NK cells constitute an innate MHC class I-reactive lymphoid population that rapidly responds to infection, injury, or cell distress. In the transplant field, NK cells have most often been associated with pro-inflammatory immunity resulting in the exacerbation of allograft injury. Despite this general view of NK cell reactivity, it has been challenging to assign unambiguous obligate roles for NK cells in the allograft response. While recent reports continue to provide evidence supporting a role for NK cells in promoting both acute and chronic rejection, there are also a growing number of studies that illustrate an alternative role for NK cells in promoting allograft survival and tolerance. This review addresses the plasticity of NK responses in transplantation by suggesting specific ‘checkpoints’ whereby NK cells can either enhance or inhibit the allograft response in vivo.
Introduction
NK cells contribute a well-appreciated role in pro-inflammatory MHC class I-associated innate immunity. These cells are known to provide rapid reactivity to varied forms of injury including pathogen infection, cell transformation, and general cellular stress. NK cells interact with target cells through a complex integration of positive and negative signals, involving inhibitory classical MHC class I molecules and an array of activating ligands, many involving MHC class I-like molecules. Though an extensive description of this varied receptor and ligands is beyond the scope of the current discussion, there are a number of excellent and detailed recent reviews available that describe the structure and function of NK-associated receptors (1-3). Importantly, unlike antigen-specific CD8 T cells that recognize and are activated by peptide ligands presented by MHC class I molecules, NK cells utilize a sort of mirror image recognition system. That is, self MHC class I interactions delivery inhibitory signals to NK cells, forming the basis of the classical model of ‘missing self’ NK surveillance for unusual cells with reduced MHC expression (4). However, the lack of self MHC class I expression is not sufficient for the activation of NK cells. In addition, NK cells require the interaction with cell-surface activating ligands that are induced by a variety of stimuli including cell transformation, infection, and stress. Taken together, NK cells respond through a complex integration of these inhibitory and activating ligands expressed on host cells. The goal of this review is to highlight recent studies regarding the biology of NK cell contributions to allograft immunity and tolerance. Special emphasis will be placed on the increasingly appreciated and somewhat unexpectedly important role that NK cells can play in regulating the allograft response and in promoting tolerance induction.
Situations whereby NK cells promote graft rejection
Much of our current understanding of NK cell biology is derived from basic cellular analyses and from studies of infectious disease and tumor immunology. The elusive aspect of NK cells in transplantation is that there are very few studies that provide definitive evidence for a required role for NK cells in most cases allograft rejection. For example, the inhibition or elimination of NK cells rarely results in significantly prolonged allograft survival. Given the emerging interest in the role of NK cells in allograft tolerance (see below) it might be tempting to underestimate the contribution of NK cells to graft injury. However, a number of studies over the past few years continue to highlight the ability of NK cells to promote several types of graft injury or rejection. In transplantation, NK cells are perhaps best known for their ability to directly reject MHC mismatched bone marrow allografts, as illustrated by recent studies (5, 6). However, there are a few examples in which NK cells can play an essential role in organ or tissue allograft rejection. NK cells can be required for cardiac allograft rejection when the costimulatory molecule CD28 is either inhibited or genetically absent (7, 8). In another model, IL-15-driven NK reactivity can result in skin allograft rejection independent of T and B cell adaptive immunity (9). NK cells can also enhance skin allograft rejection by promoting CD4 T cell-dependent ‘indirect’ (host APC-dependent) alloantigen presentation (10•, 11•). This latter result may well be related to the ability of NK cells to kill MHC mismatched donor dendritic cells (DC) (12, 13•) and so ‘seed’ antigen to self APCs. NK cells are also associated with chronic allograft injury. In a semi-allogeneic mouse model of ‘hybrid resistance’, NK cells were shown to be required for chronic cardiac allograft vascular injury (14). A recent clinical study strongly suggests that NK cells contribute to antibody-mediated rejection in kidney allograft recipients (15••). Though there are varied means by which NK cells could contribute to such injury, an obvious role for NK cells in antibody-dependent responses could be through antibody-dependent cellular cytotoxicity (ADCC) triggered by antibody Fc receptor binding and activation of host NK cells (16). Taken together, NK cells clearly have the capacity to exacerbate multiple forms of allograft injury.
While NK cells can promote allograft rejection, probably the more unexpected findings over the past few years have focused on the roles that NK cells play in regulating the immune response, including a pronounced role in allograft tolerance in some cases. In a more general sense, it has become apparent that NK cells are not merely mono-thematic but rather demonstrate considerable functional plasticity in immunity. Below is outlined a proposed series of major ‘checkpoints’ whereby NK can either enhance or regulate the allograft response.
NK ‘checkpoints’ 1 and 2: Consequence of NK interactions with donor and host antigen-presenting cells, respectively
NK cells are well known for their reciprocal interactions with antigen-presenting cells, especially DCs (17, 18). NK cells demonstrate contact-dependent activation or inhibition of DCs (19) depending on the nature of the DC and the microenvironment. DCs in turn can rapidly induce IFN-g production and cytotoxic activity in NK cells (20), in part due to the production DC-derived IL-15 (21). NK cells are an early source of IFN-γ and can rapidly ‘license’ DCs resulting in DC maturation and the subsequent promotion of T cell activation (17). Such NK cell interactions with donor DC generally promote a Th1-like alloresponse (22); without such donor NK:DC encounters, the response can default to a more Th2-like response (23). However, while NK cells can sometimes drive the activation of immature DCs (24), there is an alternative outcome whereby DCs can directly kill immature or uninfected DC (17, 19, 25). It will continue to be important to clarify those signals that trigger positive (DC activation) versus negative (DC killing) outcomes of NK:DC interactions in the setting of allograft immunity.
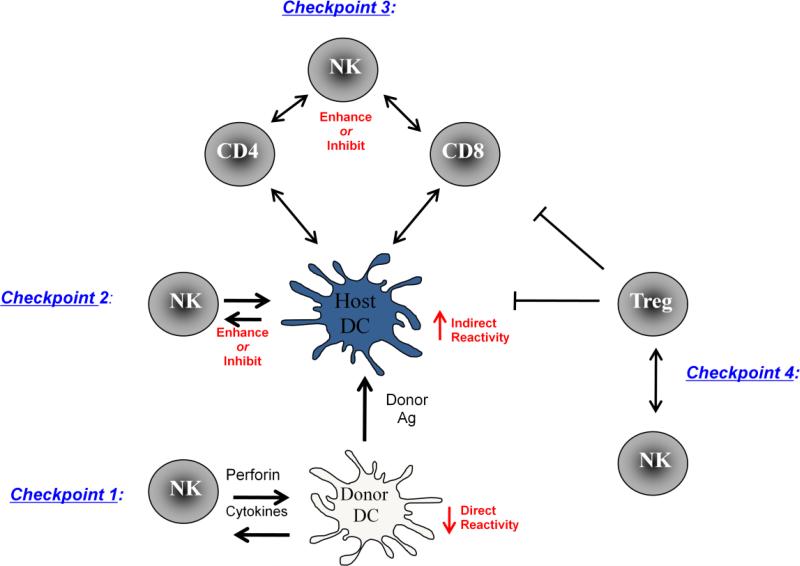
Most of the studies mentioned above involve NK interactions with self APCs, largely using infectious disease models. Over the past few years, the capacity of NK cells to kill donor-derived APCs has surfaced as a major pathway whereby NK cells can modify allograft reactivity and actually promote allograft tolerance rather than rejection. We had found that NK cells and perforin were surprisingly necessary for some forms of induced allograft tolerance (26). Soon thereafter, Yu et al made the important observation that host NK cells rapidly killed MHC-disparate allogeneic DCs (12). This DC killing appeared to be the direct result of a ‘missing self’ event since donor DCs expressing self MHC class I (i.e. host x donor F1 cells) were protected from such elimination. A more recent report connected these two studies by demonstrating the NK cells could mediate perforin-dependent killing of donor APC (13•). Thus, this initial NK:donor DC interaction represents a key primary ‘checkpoint’ in how NK cells can impact the allograft response (Figure 1). Importantly, rapid elimination of donor-type DCs would be expected to blunt the ‘direct’ (donor APC-dependent) allogeneic response that accounts for the dramatically high frequency of alloreactive T cells. Indeed, inhibition of NK cell responses can prevent donor DC elimination and actually enhance CD4 T cell alloreactivity (23).
Figure 1.
Major proposed checkpoints in NK cell-mediated impact on allograft immunity. As described in the text, we propose three key areas, or ‘checkpoints’ whereby NK cells positively or negatively impact T cell-dependent allograft immunity. (1) Checkpoint 1: NK cells can directly lyse donor DC resulting in blunted direct antigen (donor APC-dependent) presentation to host T cells and enhanced release of donor antigens acquired by host APC (indirect, or host-APC dependent presentation). (2) Checkpoint 2: NK cells can either augment or inhibit the capacity of host APC to direct donor-derived antigens to reactive T cell. (3) Checkpoint 3: NK cells can enhance or inhibit T cell reactivity through a direct interaction with activated T cells. (4) Checkpoint 4: NK cells and regulatory T cells (Treg) tend to cross-regulate one another to either promote or inhibit regulatory activity.
A secondary consequence of rapid killing of donor DC would be the enhanced availability of donor antigens to host-type APC. Recent studies illustrate this phenomenon by showing enhanced ‘indirect’ (host APC-dependent) reactivity as a result of a host NK response (10, 11). Although the alloreactive repertoire is numerically dominated by ‘direct’ allospecific T cells, NK cells responding to donor DC would serve to impact the qualitative nature of the allograft response by inhibiting the magnitude of the ‘direct’ response and diverting reactivity to promote ‘indirect’ reactivity (Figure 1). This leads to a second and potentially pivotal ‘checkpoint’ in allograft immunity whereby NK cells interact with host DC presenting donor-derived antigens. The fact that NK cells augment such ‘indirect’ donor reactive CD4 T cell responses implies that NK cells interact with host DC to promote this form of alloreactivity (10, 11•). Thus, NK cells interfacing with host DC presenting donor-derived antigens is likely to enhance graft-destructive T cell reactivity in a manner analogous with host reactivity to pathogens (17). If donor-derived DCs are eventually eliminated, then it is likely that host APC-dependent ‘indirect’ reactivity is likely to form the key branch point that dictates eventual allograft outcome. While indirect antigen presentation may certainly promote destructive allograft reactivity, this same pathway also appears to be essential for allograft tolerance induction (27, 28•, 29•). Furthermore, while regulation of alloreactivity by NK cells has largely focused on the ability of NK cells to either activate or kill DC and potentially reactive T cells, it will be important to determine if NK cells can exert other types of regulatory functions. A very intriguing recent study indicates that IL-10 producing NK cells can promote regulatory activity, suppressing host resistance to parasitic infection (30••). It will be interesting to determine if alternatively activated NK cells producing IL-10 can provide a cytokine-dependent route for regulating alloimmunity. Going forward, it will be important to clarify our understanding of the potential roles that NK cells play in modifying indirect donor antigen presentation.
NK ‘checkpoint’ 3: NK cell interactions with T cells
While NK:DC interactions have been a major focus of how NK cells mold the adaptive immune response, the ability of NK cells to directly interact with T cells is arguably an under-appreciated facet of NK cell biology. Thus, direct NK:T cell interactions form a third ‘checkpoint’ whereby NK cells can either enhance or regulate T cell alloreactivity (Figure 1). On one hand, NK cells may directly enhance T cell alloreactivity, such as by producing IFN-γ that promotes Th1-like T cell responses in a paracrine fashion (31, 32) and by direct contact via OX40-O40L interactions (33). However, there is an alternative outcome of NK-T cell interactions that can inhibit or eliminate activated T cells. T cells themselves can express activating or inhibitory NK ligands, rendering them amendable for direct NK cell recognition. On this point, it essential to emphasize that because NK cells do integrate activating and inhibitory signals on target cells, it is possible for NK cells to kill ‘stressed’ self target cells despite the expression of inhibitory self MHC class I molecules (34). A recent study showed that in persistent LCMV virus infection, reduction in T cell expression of the NK inhibitory ligand CD244 (2B4) can result in direct killing of CD8+ T cells by NK cells through a perforin-dependent process (35••). Interestingly, the direct NK cell-mediated killing of ‘stressed’ activated T cells was shown to be perforin-dependent (34, 35), consistent with our own results in vivo (26). Two more recent studies using mouse models of graft-versus-host-disease (GVHD) each suggest a role for NK cells in the direct inhibition of GVHD-inducing T cells (36, 37). In one case, results indicate that chronically activated CD4 T cells express ligands for the NK activating receptor NKG2D and that anti-NKG2D antibodies inhibited T cell elimination (36). It is intriguing to speculate that in the presence of some tolerance-inducing agents, alloreactive T cells may be inappropriately activated and express NK activating ligands, making them vulnerable to terminal ‘pruning’ by cytotoxic NK cells. Finally, in addition to a direct inhibition though cell killing, NK cells may also exert a more indirect role in controlling T cell reactivity. In an interesting recent study, NK cells were found to restrain CD8 T cell homeostatic expansion in the setting of lymphopenia, providing an additional non-antigen-specific means of NK cell-dependent T cell regulation (38•). Thus, NK cells have may have multiple routes of directly augmenting or inhibiting T cell immunity.
Checkpoint 4: Cross-regulation between NK cells and Tregs
The interaction between NK cells and regulatory T cells forms another ‘checkpoint’ of NK cell influence on allograft immunity and tolerance (Figure 1). Because NK cell activity has surprisingly been associated with some forms of allograft tolerance, it is at least plausible that NK cells might promote active regulatory T cell (Treg) activity. However, with rare exceptions (39, 40) this is not observed. Rather, there appears to be largely a cross-regulation between NK and Treg activities (at least Tregs defined by a CD4+CD25+Foxp3+ phenotype). Tregs can directly inhibit NK activity (41, 42) and a recent study found that depletion of regulatory cells resulted in pronounced pathogenic NK activity resulting in autoimmune pathology (43••). Conversely, activated NK cells can inhibit Treg induction (44) and, under some conditions of pathogen infection, NK cells can directly lyse regulatory T cells (45•), thus promoting protective effector T cell activity. Thus, this interplay between NK cells and Tregs can play a role in maintaining the balance between excessive regulation of immunity and over-exuberant immune reactivity. If such antagonistic interactions between NK cells and Tregs are generally the case, then how can NK cells promote allograft tolerance, a process that often relies on Treg function? At present, we would propose that NK cells do not primarily promote allograft tolerance through the direct induction of Tregs. Rather, by blunting APC and effector T cell activity as described above, other regulatory mechanisms may contribute to tolerance. That is, NK cells may not so much ‘induce’ tolerance, but rather simply inhibit destructive allograft immunity and so provide a more tolerance-permissive environment for other regulatory pathways to occur.
Conclusions
Clearly, the transplant field is experiencing an increasingly complex view of how NK cells impact the allograft response. A key point from this discussion is that NK cells exert a greater degree of plasticity that most previous views of allograft immunity would indicate. Specifically, NK cells are not simply innate cells that trigger a defined, programmed response leading to pro-inflammatory immunity. Rather, NK cells likely act at several checkpoints to influence graft outcome. It is highly possible that there are other key checkpoints of NK activity that were not outlined in Figure 1. For example, how NK cells directly interact over time with nonhematopoietic cells in the graft –such as with vascular endothelium and tissue parenchymal cells – may also prove to be important for dictating graft outcome. Also, finding more unexpected features of NK cells, such as their ability to produce regulatory cytokines such as IL-10 (30) and the capacity to demonstrate ‘adaptive’ properties (46•) continue to expand our view of NK cell function in vivo. Thus, rather than simply inhibit NK cells to promote allograft survival, it may be essential to consider how we can attenuate graft-destructive features of NK reactivity while preserving other regulatory properties of these cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Recommended Reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 2.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 3.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 5.Kean LS, Hamby K, Koehn B, Lee E, Coley S, Stempora L, et al. NK cells mediate costimulation blockade-resistant rejection of allogeneic stem cells during nonmyeloablative transplantation. Am J Transplant. 2006;6:292–304. doi: 10.1111/j.1600-6143.2005.01172.x. [DOI] [PubMed] [Google Scholar]
- 6.Hamby K, Trexler A, Pearson TC, Larsen CP, Rigby MR, Kean LS. NK cells rapidly reject allogeneic bone marrow in the spleen through a perforin- and Ly49D-dependent bu NKG2D-independent mechanism. Am J Transplant. 2007;7:1884–1896. doi: 10.1111/j.1600-6143.2007.01864.x. [DOI] [PubMed] [Google Scholar]
- 7.Maier S, Tertilt C, Chambron N, Gerauer K, Huser N, Heidecke C, et al. Inhibition of natural killer cells results in acceptance of cardiac allografts in CD28-/- mice. Nat Med. 2001;7:557–562. doi: 10.1038/87880. [DOI] [PubMed] [Google Scholar]
- 8.McNerney ME, Lee K-M, Zhou P, Molinero L, Mashayekhi M, Guzior D, et al. Role of natural killer cell subsets in cardiac allograft rejection. Am J Transplant. 2006;6:505–513. doi: 10.1111/j.1600-6143.2005.01226.x. [DOI] [PubMed] [Google Scholar]
- 9.Kroemer A, Xiao X, Degauque N, Edtinger K, Wei H, Demirci G, et al. The innate NK cells, allograft rejection, and a key role for IL-15. J Immunol. 2008;180:7818–7826. doi: 10.4049/jimmunol.180.12.7818. [DOI] [PubMed] [Google Scholar]
- 10 •.Ito A, Shimura H, Nitahara A, Tomiyama K, Ito M, Kanekura T, et al. NK cells contribute to the skin graft rejection promoted by CD4+ T cells activated through the indirect allorecognition pathway. Int Immunol. 2008;20:1343–1349. doi: 10.1093/intimm/dxn092. [An interesting study showing the NK cells can promote a graft-destructive ‘indirect’ allograft response. An important point here is that ‘indirect’ alloantigen presentation is not intrisically positive or negative for allograft outcome but rather depends on the consequence of this response.] [DOI] [PubMed] [Google Scholar]
- 11 •.Garrod KR, Liu FC, Forrest LE, Parker I, Kang SM, Cahalan MD. NK cell patrolling and elimination of donor-derived dendritic cells favor indirect alloreactivity. J Immunol. 2010;184:2329–2336. doi: 10.4049/jimmunol.0902748. [An important recent study that connects the concept that NK cells can kill donor-type APCs and in turn ‘seed’ antigen for indirect (host APC-dependent) alloreactivity.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu G, Xu X, Vu MD, Kilpatrick ED, Li XC. NK cells promote transplant tolerance by killing donor antigen-presenting cells. J Exp Med. 2006;203:1851–1858. doi: 10.1084/jem.20060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13 •.Laffont S, Seillet C, Ortaldo J, Coudert JD, Guery JC. Natural killer cells recruited into lymph nodes inhibit alloreactive T-cell activation through perforin-mediated killing of donor dedritic cells. Blood. 2008;112:661–671. doi: 10.1182/blood-2007-10-120089. [A study that connects the roles of NK cells, perforin activity, and donor DC killing highlighted by studies from reference numbers 11 and 26.] [DOI] [PubMed] [Google Scholar]
- 14.Uehara S, Chase CM, Kitchens WH, Rose HS, Colvin RB, Russell PS, et al. NK cells can trigger allograft vasculopathy: the role of hybrid resistance in solid organ allografts. J Immunol. 2005;175:3424–3430. doi: 10.4049/jimmunol.175.5.3424. [DOI] [PubMed] [Google Scholar]
- 15 ••.Hidalgo LG, Sis B, Sellares J, Campbell PM, Mengel M, Einecke G, et al. NK cell transcripts and NK cells in kidney biopsies from patients with donor-specific antibodies: evidence for NK cell involvment in antibody-mediated rejection. Am J Transplant. 2010;10:1812–1822. doi: 10.1111/j.1600-6143.2010.03201.x. [This is considered to be quite an important study correlating NK cells and related gene expression to clinical cases of antibody-mediated kidney allograft rejection.] [DOI] [PubMed] [Google Scholar]
- 16.Bonnema JD, Karnitz LM, Schoon RA, Abraham RT, Leibson PJ. Fc receptor stimulation of phosphatidylinositol 3-kinase in natural killer cells is associated with protein kinase C-independent granule release and cell-mediated cytotoxicity. J Exp Med. 1994;180:1427–1435. doi: 10.1084/jem.180.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Degli-Esposti MA, Smyth MJ. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat Rev Immunol. 2005;5:112–124. doi: 10.1038/nri1549. [DOI] [PubMed] [Google Scholar]
- 18 •.Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tisues. Nat Rev Immunol. 2002;2:957–264. doi: 10.1038/nri956. [Though this review was published several years ago, it is nevertheless considered an excellent discussion of this topic.] [DOI] [PubMed] [Google Scholar]
- 19.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–341. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 21.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenback A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mailliard RB, Son YI, Redlinger R, Coates PT, Giermasz A, Morel PA, et al. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J Immunol. 2003;171(5):2366–2373. doi: 10.4049/jimmunol.171.5.2366. [DOI] [PubMed] [Google Scholar]
- 23.Coudert JD, Coureau C, Guery JC. Preventing NK cell activation by donor dendritic cells enhances allospecific CD4 T cell priming and promotes Th Type 2 responses to transplantation antigens. J Immunol. 2002;169:2979–2987. doi: 10.4049/jimmunol.169.6.2979. [DOI] [PubMed] [Google Scholar]
- 24.Semino C, Angelini G, Piggi A, Rubartelli A. NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell actvation and release of DC maturation factor HMGB1. Blood. 2005;106:609–616. doi: 10.1182/blood-2004-10-3906. [DOI] [PubMed] [Google Scholar]
- 25.Ferlazzo G, Morandi B, D'Agostino A, Meazza R, Melioli G, Moretta A, et al. The interaction between NK cells and dendritic cells in bacterial infections results in rapid induction of NK cell activation and in the lysis of uninfected dendritic cells. Eur J Immunol. 2003;33:306–313. doi: 10.1002/immu.200310004. [DOI] [PubMed] [Google Scholar]
- 26.Beilke JN, Kuhl NR, Van Kaer L, Gill RG. NK cells promote islet allograft tolerance via a perforin-dependent mechanism. Nat Med. 2005;11:1059–1065. doi: 10.1038/nm1296. [DOI] [PubMed] [Google Scholar]
- 27.Yamada A, Chandraker A, Laufer TM, Gerth AJ, Sayegh MH, Auchincloss HJ. Cutting edge: recipient MHC class II expression is required to acheive long-term survival of murine cardiac allografts after costimulatory blockade. J immunol. 2001;167:5522–5526. doi: 10.4049/jimmunol.167.10.5522. [DOI] [PubMed] [Google Scholar]
- 28 •.Luo X, Pothoven KL, McCarthy D, DeGutes M, Martin A, Getts DR, et al. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proc Natl Acad Sci (USA) 2008;105:14527–14532. doi: 10.1073/pnas.0805204105. [Along with reference 29 this study provides important recent evidence that ‘indirect’ allograft reactivity plays an important role in allograft tolerance induction.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29 •.Tsang JY, Tanriver Y, Jiang S, Xue S, Ratnasothy K, Chen D, et al. Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J Clin Invest. 2008;118:3619–2628. doi: 10.1172/JCI33185. [Along with reference 28 this study provides important recent evidence that ‘indirect’ allograft reactivity plays an important role in allograft tolerance induction.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30 ••.Maroof A, Beattie L, Zubairi S, Svensson M, Stager S, Kaye PM. Postranscriptional regulation of Il10 gene expression allows natural killer cells to express immunoregulatory function. Immunity. 2008;29:295–305. doi: 10.1016/j.immuni.2008.06.012. [Very important study showing the capacity of IL-10 producing NK to cells to regulate T cell dependent immunity.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5(12):1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 32.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat Immunol. 2005;6(6):600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 33.Zingoni A, Sornasse T, Cocks BG, Tanaka Y, Santoni A, Lanier LL. Cross-talk between activated human NK cells and CD4+ T cells via OX40-OX40 ligand interactions. J Immunol. 2004;173:3716–3724. doi: 10.4049/jimmunol.173.6.3716. [DOI] [PubMed] [Google Scholar]
- 34.Rabinovich BA, Shannon J, Su RC, Miller RG. Stress renders T cell blasts sensitive to killing by activated syngeneic NK cells. J Immunol. 2000;165(5):2390–2397. doi: 10.4049/jimmunol.165.5.2390. [DOI] [PubMed] [Google Scholar]
- 35 ••.Waggoner SN, Taniguchi RT, Mathew PA, Kumer V, Welsh RM. Absence of mouse 2B4 promotes NK cell-mediated killing of activated CD8+ T cells, leading to prolonged viral persistence and altered pathogenesis. J Clin Invest. 2010;120:1925–1939. doi: 10.1172/JCI41264. [This is considered a very interesting study and demonstrates a pronounced role for T cell expression of a specific NK inhibitory ligand to prevent NK recognition. This report provides additional evidence that T cell expression of NK activating and/or inhibitory ligands can be an important ‘checkpoint’ of direct T cell regulation by NK cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rivas MN, Hazzan M, Weatherly K, Gaudray F, Salmon I, Braun MY. NK cell regulation of CD4 T cell-mediated graft-versus-host disease. J Immunol. 2010;184:6790–6798. doi: 10.4049/jimmunol.0902598. [DOI] [PubMed] [Google Scholar]
- 37.Olson JA, Levenson-Gower D, Gill S, Baker J, Beilhack A, Negrin RS. NK cells mediate reduction of GVHD by inhibitin activated, alloreactaive T cells while retaining GVL effects. Blood. 2010;115:4293–4301. doi: 10.1182/blood-2009-05-222190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38 •.Zecher D, Li Q, Oberbarnscheidt MH, Demetris AJ, Shlomchik WD, Rothstein DM, et al. NK cells delay allograft rejection in lymphopenic hosts by downregulating the homeostatic proliferation of CD8+ T cells. J Immunol. 2010;184:6649–6657. doi: 10.4049/jimmunol.0903729. [An intriguing study suggesting that NK cells may have a general role in CD8 T cell homeostasis.] [DOI] [PubMed] [Google Scholar]
- 39.Jinushi M, Takehara T, Tatsummi T, Yamaguchi S, Sakamori R, Hiramatsu N, et al. Natural killer cell and hepatic cell interaction via NKG2A leads to dendritic cell-mediated induction of CD4+CD25+ T cells with PD-1-dependent regulatory activities. Immunology. 2007;120:73–82. doi: 10.1111/j.1365-2567.2006.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vacca P, Cantoni C, Vitale M, Prato C, Canegallo F, Ragni N, et al. Crosstalk between decidual NK and CD14+ myelomonocytic cells results in induction of Tregs and immunosupppression. Proc Natl Acad Sci. 2010;107:11918–11923. doi: 10.1073/pnas.1001749107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions ian a transforming growth factor-b-dependent manner. J Exp Med. 2005;202:1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terme M, Chaput N, Combadiere B, Ma A, Ohteki T, Zitvogel L. Regulatory T cells control dendritic cell/NK cell cross-talk in lymph nodes at the steady state by inhibiting CD4+ self-reactive T cells. J Immunol. 2008;(180):4679–4686. doi: 10.4049/jimmunol.180.7.4679. [DOI] [PubMed] [Google Scholar]
- 43 ••.Feuerer M, Shen Y, Littman DR, Benoist C, Mathis D. How punctual ablation of regulatory T cells unleashes an autoimmune lesion within the pancreatic islets. . Immunity. 2009;31:654–664. doi: 10.1016/j.immuni.2009.08.023. [Though not a transplantation study, this report demonstates a dramatic role for Tregs in controlling NK activity. By eliminating Tregs, NK cells are ‘unleashed’ to initiate rapid islet injury and promote autoimmune diabetes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brillard E, Pallandre J, Chalmers D, Ryffel B, Radlovic A, Seilles E, et al. Natural killer cells prevent CD28-mediated Foxp3 transcription in CD4+CD25- T lymphoytes. Exp Hematol. 2007;(35) doi: 10.1016/j.exphem.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 45.Roy S, Barnes PF, Garg A, Wu S, Cosman D, Vankayalapati R. NK cells lyse T regulatory cells that expand in response to an intracellular pathogen. J Immunol. 2008;180:1729–1736. doi: 10.4049/jimmunol.180.3.1729. [DOI] [PubMed] [Google Scholar]
- 46 •.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [An interesting and potentially very important study indicating that NK cells can actually demonstrate a degree of memory-like features and so overalp with activities normally ascribed to the adaptive immune system. Though the relevance of this study to transplantation is not yet clear, this study greatly impacts our current paradigms regarding NK cell biology.] [DOI] [PMC free article] [PubMed] [Google Scholar]