Abstract
Transmission of antimicrobial drug resistance from resistant bacteria to non-resistant strains is an important public health issue. In this study, we have examined the possibility of multiple resistance gene transfer between Escherichia coli and Salmonella in the natural setting. Bacteria isolated from calves concurrently shedding E. coli and Salmonella showed similar antimicrobial drug resistance patterns as measured by a broth dilution method. However, microarray analysis of the antibiotic resistance at the gene level revealed several differences in resistance gene profile. Resistance profiles of E. coli isolated from different farms were closer than the profile of E. coli and Salmonella isolated from the same farm. This shows that the chance of multiple resistance gene transfers between these species is unlikely.
Keywords: Salmonella, Escherichia coli, Microarray, antibiotic resistance
Introduction
Increased prevalence of antibiotic resistant bacteria is a cause of concern for human and animal health. It is widely accepted that the use of antibiotics either for medical or agricultural purposes selects for resistant bacteria[1, 2]. Many drug resistance genes reside on self-transmissible genetic elements like conjugative plasmids and transposons, which enable the transmission of resistance to other species[3, 4]. The presence of antibiotics, even in subinhibitory concentrations, may select for resistant bacteria harboring such elements, which then have the potential to disseminate resistance to susceptible strains. Once acquired, these antibiotic resistance genes are slow to be lost even in the absence of selecting antibiotic[5, 6]. This along with transfer of resistance determinants to unrelated species, makes it a serious threat to both human and animal health. For example, evidence of extensive interspecies transfer of integron mediated antimicrobial resistance genes among multidrug-resistant enterobacteriaceae has been reported [7]. Similarly, transfer of beta-lactam resistance between Klebsiella pneumoniae and E. coli in clinical settings is also known [8]. These examples of resistance transfer between relatively distantly related species raise the possibility of much more frequent exchange of resistance determinants between closely related bacterial species. For example, E. coli and Salmonella are closely related members of enterobacteriaceae sharing a common ancestor that diverged roughly 150 million years ago[9]. In vitro experiments have demonstrated that ampicillin resistance could be transferred from Salmonella enterica serovar Typhimurium DT104 to E. coli K12[10]. However such studies do not accommodate the complexity of microbial systems in nature under which such transfers take place. High-throughput technologies like microarrays are being increasingly used for the detection and typing of their virulence factors and antibiotic resistance profile[11–13]. In this study, to investigate the possibility of multiple antibiotic resistance gene transfer between E. coli and Salmonella we have used microarrays to analyze the antimicrobial resistance and virulence gene profiles in E. coli and Salmonella isolated from bovine fecal samples from dairy farms that had concurrent shedding of both bacterial species.
Materials and Methods
Sample collection, isolation and identification of E. coli and Salmonella
To test the possibility that there could be a large scale transfer of antibiotic resistance genes between closely related species, we analyzed the antibiotic and virulence gene profile of E. coli and Salmonella isolated from bovine fecal samples with concurrent shedding of these two species. The samples (total 97 isolates) analyzed in this study were selected from a total number of 2088. Starting from September 2003, samples were collected over a period of 15 months as part of a milk replacer study from non-organic dairy farms from Michigan [14]. The antibiotic profile of isolates on the phenotypic level was analyzed using MIC testing and on the genotypic level using microarrays.
Farm selection for collection of fecal samples, selection of animals for testing, sample collection and laboratory methods for E. coli and Salmonella isolation and identification were described by Kaneene et al [14]. The details of the strains used in this study are given in Appendix 1.
| Strain ID | Organism | Strain | Serotype |
|---|---|---|---|
| 6129 | Escherichia coli | E.coli 80-53 | NA |
| 6130 | Escherichia coli | E.coli 104-69 | NA |
| 6131 | Escherichia coli | E.coli 77-67 | NA |
| 6132 | Salmonella | Sal 2-3 | reading |
| 6133 | Salmonella | Sal 3-21 | bovis-morbificans |
| 6134 | Salmonella | Sal 2-19 | bovis-morbificans |
| 6135 | Escherichia coli | E.coli 33-57 | NA |
| 6136 | Salmonella | Sal 3-77 | bovis-morbificans |
| 6137 | Escherichia coli | E.coli 26-9 | NA |
| 6138 | Salmonella | Sal 8-5 | oranienburg |
| 6139 | Escherichia coli | E.coli 101-21 | NA |
| 6140 | Escherichia coli | E.coli 28-25 | NA |
| 6141 | Salmonella | Sal 2-49 | bovis-morbificans |
| 6143 | Escherichia coli | E.coli 105-29 | NA |
| 6146 | Escherichia coli | E.coli 85-41 | NA |
| 6147 | Escherichia coli | E.coli 38-11 | NA |
| 6148 | Escherichia coli | E.coli 35-47 | NA |
| 6149 | Escherichia coli | E.coli 87-75 | NA |
| 6150 | Escherichia coli | E.coli 87-37 | NA |
| 6151 | Escherichia coli | E.coli 110-59 | NA |
| 6152 | Escherichia coli | E.coli 11-13 | NA |
| 6153 | Escherichia coli | E.coli 112-71 | NA |
| 6154 | Escherichia coli | E.coli 86-33 | NA |
| 6158 | Escherichia coli | E.coli 32-63 | NA |
| 6159 | Escherichia coli | E.coli 101-15 | NA |
| 6160 | Salmonella | Sal 9-53 | oranienburg |
| 6161 | Salmonella | Sal 3-63 | bovis-morbificans |
| 6162 | Salmonella | Sal 2-67 | oranienburg |
| 6163 | Escherichia coli | E.coli 81-9 | NA |
| 6164 | Salmonella | Sal 2-67 | bovis-morbificans |
| 6165 | Escherichia coli | E.coli 109-65 | NA |
| 6166 | Salmonella | Sal 4-59 | oranienburg |
| 6168 | Salmonella | Sal 3-41 | enteritidis |
| 6169 | Salmonella | Sal 9-53 | oranienburg |
| 6170 | Salmonella | Sal 4-79 | oranienburg |
| 6174 | Escherichia coli | E.coli 38-59 | NA |
| 6175 | Escherichia coli | E.coli 95-31 | NA |
| 6176 | Escherichia coli | E.coli 93-27 | NA |
| 6177 | Escherichia coli | E.coli 99-75 | NA |
| 6178 | Escherichia coli | E.coli 92-47 | NA |
| 6179 | Escherichia coli | E.coli 38-29 | NA |
| 6180 | Escherichia coli | E.coli 9-15 | NA |
| 6181 | Escherichia coli | E.coli 39-67 | NA |
| 6182 | Escherichia coli | E.coli 39-73 | NA |
| 6183 | Escherichia coli | E.coli 115-9 | NA |
| 6184 | Escherichia coli | E.coli 116-75 | NA |
| 6185 | Escherichia coli | E.coli 4-11 | NA |
| 6186 | Escherichia coli | E.coli 96-15 | NA |
| 6187 | Escherichia coli | E.coli 115-27 | NA |
| 6188 | Escherichia coli | E.coli 4-13 | NA |
| 6264 | Escherichia coli | E.coli 121-51 | NA |
Antimicrobial susceptibility testing
Commercially prepared microbroth dilution antimicrobial panels, with a prepared range of antibiotics were used for antimicrobial susceptibility testing for E. coli and Salmonella (Trek Diagnostics, CMV7CNCD)h. For all plates, E. coli ATCC 25922 was used as quality control (QC). QC results were reviewed with each batch of tests run and if these were not within acceptable limits, all tests were re-run. All QC results were in expected range for results reported here. Except for ceftiofur and streptomycin, isolates were classified as resistant based on Clinical Laboratory Standards Institute (CLSI) break points.
Microarray development, hybridization and data analysis
Previously we have reported development of microarray for molecular typing of Salmonella serovars [13] and for differentiation of E. coli pathotypes [12]. These two arrays contained probes (70mers) designed using genus specific, species specific and virulence genes of E. coli pathotypes and Salmonella serovars. In this study we pooled all the probes from these two arrays and added another 114 probes designed on different classes of antimicrobial resistance genes. This new array contains a total of 891 probes and in addition to detecting the virulence genes, could also detect the antimicrobial resistance profile of the strains. The complete details of the probes and genes represented in the array is given in Appendix 2. DNA isolation for microarray, microarray printing, slide processing, hybridization, scanning and data analysis was carried out as per protocols described by Scaria et al [13] and each experiment was carried out in duplicate.
| Probe name | Gene name |
|---|---|
| eco1 | F165F |
| eco3 | focF |
| eco4 | focG |
| eco5 | focH |
| eco6 | afaD1 |
| eco7 | focC |
| eco8 | focD |
| eco9 | fsoE |
| eco10 | fimB |
| eco12 | rfc |
| eco13 | afaD |
| eco14 | iucD=AerA |
| eco15 | hlyA |
| eco16 | hlyC |
| eco17 | papG1 |
| eco18 | papGII |
| eco19 | traT |
| eco20 | drbE122 |
| eco21 | cdtC |
| eco22 | afaE -5 |
| eco23 | kpsM |
| eco24 | nfaE |
| eco25 | cnF1 |
| eco26 | cdtA |
| eco27 | papC |
| eco28 | papE |
| eco29 | papG3 |
| eco30 | cdtB |
| eco31 | cvi |
| eco32 | cnf2 |
| eco33 | kfiB |
| eco34 | afaC |
| eco35 | afaB nfaE (S61968) |
| eco36 | usp |
| eco37 | F165Afimbriae |
| eco37 | focG |
| eco38 | F165G |
| eco39 | F165F |
| eco40 | hlyB |
| eco41 | hlyD |
| eco42 | papX |
| eco43 | papF |
| eco44 | papK |
| eco45 | papJ |
| eco46 | papH |
| eco47 | papA |
| eco48 | papI |
| eco49 | sat |
| eco54 | papH_2 |
| eco56 | senB |
| eco57 | senA |
Results
Antimicrobial resistance profiles from MIC data
A total of 61 E. coli and 36 Salmonella isolates were subcultured to pure culture from samples collected on three farms. For both the species, resistance to multiple antibiotics was observed (Table 1). All the E. coli strains were resistant to streptomycin. 95% of the strains were resistant to tetracycline. This was followed by 91% and 47% resistance to kanamycin and ampicillin respectively. All the strains were susceptible to amikacin, ciprofloxacin and nalidixic acid. Except for four isolates all other isolates were susceptible to all other antibiotics tested (Fig.1).
Table 1.
Common antimicrobial resistance patterns of Salmonella and E. coli isolated from calves shedding these bacteria concurrently. Antibiotic names are abbreviated as follows; Amp- Ampicillin, Amox- AmoxClav, Cef – Ceftriaxone, Cefo – Cefoxitin, Ceft- Ceftiofur, Cep – Cephalothin, Chl – Cholamphenicol, Gen- Gentamycin, Kan – Kanamycin, Strep – Streptomycin, Sulf- Sulfamethoxazole, Tet – Tetracycline, Tri- Trimethoprim
| Resistance pattern | Number of Isolates |
Organism |
|---|---|---|
| Amp, Kan, Strep, Sulf, Tet | 17 | E. coli |
| Gen, Kan, Strep, Tet | 2 | E. coli |
| Amox, Amp, Ceft, Cep, Cef, Cefo, Gen, Kan, Strep | 3 | E. coli |
| Kan, Strep, Sulf | 13 | E. coli |
| Kan, Strep, Sulf, Tet | 7 | E. coli |
| Kan, Strep, Sulf, Tet, Tri | 2 | E. coli |
| Amp, Amox, Ceph, Chl, Gen, Kan, Strep, Sulf, Tet, Ceft | 16 | Salmonella |
| Amp, Kan, Strep, Tet | 10 | Salmonella |
| Amp, Amox, Kan, Strep, Tet | 1 | Salmonella |
| Non Resistant | 7 | Salmonella |
| Ungrouped | 19 |
E.coli, Salmonella |
Fig. 1.
Heat map representation of MIC data for all E. coli (panel A) and Salmonella (panel B) strains tested. Column labels represent the antibiotic and row label represent each strain the color scheme is as follows: red – susceptible, green – resistant, black – Not interpreted.
While E. coli showed a diverse resistance pattern, Salmonella isolates showed a more skewed resistance pattern. All Salmonella isolates were susceptible to amikacin, ciprofloxacin and nalidixic acid. While 95% of E. coli isolates showed to tetracycline, streptomycin and kanamycin, only 80% of Salmonella isolates exhibited resistance to these antibiotics. The remaining 6 were susceptible to all antibiotics tested. In contrast to the 47% resistance shown in E. coli for ampicillin, all Salmonella except for the six universally susceptible isolates were resistant. Multidrug resistance in Salmonella was much more common than E. coli isolates. 33% of Salmonella isolates were resistant to ten out of sixteen antibiotics tested. The heat map representation of MIC data for E. coli and Salmonella is given in Fig.1.
Antimicrobial resistance profile of the isolates in the gene level
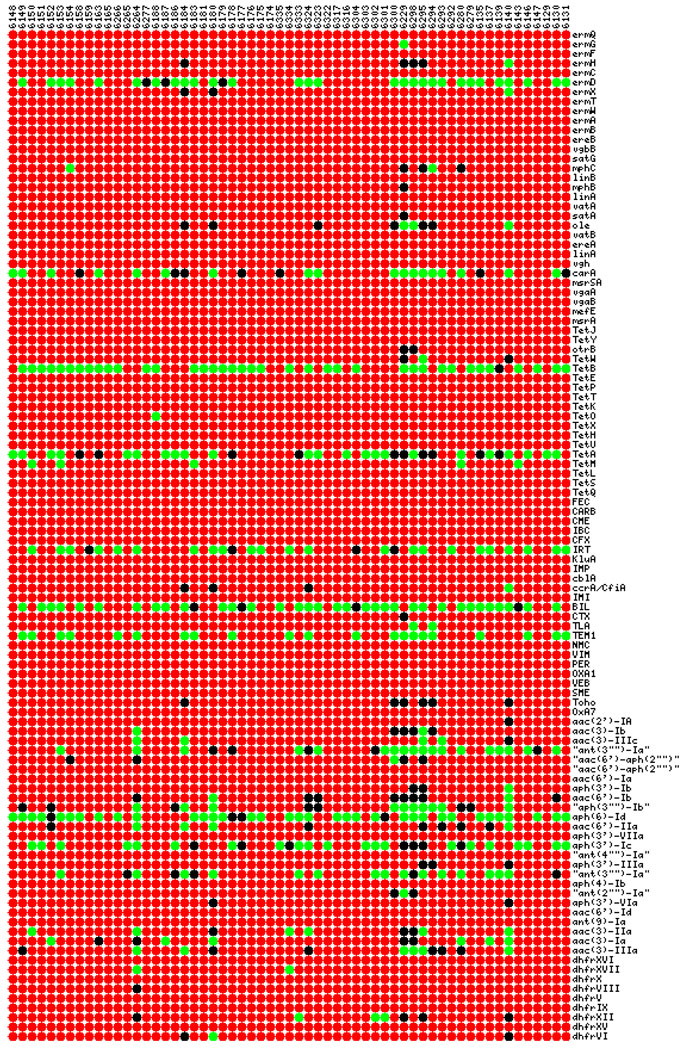
The array used in this work contained probes targeting most prevalent antibiotic resistance genes belonging to β-lactams, tetracyclines, aminoglycosides, macrolides and trimethoprim allowing higher resolution of resistance gene detection than MIC testing. In the macrolide class of resistance genes, ermD was the most prevalent gene present in E.coli (49%) and Salmonella (58%). Other genes in this class that were present in more than one E. coli isolate were oleB and mphC (<3%). One Salmonella isolate (#6170) was positive for most of the genes in all classes of antibiotics. If this isolate is excluded, all other Salmonella isolates were negative for all other macrolide genes. In the β-lactam category, BIL(55%), IRT(38%), TEM1(35%) and carA (30%) were most prevalent genes in E. coli isolates. Salmonella isolates also showed similar trend for these genes; BIL(47%), IRT(50%) and TEM1(36%). However, the striking difference was that all Salmonella isolates except for the highly resistant strain was negative for carA gene. Among tetracyclines, both species although showed similar trend in MIC level, the gene level pattern was strikingly different. Prevalent genes in E. coli for tetracycline’s were tetA(38%) and tetB(64%). tetA(80%) was prevalent in Salmonella with tetB being a rare instance; tetM was frequent (30%) in Salmonella isolates, but absent in E. coli. Among aminoglycoside resistance genes aph(6)-Id (78%), aph(3’)-Ic (63%) and ant(3”)-Ia”(8%) was most common in Salmonella. Pattern for aminoglycoside’s in E. coli was similar but more diverse; aph(6)-Id(60%), aph(3’)-Ic(35%), ant(3”)-Ia”(30%), aph(3’)-Ib(17%), aac(6’)-IIa(7%), aac(3)-IIIa(10%) and aac(3)-Ia(10%). Most common trimethoprim resistance gene was hdfrXII in both Salmonella (33%) and E. coli (5%). However, E. coli isolates contained another additional trimethoprim gene hdfrXV in equal frequency to that of hdfrXII which was absent in Salmonella isolates. A heat map representation of full antibiotic resistance gene profile of all E. coli and Salmonella isolates is given in Fig. 2 and Fig. 3, respectively. This profile, although very similar to that of MIC data shows striking differences. Most prominent genes that were present in E. coli but absent in Salmonella isolates were carA (β-lactam resistance, 30%), tetB (tetracycline resistance, 64%) and aac(3)-IIIa (10%). mefE gene conferring resistance to macrolides was very common in Salmonella (72%) but was absent in all E. coli isolates. Another difference between MIC data and microarray profile was the detection of resistance genes in the strains where MIC data showed susceptibility to all antibiotics. Microarray results were positive for several genes for the following strains which showed no resistance at MIC level; 6133 (ermD, tetA, BIL), 6134 (mefE, tetA, TEM, aph(6)-ID, aph(3)-Ic), 6136 (ermD, mefE, tetA, IRT, TEM1, aph(6)-Id, aph(3)-Ic), 6141 (ermD), mefE, tetA) and 6278(mefE, tetA, IRT, aph(6)-ID, aph(3)-Ic). However the strain 6161 was negative for all genes in array and was susceptible to all antibiotics at MIC level.
Fig. 2.
Heat map representation of microarray data for antibiotic resistance genes of all E. coli strains. Column labels represent the strains and row label represent genes. The color scheme is as follows: red – gene absent, green – gene present, black – uncertain.
Fig. 3.
Heat map representation of microarray data for antibiotic resistance genes of all Salmonella strains. Column labels represent the strains and row label represent genes. The color scheme is as follows: red – gene absent, green – gene present, black – uncertain.
Hierarchical clustering (HCL) of strains was performed with Manhattan median method as the distance matrix (Fig.4). The clustering pattern obtained did not show any correlation between location of farm and genetic resistance profile. Rather all E. coli isolates tended to cluster together while Salmonella isolates formed separate clusters (Fisher exact test p-value < 0.0001). When considering E. coli and Salmonella together, there was an association between farm and cluster (p-value =0.002). However, for E. coli alone, there was no significant association (p-value = 0.2). For Salmonella alone there was a significant association (p-value < 0.0001). This is consistent with E. coli cluster types being more evenly distributed among the farms while some Salmonella cluster types tended to be more farm specific. This shows that the resistance profiles of all E. coli isolates from different farms are closer than that of the profile of an E. coli and Salmonella isolated from the same farm. Different clustering methods did not alter the cluster configuration significantly.
Fig. 4.
Hierarchical Clustering of Salmonella and E. coli isolates based on antibiotic resistance gene profile. Genes were clustered using Manhattan median method as the distance matrix with average linkage rule. Red areas = gene absence, grey areas = uncertain, green areas = gene presence. F1E = Farm1 E. coli, F2E = Farm2 E. coli, F3E = Farm 3 E. coli, F1S = Farm 1 Salmonella, F2S = Farm 2 Salmonella, F3S = Farm 3 Salmonella.
Analysis of virulence genes
Analyzing the genetic relatedness of the isolates would be a better indicator of the strain dispersion and this could reveal clonality. When samples were hierarchically clustered based on array results from virulence genes, all the clusters formed contained isolates from all farms, indicating that these are not clonal and there is widespread strain dispersion (Fig.5). The only instance of samples from one farm forming a sub-cluster within a main cluster was observed with the group of samples 6318, 6291, 6166, 6582, 6162. These samples had also formed one tight cluster in HCL tree of antibiotic resistance genes. However, for all these samples gene variability was detected for bcf fimbrial genes, sth fimbrial genes, fels elements, phage-5 genes and among genes in SPI-3 region. This variability in multiple loci is a clear evidence for the fact that none of these isolates were clonal.
Fig. 5.
Hierarchical Clustering of E. coli isolates based on Virulence gene profile. Genes were clustered using Manhattan median method as the distance matrix with average linkage rule. Genes were clustered using Pearson absolute method as the distance matrix with average linkage rule. Red areas = gene absence, grey areas = uncertain, green areas = gene presence. F1 = Farm 1, F2 = Farm 2 and F3 = Farm 3.
The probes for E. coli genes also included common genes and virulence genes from different pathotypes. When HCL tree based on these was built for the E. coli isolates (Fig. 6), the trend was similar to that of the Salmonella. All the clusters formed were containing isolates from all the farms, again indicating that there is a large-scale dispersion of these strains. Also, there was a large number of variability with respect to the virulence gene content.
Fig. 6.
Hierarchical Clustering of Salmonella isolates based on virulence gene profile. Genes were clustered using Manhattan median method as the distance matrix with average linkage rule. Genes were clustered using Pearson absolute method as the distance matrix with average linkage rule. Red areas = gene absence, grey areas = uncertain, green areas = gene presence. F1 = Farm 1, F2 = Farm 2 and F3 = Farm 3.
Discussion
The global rise in antimicrobial resistance is not new, but the number of resistant species, the geographical locations affected and the increase in multidrug resistant bacteria is cause for concern [5]. One of the major reasons for the large scale dissemination of resistance is the horizontal transfer of resistance genes between closely related and even to unrelated bacterial species. The continuous presence of antibiotic in an ecological niche promotes the selection of resistant forms and then the possibility of resistant genes being transferred from the resistant bacteria to susceptible bacteria is very high. The large-scale agricultural use of antibiotics is such an example[15]. Reviews have suggested that of the 22.7 million Kg of antibiotics produced in United States, approximately 17.8% were used in animal production[16]. This point to the possibility of selection and dissemination of resistance genes between closely related species like Salmonella and E. coli in agricultural settings. To test this possibility, we analyzed the antimicrobial resistance profiles of E. coli and Salmonella isolates from dairy farms in Michigan. Farms were initially selected based on using oxytetracycline and neomycin in milk replacer fed to calves and the presence of tetracycline-resistant enteric bacteria. Use of these antibiotics in calf feed may select for the resistant forms of bacteria. Farm selection for collection of fecal samples was previously described [14]. All the isolates of both species were susceptible to amikacin, ciprofloxacin and nalidixic acid. Almost absolute resistance was shown for tetracycline, kanamycin and streptomycin. There were several mutlidrug resistant strains. Previous surveys of antimicrobial resistance in E. coli and Salmonella from dairy farms in USA reported mixed levels of resistance [17–20]. However, the most prevalent resistance was seen for tetracycline, streptomycin and ampicillin [19, 20] which is in consensus with our results. Analysis of MIC data showed that the overall resistance patterns for the two species were similar. A Similar conclusion was drawn after a study of E. coli and Salmonella isolates from 21 states in the US [19]. Just the phenotypic level of analysis of resistance alone would then support the theory of large-scale resistance gene transfer between E. coli and Salmonella. Although MIC testing shows the phenotypic and expressed levels of resistance, it does not provide any information about the underlying genes responsible for the resistance. Microarrays provide an excellent means of detecting this in a high throughput manner. The occurrence profile of ermD (macrolide resistance), BIL, IRT, TEM1, carA (all conferring β-lactam resistance), tetA (tetracycline resistance), aph(6)-Id,, aph(3’)-Ic) and ant(3””)-Ia(aminoglycoside resistance) genes were similar in E. coli and Salmonella. Based on such similarities, other studies have indicated the possibility of gene transfer between E. coli and Salmonella [21]. Although the resemblance in the gene profiles supported the possibility of gene exchange, the possibility of large scale transfers appeared less likely due to the differences antibiotic gene profiles detected. Most striking differences were the presence of carA, tetB and aac(3)-IIIa genes in E. coli but absent in Salmonella. mefE (macrolide resistance) which was present in 72% of Salmonella was absent in all E. coli isolates. If there was large-scale gene transfer between these two species, then it would be expected that at least one of these high prevalence genes in one species would have been transferred to the other. The three major mechanisms by which bacteria acquire genes are conjugation, transformation and transduction. However, several barriers of gene transfer even between closely related bacteria like E. coli and Salmonella is shown to be operational[22]. These barriers include surface exclusion, restriction-modification system and mismatch-repair [23]. Surface exclusion is a barrier to conjugation and is the major mechanism that prevents conjugation between unrelated species. It has been shown that mismatch-repair is major barrier to recombination between E. coli and Salmonella[22]. The success of antibiotic resistance gene transfer between these species then would depend on mechanism of exchange. For example, conjugative transfer of donor plasmid from Salmonella enterica serovar Typhimurium DT104 strain to a recipient Escherichia coli K12 strain has demonstrated [10]. Such plasmid mediated mechanisms could account for the similarities in resistance gene profile found in our study. Since we used total DNA from isolates for microarray, the difference between a plasmid and genomic DNA mediated mechanism could not be delineated. Another common route of multiple antibiotic resistance gene transfer are integrons[24]. Integrons provide bacteria with a gene-capture system perfectly adapted for the challenges of multiple-antibiotic treatment regimes[25]. However, since array used in this study did not contain probes for integrons or integron associated genes, we could not ascertain the possibility of involvement of integron mediated resistance gene transfer.
Evidence against large scale gene transfer also come from the HCL tree constructed based on the antibiotic gene profile of Salmonella and E. coli. In all the clusters formed, all E. coli grouped together while other clusters were composed of Salmonella only. Had there been large-scale gene transfers between them, E. coli and Salmonella from one farm would have had similar profiles and would have formed clusters based on origin of isolation. The HCL tree constructed based on common and virulence genes shows that there is widespread dissemination of strains across the farms and the strains are not clonal.
When compared to MIC data, use of microarray yielded higher resolution of resistance gene detection. For instance, the samples 6133, 6134, 6136, 6141 and 6278 showed no resistance at MIC level, but showed the presence of resistance genes in microarray analysis, indicating the possibility of strains harboring these genes but not expressing them. Thus this study shows that microarray could be used as a complimentary tool MIC testing to extract additional information like presence of silent resistance genes and the genetic relatedness of stains. This study also suggests that the rate of dissemination of resistant E. coli or Salmonella to different geographical locations is greater than the rate of resistance gene exchange between them.
Acknowledgements
This project was supported with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institute of Health, Department of Health and Human Services under contract, N01-AI-30054, Project No. ZC002-03, the Federal Formula Fund from the Cornell University Agricultural Experiment Station,
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walton J. In vivo transfer of infectious drug resistance. Nature. 1966;211:312–313. doi: 10.1038/211312a0. [DOI] [PubMed] [Google Scholar]
- 2.Roberts MC. Tetracycline resistance determinants: mechanisms of action, regulation of expression, genetic mobility, and distribution. FEMS Microbiol Rev. 1996;19:1–24. doi: 10.1111/j.1574-6976.1996.tb00251.x. [DOI] [PubMed] [Google Scholar]
- 3.Oyarzabal OA, Rad R, Backert S. Conjugative transfer of chromosomally encoded antibiotic resistance from Helicobacter pylori to Campylobacter jejuni. J Clin Microbiol. 2007;45:402–408. doi: 10.1128/JCM.01456-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sedgley CM, Lee EH, Martin MJ, Flannagan SE. Antibiotic resistance gene transfer between Streptococcus gordonii and Enterococcus faecalis in root canals of teeth ex vivo. J Endod. 2008;34:570–574. doi: 10.1016/j.joen.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med. 2004;10:S122–S129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 6.Pallecchi L, Bartoloni A, Paradisi F, Rossolini GM. Antibiotic resistance in the absence of antimicrobial use: mechanisms and implications. Expert Rev Anti Infect Ther. 2008;6:725–732. doi: 10.1586/14787210.6.5.725. [DOI] [PubMed] [Google Scholar]
- 7.Leverstein-van Hall MA, Box AT, Blok HE, Paauw A, Fluit AC, Verhoef J. Evidence of extensive interspecies transfer of integron-mediated antimicrobial resistance genes among multidrug-resistant Enterobacteriaceae in a clinical setting. J Infect Dis. 2002;186:49–56. doi: 10.1086/341078. [DOI] [PubMed] [Google Scholar]
- 8.Bidet P, Burghoffer B, Gautier V, et al. In vivo transfer of plasmid-encoded ACC-1 AmpC from Klebsiella pneumoniae to Escherichia coli in an infant and selection of impermeability to imipenem in K. pneumoniae. Antimicrob Agents Chemother. 2005;49:3562–3565. doi: 10.1128/AAC.49.8.3562-3565.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Euzeby JP. Revised Salmonella nomenclature: designation of Salmonella enterica (ex Kauffmann and Edwards 1952) Le Minor and Popoff 1987 sp. nov., nom. rev. as the neotype species of the genus Salmonella Lignieres 1900 (approved lists 1980), rejection of the name Salmonella choleraesuis (Smith 1894) Weldin 1927 (approved lists 1980), and conservation of the name Salmonella typhi (Schroeter 1886) Warren and Scott 1930 (approved lists 1980). Request for an opinion. Int J Syst Bacteriol. 1999;49(Pt 2):927–930. doi: 10.1099/00207713-49-2-927. [DOI] [PubMed] [Google Scholar]
- 10.Walsh C, Duffy G, Nally P, O'Mahony R, McDowell DA, Fanning S. Transfer of ampicillin resistance from Salmonella Typhimurium DT104 to Escherichia coli K12 in food. Lett Appl Microbiol. 2008;46:210–215. doi: 10.1111/j.1472-765X.2007.02288.x. [DOI] [PubMed] [Google Scholar]
- 11.Frye JG, Jesse T, Long F, et al. DNA microarray detection of antimicrobial resistance genes in diverse bacteria. Int J Antimicrob Agents. 2006;27:138–151. doi: 10.1016/j.ijantimicag.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 12.Palaniappan RU, Zhang Y, Chiu D, et al. Differentiation of Escherichia coli pathotypes by oligonucleotide spotted array. J Clin Microbiol. 2006;44:1495–1501. doi: 10.1128/JCM.44.4.1495-1501.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scaria J, Palaniappan R, Chiu D, et al. Microarray for molecular typing of Salmonella enterica serovars. Molecular and Cellular Probes. 2008 doi: 10.1016/j.mcp.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaneene JB, Warnick LD, Bolin CA, Erskine RJ, May K, Miller R. Changes in Tetracycline Susceptibility of Enteric Bacteria Following Switching to Non-Medicated Milk Replacer in Dairy Calves. J Clin Microbiol. 2008 doi: 10.1128/JCM.00169-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silbergeld EK, Graham J, Price LB. Industrial food animal production, antimicrobial resistance, and human health. Annu Rev Public Health. 2008;29:151–169. doi: 10.1146/annurev.publhealth.29.020907.090904. [DOI] [PubMed] [Google Scholar]
- 16.Mathew AG, Cissell R, Liamthong S. Antibiotic resistance in bacteria associated with food animals: a United States perspective of livestock production. Foodborne Pathog Dis. 2007;4:115–133. doi: 10.1089/fpd.2006.0066. [DOI] [PubMed] [Google Scholar]
- 17.Blau DM, McCluskey BJ, Ladely SR, et al. Salmonella in dairy operations in the United States: prevalence and antimicrobial drug susceptibility. J Food Prot. 2005;68:696–702. doi: 10.4315/0362-028x-68.4.696. [DOI] [PubMed] [Google Scholar]
- 18.Fluckey WM, Loneragan WG, Warner R, Brashears MM. Antimicrobial drug resistance of Salmonella and Escherichia coli isolates from cattle feces, hides, and carcasses. J Food Prot. 2007;70:551–556. doi: 10.4315/0362-028x-70.3.551. [DOI] [PubMed] [Google Scholar]
- 19.Lundin JI, Dargatz DA, Wagner BA, et al. Antimicrobial drug resistance of fecal Escherichia coli and Salmonella spp. isolates from United States dairy cows. Foodborne Pathog Dis. 2008;5:7–19. doi: 10.1089/fpd.2007.0018. [DOI] [PubMed] [Google Scholar]
- 20.Ray KA, Warnick LD, Mitchell RM, et al. Prevalence of antimicrobial resistance among Salmonella on midwest and northeast USA dairy farms. Prev Vet Med. 2007;79:204–223. doi: 10.1016/j.prevetmed.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 21.van Essen-Zandbergen A, Smith H, Veldman K, Mevius D. Occurrence and characteristics of class 1, 2 and 3 integrons in Escherichia coli, Salmonella and Campylobacter spp. in the Netherlands. J Antimicrob Chemother. 2007;59:746–750. doi: 10.1093/jac/dkl549. [DOI] [PubMed] [Google Scholar]
- 22.Rayssiguier C, Thaler DS, Radman M. The barrier to recombination between Escherichia coli and Salmonella typhimurium is disrupted in mismatch-repair mutants. Nature. 1989;342:396–401. doi: 10.1038/342396a0. [DOI] [PubMed] [Google Scholar]
- 23.Thomas CM, Nielsen KM. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat Rev Microbiol. 2005;3:711–721. doi: 10.1038/nrmicro1234. [DOI] [PubMed] [Google Scholar]
- 24.Stokes HW, Hall RM. A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol Microbiol. 1989;3:1669–1683. doi: 10.1111/j.1365-2958.1989.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 25.Mazel D. Integrons: agents of bacterial evolution. Nat Rev Microbiol. 2006;4:608–620. doi: 10.1038/nrmicro1462. [DOI] [PubMed] [Google Scholar]