Abstract
Aims
To identify subtypes of nonmedical opioid users, gender variations in psychiatric disorders, and quality of life in a representative sample of adults.
Methods
Analyses of data from the 2001–2002 National Epidemiologic Survey on Alcohol and Related Conditions (N=43,093). Latent class analysis (LCA) and multinomial logistic regression procedures examined subtypes of nonmedical opioid users.
Results
Approximately 5% (n=1,815) of adults used nonmedical opioids. LCA identified four subtypes: opioid–marijuana users (33%), opioid–other prescription drug users (9%), opioid–marijuana–hallucinogen users (28%), and opioid–polydrug users (30%). Subtypes were distinguished by race/ethnicity, gender, familial substance abuse, personal history of substance abuse treatment, and patterns of psychiatric disorders. Whites and men had increased odds of being in the opioid–polydrug and opioid–marijuana–hallucinogen subtypes. The opioid–other prescription drug use subtype had disproportionately affected women who were characterized by high rates of mood/anxiety disorders and low quality of life. Across all subtypes, women and men had similarly problematic substance use disorders; however, women had more major depression and disability in the mental health domain.
Conclusions
The generally high prevalence of psychiatric disorders among nonmedical opioid users, particularly women, underscores the need for comprehensive assessment and coordinated delivery of services to match needs with treatment, as well as continued monitoring of trends in opioid use and related problems.
Keywords: Comorbidity, Latent class analysis, Marijuana, Opioid use disorder, Prescription drug abuse
1. INTRODUCTION
Prescription opioids are among the most prevalent emerging drugs of nonmedical use and abuse in the United States (aSubstance Abuse and Mental Health Services Administration [SAMHSA], 2009a). National surveys of drug use, opioid-related overdose and mortality, and admissions to substance abuse treatment programs all indicate a substantial increase in nonmedical use and abuse of prescription opioids (Compton & Volkow, 2006; Johnston et al., 2009; Manchikanti, 2007; SAMHSA, 2009a; Zacny et al., 2003). According to results from the 2008 U.S. National Survey on Drug Use and Health, about 79% of past-year nonmedical users of prescription drugs (opioids, sedatives, stimulants, tranquilizers) used opioids; after cannabinoids, prescription opioid use disorders comprised the second most prevalent drug use disorder among the nine illicit and prescription-type drug classes (SAMHSA, 2009a).
These estimates demonstrate a need for research to better characterize this population because opioids have extremely high abuse potential and are associated with the highest rate of drug use-related overdose/mortality (Paulozzi et al., 2006; Veilleux et al., 2010). In particular, opioid addiction is associated with a wide range of personal, social, and medical problems, and has been identified as a chronic, relapsing disease that needs long term care (Leshner, 1998). While heroin use has been the central target for prevention and treatment, increased rates of nonmedical opioid use (i.e., nonmedical use of prescription opioids) and opioid-related admissions to substance abuse treatment programs have added a new dimension to the traditional picture of opioid users in need of treatment (SAMHSA, 2009b; Veilleux et al., 2010). Due to high societal costs of opioid addiction and some limitations of methadone treatment (e.g., it relies almost entirely on federal-approved clinics and is susceptible to abuse and overdose), the Drug Addiction Treatment Act of 2000 (DATA 2000) approved office-based treatment with buprenorphine, a Schedule III medication that requires fewer controls than methadone (Center for Substance Abuse Treatment, 2004). Thus, for the first time, physicians have the opportunity to use an opioid agonist medication for office-based treatment of opioid dependence. DATA 2000 has improved access to opioid addiction treatment by bringing it into the mainstream of medical practice, expanding the number of providers that can deliver it, and is an additional resource for treating this newer group of opioid-using individuals. Importantly, as shown by research that aimed to determine the feasibility of buprenorphine treatment (Moore et al., 2007; Sigmon, 2006; Sigmon et al., 2009), a better understanding of nonmedical opioid users is needed to inform clinicians as they develop treatment plans for nonmedical opioid users.
Recent national surveys also show that most (62%) new nonmedical opioid users are adults aged ≥ 18 years (SAMHSA, 2009). To date, adult studies have typically examined regional samples of drug users (Havens et al., 2009; Monga et al., 2007) and treatment-seeking patients (Banta-Green et al., 2009; Cicero et al., 2008; Green et al., 2009). While these studies have shown that drug use and psychiatric symptoms are relatively prevalent among nonmedical opioid users, information regarding specific DSM-IV substance use disorders (SUDs) and other mental disorders is often not available. For example, Havens et al. (2009) investigated a sample of rural stimulant users and found that other drug use (marijuana, cocaine, heroin) and symptoms of anxiety predicted nonmedical opioid use. Green et al. (2009) analyzed data from adults attending substance abuse treatment and found that all categories of drug use were associated with nonmedical opioid use even after controlling for patients’ history of medical problems and symptoms of anxiety/depression. Cicero et al. (2008) reported that adults seeking treatment for opioid analgesic abuse often initiated substance use in adolescence and reported histories of substance abuse treatment and low quality of life as seen by poorer mental and physical functioning. Further, studies have suggested considerable variability within the overall group of nonmedical opioid users. For example, a subset appears to use prescription opioids for self-medication for health-related problems; female users tend to have more mental health-related problems than males (Cicero et al., 2008; McCabe et al., 2009; Wu, Pilowsky et al., 2008); and there are at least two subgroups of nonmedical users consisting of polysubstance users and nonmedical opioid users with health-related problems (Cicero et al., 2008; McCabe et al., 2009). For these reasons, an epidemiological study of a representative sample has been recommended to more clearly describe subgroups of nonmedical opioid users in order to inform better intervention and research (Cicero et al., 2008).
For the goal of finding distinct subtypes (heterogeneous groups) within a sample, a latent class analysis (LCA) is particularly suitable because of its ability to specify unobserved (latent) subgroups of individuals (Muthén & Muthén, 2000). In LCA, observed variables are viewed as imperfect indicators of unobserved conditions, and a finite number of mutually exclusive classes are empirically derived from the response patterns of multiple variables. LCA can thus help elucidate different sets of nonmedical opioid users by classifying them into subgroups according to drug use patterns. We have demonstrated the use of LCA for enhancing understanding of subtypes of drug dependence (Wu, Blazer et al., 2009) and the extent of heterogeneity in polysubstance use among ecstasy users (Wu, Parrott et al., 2009).
Few studies have applied LCA to evaluate subtypes of nonmedical opioid users. Monga et al. (2007) analyzed 679 nonmedical opioid users recruited from fives cities in Canada. Three subtypes of users were identified that differed in drug use patterns (polydrug users, heroin–cocaine users, and injection drug users), with polydrug users showing a particularly high rate of depression and self-reported pain. Banta-Green et al. (2009) applied LCA to examine 704 chronic pain patients. They found three subtypes: a typical group that had persistent, moderate mental health and pain; an addictive group with elevated mental health symptoms and opioid problems, but with pain similar to the typical class; and a pain dysfunction group with greater pain, mental health, and opioid problems. Ghandour et al. (2008) utilized LCA to characterize opioid dependence patterns by analyzing the seven dependence criteria of opioid dependence among nonmedical opioid users in the 2002–2003 National Surveys on Drug Use and Health and found four groups of LCA-defined users. About 2% were classified as the most severe class characterized by endorsing multiple dependence symptoms, two classes (10%, 4%; respectively) reported an intermediate pattern of dependence symptoms, and a large group (84%) had a low probability of endorsing any dependence symptoms.
Together, these studies suggest the existence of different groups of nonmedical opioid users, however information about specific SUDs and other mental disorders is generally lacking. This information, nonetheless, is critical to provide a complete picture of the problems that need to be addressed by clinicians and researchers as they are often used as inclusion and exclusion criteria in clinical research and can impact treatment strategies, retention, and outcomes (Carroll, 1997; Carroll and Rounsaville, 2002; Fatséas et al., 2010; Strain, 2002; Veilleux et al., 2010). This study attempts to address this gap by investigating the presence of subtypes and their corresponding patterns of psychiatric disorders. It builds on prior research by enhancing the generalizability of results to subgroups using data from the largest national study of psychiatric comorbidity (the National Epidemiologic Survey on Alcohol and Related Conditions; NESARC); applying LCA to enhance the investigation of subtypes of nonmedical opioid users by examining drug use patterns from all drug classes complemented by multinomial logistic regression to explore external validators of LCA-defined subtypes from multiple domains; and by examining gender variations in psychiatric disorders and quality of life across subtypes as recommended by Cicero et al. (2008).
The following three questions were examined: (1) Are there subtypes of nonmedical opioid users distinguished by patterns of drug use? (2) If so, are these subtypes associated with distinct socioeconomic characteristics, SUDs and mental disorders, substance abuse treatment and familial substance abuse, and indicators of quality of life? (3) Are there gender differences in psychiatric disorders and quality of life across subtypes? The NESARC provided an excellent way to examine these issues because it measured not only substance use patterns, but also psychopathology and quality of life.
2. METHODS
2.1. Study Sample
From 2001–2002, the National Institute on Alcohol Abuse and Alcoholism conducted the NESARC, presently the largest and most ambitious comorbidity study (Grant et al., 2004). The NESARC provides prevalence rates for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Revision (DSM-IV) Axis I (substance use, mood, and anxiety disorders) and Axis II (personality) disorders, and is the first national study to assess DSM-IV personality disorders. Respondents were selected using a multistage cluster sampling design. The target population was the civilian non-institutionalized population aged ≥18 years who resided in the United States or the District of Columbia, including Alaska and Hawaii. Eligible respondents included persons living in households, military personnel living off base, and residents of group quarters (boarding houses, rooming houses, non-transient hotels and motels, shelters, facilities for housing workers, college quarters, and group homes).
Professional lay interviewers from the Bureau of the Census administered the face-to-face personal interviews using computer-assisted personal interviewing. Rights to confidentiality of NESARC participants were carefully protected. All respondents provided written informed consent and were assured that their participation was voluntary. To increase the accuracy of national estimates for demographic subgroups, Hispanics (N=8,308), non–Hispanic blacks (N=8,245), and respondents aged 18–24 years (N=5,199) were oversampled. Of the 43,093 respondents, 18,518 were male and 24,575 were female. The household and individual response rates were 89% and 93%, respectively. The overall survey response rate was 82%. Details of the survey designs are reported elsewhere (Grant et al., 2004).
2.2. Study Variables
Substance use and psychiatric disorders were assessed with the Alcohol Use Disorders and Associated Disabilities Interview Schedule–DSM-IV, an instrument of demonstrated reliability and validity (Grant et al., 2003). It assesses problems and disorders related to substance use (tobacco/nicotine, alcohol, inhalants, marijuana, cocaine, hallucinogens, sedatives, amphetamines, tranquilizers, opioid analgesics, and heroin), mood (major depression, dysthymia, mania, and hypomania), anxiety (panic disorder, social phobia, specific phobia, and generalized anxiety disorder), and personality (avoidant, dependent, obsessive-compulsive, paranoid, schizoid, histrionic, and antisocial).
For substance use, respondents were assessed for lifetime use of alcohol, tobacco/nicotine, and other drug classes. Drug use was defined as the use of substance(s) either without a doctor’s prescription; in greater amounts, more often, or longer than prescribed; or for a reason other than prescribed by a doctor. Respondents were asked to indicate whether they have ever used the following drug classes: marijuana, inhalants/solvents, cocaine/crack, hallucinogens, heroin, opioid analgesics, sedatives, amphetamines, and tranquilizers. Respondents who reported any lifetime use of the given substance then were assessed for DSM-IV abuse and dependence symptoms of the substance. Nonmedical opioid use referred to any self-reported nonmedical use of prescription opioid analgesics such as codeine, Darvon®, Percodan®, Dilaudid®, or Demerol® (not including heroin use); and age of first nonmedical use was categorized into in adolescence (<18 years) vs. in adulthood (≥ 18 years).
Substance abuse treatment use and familial substance abuse were examined as potential external validators for nonmedical opioid use subtypes (Wu, Howard et al., 2008; Wu, Parrott et al., 2009). A personal history of substance abuse treatment was defined as having ever received any treatment services for problems related to alcohol or drug use at any location (an inpatient ward, outpatient clinic, emergency room, substance abuse treatment program, mental health treatment program, jail, or self-help groups) (Grant et al., 2004). Familial substance abuse included any self-reported, positive family history of alcohol or drug use problems among any of the respondent’s biological family members (natural parents, sons, daughters, grandparents, full brothers, and full sisters).
Indicators of quality of life (SF-12 V2: Ware et al., 2002) were examined due to their association with nonmedical opioid use (Cicero et al., 2008) and inclusion as an outcome measure in treatment for opioid dependence (Veilleux et al., 2010). The 12 items reflect two summary disability measures (physical vs. mental disability) and eight sub-domains: physical functioning (engagement in moderate activities and ability to climb a flight of stairs); physical role (accomplishment and limitation); bodily pain; general health; vitality; social functioning; emotional role (accomplishment and limitation); and mental health (feeling calm and peaceful, feeling downhearted and depressed). Norm-based standardized scores (range: 0–100) were used to facilitate comparisons across groups.
Socioeconomic variables (age, gender, race/ethnicity, educational level, and total annual family income) were examined to elucidate demographic disparities in nonmedical opioid use subtypes.
2.3. Data Analysis
Because NESARC used a complex multistage survey design, data were weighted and analyzed with SUDAAN (Research Triangle Institute, 2006). Distributions of nonmedical opioid use by study variables and correlates of nonmedical opioid use were examined among all participants (N=43,093). Within the subsample of lifetime nonmedical opioid users (n=1,815), lifetime prevalence rates of prescription opioid use disorders and other drug use were calculated. LCA using Mplus (Muthén and Muthén, 2007) was then applied to eight dichotomous drug use variables (lifetime use of marijuana, inhalants, cocaine, hallucinogens, heroin, sedatives, amphetamines, and tranquilizers, respectively) to empirically determine the smallest number of subtypes (classes) with similar drug use patterns that explains their response patterns.
We evaluated the fit of LCA models using the Vuong-Lo-Mendell-Rubin likelihood ratio (VLMR) test and Bayesian Information Criterion (BIC) (Lo et al., 2001; Nylund et al., 2007). The VLMR test determines whether the data can be described by a model with K-1 classes relative to a model with K classes. A p-value < 0.05 indicates that the additional class significantly improves fit over a model with K-1 classes. BIC takes into account parsimony of the model and is commonly used to select the most parsimonious model, with smaller BIC indicating a better model and differences of 10 or more considered as evidence favoring one model over another (Raftery, 1995). We also considered the class size and interpretability in selecting a model that would be applicable to the subsequent analysis of external validators.
Multinomial logistic regression procedures (Research Triangle Institute, 2006) were then conducted to determine whether LCA-defined subtypes differed by socioeconomic characteristics, substance abuse treatment use, familial substance abuse, and prescription opioid use disorders. Finally, to explore gender differences in the profiles of various nonmedical opioid use subtypes, gender-specific prevalence rates of psychiatric disorders and indicators of quality of life were determined (Cicero et al., 2008). For ease of interpretation, 95% confidence intervals (CI) are reported. Due to multiple comparisons across subtypes of nonmedical opioid users, only results with p-values <0.01 are discussed. All results presented are weighted estimates taking into account complex survey design features, except for sample sizes (unweighted).
3. RESULTS
3.1. Nonmedical Opioid Users (Table 1)
Table 1.
Prevalence of lifetime nonmedical opioid use among adults aged 18 years or older: 2001–2002 National Epidemiologic Survey on Alcohol and Related Conditions (N=43,093)
| Selected variables | Nonmedical opioid use (yes) | Adjusted odds ratios1 (95% CI) |
|---|---|---|
| Sample size | Row % (SE) | |
| Age group, years | ||
| 18–29 | 7.4 (0.45) | 2.64 (2.25–3.10) |
| 30–44 | 5.7 (0.29) | 2.02 (1.75–2.32) |
| 45 or older | 2.9 (0.17) | 1.00 |
| Gender | ||
| Male | 6.1 (0.28) | 1.77 (1.57–2.00) |
| Female | 3.5 (0.19) | 1.00 |
| Race/ethnicity | ||
| White | 5.3 (0.23) | 1.00 |
| Black | 2.6 (0.23) | 0.48 (0.39–0.59) |
| American Indian/Alaska native | 9.1 (1.33) | 1.78 (1.30–2.43) |
| Asian/native Hawaiian | 2.7 (0.55) | 0.49 (0.32–0.75) |
| Hispanic | 3.1 (0.29) | 0.56 (0.46–0.69) |
| Educational level | ||
| < High school | 4.6 (0.33) | 0.94 (0.80–1.10) |
| High school | 4.7 (0.28) | 0.96 (0.84–1.09) |
| ≥ College | 4.8 (0.23) | 1.00 |
| Total family income | ||
| < $35,000 | 5.3 (0.26) | 1.40 (1.19–1.64) |
| $35,000–$69,999 | 4.8 (0.27) | 1.26 (1.07–1.48) |
| ≥ $70,000 | 3.8 (0.27) | 1.00 |
| Marital status | ||
| Married | 3.9 (0.19) | 1.00 |
| Widowed/separated/divorced | 5.0 (0.32) | 1.28 (1.11–1.47) |
| Never married | 7.0 (0.42) | 1.85 (1.62–2.11) |
| Family history of substance abuse | ||
| Yes | 6.9 (0.28) | 3.77 (3.23–4.41) |
| No | 1.9 (0.14) | 1.00 |
| Personal history of substance abuse treatment | ||
| Yes | 26.6 (1.19) | 9.90 (8.67–11.30) |
| No | 3.5 (0.16) | 1.00 |
Boldface: p<0.05.
CI: confidence interval; SE: standard error.
Adjusted odds ratio: The model included all variables listed in the first column.
About 5% (n=1,815) of all NESARC respondents used nonmedical prescription opioids in their lifetimes. A higher prevalence of nonmedical opioid use was found among young adults aged 18–29 years (7.4%), adults aged 30–44 years (5.7%), men (6.1%), whites (5.3%), those who had never married (7.0%), adults with a family income below $70K (4.8–5.3%), and those who reported a family history of substance abuse (6.9%) or a personal history of substance abuse treatment (26.6%). These findings are supported from adjusted logistic regression analyses.
3.2. Prescription Opioid Use Disorders
Among all respondents (N=43,093), 1.4% met criteria for a lifetime DSM-IV prescription opioid use disorder (1.1%, abuse; 0.3%, dependence). Among the 1,815 nonmedical opioid users, 22.8% met criteria for opioid abuse, and another 7.2% met criteria for opioid dependence. Men were more likely than women to have abuse (26.6% [95% CI: 23.4–30.1] vs. 16.9% [95% CI: 13.7–20.6]) and any abuse/dependence diagnosis (33.3% [95% CI: 19.6–37.3] vs. 24.8% [95% CI: 21.3–28.6]), but were as likely to have a dependence diagnosis as women (6.8% vs. 7.9%).
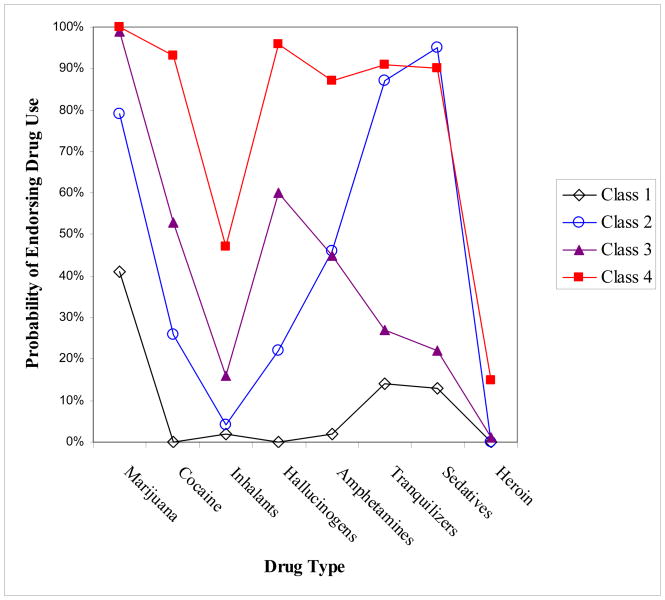
3.3. LCA-defined Subtypes of Nonmedical Opioid Users (Figure 1)
Figure 1.
Latent class analysis of subtypes of nonmedical opioid users: 2001–2002 National Epidemiologic Survey on Alcohol and Related Conditions (N=1,815)
Class 1: Opioid–marijuana users, 33%
Class 2: Opioid–other prescription drug users, 9%
Class 3: Opioid–marijuana–hallucinogen users, 28%
Class 4: Opioid–polydrug users, 30%
Marijuana (80%) was the drug most commonly used by nonmedical opioid users (n=1,815), followed by hallucinogens (49%), tranquilizers (47%), cocaine (46%), sedatives (45%), amphetamines (44%), inhalants (19%), and heroin (5%).
The 2-class model (BIC=13985; entropy=0.83) from LCA of the eight drug use variables was found to differ from a 1-class model in model fit (VLMR test, p<0.01). Next, the 2-class model was compared to a 3-class model (BIC=13644; entropy=0.77), which then was compared to a 4-class model using the VLMR test, and each comparison was significantly different (p<0.01), suggesting that a 4-class model (BIC=13559; entropy=0.78) was a better fit than others. The 4-class model did not differ from a 5-class (BIC=13582; entropy=0.74) model (VLMR test, p=0.432). These results suggested that a 4-class model was the best fit to the data, and each class size was adequate for further analysis (close to 10% for the smallest class). This model yielded the lowest BIC value (most parsimonious). The average conditional probability of correctly classifying each individual into each latent class was 0.91 for Class 1, 0.74 for Class 2, 0.89 for Class 3, and 0.90 for Class 4.
The four classes were distinguished by the extent of lifetime drug use (Figure 1). Class 1 (opioid–marijuana users, 33% of all nonmedical opioid users) manifested a moderate probability of using marijuana as their other drug of use. Class 2 (opioid–other prescription drug users, 9%) comprised primary users of marijuana and other prescription drugs (tranquilizers and sedatives). Class 3 (opioid–marijuana–hallucinogen users, 28%) mainly used other illicit drugs. Class 4 (opioid–polydrug users, 30%) manifested a comparatively high probability of using all drug classes.
3.4. Multinomial Logistics Regression Analysis of LCA-defined Subtypes (Table 2)
Table 2.
Adjusted odds ratios (AOR) and 95% confidence intervals (CI) of LCA-defined subtypes of nonmedical opioid users: 2001–2002 National Epidemiologic Survey on Alcohol and Related Conditions (N =1,815)
| Multinomial logistic model: AOR (95% CI)1 | C2 vs. C1: Opioid–other prescription drugs vs. opioid–marijuana | C3 vs. C1: Opioid– marijuana–hallucinogen vs. opioid–marijuana | C4 vs. C1: Opioid–polydrug vs. opioid–marijuana | C3 vs. C2: Opioid–marijuana–hallucinogen vs. opioid–other prescription drugs | C4 vs. C2: Opioid–polydrug vs. opioid–other prescription drugs | C4 vs. C3: Opioid–polydrug vs. opioid–marijuana–hallucinogen |
|---|---|---|---|---|---|---|
| Age group vs. ≥ 45 years | ||||||
| 18–29 years | 0.94 (0.45–197) | 1.86 (1.17–2.98)b | 0.64 (0.42–0.96)a | 1.98 (0.93–4.21) | 0.67 (0.32–1.44) | 0.34 (0.20–0.59)c |
| 30–44 years | 0.81 (0.47–1.38) | 1.35 (0.91–2.01) | 1.04 (0.74–1.47) | 1.68 (0.97–2.92) | 1.29 (0.76–2.21) | 0.77 (0.52–1.14) |
| Gender vs. female | ||||||
| Male | 1.15 (0.75–1.76) | 1.71 (1.25–2.35)c | 2.25 (1.61–3.14)c | 1.49 (0.94–2.36) | 1.96 (1.27–3.01)b | 1.31 (0.92–1.87) |
| Race/ethnicity vs. white | ||||||
| Black | 0.41 (0.20–0.83)a | 0.37 (0.22–0.63)c | 0.32 (0.19–0.56)c | 0.91 (0.40–2.09) | 0.79 (0.36–1.71) | 0.87 (0.45–1.67) |
| Hispanic | 0.66 (0.32–1.38) | 0.63 (0.37–1.07) | 0.50 (0.28–0.89)a | 0.96 (0.46–2.00) | 0.75 (0.36–1.58) | 0.79 (0.44–1.41) |
| Other | 1.01 (0.43–2.35) | 0.81 (0.43–1.55) | 0.84 (0.39–1.81) | 0.81 (0.35–1.83) | 0.83 (0.34–2.05) | 1.03 (0.50–2.12) |
| Education vs. ≥ college | ||||||
| <High school | 1.00 (0.53–1.89) | 0.93 (0.57–1.52) | 0.81 (0.50–1.29) | 0.93 (0.48–1.82) | 0.81 (0.43–1.51) | 0.87 (0.53–1.43) |
| High school | 1.18 (0.70–1.97) | 1.16 (0.80–1.70) | 0.79 (0.54–1.17) | 0.99 (0.59–1.66) | 0.67 (0.39–1.15) | 0.68 (0.46–1.01) |
| Family income vs. ≥ $70K | ||||||
| < $35,000 | 0.75 (0.36–1.54) | 0.62 (0.40–0.98)a | 0.69 (0.45–1.06) | 0.83 (0.40–1.74) | 0.92 (0.43–1.99) | 1.11 (0.72–1.72) |
| $35,000–$69,999 | 0.89 (0.44–1.84) | 0.79 (0.49–1.27) | 0.73 (0.46–1.15) | 0.88 (0.42–1.86) | 0.81 (0.39–1.70) | 0.92 (0.61–1.39) |
| Marital status vs. married | ||||||
| Wid./sep./divorced | 2.55 (1.55–4.19)c | 1.87 (1.15–3.01)a | 2.23 (1.44–3.44)c | 0.73 (0.41–1.30) | 0.87 (0.52–1.49) | 1.20 (0.74–1.93) |
| Never married | 0.93 (0.51–1.69) | 1.02 (0.69–1.51) | 1.38 (0.91–2.09) | 1.10 (0.58–2.09) | 1.49 (0.79–2.82) | 1.36 (0.89–2.06) |
| Age of first opioid use vs. ≥ 18 years | ||||||
| 17 years or younger | 1.54 (0.85–2.80) | 1.53 (1.08–2.19)a | 3.75 (2.53–5.54)c | 0.99 (0.56–1.77) | 2.43 (1.42–4.14)b | 2.44 (1.68–3.54)c |
| Familial substance abuse vs. no | ||||||
| Yes | 2.26 (1.23–4.16)c | 2.34 (1.58–3.46)c | 2.20 (1.50–3.23)c | 1.03 (0.58–1.85) | 0.97 (0.51–1.84) | 0.94 (0.61–1.46) |
| Substance abuse treatment vs. no | ||||||
| Yes | 2.95 (1.63–5.35)c | 2.17 (1.38–3.43)c | 5.54 (3.61–8.50)c | 0.74 (0.41–1.32) | 1.88 (1.12–3.15)a | 2.55 (1.81–3.58)c |
| Opioid use disorders vs. no | ||||||
| Abuse, lifetime | 1.05 (0.56–1.97) | 0.98 (0.65–1.48) | 1.88 (1.27–2.78)b | 0.93 (0.47–1.83) | 1.79 (0.96–3.31) | 1.92 (1.30–2.82)c |
| Dependence, lifetime | 2.86 (1.34–6.10)b | 1.01 (0.47–2.18) | 1.36 (0.65–2.81) | 0.35 (0.17–0.74)b | 0.47 (0.24–0.94)a | 1.34 (0.71–2.53) |
Included all variables in the first column;
p<0.05;
p<0.01;
p<0.001; due to multiple comparisons, only results with p<0.01 are discussed.
Relative to opioid–marijuana users, familial substance abuse and history of substance abuse treatment increased the odds for being in Classes 2–4; male gender and white race (vs. black race) increased odds for being in the opioid–marijuana–hallucinogen and the opioid–polydrug subtypes. Additionally, being widowed, separated, or divorced (vs. married) and opioid dependence were associated with being in the opioid–other prescription drug subtype; younger ages (18–29 years vs. 45+ years) increased the odds for being in the opioid–marijuana–hallucinogen subtype; and being widowed, separated, or divorced (vs. married), onset of nonmedical opioid use before 18 years, and opioid abuse increased odds for being in the opioid–polydrug subtype.
Supplemental comparisons among the more problematic groups (Classes 2–4) showed that opioid dependence was more likely to be found in the opioid–other prescription drug group (vs. the opioid–marijuana–hallucinogen group). Male gender and onset of nonmedical opioid use before 18 years were more likely to be in the opioid–polydrug subtype as compared with the opioid–other prescription drug subtype. Onset of nonmedical opioid use before 18 years, opioid abuse, and history of substance abuse treatment also increased odds for being in the opioid–polydrug subtype as compared with the opioid–marijuana–hallucinogen subtype.
3.5. Substance Use Disorders by LCA-defined Subtypes (Table 3)
Table 3.
Substance use disorders by LCA-defined subtypes of nonmedical opioid users (N =1,815)
| Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lifetime disorders: column % (95% CI) |
C1: Opioid– marijuana |
C2: Opioid–other prescription drugs |
C3: Opioid– marijuana– hallucinogen |
C4: Opioid– polydrug |
χ2 p-value1 |
C1: Opioid– marijuana |
C2: Opioid– other prescription drugs |
C3: Opioid– marijuana– hallucinogen |
C4: Opioid– polydrug |
χ2 p-value1 |
| Sample size | 283 | 85 | 275 | 371 | 344 | 90 | 195 | 172 | ||
| Opioid abuse | 17.8 (13.1–23.7) | 16.6 (8.2–30.7) | 21.2 (16.4–27.0) | 40.3 (34.5–46.3) | <0.01 | 14.5 (9.8–20.7) | 18.6 (11.0–29.7) | 15.4 (10.3–22.3) | 22.4 (15.9–30.7) | NS |
| Opioid dependence | 5.3 (2.4–11.0) | 12.3 (6.2–22.8) | 5.6 (2.9–10.5) | 7.6 (4.8–11.8) | NS | 3.0 (1.6–5.4) | 22.2 (13.5–34.3) | 5.4 (2.7–10.6) | 13.6 (8.8–20.2) | <0.01 |
| Opioid use disorders | 23.1 (17.9–29.2) | 28.9 (18.3–42.5) | 26.8 (21.3–33.2) | 47.8 (41.2–54.6) | <0.01 | 17.4 (12.6–23.6) | 40.8 (29.4–53.3) | 20.8 (14.9–28.1) | 36.0 (27.7–45.2) | <0.01 |
| Nicotine dependence | 37.9 (30.5–46.0) | 63.6 (50.1–75.3) | 57.4 (50.5–63.9) | 63.6 (57.4–69.4) | <0.01 | 34.5 (28.2–41.4) | 70.8 (59.7–79.9) | 55.5 (46.4–64.1) | 70.5 (61.5–78.2) | <0.01 |
| Alcohol use disorders | 71.9 (65.6–77.4) | 78.9 (66.4–87.6) | 88.2 (82.7–92.1) | 92.0 (88.4–94.6) | <0.01 | 39.5 (32.7–46.8) | 77.1 (66.7–85.0) | 79.7 (71.4–86.1) | 90.4 (85.0–94.0) | <0.01 |
| Marijuana use disorders | 23.5 (18.0–30.1) | 48.0 (34.8–61.6) | 64.0 (57.4–70.4) | 80.6 (75.8–84.7) | <0.01 | 12.8 (9.0–17.9) | 35.3 (24.3–48.2) | 62.2 (53.2–70.5) | 66.4 (57.0–74.6) | <0.01 |
| Cocaine use disorders | 0.3 (0.1–1.4) | 11.9 (6.0–22.0) | 24.8 (19.8–30.5) | 62.3 (56.1–68.1) | <0.01 | 0.0 | 23.9 (15.1–35.6) | 24.7 (18.2–32.8) | 53.9 (44.8–62.7) | <0.01 |
| Inhalant use disorders | 0.0 | 0.0 | 2.6 (1.1–6.1) | 13.9 (10.0–19.0) | <0.01 | 0.7 (0.1–4.6) | 1.9 (0.4–7.5) | 2.7 (0.8–9.0) | 7.9 (4.1–14.8) | NS |
| Hallucinogen use disorders | 0.0 | 0.7 (0.1–4.8) | 25.8 (19.6–33.0) | 45.8 (39.5–52.2) | <0.01 | 0.4 (0.1–3.2) | 2.3 (0.5–9.6) | 17.1 (11.3–25.2) | 40.3 (32.0–49.2) | <0.01 |
| Amphetamine use disorders | 0.3 (0.1–1.5) | 16.0 (9.2–26.4) | 17.6 (12.5–24.1) | 46.2 (39.9–52.6) | <0.01 | 0.6 (0.2–1.9) | 24.2 (15.1–36.6) | 21.7 (15.4–29.8) | 50.8 (41.9–59.8) | <0.01 |
| Tranquilizer use disorders | 3.0 (1.2–7.6) | 25.2 (16.1–37.2) | 6.8 (3.7–12.1) | 41.1 (34.5–48.1) | <0.01 | 0.9 (0.3–2.4) | 22.6 (14.2–33.9) | 5.1 (2.7–9.4) | 30.3 (22.8–39.1) | <0.01 |
| Sedative use disorders | 1.8 (0.5–6.2) | 18.0 (10.7–28.7) | 3.5 (1.8–6.7) | 40.7 (33.8–47.9) | <0.01 | 0.8 (0.2–3.1) | 28.3 (18.9–40.1) | 4.2 (1.9–9.0) | 33.0 (25.0–42.1) | <0.01 |
| Heroin use disorders | 0.0 | 0.0 | 2.0 (0.9–4.5) | 9.9 (7.1–13.6) | <0.01 | 0.0 | 0.0 | 0.0 | 8.5 (4.4–15.8) | NS |
| Total number of the 11 disorders, mean* | 1.6 (1.45–1.78) | 2.9 (2.45–3.38) | 3.2 (2.96–3.43) | 5.4 (5.04–5.83) | <0.01 | 1.1 (0.94–1.21) | 3.3 (2.76–3.78) | 2.9 (2.61–3.26) | 4.9 (4.37–5.39) | <0.01 |
NS: p>0.05;
due to multiple comparisons, only results with p<0.01 are discussed. CI: confidence intervals.
F-test for mean; the 11 disorders included disorders from use of opioids, nicotine, alcohol, marijuana, cocaine, inhalants, hallucinogens, amphetamines, tranquilizers, sedatives, and heroin.
Gender variations in lifetime SUDs by subtypes were determined (Table 3). Among men, opioid–polydrug users had the highest prevalence of opioid abuse (40%) and disorders related to use of opioids (48%), nicotine (64%), alcohol (92%), marijuana (81%), cocaine (62%), amphetamines (46%), hallucinogens (46%), sedatives (41%), tranquilizers (41%), inhalants (14%), and heroin (10%). Opioid–other prescription drug users also exhibited the highest prevalence of nicotine dependence (64%) and tranquilizer use disorders (25%). Opioid–marijuana–hallucinogen users differed from opioid–other prescription drug users in having a higher rate of disorders related to use of inhalants (2.6% vs. 0%), hallucinogens (26% vs. 0.7%), and heroin (2% vs. 0%). Opioid–marijuana users showed the lowest rate of disorders related to use of marijuana (24%) and other drugs (≤ 3%).
Among women, opioid–other prescription drug users resembled opioid–polydrug users in having the highest rates of opioid dependence (22%, 14%, respectively) and disorders related to use of opioids (41%, 36%), nicotine (71%, 71%), tranquilizers (23%, 30%), and sedatives (28%, 33%); opioid–marijuana–hallucinogen drug users had a higher rate of marijuana and hallucinogen use disorder than opioid–other prescription drug users. In both genders, the mean number of SUDs was highest among opioid–polydrug users (5.4 [95% CI: 5.04–5.83] in men; 4.9 [95% CI: 4.37–5.39] in women), following by opioid–other prescription drug users (2.9 [95% CI: 2.45–3.38] in men; 3.3 [95% CI: 2.76–3.78] in women), opioid–marijuana–hallucinogen users (3.2 [95% CI: 2.96–3.43] in men; 2.9 [95% CI: 2.61–3.26] in women), and opioid–marijuana users (1.6 [95% CI: 1.45–1.78] in men; 1.1 [95% CI: 0.94–1.21] in women).
3.6. Lifetime Mental Disorders by LCA-defined Subtypes (Table 4)
Table 4.
Mental disorders by LCA-defined subtypes of nonmedical opioid users (N =1,815)
| Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lifetime mental disorders: column % (95% CI) |
C1: Opioid– marijuana |
C2: Opioid– other prescription drugs |
C3: Opioid– marijuana– hallucinogen |
C4: Opioid– polydrug |
χ2 p-value |
C1: Opioid– marijuana |
C2: Opioid– other prescription drugs |
C3: Opioid– marijuana– hallucinogen |
C4: Opioid– polydrug |
χ2 p-value |
| Sample size | 283 | 85 | 275 | 371 | 344 | 90 | 195 | 172 | ||
| Mood problem/disorder | ||||||||||
| Major depression | 24.5 (19.0–30.8) | 39.2 (27.5–52.3) | 35.6 (29.5–42.1) | 42.0 (35.8–48.4) | <0.01 | 41.0 (34.6–47.7) | 66.6 (54.5–76.8) | 53.9 (45.4–62.1) | 55.7 (47.4–63.8) | <0.01 |
| Dysthymia | 3.7 (1.9–6.9) | 18.6 (10.2–31.5) | 7.1 (4.4–11.3) | 15.9 (12.1–20.7) | <0.01 | 10.5 (7.4–14.7) | 36.4 (26.8–47.2) | 20.3 (14.4–27.8) | 27.6 (20.0–36.8) | <0.01 |
| Mania | 4.2 (2.5–7.0) | 13.8 (7.7–23.4) | 13.5 (9.2–19.4) | 15.9 (12.0–20.8) | <0.01 | 11.4 (7.9–16.1) | 25.9 (16.7–37.7) | 14.8 (10.1–21.2) | 23.8 (17.0–32.4) | <0.01 |
| Hypomania | 7.8 (4.9–12.2) | 8.4 (3.5–19.0) | 6.3 (3.8–10.2) | 7.0 (4.4–11.1) | NS | 6.2 (4.0–9.4) | 8.3 (3.4–18.7) | 7.0 (3.4–14.0) | 8.7 (4.5–16.2) | NS |
| Anxiety disorder | ||||||||||
| Panic disorder | 4.5 (2.3–8.8) | 9.8 (4.8–19.0) | 8.9 (6.0–13.0) | 11.2 (8.2–15.1) | NS | 10.0 (6.8–14.4) | 16.1 (9.5–25.9) | 14.2 (8.8–21.9) | 23.7 (16.9–32.6) | <0.051 |
| Social phobia | 6.3 (3.1–12.4) | 3.8 (1.0–13.4) | 12.9 (8.1–20.0) | 7.7 (5.3–11.2) | NS | 10.5 (7.3–15.0) | 23.6 (15.2–34.7) | 17.0 (10.6–26.0) | 18.3 (12.3–26.4) | <0.051 |
| Specific phobia | 9.4 (6.2–14.0) | 20.3 (11.6–32.9) | 13.7 (9.6–19.1) | 13.2 (9.5–18.2) | NS | 21.9 (16.8–27.9) | 28.0 (18.9–39.4) | 28.8 (21.2–37.8) | 30.6 (22.6–39.9) | NS |
| Generalized anxiety | 3.5 (1.7–7.1) | 11.5 (5.5–22.6) | 5.1 (3.1–8.4) | 9.9 (7.1–13.6) | <0.051 | 9.3 (6.2–13.7) | 31.6 (22.0–43.1) | 14.4 (8.7–23.0) | 18.7 (12.5–27.1) | <0.01 |
| Personality disorder | ||||||||||
| Avoidant | 2.5 (0.9–4.3) | 12.5 (5.6–14.8) | 6.6 (3.7–11.5) | 6.7 (4.2–10.4) | NS | 6.2 (3.6–10.4) | 14.8 (8.6–24.3) | 13.8 (8.1–22.5) | 9.7 (5.5–16.5) | NS |
| Dependent | 0.5 (0.1–3.7) | 0.0 | 2.5 (0.8–8.0) | 1.8 (0.7–4.8) | NS | 0.7 (0.2–2.9) | 4.2 (1.3–12.9) | 1.8 (0.6–5.1) | 2.8 (1.2–6.5) | NS |
| Obsessive-compulsive | 11.8 (7.8–17.5) | 9.9 (4.7–19.4) | 19.1 (14.0–25.6) | 14.4 (10.6–19.1) | NS | 16.8 (12.9–21.7) | 21.6 (13.8–32.3) | 16.6 (10.6–25.2) | 23.5 (16.4–32.5) | NS |
| Paranoid | 6.0 (3.4–10.3) | 3.3 (1.0–10.6) | 10.8 (7.0–16.3) | 11.0 (7.7–15.5) | NS | 10.8 (7.8–14.8) | 23.6 (14.9–35.3) | 15.5 (9.9–23.6) | 17.6 (11.8–25.4) | <0.01 |
| Schizoid | 7.3 (4.2–12.5) | 10.9 (4.9–22.8) | 9.3 (5.9–14.3) | 9.9 (6.5–14.8) | NS | 5.9 (3.6–9.3) | 11.7 (5.9–21.9) | 11.8 (6.8–19.8) | 12.9 (8.0–20.2) | NS |
| Histrionic | 3.2 (1.7–6.0) | 5.7 (2.3–13.3) | 11.0 (7.0–16.9) | 6.6 (4.0–10.7) | NS | 3.5 (1.8–6.6) | 12.6 (6.6–22.7) | 8.4 (4.6–14.9) | 11.8 (7.1–18.9) | <0.051 |
| Antisocial | 13.3 (8.9–19.5) | 18.4 (9.1–33.6) | 26.5 (20.6–33.5) | 33.7 (28.5–39.3) | <0.01 | 6.4 (4.1–9.8) | 20.4 (12.0–32.4) | 16.1 (10.5–23.8) | 25.2 (17.9–34.2) | <0.01 |
NS: p>0.05;
due to multiple comparisons, only results with p<0.01 are discussed. CI: confidence intervals.
Among men, opioid–polydrug users had higher rates than opioid–marijuana users of major depression (42% vs. 25%), dysthymia (16% vs. 4%), mania (16% vs. 4%), and antisocial personality disorder (34% vs. 13%). Among women, the other three subtypes (Classes 2–4) had similar rates of mental disorders. However, only opioid–other prescription drug users showed higher rates than opioid–marijuana users in major depression (67% vs. 41%), dysthymia (36% vs. 11%), mania (26% vs. 11%), generalized anxiety disorder (32% vs. 9%), and paranoid personality disorder (24% vs. 11%); the three more problematic subtypes in women had higher rates of antisocial personality disorder than opioid–marijuana users (16–25% vs. 6%). For all subtypes, women tended to have a higher rate of mood or anxiety disorders than men in their corresponding subtypes.
3.7. Quality of Life by LCA-defined Subtypes (Table 5)
Table 5.
Indicators of quality of life (SF-12, version 2) by LCA-defined subtypes of nonmedical opioid users (N =1,811*)
| Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quality of life: mean score (95% CI) |
C1: Opioid– marijuana |
C2: Opioid–other prescription drugs |
C3: Opioid– marijuana– hallucinogen |
C4: Opioid– polydrug |
F-test p-value |
C1: Opioid– marijuana |
C2: Opioid–other prescription drugs |
C3: Opioid– marijuana– hallucinogen |
C4: Opioid– polydrug |
F-test p-value |
| Sample size | 283 | 85 | 274 | 370 | 343 | 90 | 194 | 172 | ||
| Physical disability | 50.41 (48.93–51.89) | 50.05 (47.78–52.32) | 51.13 (49.65–52.61) | 50.54 (49.12–51.95) | NS | 49.62 (48.23–51.01) | 48.48 (45.51–51.45) | 52.19 (50.46–53.92) | 51.68 (49.77–53.58) | <0.051 |
| Mental disability | 51.35 (49.99–52.72) | 48.43 (45.68–51.18) | 50.66 (49.21–52.12) | 49.19 (48.14–50.24) | <0.051 | 48.93 (47.60–50.27) | 42.59 (39.17–46.02) | 45.50 (43.25–47.76) | 44.62 (42.48–46.76) | <0.01 |
| Physical functioning | 52.25 (51.08–53.42) | 52.08 (49.98–54.18) | 52.51 (51.31–53.71) | 52.07 (50.96–53.17) | NS | 49.95 (48.66–51.24) | 47.78 (44.79–50.78) | 51.81 (50.36–53.26) | 51.28 (49.62–52.95) | <0.051 |
| Physical role | 50.78 (49.51–52.06) | 50.71 (48.07–53.36 | 50.97 (49.39–52.56) | 50.79 (49.30–52.29) | NS | 50.30 (49.04–51.56) | 46.53 (43.20–49.85) | 50.49 (48.93–52.05) | 50.20 (48.59–51.81) | NS |
| Bodily pain | 48.39 (46.59–50.20) | 46.95 (44.27–49.63) | 49.92 (48.35–51.49) | 47.82 (46.18–49.46) | NS | 47.40 (45.80–49.01) | 45.97 (42.78–49.16) | 49.44 (47.59–51.30) | 47.53 (45.45–49.62) | NS |
| General health | 49.86 (48.26–51.45) | 46.75 (43.86–49.63) | 49.00 (47.13–50.86) | 48.64 (47.21–50.06) | NS | 48.95 (47.38–50.53) | 45.85 (42.31–49.38) | 49.48 (47.33–51.63) | 49.03 (46.96–51.11) | NS |
| Vitality | 53.87 (52.31–55.42) | 51.11 (48.15–54.06) | 53.75 (52.41–55.09) | 52.08 (50.86–53.29) | NS | 50.81 (49.61–52.00) | 47.78 (44.38–51.19) | 49.88 (47.93–51.84) | 50.27 (48.49–52.06) | NS |
| Social functioning | 50.31 (48.86–51.75) | 49.53 (46.68–52.38) | 50.93 (49.56–52.29) | 49.33 (48.01–50.64) | NS | 49.44 (47.99–50.90) | 43.89 (40.57–47.21) | 47.71 (45.28–50.14) | 47.14 (45.18–49.09) | <0.051 |
| Emotional role | 50.69 (49.49–51.88) | 48.03 (45.30–50.75) | 50.00 (48.29–51.70) | 49.59 (48.36–50.82) | NS | 49.04 (47.58–50.50) | 42.50 (38.86–46.14) | 47.08 (45.33–48.83) | 44.85 (42.76–46.94) | <0.01 |
| Mental health | 51.06 (49.63–52.48) | 48.69 (46.07–51.32) | 50.42 (48.99–51.85) | 48.76 (47.56–49.95) | NS | 48.08 (46.65–49.52) | 43.05 (40.07–46.02) | 45.53 (43.26–47.80) | 45.12 (42.91–47.34) | <0.01 |
NS: p>0.05;
due to multiple comparisons, only results with p<0.01 are discussed. CI: confidence intervals.
A total of 4 cases with missing data on SF-12 were not included in the analysis.
Finally, there was little variation in quality of life by subtype among men. Among women, opioid–other prescription drug users exhibited lower quality of life in mental disability (mean scores: 42.59 [95% CI: 39.17–46.02] vs. 48.93 [95% CI: 47.60–50.27]), emotional role (mean scores: 42.50 [95% CI: 38.86–46.14] vs. 49.04 [95% CI: 47.58–50.50]), and mental health (mean scores: 43.05 [95% CI: 40.07–46.02] vs. 48.08 [95% CI: 46.65–49.52]) than opioid–marijuana users. For all subtypes, women reported lower quality of life in mental health than men in their corresponding subtypes.
4. DISCUSSION
This study documents the presence of considerable heterogeneity among nonmedical opioid users in a nationally representative sample of American adults. Approximately 5% of NESARC participants were nonmedical opioid users; of them, 23% and 7% met criteria for lifetime prescription opioid abuse and dependence, respectively. In both genders, opioid–polydrug users showed the highest prevalence of disorders related to use of cocaine, amphetamines, hallucinogens, and heroin; opioid–marijuana–hallucinogen users had a higher rate of hallucinogen use disorders than opioid–other prescription drug users, while the latter exhibited a higher prevalence of tranquilizer and sedative use disorders; and opioid–marijuana users showed lower rates of most psychiatric disorders than the others. The three more problematic groups were supported by their elevated odds of familial substance abuse and personal history of substance abuse treatment as compared to the milder opioid–marijuana group. These findings reveal the diversity in psychopathology among nonmedical opioid users and provide preliminary support for the four LCA-defined subtypes across gender.
4.1. Opioid–polydrug Users (30% of nonmedical users)
The study’s most salient findings concern the distinct patterns of correlates and psychiatric disorders across subtypes. Close to one third of nonmedical opioid users were classified as opioid–polydrug users who on average met criteria for five SUDs. Of this group, a high proportion had a prescription opioid use disorder (48% in men; 36% in women) and major depression (42% in men; 56% in women). Additionally, the majority (>50%) met criteria for disorders related to use of tobacco, alcohol, marijuana, and cocaine; 30–50% had disorders related to use of hallucinogens, amphetamines, tranquilizers, and sedatives; and more than one quarter had an antisocial personality disorder (34% in men; 25% in women). Their high-risk profile is supported by their significant associations with early onset of nonmedical opioid use and history of substance abuse treatment. Findings for this subtype are line with those from adults who sought treatment for prescription opioid abuse: they had high rates of alcoholism and nicotine dependence, reported history of substance abuse treatment, used multiple substances, and exhibited elevated mental health problems (Cicero et al., 2008).
Prior studies on comorbidity have been based mainly on selected samples of opioid-dependent patients who were heroin users and have documented high lifetime prevalence rates of psychiatric disorders (44–93%), major depression (4–54%), anxiety disorder (8–60%), and antisocial personality disorder (10–55%) (Strain, 2002). Comorbid SUDs and other mental disorders, unfortunately, confer an additional risk for poorer prognosis and treatment response among opioid-abusing patients (Strain, 2002; Veilleux et al., 2010). Although this study examined a national sample of primary users of prescription opioids (95% of all nonmedical opioid users had never used heroin), a disturbingly high rate of psychiatric disorders were noted in this large subtype specifically, and among all users generally. This finding has implications for research because opioid dependence treatment research has focused primarily on heroin users, but increasing rates of nonmedical opioid use indicate that clinicians are now considerably more likely to encounter prescription opioid-abusing patients (Veilleux et al., 2010). As primary nonmedical opioid users are likely to be white and younger while heroin users tend to be black and older (Mendelson et al., 2008; Rosenblum et al., 2007), further studies are needed to elucidate different, effective treatment strategies for primary nonmedical opioid users versus heroin users. Relevant areas for future research may include moderating effects of psychiatric disorders or supplemental psychiatric care on treatment retention and response, influences of pharmacological treatment (e.g., anti-depressants) on dosing and adverse effects of opioid agonists, as well as treatment strategies (e.g., methadone vs. buprenorphine; detoxification, tapering, or maintenance) (Sigmon et al., 2009; Veilleux et al., 2010; Woody et al., 2008).
4.2. Opioid–other Prescription Drug Users (9% of nonmedical users)
While this group had fewer SUDs, rates of nicotine dependence and prescription-type SUDs, including opioid (in women only), sedative (in women only), and tranquilizer use disorders, were as high as those in the opioid–polydrug group. This subtype also was distinct from opioid–marijuana–hallucinogen users in having higher rates of opioid (in women only), tranquilizer, and sedative use disorders, but lower rates of marijuana (in women only), inhalant (in men only), heroin (in men only), and hallucinogen use disorders. Additionally, members of this group had similarly high rates of mood, anxiety, and personality disorders as the opioid–polydrug group. In particular, women in this subtype not only had higher rates of several mental disorders, but also reported lower quality of life than women in the opioid–marijuana subtype. These results tend to agree with recent studies of treatment-seeking patients (Cicero et al., 2008) and a combined sample of adults with opioid or heroin use disorders (Grella et al., 2009) showing that a subset of female nonmedical opioid users experience greater mental health problems and lower quality of life than male users. Thus, these results extend from prior research by using LCA to pinpoint this prescription drug abuse subtype that disproportionately affects women, and elucidating their unique pattern of psychiatric disorders and low quality of life from mental health disability.
These findings also have implication for research. First, they suggest gender differences in risk for nonmedical opioid use (Cicero et al., 2008; Green et al., 2009). As women are reported to use more prescription opioids and have more health-related complaints than men (Cicero et al., 2008; Green et al., 2009; McCabe et al., 2009), research is needed to clarify how mental health conditions, especially depression and anxiety, moderate gender differences in risk for nonmedical opioid use and disorder; and whether self-medication of mental/medical problems explains more variance of comorbid conditions among women, while a substance-induced model (primary drug abuse and secondary mental disorders) is more related to comorbid conditions among men (Fatséas et al., 2010; Sullivan et al., 2005). This distinction is important for developing effective therapeutic plans (Strain, 2002). Second, gender differences in mental disorders and quality of life indicate that, in treatment studies for opioid use disorders, these characteristics should be carefully evaluated at intake and statistically adjusted for or examined in analyses. This potentially confounding issue is particularly important while specific SUD or mental disorders and quality of life are included as treatment outcome variables (e.g., Veilleux et al., 2010).
4.3. Opioid–marijuana–hallucinogen Users (28% of nonmedical users)
Relative to opioid–polydrug users, members of this group were less likely to initiate nonmedical opioid use in adolescence, use substance abuse treatment, and meet criteria for opioid abuse. Except for similar rates of marijuana and inhalant use disorders in women, they reported lower rates of drug use disorders than opioid–polydrug users. This subtype hence can be viewed as comprising moderately problematic nonmedical opioid users who on average had three SUDs, and about one-fourth had a prescription opioid use disorder. Moreover, members of this group were discrete from opioid–other prescription drug users because they had higher rates of hallucinogen use disorders but much lower rates of prescription tranquilizer or sedative use disorders; the reverse pattern was noted for opioid–other prescription drug users. Thus, while various subtypes may be located on a continuum of drug use problems, subgroup heterogeneity does exist as shown by different preference of drugs of abuse. This diversity justifies the need for a comprehensive assessment of SUDs to incorporate this variety into research and treatment efforts (Carroll and Rounsaville, 2002; Strain, 2002). Further, due to lesser involvement with drug abuse and treatment than the opioid–polydrug group, this group may be good candidates for brief motivational interventions or office-based treatment. However, the elevated rate of major depression among women (54%) suggests that they should be screened for depression and need for antidepressant medications or psychotherapy in addition to interventions that focus on their drug use problems (e.g., Strain, 2002).
4.4. Opioid–marijuana Users (33% of nonmedical users)
Lastly, opioid–marijuana users showed lower rates of most SUDs and other mental disorders than the other subtypes. They averaged about one SUD (1.6 in men, 1.1 in women) and were associated with African American (vs. white) race and being married (vs. married previously). Their lower risk profile was supported by reduced odds of familial substance abuse and personal history of receiving substance abuse treatment as compared with the other subtypes. Although they showed comparatively low rates of SUDs, about one fifth met criteria for an opioid use disorder, more than one fifth of men reported a marijuana use disorder, more than one third had nicotine dependence, many had an alcohol use disorder (72% in men; 40% in women), and there was a high rate of major depression in women (41% in women vs. 25% in men). Women reported lower quality of life in vitality and mental health domains than men in this group. Except for marijuana use disorder (24% in men, 13% in women), very few (≤ 3%) had other drug use disorders.
Together, women in this lower-risk group appear to manifest greater mental health problems while men tend to use more substances. Because very few had disorders related to drug use other than marijuana, opioid–marijuana users may have a low likelihood of being included in addiction treatment research where non-marijuana drug use disorders (e.g., cocaine or amphetamines) are often the focus (Wu, Blazer et al., 2009; Wu, Pan et al., 2009). Their reduced odds of using substance abuse treatment also indicate that study results from treatment-seeking samples may not generalize to this large group.
4.5. Study Limitations and Strengths
These findings should be interpreted in light of some limitations. The NESARC relies on respondents’ self-reports, which may be influenced by under-reporting and memory errors. In addition, a very small proportion of the institutionalized population (homeless, hospitalized, or incarcerated individuals) was not included in the survey. Study results cannot be applied to them. Individuals who suffered severe health consequences from their substance abuse also may have a reduced likelihood of participating in a household survey. Further, results reported here represent estimated associations between nonmedical opioid use and psychiatric conditions, not causal relationships. Lastly, researchers have suggested that assigning individuals to the most likely class membership obtained from LCA and then examining the study covariates by class membership has the potential for underestimating standard errors of the parameter (Clark and Muthén, 2010). An entropy value of 0.80 or greater is considered to indicate a high level of classification for the analysis. The entropy value from this study (0.78) is close to this recommended value. As recommended, to mitigate the potential of underestimating standard errors of the parameter, we have employed a more stringent criterion than the 0.05 level for deciding on significance of parameters (Clark and Muthén, 2010). Only results with p-values <0.01 are discussed.
The NESARC data also have noteworthy strengths. The survey includes the most comprehensive assessment of psychiatric disorders available that supports the comparison of specific psychiatric disorders among subtypes. Additionally, study results have a high level of generalizability because NESARC’s national sample represents domiciled American adults. The large sample size also permits the exploration of gender variations in psychiatric profiles across subtypes.
4.6. Conclusions
This national investigation of the heterogeneity of nonmedical opioid users adds new evidence to the field by identifying at least four subtypes that are differentiated by onset of nonmedical opioid use, patterns of psychiatric disorders, substance abuse treatment, and familial substance abuse. Across all subtypes, women were as problematic as men in their pattern of SUDs; however, women further manifested greater major depression and disability in the mental health domain. The generally high prevalence of psychiatric disorders among nonmedical opioid users underscores the need for careful assessment and coordinated delivery of services to match treatment needs when these individuals enter treatment, as well as continued monitoring of trends in nonmedical opioid use and related problems.
Acknowledgments
This work was made possible by research grants from the U.S. National Institute on Drug Abuse of the National Institutes of Health: R01DA019623, R01DA019901, and R21DA027503 to L-T Wu; R01DA026652 to WW Eaton; K05DA017009 and U10DA013043 to GE Woody; and HSN271200522071C to DG Blazer. The sponsoring agency had no further role in the study design and analysis, the writing of the report, or the decision to submit the paper for publication. Contributors: Wu contributed to the study design and data analyses, and wrote the initial draft of the paper. Yang contributed to data analyses and interpretations of results. Wu, Woody, and Blazer contributed to interpretations of results and critical revisions.
NESARC was sponsored and conducted by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health, with supplemental support from the National Institute on Drug Abuse. We thank Ms. Amanda McMillan for her editorial assistance.
Footnotes
Conflicts of Interest: GE Woody is a member of the RADARS(r) System Scientific Advisory Board whose job is to assess abuse, misuse and diversion of prescription medications. Denver Health and Hospital Authority is a non-profit public hospital that administers the RADARS(r) System, and its costs are supported by contracts with pharmaceutical companies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banta-Green CJ, Merrill JO, Doyle SR, Boudreau DM, Calsyn DA. Opioid use behaviors, mental health and pain--development of a typology of chronic pain patients. Drug Alcohol Depend. 2009;104:34–42. doi: 10.1016/j.drugalcdep.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM. New methods of treatment efficacy research: bridging clinical research and clinical practice. Alcohol Health Res World. 1997;21:352–359. [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ. On beyond urine: clinically useful assessment instruments in the treatment of drug dependence. Behav Res Ther. 2002;40:1329–1344. doi: 10.1016/s0005-7967(02)00038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Substance Abuse Treatment. Treatment Improvement Protocol (TIP) Series 40. DHHS Publication No. (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. [PubMed] [Google Scholar]
- Cicero TJ, Lynskey M, Todorov A, Inciardi JA, Surratt HL. Co-morbid pain and psychopathology in males and females admitted to treatment for opioid analgesic abuse. Pain. 2008;39:127–135. doi: 10.1016/j.pain.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Clark S, Muthén B. Relating latent class analysis results to variables not included in the analysis. Submitted for publication 2010 [Google Scholar]
- Compton WM, Volkow ND. Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend. 2006;83(suppl 1):S4–S7. doi: 10.1016/j.drugalcdep.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Fatséas M, Denis C, Lavie E, Auriacombe M. Relationship between anxiety disorders and opiate dependence--a systematic review of the literature: implications for diagnosis and treatment. J Subst Abuse Treat. 2010;38:220–230. doi: 10.1016/j.jsat.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Ghandour LA, Martins SS, Chilcoat HD. Understanding the patterns and distribution of opioid analgesic dependence symptoms using a latent empirical approach. Int J Methods Psychiatr Res. 2008;17:89–103. doi: 10.1002/mpr.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatr. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou PS, Kay W, Pickering R. The Alcohol Use Disorders and Associated Disabilities Interview Schedule–IV (AUDADIS IV): reliability of alcohol consumption, tobacco use, family history of depression and psychiatric diagnostic modules in a general population sample. Drug Alcohol Depend. 2003;71:7–16. doi: 10.1016/s0376-8716(03)00070-x. [DOI] [PubMed] [Google Scholar]
- Green TC, Grimes Serrano JM, Licari A, Budman SH, Butler SF. Women who abuse prescription opioids: findings from the Addiction Severity Index-Multimedia Version Connect prescription opioid database. Drug Alcohol Depend. 2009;103:65–73. doi: 10.1016/j.drugalcdep.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grella CE, Karno MP, Warda US, Niv N, Moore AA. Gender and comorbidity among individuals with opioid use disorders in the NESARC study. Addict Behav. 2009;34:498–504. doi: 10.1016/j.addbeh.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens JR, Stoops WW, Leukefeld CG, Garrity TF, Carlson RG, Falck R, Wang J, Booth BM. Prescription opiate misuse among rural stimulant users in a multistate community-based study. Am J Drug Alcohol Abuse. 2009;35:18–23. doi: 10.1080/00952990802326298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings, 2008 (NIH Publication No. 09-7401) National Institute on Drug Abuse; Bethesda, MD: 2009. [Google Scholar]
- Leshner AI. Drug addiction research: moving toward the 21st century. Drug Alcohol Depend. 1998;51:5–7. doi: 10.1016/s0376-8716(98)00061-1. [DOI] [PubMed] [Google Scholar]
- Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88:778. [Google Scholar]
- Manchikanti L. National drug control policy and prescription drug abuse: facts and fallacies. Pain Physician. 2007;10:399–424. [PubMed] [Google Scholar]
- McCabe SE, Boyd CJ, Teter CJ. Subtypes of nonmedical prescription drug misuse. Drug Alcohol Depend. 2009;102:63–70. doi: 10.1016/j.drugalcdep.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson J, Flower K, Pletcher MJ, Galloway GP. Addiction to prescription opioids: characteristics of the emerging epidemic and treatment with buprenorphine. Exp Clin Psychopharmacol. 2008;16:435–441. doi: 10.1037/a0013637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monga N, Rehm J, Fischer B, Brissette S, Bruneau J, El-Guebaly N, Noël L, Tyndall M, Wild C, Leri F, Fallu JS, Bahl S. Using latent class analysis (LCA) to analyze patterns of drug use in a population of illegal opioid users. Drug Alcohol Depend. 2007;88:1–8. doi: 10.1016/j.drugalcdep.2006.08.029. [DOI] [PubMed] [Google Scholar]
- Moore BA, Fiellin DA, Barry DT, Sullivan LE, Chawarski MC, O’Connor PG, Schottenfeld RS. Primary care office-based buprenorphine treatment: comparison of heroin and prescription opioid dependent patients. J Gen Intern Med. 2007;22:527–530. doi: 10.1007/s11606-007-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén B, Muthén LK. Integrating person-centered and variable-centered analyses: growth mixture modeling with latent trajectory classes. Alcohol Clin Exp Res. 2000;24:882–889. [PubMed] [Google Scholar]
- Muthén BO, Muthén LK. Mplus: Statistical Analysis with Latent Variables (Version 4.2.1) Muthén and Muthén, Inc; Los Angeles, CA: 2007. [Google Scholar]
- Nylund KL, Asparouhov T, Muthén B. Deciding on the number of classes in latent class analysis and growth mixture modeling. A Monte Carlo simulation study. Struct Equ Model. 2007;14:535–569. [Google Scholar]
- Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf. 2006;15:618–627. doi: 10.1002/pds.1276. [DOI] [PubMed] [Google Scholar]
- Raftery AE. Bayesian model selection in social research. Sociol Methodol. 1995;25:111–163. [Google Scholar]
- Research Triangle Institute. SUDAAN User’s Manual, Release 9.0. Research Triangle Institute; Research Triangle Park, NC: 2006. [Google Scholar]
- Rosenblum A, Parrino M, Schnoll SH, Fong C, Maxwell C, Cleland CM, Magura S, Haddox JD. Prescription opioid abuse among enrollees into methadone maintenance treatment. Drug Alcohol Depend. 2007;90:64–71. doi: 10.1016/j.drugalcdep.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Dunn KE, Badger GJ, Heil SH, Higgins ST. Brief buprenorphine detoxification for the treatment of prescription opioid dependence: a pilot study. Addict Behav. 2009;34:304–311. doi: 10.1016/j.addbeh.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmon SC. Characterizing the emerging population of prescription opioid abusers. Am J Addict. 2006;15:208–212. doi: 10.1080/10550490600625624. [DOI] [PubMed] [Google Scholar]
- Strain EC. Assessment and treatment of comorbid psychiatric disorders in opioid-dependent patients. Clin J Pain. 2002;18:S14–S27. doi: 10.1097/00002508-200207001-00003. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Office of Applied Studies. Results from the 2008 National Survey on Drug Use and Health: National Findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2009a. [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Office of Applied Studies. OAS Series #S-45, HHS Publication No. (SMA) 09-4360. Rockville, MD: 2009b. Treatment Episode Data Set (TEDS) Highlights—2007 National Admissions to Substance Abuse Treatment Services. [Google Scholar]
- Sullivan MD, Edlund MJ, Steffick D, Unützer J. Regular use of prescribed opioids: association with common psychiatric disorders. Pain. 2005;119:95–103. doi: 10.1016/j.pain.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Veilleux JC, Colvin PJ, Anderson J, York C, Heinz AJ. A review of opioid dependence treatment: pharmacological and psychosocial interventions to treat opioid addiction. Clin Psychol Rev. 2010;30:155–166. doi: 10.1016/j.cpr.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Turner Bowker DM, Gandek B. How to Score Version 2 of the SF-12 Health Survey. Quality Metrics; Lincoln, RI: 2002. [Google Scholar]
- Woody GE, Poole SA, Subramaniam G, Dugosh K, Bogenschutz M, Abbott P, Patkar A, Publicker M, McCain K, Potter JS, Forman R, Vetter V, McNicholas L, Blaine J, Lynch KG, Fudala P. Extended vs. short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300:2003–2011. doi: 10.1001/jama.2008.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, Pilowsky DJ, Patkar AA. Nonmedical use of pain relievers among adolescents in the United States. Drug Alcohol Depend. 2008;94:1–11. doi: 10.1016/j.drugalcdep.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, Blazer DG, Patkar AA, Stitzer ML, Wakim PG, Brooner RK. Heterogeneity of stimulant dependence: a National Drug Abuse Treatment Clinical Trials Network (CTN) study. Am J Addict. 2009;18:206–218. doi: 10.1080/10550490902787031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, Parrott AC, Ringwalt CL, Yang C, Blazer DG. The variety of ecstasy/MDMA users: results from the National Epidemiologic Survey on alcohol and related conditions. Am J Addict. 2009;18:452–461. doi: 10.3109/10550490903206049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, Howard MO, Pilowsky DJ. Substance use disorders among inhalant users: results from the National Epidemiologic Survey on alcohol and related conditions. Addict Behav. 2008;33:968–973. doi: 10.1016/j.addbeh.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, Pan JJ, Blazer DG, Tai B, Stitzer ML, Brooner RK, Woody GE, Patkar AA, Blaine JD. An item response theory modeling of alcohol and marijuana dependences: a National Drug Abuse Treatment Clinical Trials Network study. J Stud Alcohol Drugs. 2009;70:414–425. doi: 10.15288/jsad.2009.70.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny J, Bigelow G, Compton P, Foley K, Iguchi M, Sannerud C. College on Problems of Drug Dependence taskforce on prescription opioid nonmedical use and abuse: position statement. Drug Alcohol Depend. 2003;69:215–232. doi: 10.1016/s0376-8716(03)00003-6. [DOI] [PubMed] [Google Scholar]