Abstract
Loss-of-function analysis of the bHLH transcription factor Hand1 indicates critical roles in development. In an effort to generate a Hand1 cDNA knock-in reporter mouse, we generated two hypomorphic alleles, which extend embryonic survival to between E10.5 and E12.5. Heart morphogenesis appears largely normal; however, hypomorphic mice display thin left ventricular myocardium and reduction in pharyngeal mesoderm. Caudal defects, large allantois, and thickened yolk sac are observed and consistent with systemic Hand1 gene deletion. Hand1 mRNA is expressed at 30% of wild-type littermates and known Hand1-dependent genes show intermediate expression compared to wild-type and Hand1 null mice. Interestingly, putative bHLH partners, Hand2 and Twist1, show altered expression in both Hand1 null and hypomorphic backgrounds and intercrossing the Hand1 hypomorphic mice onto the Hand2 systemic null background exacerbates the cardiac and lateral mesoderm phenotypes. Together, these data define a critical threshold of Hand1 expression that is necessary for embryonic survival.
Keywords: Hand1, Hand2, heart development, ventricle, extraembryonic mesoderm
INTRODUCTION
Members of the Twist-family of basic helix-loop-helix (bHLH) transcription factors perform essential roles in embryonic development and pathological disease (Firulli, 2003; Firulli and Conway, 2008; Barnes and Firulli, 2009). The Twist-family member Hand1 not only plays essential roles in the development of the heart and sympathetic nervous system but also holds an early role in maintaining the viability of the extraembryonic mesoderm that contributes to the placenta, yolk sac, amnion, allantois, vasculature and trophoblast giant cells (Firulli et al., 1998; Riley et al., 1998; Morikawa and Cserjesi, 2004). Hand1 null mice die between E8.5 to E9.0 resulting from defects in the extraembryonic mesoderm and trophoblast giant cells in the ectoplacental cone (Firulli et al., 1998; Riley et al., 1998). Expression of Placental Lactogen I (Prld or Pl1) is greatly reduced in Hand1 null embryos (Firulli et al., 1998; Riley et al., 1998). During early mouse gestation (up to E11.0), Pl1 maintains the corpus luteum, the source of progesterone, which is required for successful pregnancy (Walker et al., 1991; Yamaguchi et al., 1994). Vascular formation within the Hand1 null yolk sac initiates, but soon after, becomes developmentally arrested. Expression of vascular endothelial growth factor (Vegf), Angiopoietin (Angpt1) and ephrin B2 are subsequently upregulated. Moreover, smooth muscle cells abnormally cluster throughout the yolk sac mesoderm (Morikawa and Cserjesi, 2004). Conditional deletion of Hand1 has revealed genetic interactions with the related family member Hand2 (McFadden et al., 2005). αMHC-Cre and Nkx2.5-Cre mediated cardiac deletion of Hand1 results in perinatal lethality due to ventricular septal defects and accompanying overriding aorta or double outlet right ventricle (McFadden et al., 2005). Ablating Hand1 in the heart on a Hand2 haploinsufficient background increases the severity of these observed phenotypes. Such genetic interactions, in part, manifest due to the broad dimerization affinities of the Twist-family bHLH proteins compared to those of other Class B bHLH factors (Firulli et al., 2003; Firulli and Conway, 2008 Firulli, 2005 #2346). Twist-family proteins can form homodimers and heterodimers with both other Class B bHLH factors and E-proteins. Thus, in gene deletion experiments, phenotypes reflect the loss-of-function as well as a consequent redistribution of the bHLH dimer pool within a cell. It is therefore not surprising that the control of Hand and Twist gene expression levels is tightly regulated.
In our efforts to generate a Hand1 maker allele that would not be haploinsufficient for Hand1, we employed a cDNA knock-in approach in conjunction with an IRES-eGFP cassette to allow for visualization of Hand1-expressing cells. Analysis shows that Hand1 homozygous cDNA knock-in mice are not viable and that the removal of the neomycin cassette does not rescue lethality. Embryonic analysis shows that eGFP epiflorescence was not detectable in heterozygous or homozygous embryos; however, mRNA expression is detectable. Interestingly, homozygous knock-in embryos die between E10–E12.5, exhibiting a clear extension of survival from that observed in the Hand1 systemic knockouts. Phenotypic analysis reveals thin dilated hearts and dysmorphic caudal development as well as the predicted yolk sac and extra-embryonic phenotypes. Cell death and proliferation analyses reveal that there is no change in apoptosis; however, a global decrease in cell proliferation as a consequence of poor placental function is observed. mRNA analysis shows that the Hand1 cDNA knock-in alleles are hypomorphic, with expression of Hand1 at 30%–40% of that observed in wildtype littermates, thus defining a threshold of necessary Hand1 expression. Accompanying this decrease in Hand1 expression is an upregulation of Hand2, which could be either compensatory or deleterious, resulting in a further destabilization of the bHLH factory stoichiometry. Intercross of the Hand1 hypomorphic allele onto a Hand2 null background largely exacerbates these observed phenotypes, indicating the importance of Twist-family gene balance in embryogenesis.
RESULTS
Generation of Hand1Hand1+Neo and Hand1Hand1ΔNeo cDNA knock-in mice
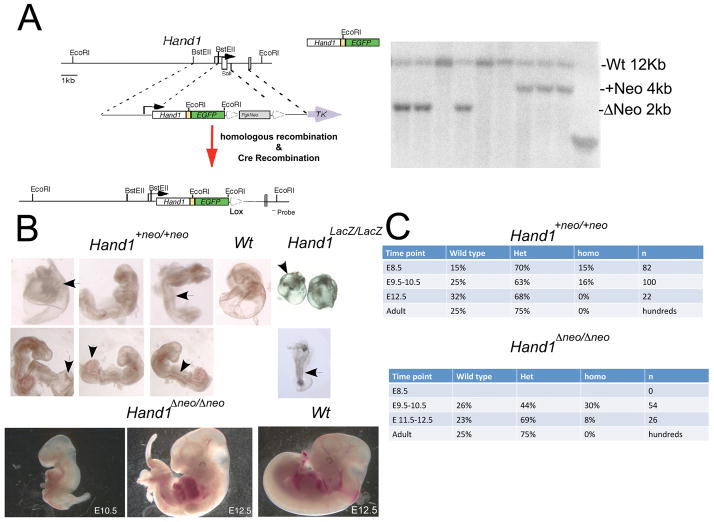
To follow real-time Hand1 expression without lowering the Hand1 gene dosage, we designed a cDNA knock-in approach, whereby the coding region of Hand1 from a 600bp cDNA initiating at the ATG and terminating at the stop codon was cloned 5′ of an IRES followed by an eGFP expression cassette. The replacement cDNA cassette was flanked by 5′ and 3′ Hand1 targeting arms that have been described previously (Firulli et al., 1998). ES cells targeted at a frequency of approximately 1:15. Two independent ES cell lines were injected into host blastocysts, which produced chimeric mice that subsequently gave rise to the germline transmission of the of the Hand1Hand1+Neo allele (Fig. 1A). Both ES lines generated mice that exhibit identical phenotypes and thus are considered identical.
Figure 1.
(A) Schematic of Hand1cDNA targeting vector and southern blot showing RFLP identification of the +Neo and ΔNeo alleles. (B) Wholemount view of Hand1Hand1+Neo/Hand1+Neo, Hand1Lacz/LacZ and wild type E9.5 day embryos. Hand1Hand1+Neo/Hand1+Neo embryos display thickened “blistered” yolk sac (arrow left panels) enlarged allantois (arrowhead) and crooked neural tube indicative of placental insufficiency. These phenotypes are observed and more severe in Hand1Lacz/LacZ embryos (right panels). Hand1Hand1+Neo/Hand1+Neo embryos display a generally normal looking looped heart as compared to Hand1Lacz/LacZ mice. E10.5 and E12.5 Hand1Hand1ΔNeo/Hand1ΔNeo embryos display caudal defects in lateral mesoderm and hypoplastic limb buds as compared to wild type littermates. Hearts of Hand1Hand1ΔNeo/Hand1ΔNeo embryos are looped and morphologically similar to wildtype. (C) Genotypic analysis at E8.5–E12.5 & adult stages for occurrence of Hand1Hand1+Neo/Hand1+Neo and Hand1Hand1ΔNeo/Hand1ΔNeo mice. Hand1Hand1+Neo/Hand1+Neo embryos are detectable at slightly lower then expected mendellian ratios up to E10.5 and are completely lost by E12.5. Hand1Hand1ΔNeo/Hand1ΔNeo embryos are detectable at expected frequencies until E12.5 where they are observed at 8% frequency. Hand1Lacz/LacZ mice are not detected beyond E9.5 (Firulli et al., 1998; Riley et al., 1998, Morikawa, 2004 #2340).
Intercross of Hand1Hand1+Neo/+ F1 mice produces no homozygous Hand1Hand1+Neo/Hand1+Neo offspring at P10, suggesting that the cDNA allele constructed is not expressing at normal levels. Subsequent eGFP whole mount and section analyses of E9.5 embryos show a lack of detectable epiflorescence; however, both heterozygous and homozygous genotypes were identified. Compared to the systemic mutants, E9.5 Hand1 knockout (Hand1LacZ/LacZ), E9.5 Hand1Hand1+Neo/Hand1+Neo embryos are larger, further developed, and had visible heartbeats. Cardiac morphology appeared normal, although hearts appear large compared to overall embryo size. The caudal region of the embryos display crooked neural tubes, overtly large allantoises, and yolks sac blistering resulting from separation of the visceral mesoderm and endoderm of the yolk sac (Fig 1B). These phenotypes are also observed, albeit to a more pronounced degree, in Hand1LacZ/LacZ mice, suggesting that the cause of death, consistent with previous findings, is placental insufficiencies (Firulli et al., 1998; Riley et al., 1998). Given that we encountered a less severe phenotype, we extended our analysis to E12.5. We detected viable (displaying regular heart beat) Hand1Hand1+Neo/Hand1+Neo embryos at E10.5 but no viable mice were observed after this time point.
To look at the influence of the PGK-neomycin cassette, we intercrossed Hand1Hand1+Neo mice with the EIIA-Cre mouse line (Lakso et al., 1996), which expresses Cre recombinase systemically within the early mouse embryo. Mice carrying successfully recombined alleles (Hand1Hand1ΔNeo/+) were readily detected by RFLP shift via Southern blotting (Fig. 1A). Similar to Hand1Hand1+Neo/Hand1+Neo mice, we were unable to detect viable homozygous Hand1Hand1ΔNeo/Hand1ΔNeo offspring. Additionally, eGFP epiflorescence was not detectable at E9.5 (data not shown). Embryonic analyses indicate that Hand1Hand1ΔNeo/Hand1ΔNeo mice exhibited similar phenotypes to those of Hand1Hand1+Neo/Hand1+Neo embryos, but at a less severe penetrance (Fig 1B &C). Although the majority of embryos are viable to E10.5, 8% of the homozygous embryos are able to develop out to E12.5 where they display pericardial hemorrhaging, hypoplastic limb buds and craniofacial defects, as well as severe caudal defects, indicating that embryonic turning is compromised.
Hand1 expression analysis shows that Hand1Hand1+Neo and Hand1Hand1ΔNeo alleles are hypomorphic
Given that the observed phenotypes associated with our Hand1 cDNA knock-in alleles show some rescue of the Hand1LacZ/LacZ phenotype, we first looked at Hand1 qualitative mRNA expression by whole mount and section in situ (Fig. 2). Whole mount analysis shows that Hand1 mRNA is detectable in all viable wild type Hand1Hand1+Neo/+ and Hand1Hand1ΔNeo/+ heterozygous mice and is indistinguishable in pattern and staining intensity. In contrast, homozygous Hand1Hand1+Neo/Hand1+Neo and Hand1Hand1ΔNeo/Hand1ΔNeo embryos appear to express lower, albeit still detectable, levels of Hand1 mRNA (Fig. 2). Section in situ analysis reveals that the levels of Hand1 expression are not uniformly reduced. For example, cardiac expression of Hand1 is barely detectable, whereas pharyngeal arch expression appears more comparable to wild-type levels (Fig. 2F-H). Hand2 mRNA expression is qualitatively similar and spatially independent of Hand1 genotype (Fig. 3I-K). Given that we were unable to detect eGFP epiflorescence, we tested both Hand1Hand1+Neo/Hand1+Neo and Hand1Hand1ΔNeo/Hand1ΔNeo homozygous embryos for eGFP mRNA expression (Fig. L & M). Expression of eGFP mRNA is clearly detectable in a pattern identical to that of Hand1 suggesting that the level of translated eGFP protein is simply below the threshold of our detection.
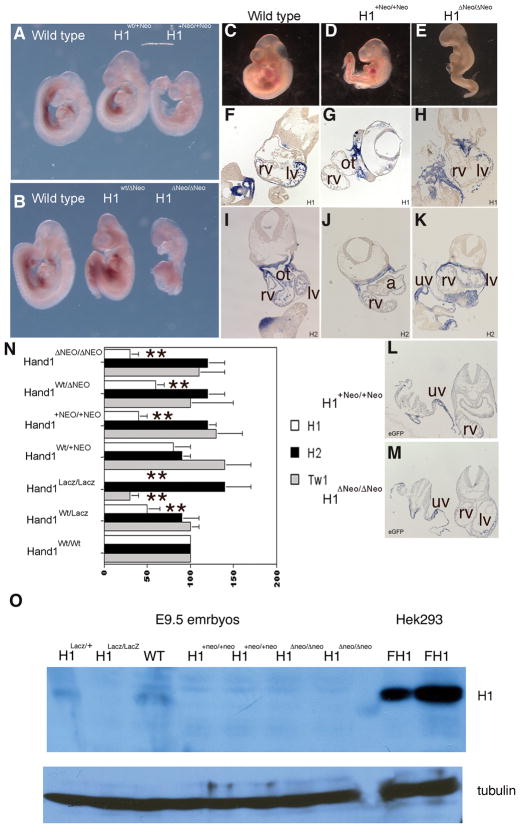
Figure 2.
(A and B) Whole mount Hand1 in situ hybridization in wildtype, heterozygous and homozygous Hand1Hand1+Neo/Hand1+Neo and Hand1Hand1ΔNeo/Hand1ΔNeo E9.5 embryos. Expression is observed in pharyngeal arches, lateral mesoderm, and left ventricle in all genotypes; however mRNA is reduced in both Hand1Hand1+Neo/Hand1+Neo and Hand1Hand1ΔNeo/Hand1ΔNeo homozygous mice. Section in situ for Hand1 (F, G, H), Hand2 (I, J, K), and eGFP (L and M). Hand1 expression appears reduced within the myocardium of the left ventricle whereas expression through pharyngeal and lateral mesoderm appears closer to wild type levels in both Hand1Hand1+Neo/Hand1+Neo and Hand1Hand1ΔNeo/Hand1ΔNeo homozygous mice. Hand2 expression is spatially unaffected in within the Hand1 cDNA homozygous mice showing expected expression within the neural crest of the outflow track, heart, pharyngeal and lateral mesoderm. Analysis of eGFP expression shows that in both Hand1Hand1+Neo/Hand1+Neo and Hand1Hand1ΔNeo/Hand1ΔNeo homozygous mice eGFP message is detectable and expressed in an identical spatial pattern to what is observed for Hand1. rv, right ventricle; lv, left ventricle; ot, outflow tract; uv umbilical vein. (N) Quantitative RT PCR using Taqman primers specific for Hand1, Hand2 and Twist1 message. In Hand1Lacz/LacZ heterozygous and homozygous null mice, a 50% and 100% reduction in Hand1 message is observed. Hand1Hand1+Neo/Hand1+Neo heterozygous mice express Hand1 at wild type levels whereas homozygous mice express Hand1 at 40% of that observed in wildtype. Hand1Hand1ΔNeo/Hand1ΔNeo heterozygous mice express Hand1 at 50% and homozygous 30% of that observed in wildtype. Hand2 expression is upregulated in Hand1Lacz/LacZ embryos as well as in Hand1Hand1+Neo/Hand1+Neo and Hand1Hand1ΔNeo/Hand1ΔNeo homozygous embryos. Twist1 expression is reduced to 40% of wildtype levels in Hand1Lacz/LacZ null mice; however, Twist1 expression is within the wildtype range in both Hand1Hand1+Neo/Hand1+Neo and Hand1Hand1ΔNeo/Hand1ΔNeo homozygous embryos. (O). Immunoblot detection of Hand1 protein in E9.5 embryos and HEK293cells transfected with pcDNA flagHand1. Protein is observed in wildtype embryos; however, the level of protein is beyond the sensitivity of the antibody in Hand1Hand1+Neo/Hand1+Neo and Hand1Hand1ΔNeo/Hand1ΔNeo homozygous embryos. Error bars denote standard error, * indicates a P value of less than or equal to 0.05 and ** indicates a P value of less than or equal to 0.01.
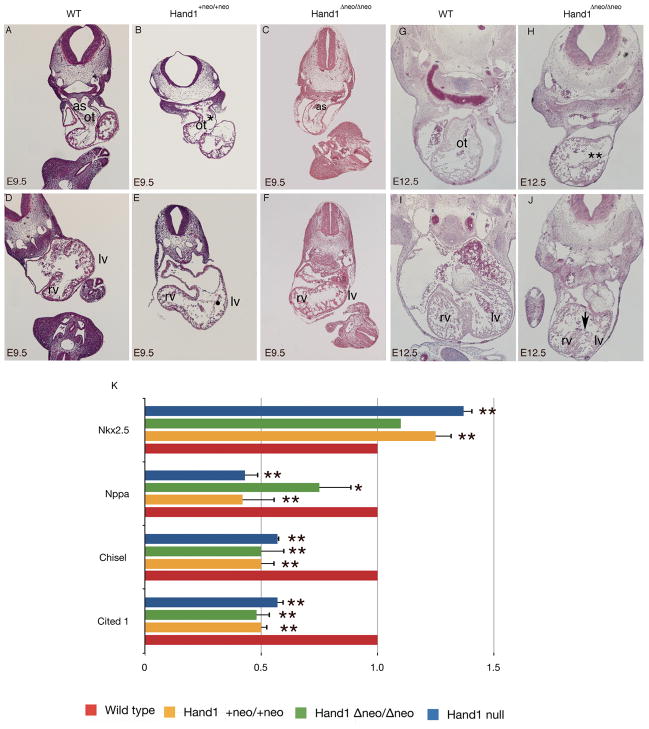
Figure 3.
H&E histological analysis of E9.5 day Hand1Hand1+Neo/Hand1+Neo (B, E) and Hand1Hand1ΔNeo/Hand1ΔNeo (C, F). Compared to wild type littermates (A,D) Hand1Hand1+Neo/Hand1+Neo embryos display thin hypotrabeculated hearts (white arrowhead) and a reduced number of mesenchymal cells within the outflow track (ot) (*). Pharyngeal mesenchyme is also reduced and lacks a forming aortic septum (as). In contrast at E9.5 Hand1Hand1ΔNeo/Hand1ΔNeo embryo hearts appear phenotypically normal. Histological analysis of E12.5 Hand1Hand1ΔNeo/Hand1ΔNeo embryos (H, J) reveals a largely acellular outflow track cushion (**) and poorly organized interventricular septum (white arrow) when compared to a wild type littermate (G, I). rv, right ventricle; lv, left ventricle. Quantitative RTPCR analysis from E9.5 day Hand1 systemic null, Hand1Hand1+Neo/Hand1+Neo and Hand1Hand1ΔNeo/Hand1ΔNeo embryos (K). Data represents the mean of at least 4 embryos and error bars denote standard error, * indicates a P value of less than or equal to 0.05 and ** indicates a P value of less than or equal to 0.01
To gain an understanding of the overall decrease in expression of the Hand1Hand1+Neo and Hand1Hand1ΔNeo alleles, we performed qRT-PCR using whole wild type, heterozygous, and homozygous Hand1Hand1+Neo and Hand1Hand1ΔNeo E9.5 embryos, along with Hand1LacZ/LacZ null controls using Taqman-labeled primers that detect Hand1, Hand2 and Twist1 messages (Fig. 2N). Setting wild type Hand1 levels as 100%, analysis of Hand1Wt/LacZ shows a significant (P≤0.01) 50% reduction in Hand1 mRNA expression and, as expected, we were unable to detect Hand1 expression in the Hand1LacZ/LacZ embryos. Expression analysis in Hand1Hand1+Neo heterozygous and homozygous mice shows higher levels of Hand1 message than those of Hand1 null mice. Heterozygous Hand1Hand1+Neo mice express approximately 90% of wild type expression levels, whereas Hand1Hand1+Neo homozygotes express Hand1 at 50% that of wildtype, similar to the viable Hand1+/LacZ mice (P≤0.01) (Fig. 3N). Hand1 expression in Hand1+/Hand1ΔNeo heterozygotes is also 50% of wild-type; however, homozygotes express Hand1 at only 30% of wild-type levels (P≤0.01). The lower level of Hand1 mRNA expression observed, in conjunction with increased viability, suggests that there are possible translational differences that are Pgk-neomycin dependent and/or that expression within extraembryonic tissues (see Fig. 5) is more robust.
We next looked at Hand2 expression in the various genotypes. It was previously reported that Hand1 null mice show an upregulation of Hand2 mRNA (Morikawa and Cserjesi, 2004). Consistent with this report, we see a reproducible 40% increase in Hand2 expression within our Hand1LacZ/LacZ embryos as compared to wild-type controls; however, this increase is not statistically significant (P≥0.05). Interestingly, in contrast to Hand1+/LacZ embryos, which express wild type levels of Hand2, Hand1Hand1+Neo and Hand1Hand1ΔNeo heterozygous and homozygous E9.5 embryos also exhibit a reproducible upregulation of Hand2 mRNA, comparable to that observed in the Hand1LacZ/LacZ mice (Fig. 2L). Given that gene dosage phenotypes are reported for a number of Twist-family proteins and, specifically, Hand1 and Hand2 (McFadden et al., 2005), this alteration in gene balance is a possible mechanism that underlies the knock-in alleles’ embryonic lethality despite that statistics suggest this increase in expression is not significant.
We also examined expression of Twist1, which codes for a potential dimer partner of Hand1 and shows genetic and functional interactions with Hand2 (Firulli et al., 2005). In Hand1LacZ/LacZ embryos, Twist1 message is significantly reduced below 50% of what is observed in wild type littermates, whereas both Hand1 homozygous hypomorphs express normal levels of Twist1 mRNA (Fig. 2N). Given that Hand1LacZ/LacZ embryos are smaller and less viable, this observed decrease in Twist1 expression could simply be a consequence of fewer mesenchymal cells within these embryos; however, collectively the changes observed in both Hand2 and Twist1 expression within the Hand1LacZ/Lacz, Hand1Hand1+Neo, and Hand1Hand1ΔNeo homozygous alleles is a shared molecular characteristic between these engineered mouse models.
To further confirm that Hand1Hand1+Neo and Hand1Hand1ΔNeo alleles were hypomorphic, we performed immunoblot analysis on E9.5 day embryos (Fig. 2O). HEK293 cells transfected with Hand1 were used to mark the size of Hand1 protein and to act as control for the Santa Cruz antisera. In wild-type and Hand1 heterozygous null embryo lysates, the Hand1 antibody detects a protein that migrates similarly to what is observed in Hand1-transfected HEK293 lysates. This Hand1 band is not observed in equally loaded Hand1LacZ/LacZ embryo lysates suggesting that indeed this antibody is recognizing Hand1 protein. Similar to Hand1 null embryos, Hand1Hand1+Neo/Hand1+Neo and Hand1Hand1ΔNeo/Hand1ΔNeo homozygous embryo lysates do not show detectable Hand1 protein expression. We conclude that there is a commensurate decrease in Hand1 translation such that levels of Hand1 protein are beyond the detection level of this antisera, but given that Hand1Hand1+Neo/Hand1+Neo and Hand1Hand1ΔNeo/Hand1ΔNeo embryos survive longer then Hand1LacZ/LacZ embryos a low level of Hand1 is indeed being translated and functionally active.
In order to gain a better understanding of these gross mutant phenotypes, a histological examination of Hand1Hand1+Neo/Hand1+Neo and Hand1Hand1ΔNeo/Hand1ΔNeo embryos was compared to wildtype littermates (Fig. 3). At E9.5, at the level of the cardiac OFT, Hand1Hand1+Neo/Hand1+Neo and Hand1Hand1ΔNeo/Hand1ΔNeo hearts are morphologically similar to what is observed in wild type littermates; however, key differences are observed. In Hand1Hand1+Neo/Hand1+Neo embryos, the heart is noticeably thinner (white arrowhead) and dilated as compared to wild-type littermates. Pharyngeal mesoderm proximal to the OFT is reduced and a forming AP septum is not visible (Fig. 3A &B). At this stage, the mesenchymal cells, the majority of which are cardiac neural crest derived, are largely absent from the forming OFT cushions when compared to wild-type mice (Fig 3B asterisk). In contrast, Hand1Hand1ΔNeo/Hand1ΔNeo embryos appear phenotypically indistinguishable from wild type littermates at this age (Fig 3A, D &E, F). Comparison of the ventricular chamber at E9.5 reveals that Hand1Hand1+Neo/Hand1+Neo hearts are thin and hypotrabeculated; (Fig. D-E), whereas Hand1Hand1ΔNeo/Hand1ΔNeo hearts show a cardiac phenotype similar to wild-type littermates (Fig 3. D and F).
As a small percentage of Hand1Hand1ΔNeo/Hand1ΔNeo mice survive to E12.5, we examined hearts at this stage. Embryos show a slightly smaller size as compared to wild type and heterozygous littermates. Histology shows that Hand1Hand1ΔNeo/Hand1ΔNeo mice display reduced OFT cushions and decreased ventricular wall thickness in (Fig. 3G & H). At the level of the forming interventricular septum, septal cardiomyocytes are disorganized as compared to wild-type mice. Given that Hand1LacZ/LacZ embryos fail to undergo cardiac looping and embryonic turning (Firulli et al., 1998) there appears to be some level of phenotypic rescue from both cDNA expression alleles; however, given that the homozygote mice are non-viable, the overall expression from these hypomorphic Hand1 alleles is insufficient.
We next compared the gene expression profiles for a number of putative Hand1 downstream targets expressed within the heart (Fig 3K). Cited1, which is expressed within the left ventricle, is down-regulated in Hand1 conditional null mice (McFadden et al., 2005). Quantitative RT-PCR analysis from whole embryo RNA shows that in Hand1LacZ/LacZ, Hand1Hand1ΔNeo/Hand1ΔNeo, and Hand1Hand1+Neo/Hand1+Neo embryos, Cited1 expression is also significantly (P≤0.01) reduced. Chisel expression, which is reported to be down-regulated in a Hand1-overexpression mouse model (Risebro et al., 2006) is also significantly down-regulated within all of our Hand1 alleles (Fig 3L). Interestingly, Atrial naturetic factor (Nppa) which is downregulated in Hand1 conditional null mice and is downregulated by more then 50% in systemic Hand1 null and Hand1Hand1+Neo/Hand1+Neo embryos (P≤0.01) is only mildly downregulated within the Hand1Hand1ΔNeo/Hand1ΔNeo mice (P≤0.05) (Fig 3L). Nkx2.5 lies directly upstream of Hand1 and Hand1 expression is downregulated in Nkx2.5 null embryos (Lyons et al., 1995). Nkx2.5 expression within Hand1 null and Hand1Hand1+Neo/Hand1+Neo embryos shows a significant (P≤0.01) up-regulation suggesting a feed back regulatory mechanism. Although Hand1Hand1ΔNeo/Hand1ΔNeo embryos do exhibit a slight increase in Nkx2.5 expression, the levels are closer to what is observed in wild-type littermates and not significantly different (P≥0.05) (Fig 3L). Collectively, these histological and expression analyses suggest that the phenotypes observed with the null alleles are partially recapitulated within the Hand1 cDNA alleles but that the less dramatic changes observed within the Hand1Hand1ΔNeo/Hand1ΔNeo homozygote embryos correlates with their extended lifespan.
Faulty mRNA processing is observed in Hand1 hypomorphic alleles
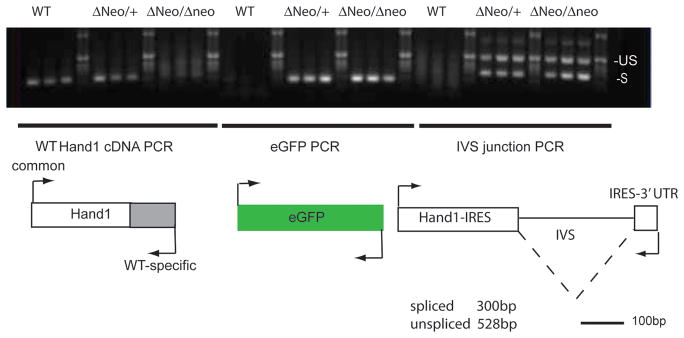
To gain a better understanding as to why the Hand1 cDNA knock-in alleles are hypomorphic, we employed RT-PCR to look specifically at eGFP and IVS IRES splicing. Defects in mRNA processing are a well-established cause of RNA degradation (Moore and Proudfoot, 2009). Primer sets that amplify only endogenously expressed Hand1 confirm that Hand1Hand1ΔNeo/Hand1ΔNeo homozygous embryo genotypes are accurate (Fig. 4). As expected, eGFP mRNA is readily detectable in both heterozygous and homozygous Hand1Hand1ΔNeo embryos but not in wild-type littermates. Interestingly, PCR across the IVS within the IRES cassette shows the presence of both unspliced and spliced Hand1 chimeric mRNA suggesting that the hypomorphic levels of expression result from faulty mRNA processing leading to message degradation and poor translation.
Figure 4.
RTPCR analysis of expressed Hand1 message from Hand1Hand1ΔNeo heterozygous and homozygous embryos. PCR primers designed to detect endogenous, eGFP and IRES-IVS splice junctions were employed to amplify cDNA pools generated from whole embryos of the indicated genotypes. IVS primers detect both spliced and un-spliced Hand1IreseGFP message.
The primary Hand1 hypomorphic phenotypes are extraembryonic and vascular insufficiency
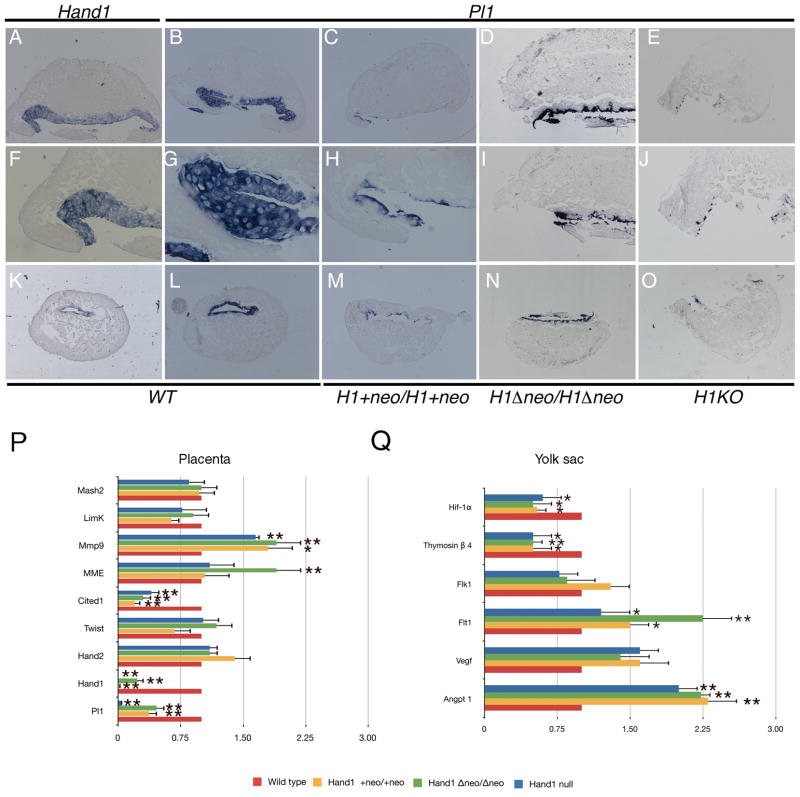
Hand1 null mice display defects within the extraembryonic membranes that lead to nutrient insufficiencies that produce numerous secondary phenotypes leading to death (Firulli et al., 1998; Riley et al., 1998). Pl1, a growth factor essential for the maintenance of the corpus luteum, is significantly downregulated in Hand1 null mice (Firulli et al., 1998; Riley et al., 1998). Therefore, we looked at Pl1 expression in E9.5 placentas to assess Pl1 expression in our hypomorphic Hand1 alleles (Fig. 5). Transverse sections of wild-type embryo decidua show that Hand1-expressing trophoblast giant cells are co-expressing Pl1 (Fig. 5A, B, F, G, K, and L). Hand1Hand1+Neo homozygous mice exhibit a marked reduction in Pl1-expressing cells (Fig. 5C, H, and M). Hand1Hand1ΔNeo homozygous mice show reduced levels of Pl1 expression as compared to wild-type mice, but visibly higher levels of expression then those observed in the Hand1Hand1+Neo homozygous embryos (Fig. 5D, I, N). As expected, Hand1LacZ/LacZ deciduas show the lowest level of PL1 expressing cells (Fig. 5E, J, O). Given the importance of Pl1 to embryonic survival, Hand1 regulation of Pl1 is the likely contributor to embryonic death in both the systemic null and hypomorphic alleles.
Figure 5.
Expression of PL1 in placentas from wildtype, Hand1Lacz/LacZ, Hand1Hand1+Neo/Hand1+Neo and Hand1Hand1ΔNeo/Hand1ΔNeo homozygous E9.5 embryos. (A,F,K) Expression of Hand1 in wild-type decidua. (B, G, L) Expression of PL1 in wild-type littermates. (C, H, M) PL1 expression in Hand1Hand1+Neo/Hand1+Neo embryos shows a reduced level of PL1 positive cells. (D, I, N) PL1 expression in Hand1Hand1ΔNeo/Hand1ΔNeo embryos is observed to be less the wild type but higher then in Hand1Hand1+Neo/Hand1+Neo embryos. (E, J, O). PL1 expression studies in Hand1Lacz/LacZ embryos show nearly a complete loss of PL1-expressing cells. (P) Comparative qRT-PCR analysis of vascular markers in wildtype, null and hypomorphic Hand1 embryos. A minimum of 4 embryos for each genotype were employed and error bars denote standard error, * indicates a P value of less than or equal to 0.05 and ** indicates a P value of less than or equal to 0.01
To quantify the differences in Pl1 gene expression, and to assay a number of other placental markers, we employed qRT-PCR analyses from E9.5 Hand1 null and hypomorphic embryo placentas (Fig. 5P). As expected, the level of Pl1 expression correlates directly with what is visibly observed by in situ analysis. The levels of Pl1 message are significantly (P≤0.01) reduced by more then 50% in both hypomorphic alleles, which is significantly higher then the levels observed within the Hand1 null placenta (Fig. 5P). Similar to the gene expression observations within the embryo, Hand1 expression is not detectable within the Hand1 null placenta where as Hand1Hand1+Neo homozygous express very low but detectable levels of Hand1 expression. Additionally, the Hand1Hand1ΔNeo/Hand1ΔNeo placenta expresses Hand1 at approximately 30% of the wild type placenta levels of Hand1 (Fig 5P). This result may clarify why Hand1Hand1ΔNeo/Hand1ΔNeo embryos survive longer then Hand1Hand1+Neo homozygous embryos even though the Hand1Hand1ΔNeo/Hand1ΔNeo embryos exhibit lower levels of embryonic Hand1 expression (Fig 2N). Consistent with the embryonic analysis, the message for Hand2 within the placenta is observed to be upregulated; however, in contrast, Twist1 mRNA levels are not down-regulated and are observed to be at the same level or at a slightly higher level of expression than is observed within the wild-type placenta (Fig. 2N & 5P). Cited1, in addition to being expressed within the heart, is also expressed within the placenta. Consistent with the cardiac expression data, Cited1 expression is markedly down in both the null and hypomorphic Hand1 placentas (P≤0.01) (Fig 5P). Membrane metallo endopeptidase (Mme; also known as 4311) is shown to exhibit expanded expression in Hand1 null conceptus (Riley et al., 1998). Consistent with these findings, we observe an increase in Mme expression within the Hand1 null and hypomorphic placenta but this increase is only significant in Hand1Hand1ΔNeo/Hand1ΔNeo placenta (Fig. 5P). Expression of matrix metallopeptidase 9 (Mmp9) is also upregulated in both Hand1 null and Hand1 hypomorphic alleles. LimK and Mash2 are reported as being down-regulated and up-regulated in Hand1 null mice respectively (Riley et al., 1998). We observe near normal levels of Limk in Hand1 null and hypomorphic mice (Fig. 5P). The expression levels of Mash2 appear indistinguishable from the wild-type placenta expression levels.
In addition to trophoblast giant cell gene expression, defects within the yolk sac vascular development, and associated changes in gene expression, are observed in Hand1 null embryos (Morikawa and Cserjesi, 2004). To determine if our Hand1 hypomorphic alleles display similar or distinct vascular expression profiles as compared to the Hand1 null mice, we performed quantitative RT-PCR to compare expression (Fig 5Q). Expression of the angiogenic growth factor Angpt1 is up-regulated within Hand1 null mice. We observe a significant (P≤0.01) 2-fold up-regulation of Angpt1 in Hand1 null embryos consistent to published reports (Fig. 5Q; (Morikawa and Cserjesi, 2004)). Both hypomorphic Hand1 alleles show comparable increases in Angpt1 expression. Upregulation of Vegf and Flt1 within in hypomorphic Hand1 mice is indistinguishable from systemic null Hand1 analysis. In contrast, we observe a decrease in the expression of Flk1 within systemic and Hand1Hand1ΔNeo homozygous embryos; however, expression in Hand1Hand1+Neo homozygous embryos is slightly elevated in a consistent but not statistically significant frequency (Fig. 5Q). Thymosin β4 is an important contributor to the coronary vasculature and was identified has a Hand1 downstream target (Smart et al., 2002). Analysis of Thymosin β4 expression in Hand1 systemic null mice validates these findings showing a significant (P≤0.01) 50% decrease in expression (Fig. 5Q). Similarly, both hypomorphic Hand1 alleles show near identical decreases in Thymosin β4 expression. Given its role in inducing angiogenesis and as hypoxia sensor, we looked at expression of the PAS-family bHLH transcription factor Hif-1α. Hif-1α is known to induce Vegf. Surprisingly, we observed a significant (P≤0.05) 50% reduction in Hif-1α mRNA in Hand1 systemic null and Hand1 hypomorphic embryos (Fig. 5Q). Given that Hif-1α should promote vessel formation, we were initially surprised to see a reduction in expression; however, Hif-1α knockout mice display abnormal vascular development and embryonic lethality, which is also associated with an upregulation of Vegf. (Ryan et al., 1998; Kotch et al., 1999). Taken together, the consistent similarity in changes observed in vascular gene expression between the Hand1 null and hypomorphic mice suggests that these defects, in combination with giant cell abnormalities, ultimately result in embryonic death. Extension of hypomorphic embryo survival is attributable to a substantial improvement in cardiac morphology and diminished alterations in cardiac gene expression.
To better define the extent of placental and vascular insufficiencies in the Hand1 hypomorphic phenotype, we performed cell death and cell proliferation analysis at E9.5 and E11.5 in wild-type, Hand1Hand1+Neo (E9.5), and Hand1Hand1ΔNeo (E 9.5 and E11.5) homozygous mice (Fig. 6). Apoptosis levels were indistinguishable between wild-type and Hand1 hypomorphic mice at E9.5 and E11.5. In contrast, BrdU incorporation analysis performed at E9.5 reveals a global decrease in the rate of cell proliferation in Hand1Hand1+Neo/Hand1+Neo Hand1Hand1ΔNeo/Hand1ΔNeo embryos compared to wildtype controls, indicating that the placental and vascular defects observed are globally affecting embryo viability. Taken together, these data correlate the severity of the observed Hand1 allelic phenotypes with the placental viability and support the hypothesis that the extraembryonic sources lead to the primary phenotypes that result in embryonic death where insufficiencies first slow and then stall embryogenesis.
Figure 6.
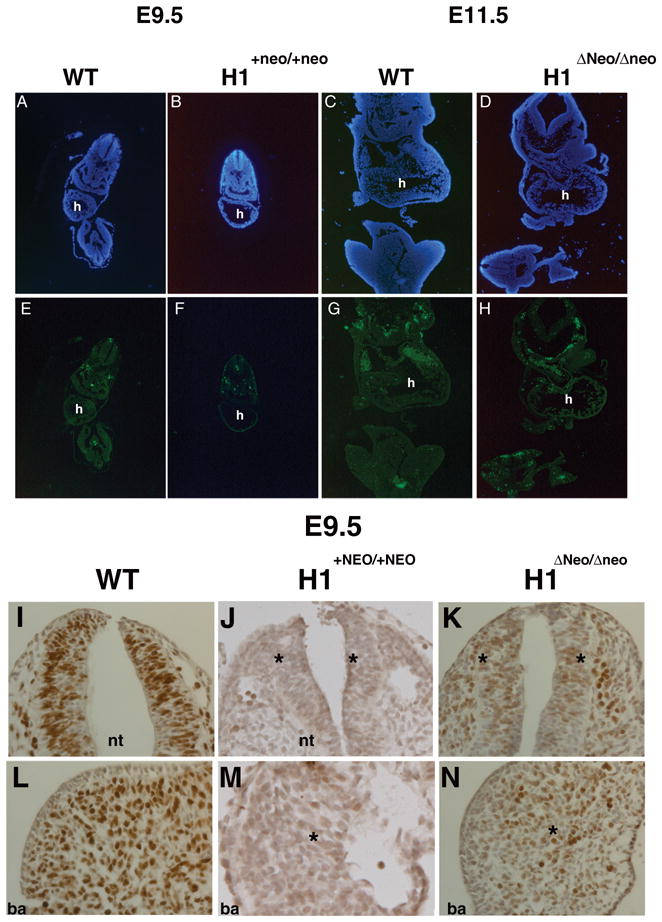
(A, B, E, F) TUNEL analysis of Hand1Hand1+Neo/Hand1+Neo (E9.5) and (C, D, G, H) Hand1Hand1ΔNeo/Hand1ΔNeo (E11.5) embryos. Detailed comparisons show no significant difference in the level of cell death compared to wild type embryos. BrdU incorporation within wild type (I, K) and Hand1Hand1ΔNeo/Hand1ΔNeo E9.5 embryos. Significant decreases in cell proliferation are observed throughout mutant embryos when compared to controls. The decrease in proliferation is observed in both non-Hand1 (neural tube I and J) and Hand1 expressing tissues (1st branchial arch K & L). h, heart; nt, neural tube; ba, first branchial arch
Loss of Hand2 enhances Hand1 hypomorphic defects
One of the key observations made in Morikawa et. al. and that we confirm is the up-regulation of the highly related transcription factor Hand2. There is a robust and growing body of data showing that members of the Twist-family of bHLH proteins have a functional sensitivity to gene dosage. In fact, Twist-family mutant phenotypes become more severe or exhibit phenotypic rescue when haploinsufficiency of other family members is introduced onto the mutant background (Firulli et al., 2005; McFadden et al., 2005). Thus, we crossed Hand1Hand1ΔNeo heterozygous mice onto the Hand2 systemic null background to deduce if a reduction in the upregulation of Hand2 would result in more severe embryonic phenotypes or further rescue the hypomorphic allele.
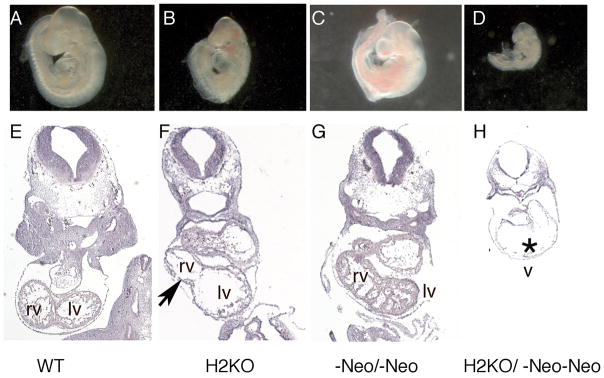
Our results show that Hand1Hand1ΔNeo/Hand1ΔNeo embryos that are also heterozygous for Hand2 are phenotypically indistinguishable from mice wild type for Hand2 (data not shown). Given the lack of phenotypic change of the hypomorphic mice on a Hand2 heterozygous null background, we next looked at Hand1Hand1ΔNeo/Hand1ΔNeo embryos on a Hand2 homozygous null (Fig. 7). Hand2 null, Hand1 hypomorph embryos are significantly smaller and display poor caudal development as compared to Hand2 null and Hand1 hypomorphic mice alone (Fig. 7A-D). Cardiac morphology shows that cardiac morphogenesis is comparable to both Hand1 hypomorph and Hand2 null littermates with regards to looping; however, sections through the myocardium show a thin ventricular wall. As discussed above, Hand1Hand1ΔNeo/Hand1ΔNeo hearts at E9.5–10.5 are similar in morphology to wild type (Fig. 7E and F). Consistent with published findings, Hand2 null embryos show a thin myocardium and a reduced/absent right ventricle (Fig. 7F) (Srivastava et al., 1997). Although it is possible that these data suggest genetic interactions within the developing heart, given that Hand factors are co-expressed within lateral mesoderm and, specifically, the vessels that connect to the placenta, these cardiac defects could be secondary to the placental insufficiencies.
Figure 7.
Histological analysis of Hand1Hand1ΔNeo/Hand1ΔNeo embryos that are heterozygous and homozygous null for Hand2 at E9.5. (A, E) Wildtype littermate; (B, F) Hand2 null; (C, G) Hand1Hand1ΔNeo/Hand1ΔNeo (D, H) Hand1Hand1ΔNeo/Hand1ΔNeo/Hand2 null. Black arrow indicates reduced right ventricle. Asterisk indicates thin myocardium. rv, right ventricle; lv, left ventricle; v, ventricle.
DISCUSSION
In our efforts to generate a Hand1 marked allele, we inadvertently generated two Hand1 hypomorphic mouse models, which partially extend the viability of Hand1 loss-of-function. The phenotypic cause of death is the result of both placental and vascular insufficiency and is further supported by our observations of decreased numbers of PL1-expressing trophoblast giant cells and changes within the vascular gene expression profile. Collectively, these defects result in a global reduction of cell proliferation within homozygous hypomorphic Hand1 mice. In Hand1 over-expressing mice, there is an observed increase of proliferation within the cardiomyocytes (Risebro et al., 2006). In both of our hypomorphic Hand1 alleles, we observe a global decrease in cell proliferation (Fig 6). Clearly, much of this reduction is a result of placental and vascular insufficiency; however, it is possible that additional decreases in proliferation within the cardiomyocytes and neural crest have a cell autonomous contribution to the phenotype.
In principle, designing targeting constructs to express a fully processed cDNA is a straightforward approach; however, in practice, unforeseen difficulties may be encountered. We show that using an IRES to allow bicistronic coexpression of both a reporter allele (eGFP) and Hand1, which appears functional in tissue culture applications, does not function well in mice (Fig 4). Although the Hand1 and eGFP mRNA is expressed, the protein expression of either Hand1 or eGFP is beyond detection with available reagents. Our only evidence that there is bona-fide translation of the Hand1 protein is the extended survival of both hypomorphic alleles when compared to what is observed in Hand1 systemic deletion. It is possible that the differences in targeting construct design could account for these observations, but we feel that this is unlikely as we employed identical 5′ and 3′ targeting arms to those that were used to generate our null allele (Fig. 1) (Firulli et al., 1998). Given that we do observe Hand1 mRNA, whereas in the Hand1 null mice we do not (Fig. 2), this is the likely mechanism for increased phenotypic viability. To achieve our intended goal of generating a Hand1 marker allele that does not effect bHLH gene dosage will possibly require knocking the reporter cassette into the intron or directly targeting the reporter into a bacterial artificial chromosome clone containing all transcriptional modules necessary to recapitulate endogenous expression.
The extension of viability observed in homozygous Hand1 hypomorphic embryos to between E10.5–12.5 reveals that cardiac morphogenesis is largely normal. Phenotypic distinctions include thin myocardium, hypo-trabeculation, and a reduction in pharyngeal mesoderm and OFT mesenchyme which are likely direct effects given the expression of Hand1 in these tissues. These phenotypes, if isolated, would likely allow for survival until birth. Cardiac specific deletion of Hand1 results in perinatal death (McFadden et al., 2005). As our hypomorphic alleles include similar placental and vascular defects to Hand1 systemic null embryos, we can deduce that the improvement in cardiac morphology contributes directly to the increased survival and maybe the result of the low level of Hand1 protein present within these tissues. Indeed, we observe differences between the three mutant Hand1 alleles in a set of cardiac expressed factors. Nppa, for example, is clearly downregulated in the Hand1 null; however, expression in Hand1Hand1ΔNeo/Hand1ΔNeo clearly shows more robust expression correlating with this alleles improved survival (Fig. 3).
The quantitative measure of Hand1 expression from both the Hand1Hand1+Neo and Hand1Hand1ΔNeo hypomorphic alleles defines the range of required Hand1 expression as between 15% and 20% from each allele of that observed from the wild type alleles. Hand1 heterozygous null mice are viable and display no obvious phenotypes. Hand1 homozygous null mice die at E8.5 and show no detectable Hand1 mRNA, confirming that Hand1LacZ is a true null allele. As hypomorphic Hand1 embryos are larger, further developed, and express 30–40% of the wild-type level of Hand1, it is clear that a precise gene dosage of Hand1 is critical and that expression slightly less then haploinsufficiency is deleterious to survival. Comparison of the Hand1 expression levels between heterozygous Hand1+/LacZ and Hand1Hand1+Neo/Hand1+Neo and Hand1Hand1ΔNeo/Hand1ΔNeo E9.5 day embryos show no significant differences, yet the former genotype is viable whereas the latter genotypes are not. It is possible that the engineered cDNA alleles lack key processing sequences that allow for efficient translation of Hand1 protein. Indeed, we have determined that allele splicing is inefficient and the lack of the complete 3′ untranslated region within these Hand1 alleles may directly point to post-transcriptional regulation such as micro RNAs that refine Hand1 expression to meet the developmental program requirements of the cell. Additionally, the improperly processed mRNA can also contribute to less-efficient translation as evidenced by the inability to detect Hand1 protein in homozygous hypomorphs where protein is detectable in wild-type and heterozygous null littermates (Fig. 2). Perhaps most interesting is the observed up-regulation of Hand2 within the null and hypomorphic mice. Early studies on Hand1 and Hand2 suggested functional redundancy (Srivastava et al., 1995). In such a biological relationship, the up-regulation of Hand2 to compensate for the loss of Hand1 is logical. Additionally, strong genetic interactions between Twist-family proteins are observed between Hand2 with Twist1 within the developing limb (Firulli et al., 2005). To test if the observed up-regulation of Hand2 is indeed a compensatory mechanism or a deleterious dysregulation, we looked at hypomorphic Hand1Hand1ΔNeo/Hand1ΔNeo mice on both a Hand2 heterozygous and homozygous null background. Hand2 heterozygousity showed no significant change to the observed Hand1-hypomorphic phenotypes suggesting genetic interactions are not significantly affected in this genotype. Evaluation of Hand1 hypomorphs devoid of Hand2 expression do show observable differences in that embryos are smaller and display reduced caudle structures and thin lateral mesoderm similar to Hand1 hypomorphs (Fig. 2 and 7). Cardiogenesis occurs but the wall of the heart is extremely thin. This result could be direct via bona fide genetic interactions within the myocardium; however, it is more likely that additive effects or genetic effects within the placenta connections exacerbate the Hand1 hypomorphic extraembryonic phenotypes.
The role of Twist-family bHLH factors in embryonic development is complex. Given that these factors function biologically within a variety of dimer complexes makes evaluation of their role in gene expression and cell specification and differentiation difficult. Dimer partner choice can result in the formation of multiple transcriptional complexes that may regulate different genes sets or the same gene sets in different ways. An example of this can be seen with Twist in drosophila where it has been shown that Twist homodimers promote somatic muscle formation, whereas Twist-daughterless (E-protein) heterodimers antagonize somatic muscle formation (Castanon et al., 2001). Considering the removal of a single bHLH gene from a cell, it is obvious that the interactive relationships of the remaining factors within that cell must change. The formation of inappropriate dimers is a likely outcome and whether these dimers are benign or functionally deleterious is likely going to be dictated by a cell type specific relationship. In summary, in our efforts to produce a Hand1 expression reporter that does not affect Hand1 gene dosage, we generated two alleles that express low levels of Hand1. Where in systemic and conditional Hand1 alleles 100% ablation is achieved, the Hand1Hand1+Neo and Hand1Hand1ΔNeo hypomorphic mice demonstrate that normal development cannot tolerate less then 30% of Hand1 within the embryo or extra-embryonic structures.
EXPERIMENTAL PROCEDURES
Plasmid Constructs
The Hand Hand1+neo targeting vector employed the identical 5′ and 3′ targeting arms employed in generation of the Hand1Laz knock-in (Firulli et al., 1998). Inserted between these arms is a Hand1 cDNA that begins 70 base pairs 5′ of the initiating methionine and ends at the translational stop site. The Hand1 cDNA is immediately 5′ of an IRESGFP cassette derived from pIRESeGFP (Clonetech), which is immediately 5′ of a PGKNeo cassette flanked by loxP sites. Homologous recombination was achieved at a frequency of one in fifteen and two independent ES lines were injected and produced germline transmission with indistinguishable phenotypes.
Mouse strains
Hand1Hand1+neo mice were generated by the IU knockout-transgenic core from ES cells targeted with the construct described above. HandHand1Δneo mice were subsequently generated via intercross of Hand Hand1+neo mice with mice heterozygous for EIIA-Cre allele (Lakso et al., 1996). Both versions of the reporter allele are viable and fertile as heterozygotes. Hand1Laz knock-in mice have been reported previously (Firulli et al., 1998) and Hand2 knockout (Srivastava et al., 1997) mice have been reported previously.
Histology
Embryos (E9.5 – E11.5) were fixed in 4% paraformaldehyde, dehydrated through an ethanol gradient and embedded in paraffin. Embryos were sectioned at 7μm unless otherwise noted. Hematoxylin and Eosin (H&E) staining was performed exactly as described (Conway et al., 2000). A minimum of 3 viable embryos (assayed via the presence of a heart beat) per genotype was used for these and all subsequent analyses.
In situ hybridization-qRT-PCR
Digoxygenin labeled section in situ hybridizations were carried out using established protocols on 10μm paraffin sections or in whole mount (Vincentz et al., 2008) using T7, T3 or SP6 polymerases (Promega) and DIG-Labeling Mix (Roche). Sense and antisense digoxygenin-labeled riboprobes were transcribed for Hand1, Hand2, eGFP, and Pl1. Hybridizations and all subsequent incubations were done concurrently on all embryos being compared. qRT-PCR was performed on a Lightcycler 480II (Roche) using Taqman labeled proprietary primer sets for Hand1, Hand2 and Gapdh as internal control. Gene expression for marker genes was performed using Taqman primers or via the Roche UPL (universal probe library, mouse) system. Whole embryos, placentas, or yolk sacs were flash frozen and genotyped via genomic DNA from the yolk sac or head of the embryo. Total RNA was isolated using a High Pure RNA Tissue kit (Roche) and cDNA was prepared using the Transcriptor First Strand cDNA synthesis kit (Roche) following the manufactures protocol. A minimum of 4 viable embryos (assayed via the presence of a heart beat) were analyzed in all experiments. Error bars denote standard error. Differences between mouse lines were examined for statistical significance by using the Students t-test. P values of less than 0.05 were regarded as significant and marked in all graphs as a single asterisk * and P values less then 0.01 are denoted with double asterisk **
Immunoblotting
Embryo lysates were collected, and equal amounts of protein were run through 12% SDS PAGE gels, electroblotted and incubated with α-Hand1 polyclonal antibody (Santa Cruz) as described (Firulli et al., 2003). Blots were visualized using the Super Signal Luminescent detection protocol (Pierce).
TUNEL and Immunohistochemistry
TUNEL analysis on sectioned embryos was performed using the ApopTag Plus Fluorescein in situ Apoptosis detection kit (S7111 Chemicon International) following the manufacturers instructions. Cell proliferation was assayed using Bromodeoxy Uridine (BrdU) incorporation and immunodetection following the manufacturer’s instructions. For embryos, time-mated females were injected IP with BrdU (100ug/g body weight) 2 hours prior to sacrifice. Embryos were processed as described above and then cut transverse in 7μm sections. Immunohistochemistry was performed using α-BrdU (Abcam), developed using a standard streptavidin-HRP method, and counterstained with Hematoxylin.
Acknowledgments
We would like to thank Danny Carney and Santiago Pineda for technical assistance. Infrastructural support at the Herman B Wells Center for Pediatric Research is in part supported by the generosity of the Riley Children’s Foundation and Division of Pediatric Cardiology. This work is supported by the NIH RO1HL061677-09 1P01HL085098-01A (ABF), and AHA 0815426G (RMB).
Grant Sponsor: NIH RO1HL061677-09 1P01HL085098-01A (ABF), and AHA 0815426G (RMB).
References
- Barnes RM, Firulli AB. A Twist of insight, the role of Twist-Family bHLH factors in development. Int J Dev Biol. 2009;53:909–924. doi: 10.1387/ijdb.082747rb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon I, Von Stetina S, Kass J, Baylies MK. Dimerization partners determine the activity of the Twist bHLH protein during Drosophila mesoderm development. Development. 2001;128:3145–3159. doi: 10.1242/dev.128.16.3145. [DOI] [PubMed] [Google Scholar]
- Conway SJ, Bundy J, Chen J, Dickman E, Rogers R, Will BM. Decreased neural crest stem cell expansion is responsible for the conotruncal heart defects within the splotch (Sp(2H))/Pax3 mouse mutant.[see comment] Cardiovascular Research. 2000;47:314–328. doi: 10.1016/s0008-6363(00)00098-5. [DOI] [PubMed] [Google Scholar]
- Firulli AB. A HANDful of questions: The molecular biology of the HAND-subclass of basic helix-loop-helix transcription factors. Gene. 2003;312C:27–40. doi: 10.1016/s0378-1119(03)00669-3. [DOI] [PubMed] [Google Scholar]
- Firulli AB, Conway SJ. Phosphoregulation of Twist1 provides a mechanism of cell fate control. Current Medicinal Chemistry. 2008;15:2641–2647. doi: 10.2174/092986708785908987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firulli AB, McFadden DG, Lin Q, Srivastava D, Olson EN. Heart and extra-embryonic mesodermal defects in mouse embryos lacking the bHLH transcription factor Hand1. Nature Genetics. 1998;18:266–270. doi: 10.1038/ng0398-266. [DOI] [PubMed] [Google Scholar]
- Firulli B, Howard MJ, McDaid JR, McIlreavey L, Dionne KM, Centonze V, Cserjesi P, Virshup DMa, Firulli AB. PKA, PKC and the Protein Phosphatase 2A Influence HAND factor function: A Mechanisms for Tissue Specific Transcriptional Regulation. Mol Cell. 2003;12:1225–1237. doi: 10.1016/s1097-2765(03)00425-8. [DOI] [PubMed] [Google Scholar]
- Firulli BA, Krawchuk D, Centonze VE, Virshup DE, Conway SJ, Cserjesi P, Laufer E, Firulli AB. Altered Twist1 and Hand2 dimerization is associated with Saethre-Chotzen syndrome and limb abnormalities. Nat Genet. 2005;37:373–381. doi: 10.1038/ng1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotch LE, Iyer NV, Laughner E, Semenza GL. Defective vascularization of HIF-1α-null embryos is not associated with VEGF deficiency but with mesenchymal cell death. Dev Biol. 1999;209:254–267. doi: 10.1006/dbio.1999.9253. [DOI] [PubMed] [Google Scholar]
- Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alts FW, Westphal H. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci USA. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons I, Parsons LM, Hartley L, Li R, Andrews JE, Robb L, Harvey RP. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2–5. Genes Dev. 1995;9:1654–1666. doi: 10.1101/gad.9.13.1654. [DOI] [PubMed] [Google Scholar]
- McFadden DG, Barbosa AC, Richardson JA, Schneider MD, Srivastava D, Olson EN. The Hand1 and Hand2 transcription factors regulate expansion of the embryonic cardiac ventricles in a gene dosage-dependent manner. Development. 2005;132:189–201. doi: 10.1242/dev.01562. [DOI] [PubMed] [Google Scholar]
- Moore MJ, Proudfoot NJ. Pre-mRNA processing reaches back to transcription and ahead to translation. Cell. 2009;136:688–700. doi: 10.1016/j.cell.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Morikawa Y, Cserjesi P. Extra-embryonic vasculature development is regulated by the transcription factor HAND1. Development. 2004;131:2195–2204. doi: 10.1242/dev.01091. [DOI] [PubMed] [Google Scholar]
- Riley P, Anson-Cartwright L, Cross JC. The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nat Genet. 1998;18:271–275. doi: 10.1038/ng0398-271. [DOI] [PubMed] [Google Scholar]
- Risebro CA, Smart N, Dupays L, Breckenridge R, Mohun TJ, Riley PR. Hand1 regulates cardiomyocyte proliferation versus differentiation in the developing heart. Development. 2006;133:4595–4606. doi: 10.1242/dev.02625. [DOI] [PubMed] [Google Scholar]
- Ryan HE, Lo J, Johnson RS. HIF-1α is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart N, Hill AA, Cross JC, Riley PR. A differential screen for putative targets of the bHLH transcription factor Hand1 in cardiac morphogenesis. Gene Expression Patterns. 2002;2:61–67. doi: 10.1016/s0925-4773(02)00380-5. [DOI] [PubMed] [Google Scholar]
- Srivastava D, Cserjesi P, Olson EN. A subclass of bHLH proteins required for cardiac morphogenesis. Science. 1995;270:1995–1999. doi: 10.1126/science.270.5244.1995. [DOI] [PubMed] [Google Scholar]
- Srivastava D, Thomas T, Lin Q, Kirby ML, Brown D, Olson EN. Regulation of cardiac mesodermal and neural crest development by the bHLH transcription factor, dHAND. Nature Genetics. 1997;16:154–160. doi: 10.1038/ng0697-154. [DOI] [PubMed] [Google Scholar]
- Vincentz JW, BArnes RM, Rodgers R, Firulli BA, Conway SJ, Firulli AB. An Absence of Twist1 results in aberrant cardiac neural crest morphogenesis. Dev Biol. 2008;320:131–139. doi: 10.1016/j.ydbio.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker WH, Fitzpatrick SL, Barrera-Saldana HA, Resendez-Perez D, Saunders GF. The human placental lactogen genes: structure, function, evolution and transcriptional regulation. Endocrine Reviews. 1991;12:316–328. doi: 10.1210/edrv-12-4-316. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Ogren L, Endo H, Soares MJ, Talamantes F. Co-localization of placental lactogen-I, placental lactogen-II, and proliferin in the mouse placenta at midpregnancy. Biology of Reproduction. 1994;51:1188–1192. doi: 10.1095/biolreprod51.6.1188. [DOI] [PubMed] [Google Scholar]