Abstract
Alcohol drinking and hepatitis C virus (HCV) infection frequently coexist in patients with chronic liver disease. There is limited information, however, about the impact of alcohol on host cell innate immunity and full cycle replication of HCV. This study investigated whether alcohol impairs the intracellular innate immunity in human hepatocytes, promoting HCV infection and replication. Alcohol treatment of human hepatocytes before, during and after viral infection significantly enhanced full cycle HCV replication. Alcohol suppressed intracellular expression of type I interferons (IFN-α/β) in human hepatocytes. Investigation of the mechanisms responsible for the alcohol action revealed that alcohol inhibited the expression of the IFN regulatory factors (IRF-5 and IRF-7), and signal transducer and activator of transcription (STAT-1 and STAT-2), the key positive regulators in type I IFN signaling pathway. In addition, alcohol induced the expression of suppressors of cytokine signaling (SOCS-2 and SOCS-3), the key negative regulators of IFN-α/β expression. These in vitro findings suggest that alcohol, through modulating the expression of key regulators in IFN signaling pathway, inhibits type I IFN-based intracellular innate immunity in hepctocytes, which may contribute to the chronicity of HCV infection and the poor efficacy of IFN-α-based therapy.
Keywords: hepatitis C virus, alcohol, innate immunity, type I interferon
1. Introduction
Hepatitis C virus (HCV) infection and alcohol represent the two most common causes of liver disease worldwide (Bhattacharya and Shuhart, 2003). HCV infection is a major cause of chronic hepatitis, liver cirrhosis and hepatocellular carcinoma and affects estimated 170 million people worldwide and about 4 million people in the United States (Alter, 2002). Alcohol is the most commonly used and abused drug in the United States. Approximately 14 million individuals in the United States suffer from alcohol abuse and dependence (Isaki and Kresina, 2000). When the etiology of liver disease in United States is categorized by single cause, HCV infection and chronic alcohol use account for 70–80% of cases, with roughly equal prevalence of each (Morgan et al., 2003). Seroprevalence studies have shown that chronic HCV infection and alcoholism coexist in a large number of people. About 70% of HCV-infected subjects have significant alcohol use history (Room et al., 2005; Schiff and Tagle, 1997), and vice versa, about one-third alcoholic individuals with clinical symptoms of liver disease are infected with HCV (Bhattacharya and Shuhart, 2003). HCV and alcohol most likely act synergistically to promote the development and progression of liver damage (Regev and Jeffers, 1999; Wiley et al., 1998).
The role of alcohol in promoting HCV disease has been suggested in clinical investigations. Although not all the studies have reported a correlation between HCV viral load and alcohol consumption (Bhattacharya and Shuhart, 2003; Corrao and Arico, 1998), majority of the studies have shown that alcohol consumption was related to higher levels of HCV RNA in blood (Bhattacharya and Shuhart, 2003; Oshita et al., 1994; Pessione et al., 1998; Prakash et al., 2002). Oshita et al (Oshita et al., 1994) reported that serum HCV RNA levels were significantly higher in habitual alcohol drinkers with chronic HCV infection than those in infrequent alcohol drinkers with chronic HCV. HCV RNA levels were also significantly higher in the alcohol drinkers than the abstainers (Oshita et al., 1994). These clinical investigations were supported by the in vitro studies. Alcohol consumption greatly impairs the efficacy of interferon-alpha (IFN-α) and ribarivin-based anti-HCV therapy, and is considered as a contraindication during HCV therapy (Loguercio et al., 2000; Safdar and Schiff, 2004). We therefore hypothesized that alcohol exposure impairs host cell innate immunity, promoting HCV infection/replication. Using the HCV replicon system, our group (Zhang et al., 2003) and others (McCartney et al., 2008) have reported that alcohol potentiates HCV replicon expression. The HCV subgenomic replicon systems, however, only support continuous HCV RNA genome replication without producing infectious virus particles. Therefore, there is still a need to further determine the interactions between alcohol and HCV in infectious cell system in vitro. Taking advantage of HCV Japanese Fulminant Hepatitis-1 (JFH-1) infectious system (Lindenbach et al., 2005; Wakita et al., 2005; Zhong et al., 2005), we examined whether alcohol enhances full cycle HCV infection/replication. We also explored the mechanism(s) at cellular and molecular levels for the alcohol action on HCV infection of and replication in human hepatocytes.
2. Materials and Methods
2.1. Reagents
Alcohol was purchased from Aaper Alcohol and Chemical Company (Shelbyville, KY). Recombinant IFN-α was purchased from R&D Systems Inc. (Minneapolis, MN). Mouse anti-HCV NS3 antibody was generated and provided by Dr. Guangxiang Luo (University of Kentucky, Lexington, KY). Rabbit anti-signal transducer and activator of transcription (STAT)-1, IFN regulatory factor (IRF)-3, IRF-7, and suppressors of cytokine signaling (SOCS)-2, mouse anti-STAT-2, goat anti-IRF-5, SOCS-3 and GAPDH antibodies were purchased from Santa Cruz Biotechnology Inc (Santa Cruz, CA). The secondary antibodies used for Western blot (horseradish peroxidase (HRP)-conjugated goat-anti-rabbit IgG, goat-anti-mouse IgG) were purchased from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA).
2.2. Cell Cultures
Freshly isolated primary human hepatocytes from healthy donors (Clonetics® Normal Human Hepatocytes) were purchased from Lonza Walkersville Inc. (Walkersville, MD). Cells were cultured on collagen-coated plates and maintained with Hepatocyte Culture Medium (Lonza Walkersville Inc.) according to the manufacturer’s protocol. Huh7 and 293T Cells were maintained in Dulbecco’s modified Eagle’s Medium (DMEM) with 10% fetal calf serum (FCS). For the long-term alcohol treatment, the growth-arrested Huh7 cells, which are also permissive for HCV JFH-1 infection and replication, were achieved by supplementing the culture medium with 1% dimethyl sulfoxide (DMSO) (vol/vol) as described (Sainz and Chisari, 2006).
2.3. HCV JFH-1 Infection of Hepatocytes
To generate infectious HCV JFH-1, in vitro transcribed genomic JFH-1 RNA was transfected into Huh7 cells as described (Wakita et al., 2005). The cell-free media collected at day 10 post transfection were centrifuged, passed through 0.22µm filter and used as HCV JFH-1 stocks. Infection of hepatocytes (primary human hepatocytes and Huh7 cells) with HCV JFH-1 was carried out at a multiplicity of infection (MOI) of 0.01.
2.4. HCV pseudovirus
HCV pseudovirus was generated by coexpression of an envelope-negative, luciferase-expressing HIV-1 genome (NLluc+env−) and HCV E1/E2 proteins from H77 subtype 1a isolate (Kolykhalov et al., 1997) as described previously (Bertaux and Dragic, 2006; Meertens et al., 2006). Briefly, 293T cells were lipofected with a 1:2 ratio of NLluc+env− vector and HCV E1/E2 vector. Supernatants containing HCV pseudovirus were collected 36h postlipofection and filtered (0.45µm). Target Huh7 cells (2× 104) were incubated with 200µl of supernatants overnight, then washed and placed in fresh medium for another 36h. Luciferase activity (relative lights units, RLU) was measured in cell lysates using the Promega luciferase kit, as previously described (Bertaux and Dragic, 2006; Meertens et al., 2006).
2.5. Alcohol Treatment
Human hepatocytes or HCV JFH-1-infected cells plated in 24-well plates were incubated in the presence or absence of alcohol (20–80mM) for up to 8 days. The alcohol concentrations selected for the study were based on our earlier study (Zhang et al., 2003), which showed that alcohol at 100mM or lower concentrations had little or no cytotoxic effect on human hepatocytes. For multiple time-point treatment, alcohol was added to cell cultures every 24h. To minimize alcohol evaporation that diminishes alcohol concentration in the plates, the alcohol-treated cells were maintained in the plates sealed with Parafilm (American National Can, Greenwich, CT). The control plates were also maintained in the plates sealed with Parafilm.
2.6. Quantitative Real-Time RT PCR
The quantitative real-time RT PCR was performed to measure mRNAs of HCV, IFN-α, IFN-β, IRF-3, IRF-5, IRF-7, STAT-1, STAT-2, SOCS-1, SOCS-2, SOCS-3, protein inhibitor of activated STAT (PIAS)-1, PIAS-3, HCV entry receptors (CD81, claudin-1, LDLR, SB-RI, and occludin), and GAPDH using the iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA) as previously described (Li et al., 2005). Briefly, Total cellular RNA was extracted from cells using Tri-Reagent (Molecular Research Center, Cincinnati, OH) and then subjected to the reverse transcription system from Promega (Madison, WI). The levels of GAPDH mRNA were used as an endogenous reference to normalize the quantities of target mRNA. The special oligonucleotide primers used in this study were listed as Table 1. The oligonucleotide primers were synthesized by Integrated DNA Technologies Inc. (Coralville, IA).
Table 1.
Primer Pairs for the Real-time RT-PCR
| Primer | Orientation | Sequence (5’-3’) |
|---|---|---|
| HCV | Sense: | RAY CACTCCCCTGTGAGGACC |
| Antisense: | TGR TGCACGGTCTACGAGACCTC | |
| CD81 | Sense: | CGCCAAGGATGTGAAGCAGTTC |
| Antisense: | TCCCGGAGAAGAGGTCATCGAT | |
| Claudin-1 | Sense: | GGCAGATCCAGTGCAAAGTC |
| Antisense: | TCTTCTGCACCTCATCGTCTT | |
| LDLR | Sense: | ACTGGTGTGAGAGGACCACC |
| Antisense: | CAAAGGAAGACGAGGAGCAC | |
| SB-R I | Sense: | GGTCCCTGTCATCTGCCAA |
| Antisense: | CTCCTTATCCTTTGAGCCCTTT | |
| Occludin | Sense: | AAGCAAGTGAAGGGATCTGC |
| Antisense: | GGGGTTATGGTCCAAAGTCA | |
| IFN-α* | Sense: | TTTCTCCTGCCTGAAGGACAG |
| Antisense: | GCTCATGATTTCTGCTCTGACA | |
| IFN-β | Sense: | GCCGCATTGACCATCTATGAGA |
| Antisense: | GAGATCTTCAGTTTCGGAGGTAAC | |
| IRF-3 | Sense: | ACCAGCCGTGGACCAAGAG |
| Antisense: | TACCAAGGCCCTGAGGCAC | |
| IRF-5 | Sense: | AAGCCGATCCGGCCAA |
| Antisense: | GGAAGTCCCGGCTCTTGTTAA | |
| IRF-7 | Sense: | TGGTCCTGGTGAAGCTGGAA |
| Antisense: | GATGTCGTCATAGAGGCTGTTGG | |
| STAT-1 | Sense: | GTGGAAAGACAGCCCTGCAT |
| Antisense: | ACTGGACCCCTGTCTTCAAGAC | |
| STAT-2 | Sense: | CCCCATCGACCCCTCATC |
| Antisense: | GAGTCTCACCAGCAGCCTTGT | |
| SOCS-1 | Sense: | GACGCCTGCGGATTCTACTG |
| Antisense: | GGCCATCTTCACGCTAAGGG | |
| SOCS-2 | Sense: | TGCAAGGATAAGCGGACAGG |
| Antisense: | CAGAGATGCTGCAGAGATGG | |
| SOCS-3 | Sense: | TGCGCCTCAAGACCTTCAGC |
| Antisense: | GATGCGCAGGTTCTTGGTCC | |
| PIAS-1 | Sense: | TCCCACCCAATCTTTGTGTG |
| Antisense: | GCCGCATTTTACCAAGTGGA | |
| PIAS-3 | Sense: | TGCTGGCCGGAACAAGAGTG |
| Antisense: | AGGGGGCAAAGAGAGAAGGG | |
| GAPDH | Sense: | GGTGGTCTCCTCTGACTTCAACA |
| Antisense | GTTGCTGTAGCCAAATTCGTTGT |
The IFN-α primer pair matches 11 IFN-α subtypes: IFNA2, IFNA4, IFNA5, IFNA6, IFNA7, IFNA8, IFNA10, IFNA14, IFNA16, IFNA17, and IFNA21.
2.7. Titration of extracellular and intracellular infectious HCV JFH-1
The HCV infectivity titer was determined in Huh7 cells by end point dilution and immunofluorescence as described (Gastaminza et al., 2006; Zhong et al., 2005) with minor modifications. For titration of extracellular infectious HCV, 25µL supernatant was serially diluted 10-fold in DMEM-10% FCS and used to infect Huh7 cells in 96-well plates (104 cells/well). Infection was examined at 72h postinoculation by immunofluorescence using 1:1500 dilution of anti-NS3 antibody and appropriate secondary Alexa 488-conjugated antibody. The virus titer is expressed as focus forming units per milliliter (FFU/mL) of supernatant. For titration of intracellular infectious HCV, the infected cells were washed once with PBS and then incubated with trypsin-EDTA for 5 min at 37°C. Cells were resuspended in DMEM-10%FCS and collected by centrifugation at 1,500 rpm for 3min. The cell pellets resuspended in DMEM-10% FCS (100uL for one well cells from 24-well plates) were lysed by 4 freeze-thaw cycles in dry ice and a 37°C water bath, respectively. Cell debris was pelleted by centrifugation for 5 min at 4,000rpm. The supernatants were then collected for infectivity titration assay. 25 µL cell lysate was used to measure the infectious virus titer, which is expressed as FFU/mL of cell lysates.
2.8. Western Blot Assay
Total cell lysates from the hepatocytes were prepared by radioimmune precipitation assay (RIPA) buffer (Promega, Madison, WI) with 1% protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Western blot were carried out as previously described (Li et al., 2005; Li et al., 2003). The primary antibodies for Western blot were used as follows: mouse anti-HCV NS3 (1:1000), rabbit anti-IRF-3 (1:500), goat anti-IRF-5 (1:200), rabbit anti-IRF-7 (1:400), rabbit anti-STAT-1 (1:1000), mouse anti-STAT-2 (1:200), rabbit anti-SOCS-2 (1:500), goat anti-SOCS-3 (1:300) and goat anti-GAPDH (1:500). The secondary antibodies were HRP-conjugated goat anti-rabbit IgG (1:10000), goat anti-mouse IgG (1:5000) or donkey anti-goat IgG (1:5000). The bound secondary antibodies were visualized with SuperSignal West Pico Chemiluminescent Substrate Kit (Pierce, Rockford, IL).
2.9. Statistical Analysis
All variables were tested in triplicate, and experiments were repeated at least 3 times. An unpaired student t test was used to evaluate the significance between two groups. Multiple-group comparisons were performed by one-way analysis of variance. All data are presented as mean ±SD. Statistical analyses were performed with GraphPad Instat Statistical Software. Statistical significance was defined as P<0.05.
3. Results
3.1. Alcohol enhances full cycle HCV JFH-1 replication in human hepatocytes
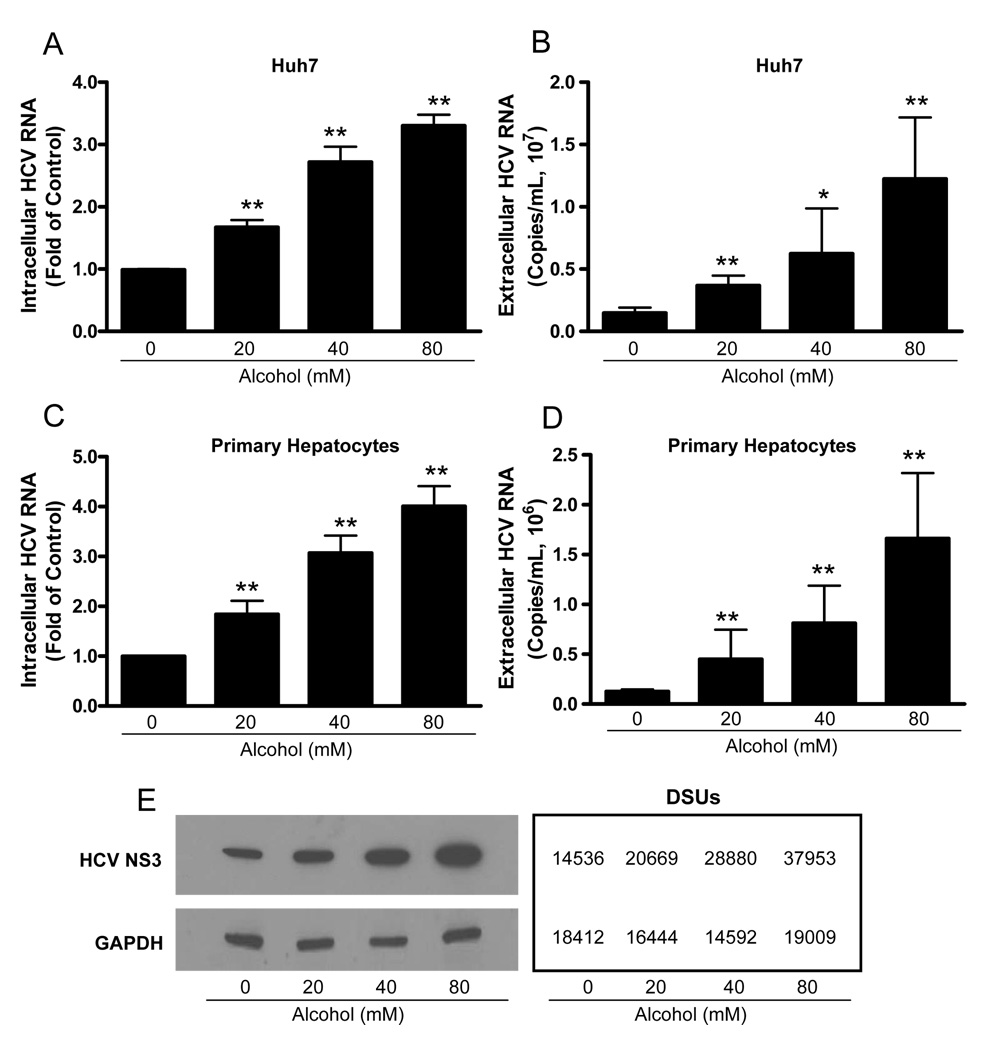
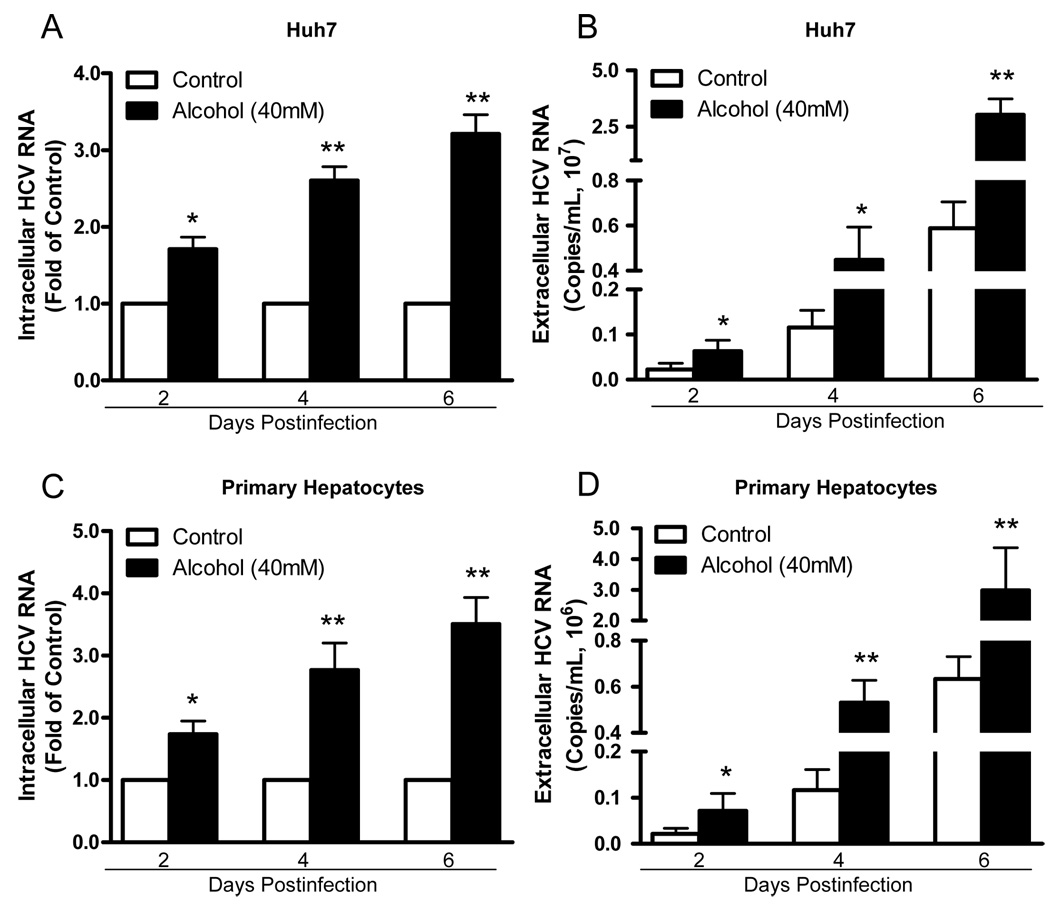
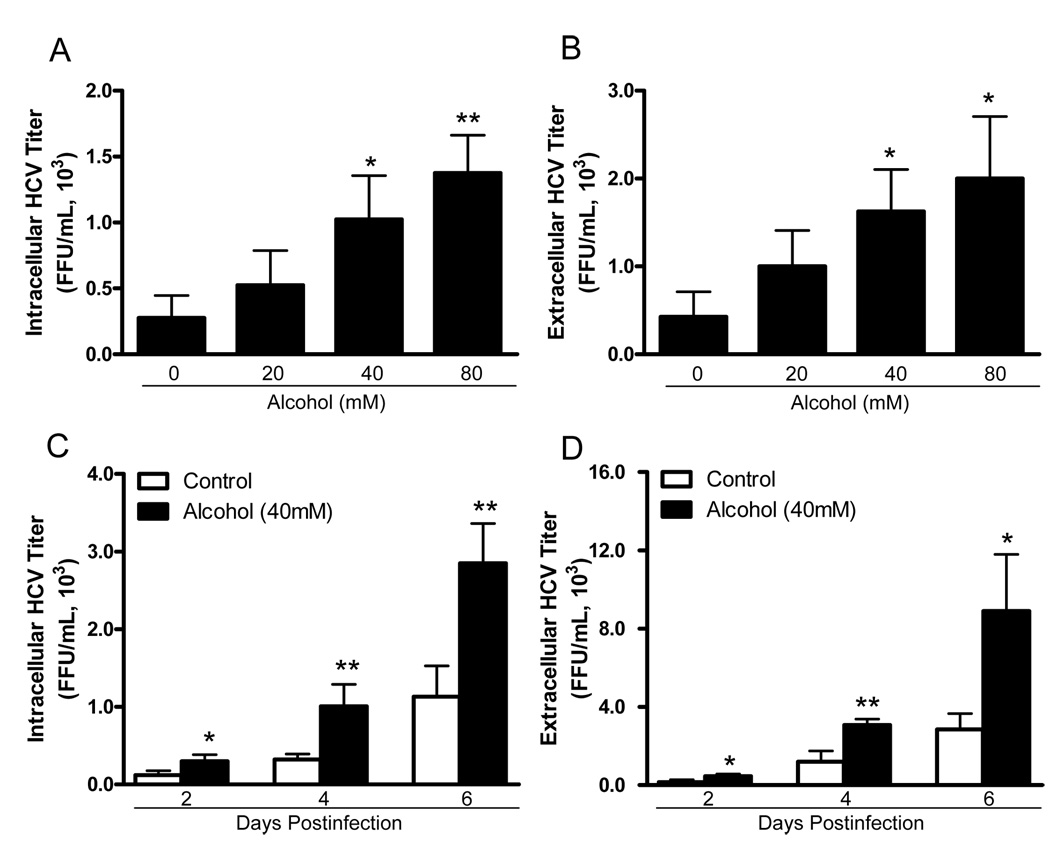
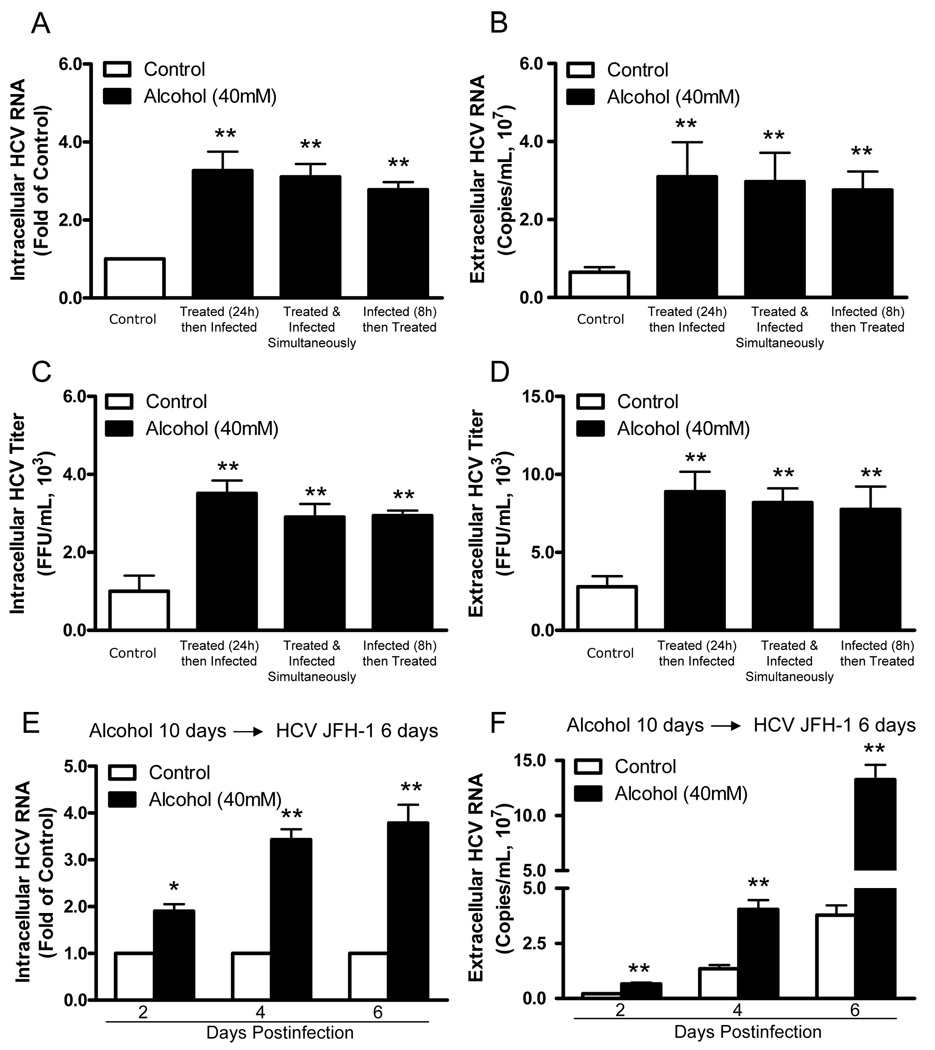
To examine whether alcohol enhances HCV JFH-1 replication in human hepatocytes, HCV JFH-1-infected Huh7 cells and primary human hepatocytes were cultured in the presence or absence of alcohol at different concentrations. Alcohol treatment of Huh7 cells and primary hepatocytes significantly increased HCV RNA expression, either within cells (Fig. 1A and 1C) or in culture supernatants (Fig. 1B and 1D). This enhancing effect of alcohol on HCV RNA expression was dose-dependent, and the maximum HCV RNA expression was observed in cell cultures treated with 80mM alcohol (Fig. 1). In comparison with untreated Huh7 cells, alcohol-treated cells also express higher levels of HCV NS3 protein at day 6 postinfection as determined by Western blot assay (Fig. 1E). We also examined the time course effect of alcohol on HCV JFH-1 RNA expression in human hepatocytes. The effect of alcohol on HCV RNA expression was time-dependent in both Huh7 cells (Fig. 2A and 2B) and primary human hepatocytes (Fig. 2C and 2D) at both intracellular (Fig. 2A and 2C) and extracellular levels (Fig. 2B and 2D). To determine whether alcohol treatment enhances the production of infectious HCV, we examined intracellular and extracellular HCV titers in cell cultures treated with or without alcohol. Cell lysates and supernatants collected from JFH-1-infected Huh7 cultures treated with alcohol had higher virus titers than those from the untreated cell cultures (Fig. 3). The effect of alcohol on the production of infectious HCV was dose- (Fig. 3A and 3B) and time- (Fig. 3C and 3D) dependent. To mimic in vivo situation where alcohol intake can occur in individuals either before, during or after HCV infection, we determined the impact of alcohol on HCV infection under three different conditions: Huh7 cells were cultured in the presence or absence of alcohol for either 24h prior to HCV infection, or simultaneously or 8h postinfection. Cell cultures that pretreated for 24h with alcohol and then infected had significantly higher levels of HCV RNA and viral titers than untreated infected cells at both intracellular (Fig. 4A and 4C) and extracellular (Fig. 4B and 4D) levels. Similarly, cell cultures infected with HCV JFH-1 and treated with alcohol simultaneously or 8h postinfection also had higher levels of HCV RNA and viral titers than the untreated cells at both intracellular (Fig. 4A and 4C) and extracellular (Fig. 4B and 4D) levels. In addition, chronic alcohol treatment (16 days’ treatment: 10 days before infection and 6 days after infection) of Huh7 cells resulted in significantly higher levels of HCV RNA, either within cells (Fig. 4E) or in supernatants (Fig. 4F), than those of untreated cells.
Fig. 1.
Alcohol enhances HCV replication in human hepatocytes. HCV JFH-1-infected Huh7 cells or primary hepatocytes were cultured in the presence or absence of alcohol at indicated concentrations. Alcohol was added to the cell cultures every 24h. Cell lysates and culture supernatants were collected at day 6 postinfection. (A–D) The levels of intracellular or extracellular HCV RNA were determined by real-time RT-PCR. (A) The intracellular HCV RNA in Huh7 cells; (B) The extracellular HCV RNA in Huh7 cell cultures; (C) The intracellular HCV RNA in primary human hepatocytes; (D) The extracellular HCV RNA in primary human hepatocyte cultures. The levels of intracellular HCV RNA (A, C), with normalization to corresponding GAPDH mRNA, are expressed as the fold of control (without alcohol treatment, which was defined as 1). The levels of extracellular HCV RNA (B, D) are expressed as copies/mL. The results (A–D) shown are the mean ± SD of three repeated experiments. All variables in each experiment were tested in triplicate (*, P<0.05, **, P<0.01, alcohol treated vs untreated). (E) Alcohol enhances HCV NS3 protein expression. HCV JFH-1-infected Huh7 cells were cultured in the presence or absence of alcohol (40mM) for 6 days. Total cellular proteins were collected and subjected to Western blot using the antibodies against to HCV NS3 and GAPDH. The numbers in the right panel are the signal intensities of protein bands of western blot shown in the left panel, which are expressed as densitometry scanning units (DSUs).
Fig. 2.
Time course effect of alcohol on HCV replication in human hepatocytes. HCV JFH-1-infected Huh7 cells or primary human hepatocytes were cultured in the presence or absence of alcohol (40mM). Alcohol was added to the cultures every 24h. Cell lysates and culture supernatants were collected at different time points as indicated. The levels of intracellular or extracellular HCV RNA were determined by real-time RT-PCR. (A) The intracellular HCV RNA in Huh7 cells; (B) The extracellular HCV RNA in Huh7 cell cultures; (C) The intracellular HCV RNA in primary human hepatocytes; (D) The extracellular HCV RNA in primary human hepatocyte cultures. The levels of intracellular HCV RNA (A, C), with normalization to corresponding GAPDH mRNA, are expressed as the fold of control (without alcohol treatment, which was defined as 1). The levels of extracellular HCV RNA (B, D) are expressed as copies/mL. The results (A–D) shown are the mean ± SD of three repeated experiments. All variables in each experiment were tested in triplicate (*, P<0.05, **, P<0.01, alcohol treated vs untreated).
Fig. 3.
Alcohol treatment increases infectious HCV JFH-1 production in human hepatocytes. (A, B) Dose-dependent effect of alcohol on production of infectious HCV. HCV JFH-1-infected Huh7 cells were cultured in the presence or absence of alcohol at indicated concentrations. Alcohol was added to the cell cultures every 24h. Cell lysates and culture supernatants were collected at day 6 postinfection. Intracellular (A) or extracellular (B) HCV infectious titers were determined by serial dilution and immunofluorescence. (C, D) Time course effect of alcohol on production of infectious HCV. HCV JFH-1-infected Huh7 cells were cultured in the presence or absence of alcohol (40mM). Alcohol was added to the cultures every 24h. Cell lysates and culture supernatants were collected at different time points as indicated. Intracellular (C) or extracellular (D) HCV infectious titers were determined by serial dilution and immunofluorescence. The HCV titers (A–D) are expressed as foci form units/mL (FFU/mL) and presented as the mean ± SD of three repeated experiments. All variables in each experiment were tested in triplicate (*, P<0.05, **, P<0.01, alcohol treated vs untreated).
Fig. 4.
Alcohol enhances HCV JFH-1 infection of human hepatocytes under different situations. Huh7 cells were cultured in the presence or absence of alcohol for either 24h prior to HCV infection, or simultaneously or 8h postinfection. After HCV infection, the infected cells were then washed 5 times with plain DMEM to remove input HCV and then cultured in the presence or absence of alcohol (40mM) for 6 days. Cell lysates and culture supernatants were collected at day 6 postinfection. The levels of intracellular (A) or extracellular (B) HCV RNA were determined by real-time RT-PCR. The levels of intracellular (C) or extracellular (D) HCV infectious titers were determined by serial dilution and immunofluorescence. (E, F) Effect of long-term alcohol treatment on HCV infection/replication. 1% DMSO (vol/vol) treated-Huh7 cells were cultured in the presence or absence of alcohol (40mM) and the cells were replenished alcohol (40mM) every 24h thereafter. After cultured for 10 days, Huh7 cells treated with or without alcohol were infected with HCV JFH-1. Cell lysates and culture supernatants were collected at day 6 postinfection. The levels of intracellular (E) or extracellular (F) HCV RNA were determined by real-time RT-PCR. The levels of intracellular HCV RNA (A, E), with normalization to corresponding GAPDH mRNA, are expressed as the fold of control (without alcohol treatment, which was defined as 1). The levels of extracellular HCV RNA (B, F) are expressed as copies/mL. The intracellular (C) and extracellular (D) HCV titers are expressed as FFU/mL. The results shown (A–F) are the mean ± SD of three repeated experiments. All variables in each experiment were tested in triplicate (*, P<0.05, **, P<0.01, alcohol treated vs untreated).
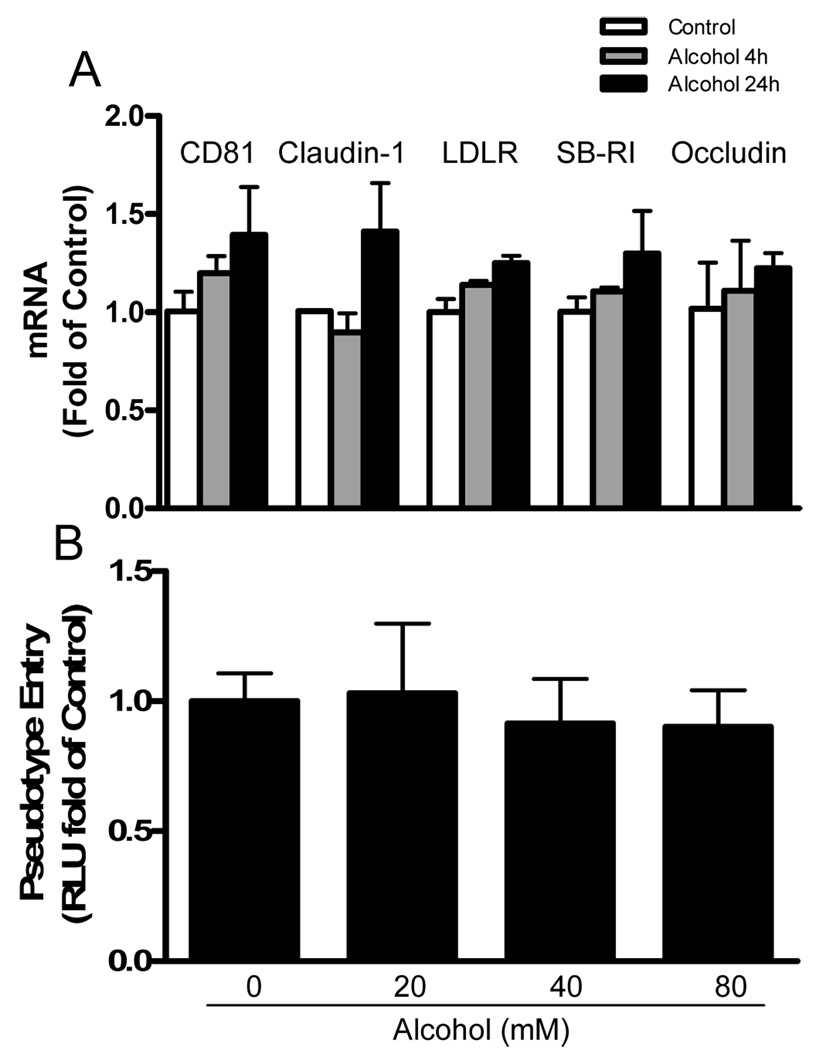
3.2. Alcohol has little effect on HCV entry into hepatocytes
To examine whether alcohol affects HCV entry into human hepatocytes, we investigated whether alcohol has the ability to modulate the expression of HCV entry receptors in Huh7 cells. Alcohol treatment had little effect on the expression of HCV receptors (CD81, claudin-1, LDLR, SB-RI, and occludin) in Huh7 cells (Fig. 5A). To confirm there is lack of the involvement of the known HCV receptors in alcohol-mediated action on HCV infection, we used the pseudovirus enveloped with HCV E1/E2 proteins to evaluate the effect of alcohol on HCV entry into hepatocytes. Alcohol treatment of Huh7 cells has little effect on pseudotyped HCV entry as determined by luciferase activity (Fig. 5B).
Fig. 5.
Effect of alcohol on HCV entry of hepatocytes. (A) Effect of alcohol treatment on HCV receptor expression. Huh7 cells were cultured in the presence or absence of alcohol for 4h or 24h. Total cellular RNA extracted from the cell cultures was subjected to the real-time PCR for quantification of HCV entry cellular receptor (CD81, claudin-1, LDLR, SB-RI, and occludin) and GAPDH mRNA. The data, with normalization to GAPDH mRNA, are expressed as the fold of control (without alcohol treatment, which was defined as 1). (B) Effect of alcohol treatment on HCV pseudovirus entry into Huh7 cells. Huh7 cells were cultured in the presence or absence of alcohol at indicated concentrations for 24h, and then infected with NLluc+env− virus pseudotyped with HCV E1E2 glycoproteins in the presence or absence of alcohol. Luciferase activities (relative light units, RLU) were measure at 36h postinfection. The data are expressed as the RLU fold of control (without alcohol treatment, which was defined as 1).
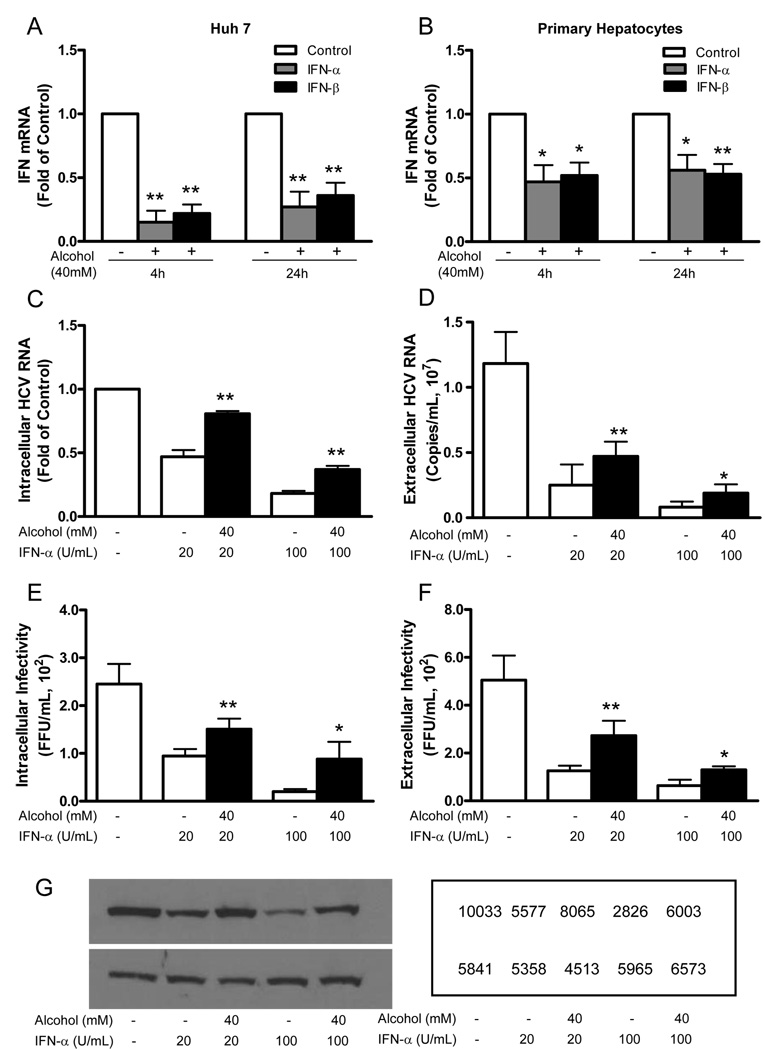
3.3. Alcohol suppresses intracellular type I IFN expression
Type I IFNs play a key role in host cell innate immunity against viral infections. Clinically, IFN-α is used for the treatment of HCV-infected patients. We thus examined whether alcohol has the ability to inhibit intracellular type I IFN gene expression in human hepatic cells. Alcohol treatment significantly suppressed IFN-α and IFN-β expression in both Huh 7 cells (Fig. 6A) and primary human hepatocytes (Fig. 6B). We then examined whether alcohol has a negative impact on the anti-HCV activity of recombinant IFN-α in HCV JFH-1-infected hepatocytes. As expected, recombinant IFN-α, when added to cell cultures, significantly inhibited HCV RNA expression and decreased the production of infectious HCV at both intracellular (Fig. 6C and 6E) and extracellular (Fig. 6D and 6F) levels. This anti-HCV activity of recombinant IFN-α, however, was compromised by alcohol treatment (Fig. 6C–6F). The negative impact of alcohol on the anti-HCV activity of IFN-α was also confirmed at protein levels by Western blot assay, showing that the inhibitory effect of IFN-α on HCV NS3 protein expression was reduced in alcohol-treated hepatocytes (Fig. 6G)
Fig. 6.
Alcohol suppresses IFN-α/β expression and compromises anti-HCV activity of recombinant IFN-α. (A, B) Alcohol suppresses IFN-α/β expression in Huh7 cells (A) and primary human hepatocytes (B). Human hepatocytes were incubated in the presence or absence of alcohol (40mM) for 4h or 24h. Total cellular RNA extracted from the cell cultures was subjected to the real-time PCR for IFN-α/β and GAPDH mRNA quantification. The data, with normalization to GAPDH mRNA, are expressed as the fold of control (without alcohol treatment, which was defined as 1). (C–G) Alcohol compromises the anti-HCV activity of IFN-α. HCV JFH-1-infected Huh7 cells (at day 3 postinfection) were incubated with or without alcohol (40mM) and/or IFN-α (20U/ml, 100U/mL) for 48 h. The levels of intracellular (C) and extracellular (D) HCV RNA were determined by real-time RT-PCR. The data are expressed as copies/mL. The levels of intracellular or extracellular (F) HCV infectious titers were determined by serial dilution and immunofluorescence. The data are expressed as FFU/mL. The results (A–F) shown are the mean ± SD of three repeated experiments. All variables in each experiment were tested in triplicate (*, P<0.05, **, P<0.01, alcohol treated vs untreated). (G) Total cellular proteins were subjected to Western blot using the antibodies against to HCV NS3 and GAPDH. The numbers in the right panel are the signal intensities of protein bands of western blot shown in the left panel, which are expressed as densitometry scanning units (DSUs).
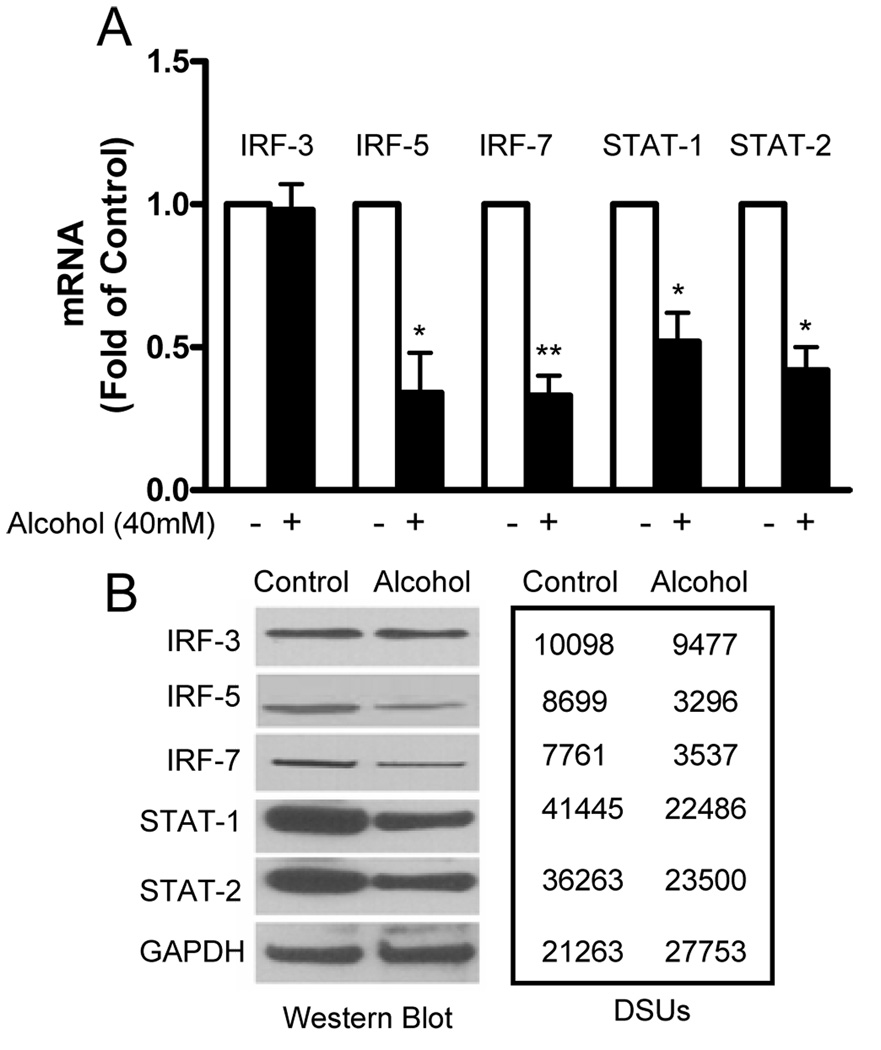
3.4. Alcohol suppresses IFN regulator factors and STATs
In order to investigate the mechanisms involved in alcohol-mediated suppression of intracellular IFN-α/β, we examined whether alcohol has the ability to inhibit the expression of IRF-3, IRF-5 and IRF-7 that have a crucial role in the activation of type I IFNs (Mamane et al., 1999; Nguyen et al., 1997). Alcohol treatment resulted in a significant decrease of IRF-5 and IRF-7 mRNA expression in Huh7 cells (Fig. 7A). However, alcohol had no effect on the expression of IRF-3 mRNA (Fig. 7A). This selective inhibition of IRF-5 and IRF-7 expression was confirmed by the Western blot assay, showing that alcohol-treated hepatocytes had lower levels of IRF-5 and IRF-7 proteins than the control cells (Fig. 7B). Similar to mRNA results, the IRF-3 protein was not affected by alcohol treatment (Fig. 7B). We also examined whether alcohol treatment has the ability to inhibit the expression of STAT-1 and -2, the key regulators in IFN-mediated biological responses, including activation of the antiviral state and induction of type I IFNs. As shown in Fig. 7, the expression of STAT-1/STAT-2 mRNAs and proteins in human hepatocytes was also significantly inhibited by alcohol treatment.
Fig. 7.
Alcohol suppresses the expression of IFN regulatory factors (IRFs) and signal transducers and activators of transcription (STATs) in human hepatocytes. Huh7 cells were cultured in the presence or absence of alcohol (40mM) for 24h. (A) Total cellular RNA extracted from the cell culture was subjected to the real-time RT PCR for IRF, STAT, and GAPDH mRNA quantification. The data, with normalization to GAPDG, are expressed as the fold of control (without alcohol treatment, which is defined as 1). The results shown are mean ± SD of three repeated experiments. All variables in each experiment were tested in triplicate (*, P<0.05, **, P<0.01, alcohol treated vs untreated). (B) Huh7 cells were cultured in the presence or absence of alcohol (40mM) for 72h. Total cellular proteins were subjected to Western blot assay using the antibodies against IRF-3, IRF-5, IRF-7, STAT-1, STAT-2, and GAPDH. The numbers in the right panel are the signal intensities of protein bands of Western blot shown in the left panel, which are expressed as densitometry scanning units (DSUs).
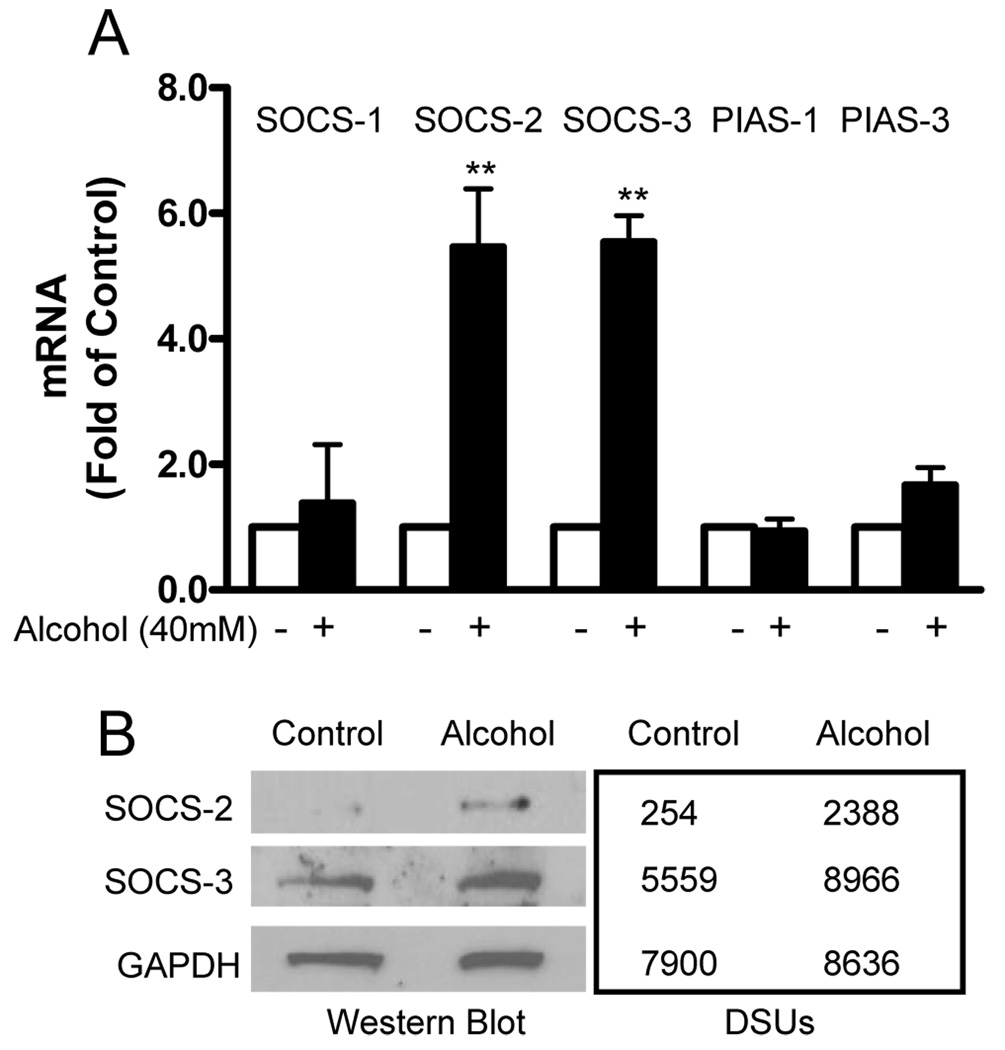
3.5. Alcohol induces SOCS-2 and SOCS-3 expression
SOCS and PIAS are two major families of negative regulators of signal transduction induced by cytokines (Norkina et al., 2008; Rakesh and Agrawal, 2005). SOCS members form a classical negative feedback loop with key actions involving in inhibition of the JAK-STAT signaling cascade. PIASs are specific inhibitors of STAT signaling. Therefore, we investigated whether alcohol treatment can induce SOCS and PIAS expression in human hepatocytes. As demonstrated in Fig. 8, alcohol treatment resulted in a significant increase of SOCS-2 and SOCS-3 expression in Huh7 cells at both mRNA (Fig. 8A) and protein (Fig. 8B) levels. However, alcohol had little effect on the expression of other members (SOCS-1, PIAS-1 and PIAS-3) in SOCS and PIAS families (Fig. 8).
Fig. 8.
Alcohol induces the expression of suppressors of cytokine signaling (SOCSs) in human hepatocytes. (A) Huh7 cells were cultured in the presence or absence of alcohol (40mM) for 24h. Total cellular RNA extracted from the cell culture was subjected to the real-time RT PCR for SOCS, protein inhibitor of activated STAT (PIAS), and GAPDH mRNA quantification. The data, with normalization to GAPDH mRNA, are expressed as the fold of control (without alcohol treatment, which is defined as 1). The results shown are mean ± SD of three repeated experiments. All variables in each experiment were tested in triplicate (**, P<0.01, alcohol treated vs untreated). (B) Huh7 cells were cultured in the presence or absence of alcohol (40mM) for 72h. Total cellular proteins were subjected to Western blot using the antibodies against to SOCS-2, -3 and GAPDH. The numbers in the right panel are the signal intensities of protein bands of Western blot shown in the left panel, which are expressed as densitometry scanning units (DSUs).
4. Discussion
In this study, we used HCV JFH-1 system (Lindenbach et al., 2005; Wakita et al., 2005; Zhong et al., 2005) to examine the ability of alcohol to enhance HCV replication in human hepatocytes. In contrast to HCV replicon system where we were only able to examine the impact of alcohol on intracellular HCV replicon expression, the HCV JFH-1 cell model allows us to determine whether alcohol treatment enhances full cycle HCV infection of human hepatocytes. Using this system, we demonstrated that alcohol treatment at physiologically achievable concentrations enhanced HCV JFH-1 replication in both Huh7 cells (Figs. 1 and 2) and primary hepatocytes (Figs. 1 and 2). It is reported that the in vitro 25mM alcohol concentration is equivalent to a 0.1 g/dl blood alcohol level achieved after consumption of four or five standard drinks in nonalcoholic individuals, and the 100mM in vitro alcohol concentration corresponds to a 0.4 g/dl blood alcohol level often seen in chronic alcoholics after an acute alcohol intake (Szabo et al., 2001). In addition, our earlier study (Zhang et al., 2003) and studies by others (Ahluwalia et al., 2000; Deitrich and Harris, 1996) suggested that the highest level of alcohol in vitro should not be above 100mM. In our study, we used alcohol at the concentration of 80mM or lower. The alcohol not only elevated the levels of HCV RNA both intracellularly and extracellularly, but also increased the production of infectious viruses within cells and in culture supernatants (Fig. 3). With HCV JFH-1 system, we were also able to examine whether alcohol treatment increased the susceptibility of human hepatocytes to HCV infection. We demonstrated that the cells pretreated with alcohol for 24h became more susceptible to HCV JFH-1 infection (Fig. 4A and 4B) and produced higher titers of infectious viruses than untreated cells (Fig. 4C and 4D). The enhancement of HCV replication by alcohol was also observed even after HCV infection has taken place (Fig. 4A–4D). In addition to the demonstration of the acute effect of alcohol on HCV replication, we also showed the chronic alcohol treatment (16 days) significantly increased HCV replication in human hepatocytes (Fig. 4E and 4F).
The underlying mechanisms responsible for the alcohol effect on HCV replication remain to be determined. Several mechanisms have been suggested, including alcohol-induced changes in oxidative stress and mitochondrial function (McCartney et al., 2008; Otani et al., 2005; Rigamonti et al., 2003), and alcohol-induced immune dysregulation (Szabo, 2003). McCartney et al. indicated that cytochrome P450-2E1 (CYP2E1) metabolism of alcohol, stimulating the microsomal production of reactive oxygen species (ROS), may play the key role in the increase of HCV replication by alcohol (McCartney et al., 2008). In our study, we first examined the impact of alcohol on HCV infection at the entry level, demonstrating that alcohol had little effect on HCV entry (Fig. 5). We then investigated whether alcohol affects intracellular type I IFN-mediated innate immunity, as type I IFNs have a crucial role in protecting host cells from viral infections and in controlling viral replication (Zhang et al., 2005). Our data that the expression of endogenous type I IFNs (both IFN-α and IFN-β) in human hepatocytes was significantly inhibited by alcohol treatment (Fig. 6A and 6B) provide a mechanism for the alcohol-mediated enhancement of HCV infection and replication. Our further investigations showed that alcohol inhibited the expression of STAT-1 and STAT-2 (Fig. 7). It is known that the Janus Kinase-STAT (JAK-STAT) signaling pathway is the major pathway for IFN-mediated signaling and activation of gene expression (Velazquez et al., 1992). STAT-1 plays a crucial role in mediating IFN-dependent biological responses including activation of the antiviral state and induction of type I IFN expression (Mbow and Sarisky, 2004; O'Shea et al., 2002; van Boxel-Dezaire et al., 2006) that is directly modulated by a family of transcription factors, IRFs. Among nine known IRFs, IRF3, IRF5, and IRF7 have been implicated as key positive regulators of type I IFNs (Honda et al., 2006). These IRFs not only recognize the elements of the IFN promoter to modulate the expression of type I IFN genes selectively, but also regulate the IFN-stimulated response element (ISRE) in some of IFN-stimulated genes (ISGs), leading to induction of an antiviral state (Barnes et al., 2001; Marie et al., 1998). IRF3 and IRF7, which are highly homologous, are considered as the key regulators of type I IFN gene expression induced by viruses (Honda et al., 2006). It has been demonstrated that IRF-7 is the master regulator of type I IFN-dependent immune response, as it not only induces IFN-α expression, but also activates many ISGs that have antiviral activities (Honda and Taniguchi, 2006; Honda et al., 2005). In addition to IRF-3 and IRF-7, IRF-5 has also been characterized as an important factor participating in the induction of IFN-α/β (Barnes et al., 2002; Barnes et al., 2001; Honda and Taniguchi, 2006). Like IRF-3 and IRF-7, IRF-5 requires phosphorylation-induced activation in order to translocate to the nucleus, activating type I IFNs. Therefore, the suppression of IRF-5 and IRF-7 expression in the hepatocytes by alcohol treatment provides a plausible mechanism for the alcohol action on type I IFNs.
In addition to its inhibitory effect on the type I IFN positive regulators in the JAK-STAT pathway, alcohol treatment also selectively induced the expression of SOCS-2 and SOCS-3 (Fig. 8), the two potent suppressors of the JAK-STAT signaling cascade (Murray, 2007; Norkina et al., 2008; Rakesh and Agrawal, 2005). This effect of alcohol on SOCS-2 and SOCS-3 appears to be selective, as other negative regulators (SOCS-1, PIAS-1 and PIAS-3) of the JAK-STAT pathway were not affected by alcohol treatment. This finding not only provides additional mechanisms for the alcohol action on endogenous IFN-α/β expression, but also explains the observation that alcohol had the ability to compromise IFN-α treatment of HCV JFH-1 infected cells (Fig. 6). This in vitro observation is highly significant, as it supports the in vivo studies, showing that that increased hepatic expression of SOCS-3 is associated with non-response to antiviral therapy by IFN-α in patients with chronic HCV genotype 1 infection (Walsh et al., 2006) and that the overexpression of SOCS-3 in human hepatocytes can inhibit IFN-α induced antiviral actions (Vlotides et al., 2004).
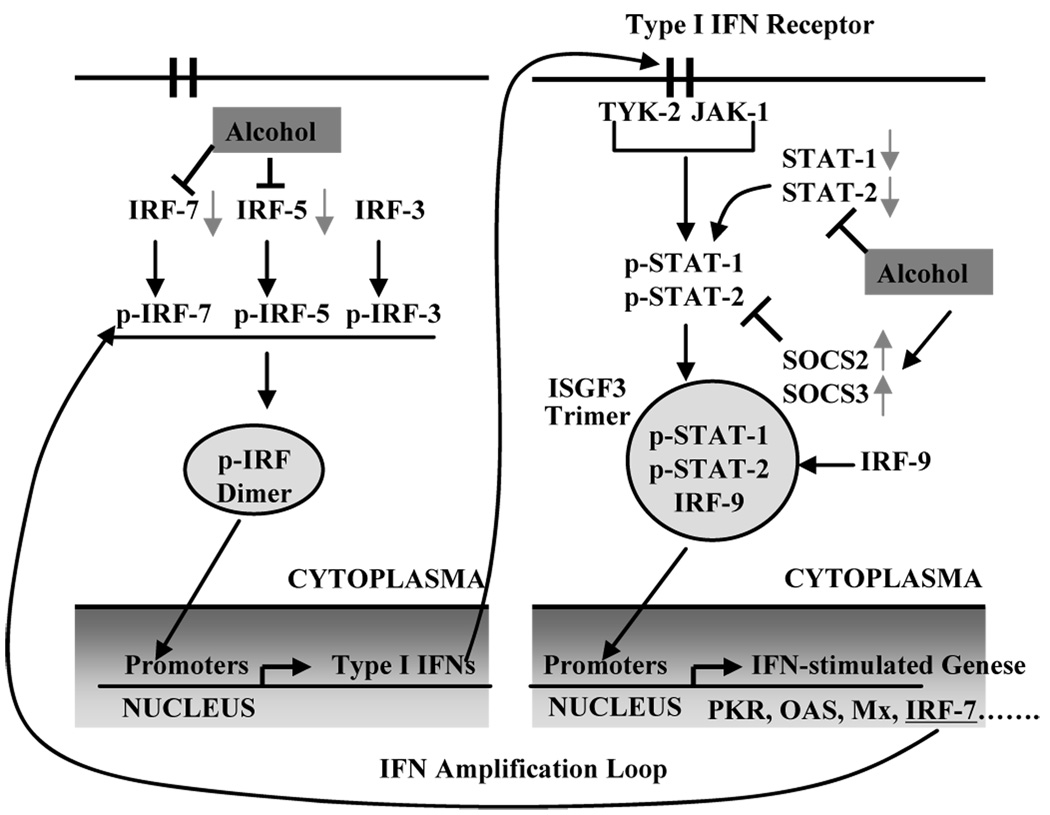
In summary, we have for the first time documented the impact of alcohol on full cycle HCV replication in infectious JFH-1 system. Our study provides experimental evidence that alcohol has the ability to enhance HCV JFH-1 infection and replication, through the impairment of host cell innate antiviral immunity. Although other mechanisms might also be involved, the suppression of type I IFN signaling pathway, as illustrated in the schematic diagram (Fig. 9), may play a role in the alcohol actions. Because type I IFNs and associated antiviral factors can directly inhibit HCV at different steps of the viral replication cycle, alcohol-mediated suppression of these antiviral factors provides a favorable microenvironment for HCV growth in hepatocytes. Our results in conjunction with the studies by others (McCartney et al., 2008; Otani et al., 2005; Zhang et al., 2003) indicate that alcohol abusers may have a compromised innate immunity within hepatocytes, which may contribute to the chronicity of HCV infection and the poor efficacy of IFN-α-based therapy.
Fig. 9.
Schematic diagram of mechanisms involved in alcohol-mediated suppression of type I IFN pathway in human hepatocytes. Alcohol not only inhibits the expression of the IRF-5 and IRF-7 (the key positive regulators of type I IFN production), but also suppresses the expression of STAT-1 and -2 (two key elements in JAK-STAT pathway). In addition, alcohol induces the expression of SOCS-2 and -3 (the negative regulators of JAK-STAT pathway). Abbreviations: IRF, interferon regulatory factor; ISGF3, IFN-stimulated gene factor-3; JAK, Janus kinase; Mx, myxovirus-resistance proteins; OAS, oligoadenylate synthetase; PKR, protein kinase; SOCS, suppressors of cytokine signaling; STAT, signal transducer and activator of transcription.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Ahluwalia B, Wesley B, Adeyiga O, Smith DM, Da-Silva A, Rajguru S. Alcohol modulates cytokine secretion and synthesis in human fetus: an in vivo and in vitro study. Alcohol. 2000;21:207–213. doi: 10.1016/s0741-8329(00)00076-8. [DOI] [PubMed] [Google Scholar]
- Alter MJ. Prevention of spread of hepatitis C. Hepatology. 2002;36:S93–S98. doi: 10.1053/jhep.2002.36389. [DOI] [PubMed] [Google Scholar]
- Barnes BJ, Kellum MJ, Field AE, Pitha PM. Multiple regulatory domains of IRF-5 control activation, cellular localization, and induction of chemokines that mediate recruitment of T lymphocytes. Mol Cell Biol. 2002;22:5721–5740. doi: 10.1128/MCB.22.16.5721-5740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes BJ, Moore PA, Pitha PM. Virus-specific activation of a novel interferon regulatory factor, IRF-5, results in the induction of distinct interferon alpha genes. J Biol Chem. 2001;276:23382–23390. doi: 10.1074/jbc.M101216200. [DOI] [PubMed] [Google Scholar]
- Bertaux C, Dragic T. Different domains of CD81 mediate distinct stages of hepatitis C virus pseudoparticle entry. J Virol. 2006;80:4940–4948. doi: 10.1128/JVI.80.10.4940-4948.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya R, Shuhart MC. Hepatitis C and alcohol: interactions, outcomes, and implications. J Clin Gastroenterol. 2003;36:242–252. doi: 10.1097/00004836-200303000-00012. [DOI] [PubMed] [Google Scholar]
- Corrao G, Arico S. Independent and combined action of hepatitis C virus infection and alcohol consumption on the risk of symptomatic liver cirrhosis. Hepatology. 1998;27:914–919. doi: 10.1002/hep.510270404. [DOI] [PubMed] [Google Scholar]
- Deitrich RA, Harris RA. How much alcohol should I use in my experiments? Alcohol Clin Exp Res. 1996;20:1–2. doi: 10.1111/j.1530-0277.1996.tb01033.x. [DOI] [PubMed] [Google Scholar]
- Gastaminza P, Kapadia SB, Chisari FV. Differential biophysical properties of infectious intracellular and secreted hepatitis C virus particles. J Virol. 2006;80:11074–11081. doi: 10.1128/JVI.01150-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Takaoka A, Taniguchi T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6:644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, Taniguchi T. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- Isaki L, Kresina TF. Directions for biomedical research in alcohol and HIV: where are we now and where can we go? AIDS Res Hum Retroviruses. 2000;16:1197–1207. doi: 10.1089/08892220050116961. [DOI] [PubMed] [Google Scholar]
- Kolykhalov AA, Agapov EV, Blight KJ, Mihalik K, Feinstone SM, Rice CM. Transmission of hepatitis C by intrahepatic inoculation with transcribed RNA. Science. 1997;277:570–574. doi: 10.1126/science.277.5325.570. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang X, Douglas SD, Metzger DS, Woody G, Zhang T, Song L, Ho WZ. CD8+ T cell depletion amplifies hepatitis C virus replication in peripheral blood mononuclear cells. J Infect Dis. 2005;192:1093–1101. doi: 10.1086/432957. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang T, Douglas SD, Lai JP, Xiao WD, Pleasure DE, Ho WZ. Morphine enhances hepatitis C virus (HCV) replicon expression. Am J Pathol. 2003;163:1167–1175. doi: 10.1016/S0002-9440(10)63476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbach BD, Evans MJ, Syder AJ, Wolk B, Tellinghuisen TL, Liu CC, Maruyama T, Hynes RO, Burton DR, McKeating JA, Rice CM. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623–626. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- Loguercio C, Di Pierro M, Di Marino MP, Federico A, Disalvo D, Crafa E, Tuccillo C, Baldi F, del VecchioBlanco C. Drinking habits of subjects with hepatitis C virus-related chronic liver disease: prevalence and effect on clinical, virological and pathological aspects. Alcohol Alcohol. 2000;35:296–301. doi: 10.1093/alcalc/35.3.296. [DOI] [PubMed] [Google Scholar]
- Mamane Y, Heylbroeck C, Genin P, Algarte M, Servant MJ, LePage C, DeLuca C, Kwon H, Lin R, Hiscott J. Interferon regulatory factors: the next generation. Gene. 1999;237:1–14. doi: 10.1016/s0378-1119(99)00262-0. [DOI] [PubMed] [Google Scholar]
- Marie I, Durbin JE, Levy DE. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. Embo J. 1998;17:6660–6669. doi: 10.1093/emboj/17.22.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbow ML, Sarisky RT. What is disrupting IFN-alpha's antiviral activity? Trends Biotechnol. 2004;22:395–399. doi: 10.1016/j.tibtech.2004.06.002. [DOI] [PubMed] [Google Scholar]
- McCartney EM, Semendric L, Helbig KJ, Hinze S, Jones B, Weinman SA, Beard MR. Alcohol metabolism increases the replication of hepatitis C virus and attenuates the antiviral action of interferon. J Infect Dis. 2008;198:1766–1775. doi: 10.1086/593216. [DOI] [PubMed] [Google Scholar]
- Meertens L, Bertaux C, Dragic T. Hepatitis C virus entry requires a critical postinternalization step and delivery to early endosomes via clathrin-coated vesicles. J Virol. 2006;80:11571–11578. doi: 10.1128/JVI.01717-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan TR, Brenner D, Everhart J, French SW, Fried MW, Gretch DR, Koziel MJ, McClain CJ, Peters MG, Weinman SA, Lucas DL. Hepatitis C and alcohol: fundamental and translational research directions. Alcohol Clin Exp Res. 2003;27:726–731. doi: 10.1097/01.ALC.0000062741.58464.19. [DOI] [PubMed] [Google Scholar]
- Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- Nguyen H, Hiscott J, Pitha PM. The growing family of interferon regulatory factors. Cytokine Growth Factor Rev. 1997;8:293–312. doi: 10.1016/s1359-6101(97)00019-1. [DOI] [PubMed] [Google Scholar]
- Norkina O, Dolganiuc A, Catalano D, Kodys K, Mandrekar P, Syed A, Efros M, Szabo G. Acute alcohol intake induces SOCS1 and SOCS3 and inhibits cytokine-induced STAT1 and STAT3 signaling in human monocytes. Alcohol Clin Exp Res. 2008;32:1565–1573. doi: 10.1111/j.1530-0277.2008.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109 Suppl:S121–S131. doi: 10.1016/s0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- Oshita M, Hayashi N, Kasahara A, Hagiwara H, Mita E, Naito M, Katayama K, Fusamoto H, Kamada T. Increased serum hepatitis C virus RNA levels among alcoholic patients with chronic hepatitis C. Hepatology. 1994;20:1115–1120. [PubMed] [Google Scholar]
- Otani K, Korenaga M, Beard MR, Li K, Qian T, Showalter LA, Singh AK, Wang T, Weinman SA. Hepatitis C virus core protein, cytochrome P450 2E1, and alcohol produce combined mitochondrial injury and cytotoxicity in hepatoma cells. Gastroenterology. 2005;128:96–107. doi: 10.1053/j.gastro.2004.10.045. [DOI] [PubMed] [Google Scholar]
- Pessione F, Degos F, Marcellin P, Duchatelle V, Njapoum C, Martinot-Peignoux M, Degott C, Valla D, Erlinger S, Rueff B. Effect of alcohol consumption on serum hepatitis C virus RNA and histological lesions in chronic hepatitis C. Hepatology. 1998;27:1717–1722. doi: 10.1002/hep.510270635. [DOI] [PubMed] [Google Scholar]
- Prakash O, Mason A, Luftig RB, Bautista AP. Hepatitis C virus (HCV) and human immunodeficiency virus type 1 (HIV-1) infections in alcoholics. Front Biosci. 2002;7:e286–e300. doi: 10.2741/A924. [DOI] [PubMed] [Google Scholar]
- Rakesh K, Agrawal DK. Controlling cytokine signaling by constitutive inhibitors. Biochem Pharmacol. 2005;70:649–657. doi: 10.1016/j.bcp.2005.04.042. [DOI] [PubMed] [Google Scholar]
- Regev A, Jeffers LJ. Hepatitis C and alcohol. Alcohol Clin Exp Res. 1999;23:1543–1551. [PubMed] [Google Scholar]
- Rigamonti C, Mottaran E, Reale E, Rolla R, Cipriani V, Capelli F, Boldorini R, Vidali M, Sartori M, Albano E. Moderate alcohol consumption increases oxidative stress in patients with chronic hepatitis C. Hepatology. 2003;38:42–49. doi: 10.1053/jhep.2003.50275. [DOI] [PubMed] [Google Scholar]
- Room R, Babor T, Rehm J. Alcohol and public health. Lancet. 2005;365:519–530. doi: 10.1016/S0140-6736(05)17870-2. [DOI] [PubMed] [Google Scholar]
- Safdar K, Schiff ER. Alcohol and hepatitis C. Semin Liver Dis. 2004;24:305–315. doi: 10.1055/s-2004-832942. [DOI] [PubMed] [Google Scholar]
- Sainz B, Jr, Chisari FV. Production of infectious hepatitis C virus by well-differentiated, growth-arrested human hepatoma-derived cells. J Virol. 2006;80:10253–10257. doi: 10.1128/JVI.01059-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff ER, Tagle FM. Treatment of HCV: approach to difficult cases. Clin Liver Dis. 1997;1:647–662. viii–ix. doi: 10.1016/s1089-3261(05)70327-5. [DOI] [PubMed] [Google Scholar]
- Szabo G. Pathogenic interactions between alcohol and hepatitis C. Curr Gastroenterol Rep. 2003;5:86–92. doi: 10.1007/s11894-003-0014-x. [DOI] [PubMed] [Google Scholar]
- Szabo G, Catalano D, Bellerose G, Mandrekar P. Interferon alpha and alcohol augment nuclear regulatory factor-kappaB activation in HepG2 cells, and interferon alpha increases pro-inflammatory cytokine production. Alcohol Clin Exp Res. 2001;25:1188–1197. [PubMed] [Google Scholar]
- van Boxel-Dezaire AH, Rani MR, Stark GR. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity. 2006;25:361–372. doi: 10.1016/j.immuni.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Velazquez L, Fellous M, Stark GR, Pellegrini S. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell. 1992;70:313–322. doi: 10.1016/0092-8674(92)90105-l. [DOI] [PubMed] [Google Scholar]
- Vlotides G, Sorensen AS, Kopp F, Zitzmann K, Cengic N, Brand S, Zachoval R, Auernhammer CJ. SOCS-1 and SOCS-3 inhibit IFN-alpha-induced expression of the antiviral proteins 2,5-OAS and MxA. Biochem Biophys Res Commun. 2004;320:1007–1014. doi: 10.1016/j.bbrc.2004.06.051. [DOI] [PubMed] [Google Scholar]
- Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, Murthy K, Habermann A, Krausslich HG, Mizokami M, Bartenschlager R, Liang TJ. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MJ, Jonsson JR, Richardson MM, Lipka GM, Purdie DM, Clouston AD, Powell EE. Non-response to antiviral therapy is associated with obesity and increased hepatic expression of suppressor of cytokine signalling 3 (SOCS-3) in patients with chronic hepatitis C, viral genotype 1. Gut. 2006;55:529–535. doi: 10.1136/gut.2005.069674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley TE, McCarthy M, Breidi L, McCarthy M, Layden TJ. Impact of alcohol on the histological and clinical progression of hepatitis C infection. Hepatology. 1998;28:805–809. doi: 10.1002/hep.510280330. [DOI] [PubMed] [Google Scholar]
- Zhang T, Li Y, Lai JP, Douglas SD, Metzger DS, O'Brien CP, Ho WZ. Alcohol potentiates hepatitis C virus replicon expression. Hepatology. 2003;38:57–65. doi: 10.1053/jhep.2003.50295. [DOI] [PubMed] [Google Scholar]
- Zhang T, Lin RT, Li Y, Douglas SD, Maxcey C, Ho C, Lai JP, Wang YJ, Wan Q, Ho WZ. Hepatitis C virus inhibits intracellular interferon alpha expression in human hepatic cell lines. Hepatology. 2005;42:819–827. doi: 10.1002/hep.20854. [DOI] [PubMed] [Google Scholar]
- Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, Burton DR, Wieland SF, Uprichard SL, Wakita T, Chisari FV. Robust hepatitis C virus infection in vitro. PNAS. 2005;102:9294–9299. doi: 10.1073/pnas.0503596102. [DOI] [PMC free article] [PubMed] [Google Scholar]