics, antiepileptics, and drugs approved for Alzheimer’s disease 266,267. Two atypical antipsychotic drugs are FDA approved for irritability associated with autism: risperidone 268 (Risperdal™, Janssen Pharmaceutica, Beerse, Belgium) and aripiprazole 269 (Abilify™, Bristol-Myers Squibb, New York, NY and Otsuka America Pharmaceutical, Inc., Rockville, MD). Clinical development efforts for monogenic forms of autism, including Rett syndrome, fragile X syndrome, and tuberous sclerosis, may have benefits for some individuals with idiopathic autism. Additional clinical research is required to determine whether these drugs are safe and efficacious for some or all individuals with ASD.
CONCLUSION
Factors that affect amenability of pharmacotherapy
Several factors influence the potential for successful pharmacotherapy for neurodevelopmental disorders (NDDs). For instance, in some cases, effective therapy may require drugs to be given beginning in infants or young children, as is the case for phenylketonuria 1. To do so requires timely diagnosis. Diseases with a simple form of inheritance and low rates of de novo mutations are easier to diagnosis prenatally or perinatally based on family history and genetic testing. The genes responsible for neurofibromatosis type 1 (NF-1) and tuberous sclerosis (TS) exhibit among the highest recorded rates of de novo mutations 161,185. Children with these disorders generally do not have a family history of disease, and overt disease symptoms occur later in childhood or adolescence. If pharmacotherapies are developed that require early intervention, such as antiepileptics for infantile seizures 191, more widespread genetic testing for early diagnosis will be required.
The nature of genetic insult responsible for a particular NDD affects the ease with which a disease can be recreated in an animal model, characterized biologically, and addressed therapeutically. NDDs caused by monogenic disorders are generally easier to understand mechanistically and thus easier to treat. Monogenic disorders such as FXS and Rett syndrome (RTT) permit concentrated study of FMR1 and MECP2 genes, respectively. However, epigenetic factors may have a larger impact in man than for inbred mouse strains. Disorders such as Down syndrome (DS) or autism in which many genes are affected require a more holistic approach for study and treatment due to the potential for complex interactions between numerous gene products.
The consistency of genetic mutation across tissues also affects the ease of translational research in animal models of NDDs. For animal studies that require comparison among a large number of animals for statistical power, random X chromosome inactivation (XCI) is unacceptable. Studies of animal models of FXS and RTT generally use males to ease comparison across subjects 270,271. RTT occurs most commonly in females 66,118, so mouse studies must be considered with this caveat in mind.
A second consideration concerns the propensity for second-hit mutations that underlie hamartoma formation in TS and NF-1. Second hit mutations occur randomly, and the exact size, distribution, and developmental time-point of these mutations influence disease presentation. Mouse models of these disorders have used inducible and cell-type specific genetic constructs to consistently reproduce mutations in time and tissue. While this aids in comparison across subjects, any therapies developed for use in man must consider variability caused by second-hit mutations.
Pharmacotherapies that target disrupted equilibrium of neurotransmitter systems offer promise for NDDs. Drugs that counteract such imbalances affect ongoing neural circuit dynamics rather than established gross anatomical abnormalities. Therapeutic strategies in this category include GABA antagonists for DS 35 and NF-1 178, mGluR antagonists for FXS 98, and norepinephrine reuptake inhibitors for RTT 156,157. Functional homeostatic mechanisms may contribute to the rebalancing of neurotransmitter function after drug therapy 35.
Different therapeutic strategies for NDDs may be required at different stages of life. For example, infantile spasms in DS and TS are treated with antiepileptics 193,272. If these treatments are unsuccessful, homeostatic mechanisms can be recruited that enhance inhibition to counteract the excitability responsible for spasms and lead to memory deficits 30,31. Once neural circuits have stabilized with enhanced inhibition, drugs that reduce inhibition are more appropriate therapeutically 35. As more is learned about the time-course of dysfunction in NDDs, targeting of therapies to the existing brain state may be improved. Moreover, individuals with NDDs have multiple cognitive and behavioral disabilities, and a particular drug therapy may improve only a subset of cognitive functions. Thus, a combination of complementary drugs may offer the most benefit by addressing deficits in attention, arousal, information processing, or depression.
Common mechanisms and therapeutic targets
The NDDs discussed here are phenotypically diverse yet linked by common mechanisms of dysfunction, including abnormal gene dosage, imbalance among neurotransmitter systems, and local protein translation (Fig. 2). A particular NDD can be caused by mutations in multiple genes, underscoring the convergence of dysfunction in key biochemical pathways.
Imbalances between excitatory and inhibitory networks are present in NDDs, as well as other psychiatric and neurodegenerative disorders (Fig 2A–B). Inhibitory networks dominate in DS 35, RTT 138,139 and NF-1 173, while overexcitation occurs in FXS 98,273 and TS 274. Moreover, imbalances of excitation and inhibition can shift during development. In DS and TS, infantile spasms are common, while inhibition dominates later in development. However, similar homeostatic mechanisms can be recruited in adult mice to rebalance circuit excitability 3. Neurotransmitters such as norepinephrine, acetylcholine, and serotonin can modify the balance and effect of inhibition and excitation. Drugs that target these systems represent alternatives for resolving imbalances.
Altered gene dosage is a common theme in NDDs(Fig. 2C–D). Hundreds of genes are triplicated in DS 20, and microdeletions or microduplications of regions on a number of chromosomes affects susceptibility to autism 226. Such copy number variation can increase or decrease the expression of genes, often in an unexpected or nonlinear fashion due to gene-gene interactions. For instance, many genes triplicated in DS are expressed at levels above or below the predicted 1.5-fold overexpression 23. In NF-1 and TS, a single copy of a mutant gene appears to be insufficient to produce hamartoma growth, which requires loss-of-heterozygosity due to a second hit somatic mutation 8,275.
Abnormal translation of proteins near synapses is a third common cause of dysfunction in NDDs (Fig. 2E–H). Altered translation can cause abnormal cell proliferation, dendritic spine anatomy, and synaptic plasticity. For instance, fragile X mental retardation protein (FMRP) normally inhibits protein translation, so reduced FMRP function causes excessive translation responsible for enhanced plasticity in mouse models of FXS 276 (Fig. 2G–H). Similarly, loss-of-function of MeCP2 in RTT reduces activity-dependent local translation responsible for long-term plasticity 130. Genes mutated in NF-1, TS, and some forms of autism affect translation, such as via the mammalian target of rapamycin (mTOR) cell proliferation pathway 8,274 (Fig. 2E–F). Thus, therapeutic strategies that recover normal levels of translation under appropriate circumstances are promising for several NDDs.
Translational cycle future directions
Discoveries about the molecular, genetic, anatomic, and neurophysiological mechanisms underlying NDDs have increased substantially in recent years. As our understanding of the neurobiology of NDDs grows, so does the spectrum of viable clinical strategies. NDDs reduce learning abilities during a period rich in intellectual, social, and emotional development, so even small improvements in cognitive function could provide significant benefits. There is still much more to learn about NDDs through each stage of the translational cycle. However, recent research findings suggest that new, effective, and principled therapies are possible for NDDs. Designing and executing clinical trials to test therapies will require significant effort and resources from government, philanthropic foundations, academic institutions, and the pharmaceutical and biotechnology industry. The repeated finding that cognitive function can be improved in adult animals in animal models of NDDs suggests that pharmacotherapies may help individuals with NDDs throughout their lives.
Acknowledgments
Sources of support: DZW is supported by a Neuro-innovation and Translational Neurosciences fellowship from Stanford University. Work in the Garner lab on neurodevelopmental disorders is supported by the Coulter, Fidelity, and Down Syndrome Research and Treatment foundations and NIH grant NS353862 to CCG.
References
- 1.Hanley WB. Adult phenylketonuria. Am J Med. 2004;117:590–595. doi: 10.1016/j.amjmed.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 2.Dobyns WB. Developmental aspects of lissencephaly and the lissencephaly syndromes. Birth Defects Orig Artic Ser. 1987;23:225–241. [PubMed] [Google Scholar]
- 3.Fernandez F, Garner CC. Over-inhibition: a model for developmental intellectual disability. Trends Neurosci. 2007;30:497–503. doi: 10.1016/j.tins.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 5.Ehninger D, Li W, Fox K, et al. Reversing neurodevelopmental disorders in adults. Neuron. 2008;60:950–960. doi: 10.1016/j.neuron.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva AJ, Ehninger D. Adult reversal of cognitive phenotypes in neurodevelopmental disorders. J Neurodev Disord. 2009;1:150–157. doi: 10.1007/s11689-009-9018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourneville DM. Sclérose tubéreuse des circonvolutions cérébrales. Arch Int Neurol. 1880;1:81–91. [Google Scholar]
- 8.Boyd KP, Korf BR, Theos A. Neurofibromatosis type 1. J Am Acad Dermatol. 2009;61:1–14. doi: 10.1016/j.jaad.2008.12.051. quiz 15–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz RA, Fernández G, Kotulska K, et al. Tuberous sclerosis complex: advances in diagnosis, genetics, and management. J Am Acad Dermatol. 2007;57:189–202. doi: 10.1016/j.jaad.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Down JLH. Observations on an Ethnic Classification of Idiots. London Hospital Reports. 1866;3:259–262. [Google Scholar]
- 11.Lejeune J, Turpin R, Gautier M. Mongolism; a chromosomal disease (trisomy) Bull Acad Natl Med. 1959;143:256–265. [PubMed] [Google Scholar]
- 12.Bockamp E, Maringer M, Spangenberg C, et al. Of mice and models: improved animal models for biomedical research. Physiol Genomics. 2002;11:115–132. doi: 10.1152/physiolgenomics.00067.2002. [DOI] [PubMed] [Google Scholar]
- 13.Egan JFX, Benn PA, Zelop CM, et al. Down syndrome births in the United States from 1989 to 2001. Am J Obstet Gynecol. 2004;191:1044–1048. doi: 10.1016/j.ajog.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 14.Morris JK, Alberman E. Trends in Down’s syndrome live births and antenatal diagnoses in England and Wales from 1989 to 2008: analysis of data from the National Down Syndrome Cytogenetic Register. BMJ. 2009;339:b3794. doi: 10.1136/bmj.b3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roizen NJ, Patterson D. Down’s syndrome. Lancet. 2003;361:1281–1289. doi: 10.1016/S0140-6736(03)12987-X. [DOI] [PubMed] [Google Scholar]
- 16.Silverman W. Down syndrome: cognitive phenotype. Ment Retard Dev Disabil Res Rev. 2007;13:228–236. doi: 10.1002/mrdd.20156. [DOI] [PubMed] [Google Scholar]
- 17.Nadel L. Down’s syndrome: a genetic disorder in biobehavioral perspective. Genes Brain Behav. 2003;2:156–166. doi: 10.1034/j.1601-183x.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- 18.Pennington BF, Moon J, Edgin J, et al. The neuropsychology of Down syndrome: evidence for hippocampal dysfunction. Child Dev. 2003;74:75–93. doi: 10.1111/1467-8624.00522. [DOI] [PubMed] [Google Scholar]
- 19.Yang Q, Rasmussen SA, Friedman JM. Mortality associated with Down’s syndrome in the USA from 1983 to 1997: a population-based study. Lancet. 2002;359:1019–1025. doi: 10.1016/s0140-6736(02)08092-3. [DOI] [PubMed] [Google Scholar]
- 20.Antonarakis SE, Lyle R, Dermitzakis ET, et al. Chromosome 21 and down syndrome: from genomics to pathophysiology. Nat Rev Genet. 2004;5:725–738. doi: 10.1038/nrg1448. [DOI] [PubMed] [Google Scholar]
- 21.Nikolaienko O, Nguyen C, Crinc LS, et al. Human chromosome 21/Down syndrome gene function and pathway database. Gene. 2005;364:90–98. doi: 10.1016/j.gene.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 22.Patterson D. Molecular genetic analysis of Down syndrome. Hum Genet. 2009;126:195–214. doi: 10.1007/s00439-009-0696-8. [DOI] [PubMed] [Google Scholar]
- 23.Lyle R, Gehrig C, Neergaard-Henrichsen C, et al. Gene expression from the aneuploid chromosome in a trisomy mouse model of down syndrome. Genome Res. 2004;14:1268–1274. doi: 10.1101/gr.2090904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gardiner KJ. Molecular basis of pharmacotherapies for cognition in Down syndrome. Trends Pharmacol Sci. 2009 doi: 10.1016/j.tips.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salehi A, Faizi M, Belichenko PV, et al. Using mouse models to explore genotype-phenotype relationship in Down syndrome. Ment Retard Dev Disabil Res Rev. 2007;13:207–214. doi: 10.1002/mrdd.20164. [DOI] [PubMed] [Google Scholar]
- 26.Davisson MT, Schmidt C, Akeson EC. Segmental trisomy of murine chromosome 16: a new model system for studying Down syndrome. Prog Clin Biol Res. 1990;360:263–280. [PubMed] [Google Scholar]
- 27.Belichenko NP, Belichenko PV, Kleschevnikov AM, et al. The “Down syndrome critical region” is sufficient in the mouse model to confer behavioral, neurophysiological, and synaptic phenotypes characteristic of Down syndrome. J Neurosci. 2009;29:5938–5948. doi: 10.1523/JNEUROSCI.1547-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olson LE, Roper RJ, Sengstaken CL, et al. Trisomy for the Down syndrome ‘critical region’ is necessary but not sufficient for brain phenotypes of trisomic mice. Hum Mol Genet. 2007;16:774–782. doi: 10.1093/hmg/ddm022. [DOI] [PubMed] [Google Scholar]
- 29.Kurt MA, Davies DC, Kidd M, et al. Synaptic deficit in the temporal cortex of partial trisomy 16 (Ts65Dn) mice. Brain Res. 2000;858:191–197. doi: 10.1016/s0006-8993(00)01984-3. [DOI] [PubMed] [Google Scholar]
- 30.Belichenko PV, Kleschevnikov AM, Masliah E, et al. Excitatory-inhibitory relationship in the fascia dentata in the Ts65Dn mouse model of Down syndrome. J Comp Neurol. 2009;512:453–466. doi: 10.1002/cne.21895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belichenko PV, Masliah E, Kleschevnikov AM, et al. Synaptic structural abnormalities in the Ts65Dn mouse model of Down Syndrome. J Comp Neurol. 2004;480:281–298. doi: 10.1002/cne.20337. [DOI] [PubMed] [Google Scholar]
- 32.Belichenko PV, Kleschevnikov AM, Salehi A, et al. Synaptic and cognitive abnormalities in mouse models of Down syndrome: exploring genotype-phenotype relationships. J Comp Neurol. 2007;504:329–345. doi: 10.1002/cne.21433. [DOI] [PubMed] [Google Scholar]
- 33.Costa ACS, Scott-McKean JJ, Stasko MR. Acute injections of the NMDA receptor antagonist memantine rescue performance deficits of the Ts65Dn mouse model of Down syndrome on a fear conditioning test. Neuropsychopharmacology. 2008;33:1624–1632. doi: 10.1038/sj.npp.1301535. [DOI] [PubMed] [Google Scholar]
- 34.Costa ACS, Grybko MJ. Deficits in hippocampal CA1 LTP induced by TBS but not HFS in the Ts65Dn mouse: a model of Down syndrome. Neurosci Lett. 2005;382:317–322. doi: 10.1016/j.neulet.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez F, Morishita W, Zuniga E, et al. Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat Neurosci. 2007;10:411–413. doi: 10.1038/nn1860. [DOI] [PubMed] [Google Scholar]
- 36.Kleschevnikov AM, Belichenko PV, Villar AJ, et al. Hippocampal long-term potentiation suppressed by increased inhibition in the Ts65Dn mouse, a genetic model of Down syndrome. J Neurosci. 2004;24:8153–8160. doi: 10.1523/JNEUROSCI.1766-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rueda N, Flórez J, Martínez-Cué C. Chronic pentylenetetrazole but not donepezil treatment rescues spatial cognition in Ts65Dn mice, a model for Down syndrome. Neurosci Lett. 2008;433:22–27. doi: 10.1016/j.neulet.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 38.Siarey RJ, Carlson EJ, Epstein CJ, et al. Increased synaptic depression in the Ts65Dn mouse, a model for mental retardation in Down syndrome. Neuropharmacology. 1999;38:1917–1920. doi: 10.1016/s0028-3908(99)00083-0. [DOI] [PubMed] [Google Scholar]
- 39.Siarey RJ, Kline-Burgess A, Cho M, et al. Altered signaling pathways underlying abnormal hippocampal synaptic plasticity in the Ts65Dn mouse model of Down syndrome. J Neurochem. 2006;98:1266–1277. doi: 10.1111/j.1471-4159.2006.03971.x. [DOI] [PubMed] [Google Scholar]
- 40.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol (Lond) 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mason CR, Cooper RM. A permanent change in convulsive threshold in normal and brain-damaged rats with repeated small doses of pentylenetetrazol. Epilepsia. 1972;13:663–674. doi: 10.1111/j.1528-1157.1972.tb04401.x. [DOI] [PubMed] [Google Scholar]
- 42.Kruger L. Treatment of schizophrenia by Meduna’s method. Psych Neurol Wchnschr. 1936;38:721. [Google Scholar]
- 43.Lu L, Stotsky BA, Cole JO. A controlled study of drugs in long-term geriatric psychiatric patients. Arch Gen Psych. 1971;25:284–288. [Google Scholar]
- 44.BERMAN HH, LAZAR M, NOE O, et al. Pentylenetetrazol (metrazol) in mental deficiency. AMA journal of diseases of children. 1957;94:231–233. doi: 10.1001/archpedi.1957.04030040013003. [DOI] [PubMed] [Google Scholar]
- 45.Delatour B, Braudeau J, Dodd RH, et al. Program No. 829.16. 2009 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience; 2009. Alleviation of cognitive deficits in Ts65Dn mice modeling Down syndrome by pharmacological inhibition of GABAergic transmission. Online.. 2009. [Google Scholar]
- 46.Atack JR. Preclinical and clinical pharmacology of the GABA(A) receptor alpha5 subtype-selective inverse agonist alpha5IA. Pharmacol Ther. 2010;125:11–26. doi: 10.1016/j.pharmthera.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Harashima C, Jacobowitz DM, Witta J, et al. Abnormal expression of the G-protein-activated inwardly rectifying potassium channel 2 (GIRK2) in hippocampus, frontal cortex, and substantia nigra of Ts65Dn mouse: a model of Down syndrome. J Comp Neurol. 2006;494:815–833. doi: 10.1002/cne.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Best TK, Siarey RJ, Galdzicki Z. Ts65Dn, a mouse model of Down syndrome, exhibits increased GABAB-induced potassium current. J Neurophysiol. 2007;97:892–900. doi: 10.1152/jn.00626.2006. [DOI] [PubMed] [Google Scholar]
- 49.Kleschevnikov AM, Belichenko PV, Mobley WC. Program No. 348.4. 2008 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2008. Antagonists of the GABAB receptors enhance LTP and reduce pro-epileptiform activity in Ts65Dn mouse model of Down syndrome. Online.. 2008. [Google Scholar]
- 50.Cooper JD, Salehi A, Delcroix JD, et al. Failed retrograde transport of NGF in a mouse model of Down’s syndrome: reversal of cholinergic neurodegenerative phenotypes following NGF infusion. Proc Natl Acad Sci USA. 2001;98:10439–10444. doi: 10.1073/pnas.181219298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salehi A, Delcroix J-D, Belichenko PV, et al. Increased App expression in a mouse model of Down’s syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron. 2006;51:29–42. doi: 10.1016/j.neuron.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 52.Granholm AC, Sanders LA, Crnic LS. Loss of cholinergic phenotype in basal forebrain coincides with cognitive decline in a mouse model of Down’s syndrome. Exp Neurol. 2000;161:647–663. doi: 10.1006/exnr.1999.7289. [DOI] [PubMed] [Google Scholar]
- 53.Lockrow J, Prakasam A, Huang P, et al. Cholinergic degeneration and memory loss delayed by vitamin E in a Down syndrome mouse model. Exp Neurol. 2009;216:278–289. doi: 10.1016/j.expneurol.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lai F, Williams RS. A prospective study of Alzheimer disease in Down syndrome. Arch Neurol. 1989;46:849–853. doi: 10.1001/archneur.1989.00520440031017. [DOI] [PubMed] [Google Scholar]
- 55.Wisniewski KE, Dalton AJ, McLachlan C, et al. Alzheimer’s disease in Down’s syndrome: clinicopathologic studies. Neurology. 1985;35:957–961. doi: 10.1212/wnl.35.7.957. [DOI] [PubMed] [Google Scholar]
- 56.Kishnani PS, Sommer BR, Handen BL, et al. The efficacy, safety, and tolerability of donepezil for the treatment of young adults with Down syndrome. Am J Med Genet A. 2009;149A:1641–1654. doi: 10.1002/ajmg.a.32953. [DOI] [PubMed] [Google Scholar]
- 57.Lockrow J, Boger H, Bimonte-Nelson H, et al. Effects of long-term memantine on memory and neuropathology in Ts65Dn mice, a model for Down syndrome. Behav Brain Res. 2010 doi: 10.1016/j.bbr.2010.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salehi A, Faizi M, Colas D, et al. Restoration of Norepinephrine-Modulated Contextual Memory in a Mouse Model of Down Syndrome. Sci Transl Med. 2009;1:7ra17–17ra17. doi: 10.1126/scitranslmed.3000258. [DOI] [PubMed] [Google Scholar]
- 59.Clark S, Schwalbe J, Stasko MR, et al. Fluoxetine rescues deficient neurogenesis in hippocampus of the Ts65Dn mouse model for Down syndrome. Exp Neurol. 2006;200:256–261. doi: 10.1016/j.expneurol.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 60.Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 61.Baxter LL, Moran TH, Richtsmeier JT, et al. Discovery and genetic localization of Down syndrome cerebellar phenotypes using the Ts65Dn mouse. Hum Mol Genet. 2000;9:195–202. doi: 10.1093/hmg/9.2.195. [DOI] [PubMed] [Google Scholar]
- 62.Roper RJ, Baxter LL, Saran NG, et al. Defective cerebellar response to mitogenic Hedgehog signaling in Down [corrected] syndrome mice. Proc Natl Acad Sci USA. 2006;103:1452–1456. doi: 10.1073/pnas.0510750103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roper RJ, Vanhorn JF, Cain CC, et al. A neural crest deficit in Down syndrome mice is associated with deficient mitotic response to Sonic hedgehog. Mech Dev. 2009;126:212–219. doi: 10.1016/j.mod.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salman M. Systematic review of the effect of therapeutic dietary supplements and drugs on cognitive function in subjects with Down syndrome. Eur J Paediatr Neurol. 2002;6:213–219. doi: 10.1053/ejpn.2002.0596. [DOI] [PubMed] [Google Scholar]
- 65.Rueda N, Flórez J, Martínez-Cué C. Effects of chronic administration of SGS-111 during adulthood and during the pre-and post-natal periods on the cognitive deficits of Ts65Dn mice, a model of Down syndrome. Behav Brain Res. 2008;188:355–367. doi: 10.1016/j.bbr.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 66.Hagerman PJ. The fragile X prevalence paradox. J Med Genet. 2008;45:498–499. doi: 10.1136/jmg.2008.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turner G, Webb T, Wake S, et al. Prevalence of fragile X syndrome. Am J Med Genet. 1996;64:196–197. doi: 10.1002/(SICI)1096-8628(19960712)64:1<196::AID-AJMG35>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 68.Cornish K, Sudhalter V, Turk J. Attention and language in fragile X. Ment Retard Dev Disabil Res Rev. 2004;10:11–16. doi: 10.1002/mrdd.20003. [DOI] [PubMed] [Google Scholar]
- 69.Hagerman RJ, Berry-Kravis E, Kaufmann WE, et al. Advances in the treatment of fragile X syndrome. Pediatrics. 2009;123:378–390. doi: 10.1542/peds.2008-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cornish K, Turk J, Hagerman R. The fragile X continuum: new advances and perspectives. J Intellect Disabil Res. 2008;52:469–482. doi: 10.1111/j.1365-2788.2008.01056.x. [DOI] [PubMed] [Google Scholar]
- 71.Verkerk AJ, Pieretti M, Sutcliffe JS, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 72.Heitz D, Rousseau F, Devys D, et al. Isolation of sequences that span the fragile X and identification of a fragile X-related CpG island. Science. 1991;251:1236–1239. doi: 10.1126/science.2006411. [DOI] [PubMed] [Google Scholar]
- 73.Sutcliffe JS, Nelson DL, Zhang F, et al. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum Mol Genet. 1992;1:397–400. doi: 10.1093/hmg/1.6.397. [DOI] [PubMed] [Google Scholar]
- 74.Jin P, Warren ST. New insights into fragile X syndrome: from molecules to neurobehaviors. Trends Biochem Sci. 2003;28:152–158. doi: 10.1016/S0968-0004(03)00033-1. [DOI] [PubMed] [Google Scholar]
- 75.Lu R, Wang H, Liang Z, et al. The fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc Natl Acad Sci USA. 2004;101:15201–15206. doi: 10.1073/pnas.0404995101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Todd PK, Mack KJ, Malter JS. The fragile X mental retardation protein is required for type-I metabotropic glutamate receptor-dependent translation of PSD-95. Proc Natl Acad Sci USA. 2003;100:14374–14378. doi: 10.1073/pnas.2336265100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zalfa F, Eleuteri B, Dickson KS, et al. A new function for the fragile X mental retardation protein in regulation of PSD-95 mRNA stability. Nat Neurosci. 2007;10:578–587. doi: 10.1038/nn1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hinton VJ, Brown WT, Wisniewski K, et al. Analysis of neocortex in three males with the fragile X syndrome. Am J Med Genet. 1991;41:289–294. doi: 10.1002/ajmg.1320410306. [DOI] [PubMed] [Google Scholar]
- 79.Comery TA, Harris JB, Willems PJ, et al. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci USA. 1997;94:5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Galvez R, Greenough WT. Sequence of abnormal dendritic spine development in primary somatosensory cortex of a mouse model of the fragile X mental retardation syndrome. Am J Med Genet A. 2005;135:155–160. doi: 10.1002/ajmg.a.30709. [DOI] [PubMed] [Google Scholar]
- 81.Galvez R, Gopal AR, Greenough WT. Somatosensory cortical barrel dendritic abnormalities in a mouse model of the fragile X mental retardation syndrome. Brain Res. 2003;971:83–89. doi: 10.1016/s0006-8993(03)02363-1. [DOI] [PubMed] [Google Scholar]
- 82.Koekkoek SKE, Yamaguchi K, Milojkovic BA, et al. Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in Fragile X syndrome. Neuron. 2005;47:339–352. doi: 10.1016/j.neuron.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 83.McKinney BC, Grossman AW, Elisseou NM, et al. Dendritic spine abnormalities in the occipital cortex of C57BL/6 Fmr1 knockout mice. Am J Med Genet B Neuropsychiatr Genet. 2005;136B:98–102. doi: 10.1002/ajmg.b.30183. [DOI] [PubMed] [Google Scholar]
- 84.Nimchinsky EA, Oberlander AM, Svoboda K. Abnormal development of dendritic spines in FMR1 knock-out mice. J Neurosci. 2001;21:5139–5146. doi: 10.1523/JNEUROSCI.21-14-05139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jin P, Zarnescu DC, Zhang F, et al. RNA-mediated neurodegeneration caused by the fragile X premutation rCGG repeats in Drosophila. Neuron. 2003;39:739–747. doi: 10.1016/s0896-6273(03)00533-6. [DOI] [PubMed] [Google Scholar]
- 86.Morales J, Hiesinger PR, Schroeder AJ, et al. Drosophila fragile X protein, DFXR, regulates neuronal morphology and function in the brain. Neuron. 2002;34:961–972. doi: 10.1016/s0896-6273(02)00731-6. [DOI] [PubMed] [Google Scholar]
- 87.Tucker B, Richards RI, Lardelli M. Contribution of mGluR and Fmr1 functional pathways to neurite morphogenesis, craniofacial development and fragile X syndrome. Hum Mol Genet. 2006;15:3446–3458. doi: 10.1093/hmg/ddl422. [DOI] [PubMed] [Google Scholar]
- 88.Consortium TD-BFX. Fmr1 knockout mice: a model to study fragile X mental retardation. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- 89.Moon J, Ota KT, Driscoll LL, et al. A mouse model of fragile X syndrome exhibits heightened arousal and/or emotion following errors or reversal of contingencies. Dev Psychobiol. 2008;50:473–485. doi: 10.1002/dev.20308. [DOI] [PubMed] [Google Scholar]
- 90.Paradee W, Melikian HE, Rasmussen DL, et al. Fragile X mouse: strain effects of knockout phenotype and evidence suggesting deficient amygdala function. Neuroscience. 1999;94:185–192. doi: 10.1016/s0306-4522(99)00285-7. [DOI] [PubMed] [Google Scholar]
- 91.Peier AM, McIlwain KL, Kenneson A, et al. (Over)correction of FMR1 deficiency with YAC transgenics: behavioral and physical features. Hum Mol Genet. 2000;9:1145–1159. doi: 10.1093/hmg/9.8.1145. [DOI] [PubMed] [Google Scholar]
- 92.Yan QJ, Asafo-Adjei PK, Arnold HM, et al. A phenotypic and molecular characterization of the fmr1-tm1Cgr fragile X mouse. Genes Brain Behav. 2004;3:337–359. doi: 10.1111/j.1601-183X.2004.00087.x. [DOI] [PubMed] [Google Scholar]
- 93.Chen L, Toth M. Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience. 2001;103:1043–1050. doi: 10.1016/s0306-4522(01)00036-7. [DOI] [PubMed] [Google Scholar]
- 94.Liu Z-H, Smith CB. Dissociation of social and nonsocial anxiety in a mouse model of fragile X syndrome. Neurosci Lett. 2009;454:62–66. doi: 10.1016/j.neulet.2009.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McNaughton CH, Moon J, Strawderman MS, et al. Evidence for social anxiety and impaired social cognition in a mouse model of fragile X syndrome. Behav Neurosci. 2008;122:293–300. doi: 10.1037/0735-7044.122.2.293. [DOI] [PubMed] [Google Scholar]
- 96.Musumeci SA, Calabrese G, Bonaccorso CM, et al. Audiogenic seizure susceptibility is reduced in fragile X knockout mice after introduction of FMR1 transgenes. Exp Neurol. 2007;203:233–240. doi: 10.1016/j.expneurol.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 97.Gantois I, Bakker CE, Reyniers E, et al. Restoring the phenotype of fragile X syndrome: insight from the mouse model. Curr Mol Med. 2001;1:447–455. doi: 10.2174/1566524013363492. [DOI] [PubMed] [Google Scholar]
- 98.Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 99.D’Hulst C, Kooy RF. The GABAA receptor: a novel target for treatment of fragile X? Trends Neurosci. 2007;30:425–431. doi: 10.1016/j.tins.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 100.Huber KM, Gallagher SM, Warren ST, et al. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci USA. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- 102.Chuang S-C, Zhao W, Bauchwitz R, et al. Prolonged epileptiform discharges induced by altered group I metabotropic glutamate receptor-mediated synaptic responses in hippocampal slices of a fragile X mouse model. J Neurosci. 2005;25:8048–8055. doi: 10.1523/JNEUROSCI.1777-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qiu L-F, Lu T-J, Hu X-L, et al. Limbic epileptogenesis in a mouse model of fragile X syndrome. Cereb Cortex. 2009;19:1504–1514. doi: 10.1093/cercor/bhn163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dölen G, Osterweil E, Rao BSS, et al. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McBride SMJ, Choi CH, Wang Y, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron. 2005;45:753–764. doi: 10.1016/j.neuron.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 106.de Vrij FMS, Levenga J, van der Linde HC, et al. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol Dis. 2008;31:127–132. doi: 10.1016/j.nbd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yan QJ, Rammal M, Tranfaglia M, et al. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 108.Berry-Kravis E, Hessl D, Coffey S, et al. Apilot open label, single dose trial of fenobam in adults with fragile X syndrome. J Med Genet. 2009;46:266–271. doi: 10.1136/jmg.2008.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Berry-Kravis E, Sumis A, Hervey C, et al. Open-label treatment trial of lithium to target the underlying defect in fragile X syndrome. J Dev Behav Pediatr. 2008;29:293–302. doi: 10.1097/DBP.0b013e31817dc447. [DOI] [PubMed] [Google Scholar]
- 110.Berry-Kravis E, Krause SE, Block SS, et al. Effect of CX516, an AMPA-modulating compound, on cognition and behavior in fragile X syndrome: a controlled trial. J Child Adolesc Psychopharmacol. 2006;16:525–540. doi: 10.1089/cap.2006.16.525. [DOI] [PubMed] [Google Scholar]
- 111.D’Hulst C, De Geest N, Reeve SP, et al. Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res. 2006;1121:238–245. doi: 10.1016/j.brainres.2006.08.115. [DOI] [PubMed] [Google Scholar]
- 112.El Idrissi A, Ding X-H, Scalia J, et al. Decreased GABA(A) receptor expression in the seizure-prone fragile X mouse. Neurosci Lett. 2005;377:141–146. doi: 10.1016/j.neulet.2004.11.087. [DOI] [PubMed] [Google Scholar]
- 113.Selby L, Zhang C, Sun QQ. Major defects in neocortical GABAergic inhibitory circuits in mice lacking the fragile X mental retardation protein. Neurosci Lett. 2007;412:227–232. doi: 10.1016/j.neulet.2006.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Curia G, Papouin T, Séguéla P, et al. Downregulation of tonic GABAergic inhibition in a mouse model of fragile X syndrome. Cereb Cortex. 2009;19:1515–1520. doi: 10.1093/cercor/bhn159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reddy DS. Role of neurosteroids in catamenial epilepsy. Epilepsy Res. 2004;62:99–118. doi: 10.1016/j.eplepsyres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 116.Bilousova TV, Dansie L, Ngo M, et al. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J Med Genet. 2009;46:94–102. doi: 10.1136/jmg.2008.061796. [DOI] [PubMed] [Google Scholar]
- 117.Lauterborn JC, Rex CS, Kramár E, et al. Brain-derived neurotrophic factor rescues synaptic plasticity in a mouse model of fragile X syndrome. J Neurosci. 2007;27:10685–10694. doi: 10.1523/JNEUROSCI.2624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Percy AK. Rett syndrome: recent research progress. J Child Neurol. 2008;23:543–549. doi: 10.1177/0883073807309786. [DOI] [PubMed] [Google Scholar]
- 119.Hagberg B, Aicardi J, Dias K, et al. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: report of 35 cases. Ann Neurol. 1983;14:471–479. doi: 10.1002/ana.410140412. [DOI] [PubMed] [Google Scholar]
- 120.Rett A. On a unusual brain atrophy syndrome in hyperammonemia in childhood. Wien Med Wochenschr. 1966;116:723–726. [PubMed] [Google Scholar]
- 121.Weaving LS, Ellaway CJ, Gécz J, et al. Rett syndrome: clinical review and genetic update. J Med Genet. 2005;42:1–7. doi: 10.1136/jmg.2004.027730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Williamson SL, Christodoulou J. Rett syndrome: new clinical and molecular insights. Eur J Hum Genet. 2006;14:896–903. doi: 10.1038/sj.ejhg.5201580. [DOI] [PubMed] [Google Scholar]
- 123.Lewis JD, Meehan RR, Henzel WJ, et al. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell. 1992;69:905–914. doi: 10.1016/0092-8674(92)90610-o. [DOI] [PubMed] [Google Scholar]
- 124.Amir RE, Van den Veyver IB, Wan M, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 125.Huppke P, Maier EM, Warnke A, et al. Very mild cases of Rett syndrome with skewed X inactivation. J Med Genet. 2006;43:814–816. doi: 10.1136/jmg.2006.042077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ishii T, Makita Y, Ogawa A, et al. The role of different X-inactivation pattern on the variable clinical phenotype with Rett syndrome. Brain Dev. 2001;23 (Suppl 1):S161–164. doi: 10.1016/s0387-7604(01)00344-8. [DOI] [PubMed] [Google Scholar]
- 127.Young JI, Zoghbi HY. X-chromosome inactivation patterns are unbalanced and affect the phenotypic outcome in a mouse model of rett syndrome. Am J Hum Genet. 2004;74:511–520. doi: 10.1086/382228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Erlandson A, Hagberg B. MECP2 abnormality phenotypes: clinicopathologic area with broad variability. J Child Neurol. 2005;20:727–732. doi: 10.1177/08830738050200090501. [DOI] [PubMed] [Google Scholar]
- 129.Hendrich B, Bird A. Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol Cell Biol. 1998;18:6538–6547. doi: 10.1128/mcb.18.11.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 131.Guy J, Hendrich B, Holmes M, et al. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- 132.Shahbazian M, Young J, Yuva-Paylor L, et al. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–254. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- 133.Lawson-Yuen A, Liu D, Han L, et al. Ube3a mRNA and protein expression are not decreased in Mecp2R168X mutant mice. Brain Res. 2007;1180:1–6. doi: 10.1016/j.brainres.2007.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen RZ, Akbarian S, Tudor M, et al. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- 135.Pelka GJ, Watson CM, Radziewic T, et al. Mecp2 deficiency is associated with learning and cognitive deficits and altered gene activity in the hippocampal region of mice. Brain. 2006;129:887–898. doi: 10.1093/brain/awl022. [DOI] [PubMed] [Google Scholar]
- 136.Moretti P, Bouwknecht JA, Teague R, et al. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum Mol Genet. 2005;14:205–220. doi: 10.1093/hmg/ddi016. [DOI] [PubMed] [Google Scholar]
- 137.Nelson ED, Kavalali ET, Monteggia LM. MeCP2-dependent transcriptional repression regulates excitatory neurotransmission. Curr Biol. 2006;16:710–716. doi: 10.1016/j.cub.2006.02.062. [DOI] [PubMed] [Google Scholar]
- 138.Dani VS, Chang Q, Maffei A, et al. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc Natl Acad Sci USA. 2005;102:12560–12565. doi: 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Medrihan L, Tantalaki E, Aramuni G, et al. Early defects of GABAergic synapses in the brain stem of a MeCP2 mouse model of Rett syndrome. J Neurophysiol. 2008;99:112–121. doi: 10.1152/jn.00826.2007. [DOI] [PubMed] [Google Scholar]
- 140.Chao H-T, Zoghbi HY, Rosenmund C. MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron. 2007;56:58–65. doi: 10.1016/j.neuron.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen WG, Chang Q, Lin Y, et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- 142.Asaka Y, Jugloff DGM, Zhang L, et al. Hippocampal synaptic plasticity is impaired in the Mecp2-null mouse model of Rett syndrome. Neurobiol Dis. 2006;21:217–227. doi: 10.1016/j.nbd.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 143.Moretti P, Levenson JM, Battaglia F, et al. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J Neurosci. 2006;26:319–327. doi: 10.1523/JNEUROSCI.2623-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Collins AL, Levenson JM, Vilaythong AP, et al. Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Hum Mol Genet. 2004;13:2679–2689. doi: 10.1093/hmg/ddh282. [DOI] [PubMed] [Google Scholar]
- 145.Jugloff DGM, Vandamme K, Logan R, et al. Targeted delivery of an Mecp2 transgene to forebrain neurons improves the behavior of female Mecp2-deficient mice. Hum Mol Genet. 2008;17:1386–1396. doi: 10.1093/hmg/ddn026. [DOI] [PubMed] [Google Scholar]
- 146.Luikenhuis S, Giacometti E, Beard CF, et al. Expression of MeCP2 in postmitotic neurons rescues Rett syndrome in mice. Proc Natl Acad Sci USA. 2004;101:6033–6038. doi: 10.1073/pnas.0401626101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Alvarez-Saavedra M, Sáez MA, Kang D, et al. Cell-specific expression of wild-type MeCP2 in mouse models of Rett syndrome yields insight about pathogenesis. Hum Mol Genet. 2007;16:2315–2325. doi: 10.1093/hmg/ddm185. [DOI] [PubMed] [Google Scholar]
- 148.Giacometti E, Luikenhuis S, Beard C, et al. Partial rescue of MeCP2 deficiency by postnatal activation of MeCP2. Proc Natl Acad Sci USA. 2007;104:1931–1936. doi: 10.1073/pnas.0610593104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Guy J, Gan J, Selfridge J, et al. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tropea D, Giacometti E, Wilson NR, et al. Partial reversal of Rett Syndrome-like symptoms in MeCP2 mutant mice. Proc Natl Acad Sci USA. 2009;106:2029–2034. doi: 10.1073/pnas.0812394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mount RH, Hastings RP, Reilly S, et al. Behavioural and emotional features in Rett syndrome. Disabil Rehabil. 2001;23:129–138. doi: 10.1080/09638280150504207. [DOI] [PubMed] [Google Scholar]
- 152.Nuber UA, Kriaucionis S, Roloff TC, et al. Up-regulation of glucocorticoid-regulated genes in a mouse model of Rett syndrome. Hum Mol Genet. 2005;14:2247–2256. doi: 10.1093/hmg/ddi229. [DOI] [PubMed] [Google Scholar]
- 153.McGill BE, Bundle SF, Yaylaoglu MB, et al. Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proc Natl Acad Sci USA. 2006;103:18267–18272. doi: 10.1073/pnas.0608702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Samaco RC, Mandel-Brehm C, Chao H-T, et al. Loss of MeCP2 in aminergic neurons causes cell-autonomous defects in neurotransmitter synthesis and specific behavioral abnormalities. Proc Natl Acad Sci USA. 2009 doi: 10.1073/pnas.0912257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Viemari J-C, Roux J-C, Tryba AK, et al. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J Neurosci. 2005;25:11521–11530. doi: 10.1523/JNEUROSCI.4373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Roux J-C, Dura E, Moncla A, et al. Treatment with desipramine improves breathing and survival in a mouse model for Rett syndrome. Eur J Neurosci. 2007;25:1915–1922. doi: 10.1111/j.1460-9568.2007.05466.x. [DOI] [PubMed] [Google Scholar]
- 157.Zanella S, Mebarek S, Lajard A-M, et al. Oral treatment with desipramine improves breathing and life span in Rett syndrome mouse model. Respir Physiol Neurobiol. 2008;160:116–121. doi: 10.1016/j.resp.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 158.Ogier M, Wang H, Hong E, et al. Brain-derived neurotrophic factor expression and respiratory function improve after ampakine treatment in a mouse model of Rett syndrome. J Neurosci. 2007;27:10912–10917. doi: 10.1523/JNEUROSCI.1869-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Friedman JM. Epidemiology of neurofibromatosis type 1. Am J Med Genet. 1999;89:1–6. [PubMed] [Google Scholar]
- 160.Wallace MR, Marchuk DA, Andersen LB, et al. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science. 1990;249:181–186. doi: 10.1126/science.2134734. [DOI] [PubMed] [Google Scholar]
- 161.Upadhyaya M, Ruggieri M, Maynard J, et al. Gross deletions of the neurofibromatosis type 1 (NF1) gene are predominantly of maternal origin and commonly associated with a learning disability, dysmorphic features and developmental delay. Hum Genet. 1998;102:591–597. doi: 10.1007/s004390050746. [DOI] [PubMed] [Google Scholar]
- 162.Tonsgard JH. Clinical manifestations and management of neurofibromatosis type 1. Semin Pediatr Neurol. 2006;13:2–7. doi: 10.1016/j.spen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 163.Hyman SL, Shores A, North KN. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology. 2005;65:1037–1044. doi: 10.1212/01.wnl.0000179303.72345.ce. [DOI] [PubMed] [Google Scholar]
- 164.North KN, Riccardi V, Samango-Sprouse C, et al. Cognitive function and academic performance in neurofibromatosis. 1: consensus statement from the NF1 Cognitive Disorders Task Force. Neurology. 1997;48:1121–1127. doi: 10.1212/wnl.48.4.1121. [DOI] [PubMed] [Google Scholar]
- 165.Daston MM, Scrable H, Nordlund M, et al. The protein product of the neurofibromatosis type 1 gene is expressed at highest abundance in neurons, Schwann cells, and oligodendrocytes. Neuron. 1992;8:415–428. doi: 10.1016/0896-6273(92)90270-n. [DOI] [PubMed] [Google Scholar]
- 166.Malhotra R, Ratner N. Localization of neurofibromin to keratinocytes and melanocytes in developing rat and human skin. J Invest Dermatol. 1994;102:812–818. doi: 10.1111/1523-1747.ep12379925. [DOI] [PubMed] [Google Scholar]
- 167.Xu GF, O’Connell P, Viskochil D, et al. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell. 1990;62:599–608. doi: 10.1016/0092-8674(90)90024-9. [DOI] [PubMed] [Google Scholar]
- 168.Cichowski K, Shih TS, Schmitt E, et al. Mouse models of tumor development in neurofibromatosis type 1. Science. 1999;286:2172–2176. doi: 10.1126/science.286.5447.2172. [DOI] [PubMed] [Google Scholar]
- 169.Guo HF, The I, Hannan F, et al. Requirement of Drosophila NF1 for activation of adenylyl cyclase by PACAP38-like neuropeptides. Science. 1997;276:795–798. doi: 10.1126/science.276.5313.795. [DOI] [PubMed] [Google Scholar]
- 170.The I, Hannigan GE, Cowley GS, et al. Rescue of a Drosophila NF1 mutant phenotype by protein kinase A. Science. 1997;276:791–794. doi: 10.1126/science.276.5313.791. [DOI] [PubMed] [Google Scholar]
- 171.Tong J, Hannan F, Zhu Y, et al. Neurofibromin regulates G protein-stimulated adenylyl cyclase activity. Nat Neurosci. 2002;5:95–96. doi: 10.1038/nn792. [DOI] [PubMed] [Google Scholar]
- 172.Guo HF, Tong J, Hannan F, et al. A neurofibromatosis-1-regulated pathway is required for learning in Drosophila. Nature. 2000;403:895–898. doi: 10.1038/35002593. [DOI] [PubMed] [Google Scholar]
- 173.Silva AJ, Frankland PW, Marowitz Z, et al. A mouse model for the learning and memory deficits associated with neurofibromatosis type I. Nat Genet. 1997;15:281–284. doi: 10.1038/ng0397-281. [DOI] [PubMed] [Google Scholar]
- 174.Rizvi TA, Akunuru S, de Courten-Myers G, et al. Region-specific astrogliosis in brains of mice heterozygous for mutations in the neurofibromatosis type 1 (Nf1) tumor suppressor. Brain Res. 1999;816:111–123. doi: 10.1016/s0006-8993(98)01133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhu Y, Romero MI, Ghosh P, et al. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 2001;15:859–876. doi: 10.1101/gad.862101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Costa RM, Yang T, Huynh DP, et al. Learning deficits, but normal development and tumor predisposition, in mice lacking exon 23a of Nf1. Nat Genet. 2001;27:399–405. doi: 10.1038/86898. [DOI] [PubMed] [Google Scholar]
- 177.Costa RM, Federov NB, Kogan JH, et al. Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature. 2002;415:526–530. doi: 10.1038/nature711. [DOI] [PubMed] [Google Scholar]
- 178.Cui Y, Costa RM, Murphy GG, et al. Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell. 2008;135:549–560. doi: 10.1016/j.cell.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li W, Cui Y, Kushner SA, et al. The HMG-CoA reductase inhibitor lovastatin reverses the learning and attention deficits in a mouse model of neurofibromatosis type 1. Curr Biol. 2005;15:1961–1967. doi: 10.1016/j.cub.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 180.Krab LC, De Goede-Bolder A, Aarsen FK, et al. Effect of Simvastatin on Cognitive Functioning in Children With Neurofibromatosis Type 1: A Randomized Controlled Trial. JAMA. 2008;300:287–294. doi: 10.1001/jama.300.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Johannessen CM, Reczek EE, James MF, et al. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci USA. 2005;102:8573–8578. doi: 10.1073/pnas.0503224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.van Slegtenhorst M, de Hoogt R, Hermans C, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 183.Consortium ECTS. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75:1305–1315. doi: 10.1016/0092-8674(93)90618-z. [DOI] [PubMed] [Google Scholar]
- 184.Osborne JP, Fryer A, Webb D. Epidemiology of tuberous sclerosis. Ann N Y Acad Sci. 1991;615:125–127. doi: 10.1111/j.1749-6632.1991.tb37754.x. [DOI] [PubMed] [Google Scholar]
- 185.Langkau N, Martin N, Brandt R, et al. TSC1 and TSC2 mutations in tuberous sclerosis, the associated phenotypes and a model to explain observed TSC1/TSC2 frequency ratios. Eur J Pediatr. 2002;161:393–402. doi: 10.1007/s00431-001-0903-7. [DOI] [PubMed] [Google Scholar]
- 186.Webb DW, Fryer AE, Osborne JP. Morbidity associated with tuberous sclerosis: a population study. Dev Med Child Neurol. 1996;38:146–155. doi: 10.1111/j.1469-8749.1996.tb12086.x. [DOI] [PubMed] [Google Scholar]
- 187.Luat AF, Makki M, Chugani HT. Neuroimaging in tuberous sclerosis complex. Curr Opin Neurol. 2007;20:142–150. doi: 10.1097/WCO.0b013e3280895d93. [DOI] [PubMed] [Google Scholar]
- 188.Harrison JE, O’Callaghan FJ, Hancock E, et al. Cognitive deficits in normally intelligent patients with tuberous sclerosis. Am J Med Genet. 1999;88:642–646. [PubMed] [Google Scholar]
- 189.Prather P, de Vries PJ. Behavioral and cognitive aspects of tuberous sclerosis complex. J Child Neurol. 2004;19:666–674. doi: 10.1177/08830738040190090601. [DOI] [PubMed] [Google Scholar]
- 190.Gillberg IC, Gillberg C, Ahlsén G. Autistic behaviour and attention deficits in tuberous sclerosis: a population-based study. Dev Med Child Neurol. 1994;36:50–56. doi: 10.1111/j.1469-8749.1994.tb11765.x. [DOI] [PubMed] [Google Scholar]
- 191.Joinson C, O’Callaghan FJ, Osborne JP, et al. Learning disability and epilepsy in an epidemiological sample of individuals with tuberous sclerosis complex. Psychol Med. 2003;33:335–344. doi: 10.1017/s0033291702007092. [DOI] [PubMed] [Google Scholar]
- 192.Bolton PF, Park RJ, Higgins JNP, et al. Neuro-epileptic determinants of autism spectrum disorders in tuberous sclerosis complex. Brain. 2002;125:1247–1255. doi: 10.1093/brain/awf124. [DOI] [PubMed] [Google Scholar]
- 193.Curatolo P, Seri S, Verdecchia M, et al. Infantile spasms in tuberous sclerosis complex. Brain Dev. 2001;23:502–507. doi: 10.1016/s0387-7604(01)00300-x. [DOI] [PubMed] [Google Scholar]
- 194.Ehninger D, de Vries PJ, Silva AJ. From mTOR to cognition: molecular and cellular mechanisms of cognitive impairments in tuberous sclerosis. J Intellect Disabil Res. 2009;53:838–851. doi: 10.1111/j.1365-2788.2009.01208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rosner M, Hanneder M, Siegel N, et al. The tuberous sclerosis gene products hamartin and tuberin are multifunctional proteins with a wide spectrum of interacting partners. Mutat Res. 2008;658:234–246. doi: 10.1016/j.mrrev.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 196.Kwiatkowski DJ, Manning BD. Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Hum Mol Genet. 2005;14(Spec No 2):R251–258. doi: 10.1093/hmg/ddi260. [DOI] [PubMed] [Google Scholar]
- 197.Jaworski J, Sheng M. The growing role of mTOR in neuronal development and plasticity. Mol Neurobiol. 2006;34:205–219. doi: 10.1385/MN:34:3:205. [DOI] [PubMed] [Google Scholar]
- 198.Tee AR, Fingar DC, Manning BD, et al. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci USA. 2002;99:13571–13576. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tee AR, Manning BD, Roux PP, et al. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003;13:1259–1268. doi: 10.1016/s0960-9822(03)00506-2. [DOI] [PubMed] [Google Scholar]
- 200.Ito N, Rubin GM. gigas, a Drosophila homolog of tuberous sclerosis gene product-2, regulates the cell cycle. Cell. 1999;96:529–539. doi: 10.1016/s0092-8674(00)80657-1. [DOI] [PubMed] [Google Scholar]
- 201.Tapon N, Ito N, Dickson BJ, et al. The Drosophila tuberous sclerosis complex gene homologs restrict cell growth and cell proliferation. Cell. 2001;105:345–355. doi: 10.1016/s0092-8674(01)00332-4. [DOI] [PubMed] [Google Scholar]
- 202.Gao X, Pan D. TSC1 and TSC2 tumor suppressors antagonize insulin signaling in cell growth. Genes Dev. 2001;15:1383–1392. doi: 10.1101/gad.901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ehninger D, Han S, Shilyansky C, et al. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med. 2008;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Meikle L, Talos DM, Onda H, et al. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci. 2007;27:5546–5558. doi: 10.1523/JNEUROSCI.5540-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Uhlmann EJ, Wong M, Baldwin RL, et al. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann Neurol. 2002;52:285–296. doi: 10.1002/ana.10283. [DOI] [PubMed] [Google Scholar]
- 206.Wang Y, Greenwood JSF, Calcagnotto ME, et al. Neocortical hyperexcitability in a human case of tuberous sclerosis complex and mice lacking neuronal expression of TSC1. Ann Neurol. 2007;61:139–152. doi: 10.1002/ana.21058. [DOI] [PubMed] [Google Scholar]
- 207.Zeng L-H, Ouyang Y, Gazit V, et al. Abnormal glutamate homeostasis and impaired synaptic plasticity and learning in a mouse model of tuberous sclerosis complex. Neurobiol Dis. 2007;28:184–196. doi: 10.1016/j.nbd.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Goorden SMI, van Woerden GM, van der Weerd L, et al. Cognitive deficits in Tsc1+/− mice in the absence of cerebral lesions and seizures. Ann Neurol. 2007;62:648–655. doi: 10.1002/ana.21317. [DOI] [PubMed] [Google Scholar]
- 209.von der Brelie C, Waltereit R, Zhang L, et al. Impaired synaptic plasticity in a rat model of tuberous sclerosis. Eur J Neurosci. 2006;23:686–692. doi: 10.1111/j.1460-9568.2006.04594.x. [DOI] [PubMed] [Google Scholar]
- 210.Waltereit R, Welzl H, Dichgans J, et al. Enhanced episodic-like memory and kindling epilepsy in a rat model of tuberous sclerosis. J Neurochem. 2006;96:407–413. doi: 10.1111/j.1471-4159.2005.03538.x. [DOI] [PubMed] [Google Scholar]
- 211.Hancock E, Osborne JP. Vigabatrin in the treatment of infantile spasms in tuberous sclerosis: literature review. J Child Neurol. 1999;14:71–74. doi: 10.1177/088307389901400201. [DOI] [PubMed] [Google Scholar]
- 212.Jambaqué I, Chiron C, Dumas C, et al. Mental and behavioural outcome of infantile epilepsy treated by vigabatrin in tuberous sclerosis patients. Epilepsy Res. 2000;38:151–160. doi: 10.1016/s0920-1211(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 213.Collins JJ, Tudor C, Leonard JM, et al. Levetiracetam as adjunctive antiepileptic therapy for patients with tuberous sclerosis complex: a retrospective open-label trial. J Child Neurol. 2006;21:53–57. doi: 10.1177/08830738060210011201. [DOI] [PubMed] [Google Scholar]
- 214.Jozwiak J, Jozwiak S, Oldak M. Molecular activity of sirolimus and its possible application in tuberous sclerosis treatment. Med Res Rev. 2006;26:160–180. doi: 10.1002/med.20049. [DOI] [PubMed] [Google Scholar]
- 215.Zeng L-H, Xu L, Gutmann DH, et al. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Meikle L, Pollizzi K, Egnor A, et al. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci. 2008;28:5422–5432. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rauktys A, Lee N, Lee L, et al. Topical rapamycin inhibits tuberous sclerosis tumor growth in a nude mouse model. BMC Dermatol. 2008;8:1. doi: 10.1186/1471-5945-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kenerson H, Dundon TA, Yeung RS. Effects of rapamycin in the Eker rat model of tuberous sclerosis complex. Pediatr Res. 2005;57:67–75. doi: 10.1203/01.PDR.0000147727.78571.07. [DOI] [PubMed] [Google Scholar]
- 219.Messina MP, Rauktys A, Lee L, et al. Tuberous sclerosis preclinical studies: timing of treatment, combination of a rapamycin analog (CCI-779) and interferon-gamma, and comparison of rapamycin to CCI-779. BMC Pharmacol. 2007;7:14. doi: 10.1186/1471-2210-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lee L, Sudentas P, Dabora SL. Combination of a rapamycin analog (CCI-779) and interferon-gamma is more effective than single agents in treating a mouse model of tuberous sclerosis complex. Genes Chromosomes Cancer. 2006;45:933–944. doi: 10.1002/gcc.20357. [DOI] [PubMed] [Google Scholar]
- 221.Dabora SL, Jozwiak S, Franz DN, et al. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet. 2001;68:64–80. doi: 10.1086/316951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Courchesne E. Brain development in autism: early overgrowth followed by premature arrest of growth. Ment Retard Dev Disabil Res Rev. 2004;10:106–111. doi: 10.1002/mrdd.20020. [DOI] [PubMed] [Google Scholar]
- 223.Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 224.Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr Opin Neurobiol. 2005;15:225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 225.Fombonne E. Epidemiology of pervasive developmental disorders. Pediatr Res. 2009;65:591–598. doi: 10.1203/PDR.0b013e31819e7203. [DOI] [PubMed] [Google Scholar]
- 226.Kumar RA, Christian SL. Genetics of autism spectrum disorders. Curr Neurol Neurosci Rep. 2009;9:188–197. doi: 10.1007/s11910-009-0029-2. [DOI] [PubMed] [Google Scholar]
- 227.Folstein SE, Rosen-Sheidley B. Genetics of autism: complex aetiology for a heterogeneous disorder. Nat Rev Genet. 2001;2:943–955. doi: 10.1038/35103559. [DOI] [PubMed] [Google Scholar]
- 228.Consortium AGP, Szatmari P, Paterson AD, et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Persico AM, Bourgeron T. Searching for ways out of the autism maze: genetic, epigenetic and environmental clues. Trends Neurosci. 2006;29:349–358. doi: 10.1016/j.tins.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 230.Polleux F, Lauder JM. Toward a developmental neurobiology of autism. Ment Retard Dev Disabil Res Rev. 2004;10:303–317. doi: 10.1002/mrdd.20044. [DOI] [PubMed] [Google Scholar]
- 231.Herbert MR. Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Curr Opin Neurol. 2010 doi: 10.1097/WCO.0b013e328336a01f. [DOI] [PubMed] [Google Scholar]
- 232.Landrigan PJ. What causes autism? Exploring the environmental contribution. Curr Opin Pediatr. 2010 doi: 10.1097/MOP.0b013e328336eb9a. [DOI] [PubMed] [Google Scholar]
- 233.Freitag CM. The genetics of autistic disorders and its clinical relevance: a review of the literature. Mol Psychiatry. 2007;12:2–22. doi: 10.1038/sj.mp.4001896. [DOI] [PubMed] [Google Scholar]
- 234.Pardo CA, Eberhart CG. The neurobiology of autism. Brain Pathol. 2007;17:434–447. doi: 10.1111/j.1750-3639.2007.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Bourgeron T. A synaptic trek to autism. Curr Opin Neurobiol. 2009;19:231–234. doi: 10.1016/j.conb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 236.Lam KSL, Aman MG, Arnold LE. Neurochemical correlates of autistic disorder: a review of the literature. Res Dev Disabil. 2006;27:254–289. doi: 10.1016/j.ridd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 237.Moy SS, Nadler JJ. Advances in behavioral genetics: mouse models of autism. Mol Psychiatry. 2008;13:4–26. doi: 10.1038/sj.mp.4002082. [DOI] [PubMed] [Google Scholar]
- 238.Belmonte MK, Bourgeron T. Fragile X syndrome and autism at the intersection of genetic and neural networks. Nat Neurosci. 2006;9:1221–1225. doi: 10.1038/nn1765. [DOI] [PubMed] [Google Scholar]
- 239.Chubykin AA, Liu X, Comoletti D, et al. Dissection of synapse induction by neuroligins: effect of a neuroligin mutation associated with autism. J Biol Chem. 2005;280:22365–22374. doi: 10.1074/jbc.M410723200. [DOI] [PubMed] [Google Scholar]
- 240.Dean C, Dresbach T. Neuroligins and neurexins: linking cell adhesion, synapse formation and cognitive function. Trends Neurosci. 2006;29:21–29. doi: 10.1016/j.tins.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 241.Südhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Jamain S, Quach H, Betancur C, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Laumonnier F, Bonnet-Brilhault F, Gomot M, et al. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet. 2004;74:552–557. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zhang C, Milunsky JM, Newton S, et al. A neuroligin-4 missense mutation associated with autism impairs neuroligin-4 folding and endoplasmic reticulum export. J Neurosci. 2009;29:10843–10854. doi: 10.1523/JNEUROSCI.1248-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Song JY, Ichtchenko K, Südhof TC, et al. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci USA. 1999;96:1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Varoqueaux F, Jamain S, Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol. 2004;83:449–456. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- 247.Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- 248.Prange O, Wong TP, Gerrow K, et al. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc Natl Acad Sci USA. 2004;101:13915–13920. doi: 10.1073/pnas.0405939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Chubykin AA, Atasoy D, Etherton MR, et al. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54:919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hines RM, Wu L, Hines DJ, et al. Synaptic imbalance, stereotypies, and impaired social interactions in mice with altered neuroligin 2 expression. J Neurosci. 2008;28:6055–6067. doi: 10.1523/JNEUROSCI.0032-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Blundell J, Blaiss CA, Etherton MR, et al. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J Neurosci. 2010;30:2115–2129. doi: 10.1523/JNEUROSCI.4517-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kim J, Jung S-Y, Lee YK, et al. Neuroligin-1 is required for normal expression of LTP and associative fear memory in the amygdala of adult animals. Proc Natl Acad Sci USA. 2008;105:9087–9092. doi: 10.1073/pnas.0803448105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Radyushkin K, Hammerschmidt K, Boretius S, et al. Neuroligin-3-deficient mice: model of a monogenic heritable form of autism with an olfactory deficit. Genes Brain Behav. 2009;8:416–425. doi: 10.1111/j.1601-183X.2009.00487.x. [DOI] [PubMed] [Google Scholar]
- 254.Boeckers TM, Bockmann J, Kreutz MR, et al. ProSAP/Shank proteins - a family of higher order organizing molecules of the postsynaptic density with an emerging role in human neurological disease. J Neurochem. 2002;81:903–910. doi: 10.1046/j.1471-4159.2002.00931.x. [DOI] [PubMed] [Google Scholar]
- 255.Durand CM, Betancur C, Boeckers TM, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39:25–27. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Gauthier J, Spiegelman D, Piton A, et al. Novel de novo SHANK3 mutation in autistic patients. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:421–424. doi: 10.1002/ajmg.b.30822. [DOI] [PubMed] [Google Scholar]
- 257.Moessner R, Marshall CR, Sutcliffe JS, et al. Contribution of SHANK3 mutations to autism spectrum disorder. Am J Hum Genet. 2007;81:1289–1297. doi: 10.1086/522590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Hung AY, Futai K, Sala C, et al. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. J Neurosci. 2008;28:1697–1708. doi: 10.1523/JNEUROSCI.3032-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Alarcón M, Abrahams BS, Stone JL, et al. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008;82:150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Prasad HC, Steiner JA, Sutcliffe JS, et al. Enhanced activity of human serotonin transporter variants associated with autism. Philos Trans R Soc Lond, B, Biol Sci. 2009;364:163–173. doi: 10.1098/rstb.2008.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Sutcliffe JS, Delahanty RJ, Prasad HC, et al. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. Am J Hum Genet. 2005;77:265–279. doi: 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.McCauley JL, Olson LM, Dowd M, et al. Linkage and association analysis at the serotonin transporter (SLC6A4) locus in a rigid-compulsive subset of autism. Am J Med Genet B Neuropsychiatr Genet. 2004;127B:104–112. doi: 10.1002/ajmg.b.20151. [DOI] [PubMed] [Google Scholar]
- 263.Schain RJ, Freedman DX. Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children. J Pediatr. 1961;58:315–320. doi: 10.1016/s0022-3476(61)80261-8. [DOI] [PubMed] [Google Scholar]
- 264.Bourgeron T. The possible interplay of synaptic and clock genes in autism spectrum disorders. Cold Spring Harb Symp Quant Biol. 2007;72:645–654. doi: 10.1101/sqb.2007.72.020. [DOI] [PubMed] [Google Scholar]
- 265.King BH, Hollander E, Sikich L, et al. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch Gen Psychiatry. 2009;66:583–590. doi: 10.1001/archgenpsychiatry.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.King BH, Bostic JQ. An update on pharmacologic treatments for autism spectrum disorders. Child Adolesc Psychiatr Clin N Am. 2006;15:161–175. doi: 10.1016/j.chc.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 267.Leskovec TJ, Rowles BM, Findling RL. Pharmacological treatment options for autism spectrum disorders in children and adolescents. Harv Rev Psychiatry. 2008;16:97–112. doi: 10.1080/10673220802075852. [DOI] [PubMed] [Google Scholar]
- 268.Network RUoPPA. Risperidone treatment of autistic disorder: longer-term benefits and blinded discontinuation after 6 months. Am J Psychiatry. 2005;162:1361–1369. doi: 10.1176/appi.ajp.162.7.1361. [DOI] [PubMed] [Google Scholar]
- 269.Marcus RN, Owen R, Kamen L, et al. A placebo-controlled, fixed-dose study of aripiprazole in children and adolescents with irritability associated with autistic disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:1110–1119. doi: 10.1097/CHI.0b013e3181b76658. [DOI] [PubMed] [Google Scholar]
- 270.D’Hulst C, Kooy RF. Fragile X syndrome: from molecular genetics to therapy. J Med Genet. 2009;46:577–584. doi: 10.1136/jmg.2008.064667. [DOI] [PubMed] [Google Scholar]
- 271.Ricceri L, De Filippis B, Laviola G. Mouse models of Rett syndrome: from behavioural phenotyping to preclinical evaluation of new therapeutic approaches. Behav Pharmacol. 2008;19:501–517. doi: 10.1097/FBP.0b013e32830c3645. [DOI] [PubMed] [Google Scholar]
- 272.Prasher VP. Epilepsy and associated effects on adaptive behaviour in adults with Down syndrome. Seizure. 1995;4:53–56. doi: 10.1016/s1059-1311(05)80079-2. [DOI] [PubMed] [Google Scholar]
- 273.Davis JD, Tremont G. Neuropsychiatric aspects of hypothyroidism and treatment reversibility. Minerva Endocrinol. 2007;32:49–65. [PubMed] [Google Scholar]
- 274.Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. 2008;372:657–668. doi: 10.1016/S0140-6736(08)61279-9. [DOI] [PubMed] [Google Scholar]
- 275.Ess KC. Tuberous sclerosis complex: a brave new world? Curr Opin Neurol. 2010 doi: 10.1097/WCO.0b013e32832c4ff5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Oostra BA, Willemsen R. FMR1: a gene with three faces. Biochim Biophys Acta. 2009;1790:467–477. doi: 10.1016/j.bbagen.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]