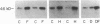Abstract
Previous studies have suggested that alteration in the expression of the insulin-regulatable glucose transporter of muscle (GLUT-4 protein) may be an important determinant of insulin action. In the present studies, we have examined GLUT-4 mRNA and protein concentrations in muscle after variations in the metabolic status of the intact animal (i.e., 7 d streptozotocin-induced diabetes, 7 d insulin-induced hypoglycemia, and 3 d fasting). These changes in glucose homeostasis were associated with the following changes in GLUT-4 gene products: a decrease of approximately 30% in both mRNA and protein with diabetes; a 50% increase in mRNA and a 2.4-fold increase in protein with insulin injection; and normal mRNA in spite of a 2.7-fold increase in protein with fasting. Fasted diabetics exhibited an increase of 50% in GLUT-4 mRNA and a 2.4-fold increase in protein relative to fed diabetics. In diabetic and insulin-injected groups, the changes in GLUT-4 protein were similar to changes in mRNA, but in fasting, GLUT-4 protein increased without a concomitant change in mRNA. Overall there was no correlation between muscle concentrations of GLUT-4 protein and mRNA. Muscle GLUT-4 protein concentration tended to correlate with plasma glucose (r = -0.57, P less than 0.001), but not with plasma insulin. These results indicate that (a) chronic changes in glucose homeostasis are associated with changes in expression of GLUT-4 protein in muscle; (b) GLUT-4 protein increased in fasted soleus muscle without change in mRNA, thereby differing from fasted adipocytes in which both GLUT-4 products diminish; and (c) no simple relationship exists between total muscle GLUT-4 protein content and whole-body insulin sensitivity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger J., Biswas C., Vicario P. P., Strout H. V., Saperstein R., Pilch P. F. Decreased expression of the insulin-responsive glucose transporter in diabetes and fasting. Nature. 1989 Jul 6;340(6228):70–72. doi: 10.1038/340070a0. [DOI] [PubMed] [Google Scholar]
- Birnbaum M. J. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989 Apr 21;57(2):305–315. doi: 10.1016/0092-8674(89)90968-9. [DOI] [PubMed] [Google Scholar]
- Charron M. J., Brosius F. C., 3rd, Alper S. L., Lodish H. F. A glucose transport protein expressed predominately in insulin-responsive tissues. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2535–2539. doi: 10.1073/pnas.86.8.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Gunnarsson R., Björkman O., Olsson M., Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985 Jul;76(1):149–155. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohm G. L., Tapscott E. B., Pories W. J., Dabbs D. J., Flickinger E. G., Meelheim D., Fushiki T., Atkinson S. M., Elton C. W., Caro J. F. An in vitro human muscle preparation suitable for metabolic studies. Decreased insulin stimulation of glucose transport in muscle from morbidly obese and diabetic subjects. J Clin Invest. 1988 Aug;82(2):486–494. doi: 10.1172/JCI113622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey W. T., Huecksteadt T. P., Birnbaum M. J. Pretranslational suppression of an insulin-responsive glucose transporter in rats with diabetes mellitus. Science. 1989 Jul 7;245(4913):60–63. doi: 10.1126/science.2662408. [DOI] [PubMed] [Google Scholar]
- Issad T., Pénicaud L., Ferré P., Kandé J., Baudon M. A., Girard J. Effects of fasting on tissue glucose utilization in conscious resting rats. Major glucose-sparing effect in working muscles. Biochem J. 1987 Aug 15;246(1):241–244. doi: 10.1042/bj2460241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D. E., Brown R., Navarro J., Pilch P. F. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature. 1988 May 12;333(6169):183–185. doi: 10.1038/333183a0. [DOI] [PubMed] [Google Scholar]
- James D. E., Strube M., Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989 Mar 2;338(6210):83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- Kahn B. B., Charron M. J., Lodish H. F., Cushman S. W., Flier J. S. Differential regulation of two glucose transporters in adipose cells from diabetic and insulin-treated diabetic rats. J Clin Invest. 1989 Aug;84(2):404–411. doi: 10.1172/JCI114180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn B. B., Cushman S. W., Flier J. S. Regulation of glucose transporter-specific mRNA levels in rat adipose cells with fasting and refeeding. Implications for in vivo control of glucose transporter number. J Clin Invest. 1989 Jan;83(1):199–204. doi: 10.1172/JCI113859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn B. B., Horton E. S., Cushman S. W. Mechanism for enhanced glucose transport response to insulin in adipose cells from chronically hyperinsulinemic rats. Increased translocation of glucose transporters from an enlarged intracellular pool. J Clin Invest. 1987 Mar;79(3):853–858. doi: 10.1172/JCI112894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley D., Mitrakou A., Marsh H., Schwenk F., Benn J., Sonnenberg G., Arcangeli M., Aoki T., Sorensen J., Berger M. Skeletal muscle glycolysis, oxidation, and storage of an oral glucose load. J Clin Invest. 1988 May;81(5):1563–1571. doi: 10.1172/JCI113489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Olefsky J. M. Long-term regulation of adipocyte glucose transport capacity by circulating insulin in rats. J Clin Invest. 1978 Jul;62(1):73–81. doi: 10.1172/JCI109116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koranyi L., James D., Mueckler M., Permutt M. A. Glucose transporter levels in spontaneously obese (db/db) insulin-resistant mice. J Clin Invest. 1990 Mar;85(3):962–967. doi: 10.1172/JCI114526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koranyi L., Permutt M. A., Chirgwin J. M., Giddings S. J. Proinsulin I and II gene expression in inbred mouse strains. Mol Endocrinol. 1989 Nov;3(11):1895–1902. doi: 10.1210/mend-3-11-1895. [DOI] [PubMed] [Google Scholar]
- Kruszynska Y. T., Home P. D., Alberti K. G. Insulin insensitivity and skeletal muscle enzyme activities in response to overinsulinization in the rat. Metabolism. 1987 Mar;36(3):281–285. doi: 10.1016/0026-0495(87)90189-2. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pénicaud L., Kandé J., Le Magnen J., Girard J. R. Insulin action during fasting and refeeding in rat determined by euglycemic clamp. Am J Physiol. 1985 Nov;249(5 Pt 1):E514–E518. doi: 10.1152/ajpendo.1985.249.5.E514. [DOI] [PubMed] [Google Scholar]
- Ramlal T., Rastogi S., Vranic M., Klip A. Decrease in glucose transporter number in skeletal muscle of mildly diabetic (streptozotocin-treated) rats. Endocrinology. 1989 Aug;125(2):890–897. doi: 10.1210/endo-125-2-890. [DOI] [PubMed] [Google Scholar]
- Restrepo D., Kozody D. J., Knauf P. A. Synthetic diacylglycerols trigger an increase of intracellular free calcium in promyelocytic HL60 cells. J Biol Chem. 1989 Jan 15;264(2):776–781. [PubMed] [Google Scholar]
- Rossetti L., Smith D., Shulman G. I., Papachristou D., DeFronzo R. A. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987 May;79(5):1510–1515. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivitz W. I., DeSautel S. L., Kayano T., Bell G. I., Pessin J. E. Regulation of glucose transporter messenger RNA in insulin-deficient states. Nature. 1989 Jul 6;340(6228):72–74. doi: 10.1038/340072a0. [DOI] [PubMed] [Google Scholar]
- Stirewalt W. S., Low R. B., Slaiby J. M. Insulin sensitivity and responsiveness of epitrochlearis and soleus muscles from fed and starved rats. Recognition of differential changes in insulin sensitivities of protein synthesis and glucose incorporation into glycogen. Biochem J. 1985 Apr 15;227(2):355–362. doi: 10.1042/bj2270355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordjman K. M., Leingang K. A., James D. E., Mueckler M. M. Differential regulation of two distinct glucose transporter species expressed in 3T3-L1 adipocytes: effect of chronic insulin and tolbutamide treatment. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7761–7765. doi: 10.1073/pnas.86.20.7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallberg-Henriksson H., Holloszy J. O. Activation of glucose transport in diabetic muscle: responses to contraction and insulin. Am J Physiol. 1985 Sep;249(3 Pt 1):C233–C237. doi: 10.1152/ajpcell.1985.249.3.C233. [DOI] [PubMed] [Google Scholar]
- Wardzala L. J., Hirshman M., Pofcher E., Horton E. D., Mead P. M., Cushman S. W., Horton E. S. Regulation of glucose utilization in adipose cells and muscle after long-term experimental hyperinsulinemia in rats. J Clin Invest. 1985 Aug;76(2):460–469. doi: 10.1172/JCI111994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziel F. H., Venkatesan N., Davidson M. B. Glucose transport is rate limiting for skeletal muscle glucose metabolism in normal and STZ-induced diabetic rats. Diabetes. 1988 Jul;37(7):885–890. doi: 10.2337/diab.37.7.885. [DOI] [PubMed] [Google Scholar]