Abstract
Background & Aims
Stress signaling, both within and outside the endoplasmic reticulum, has been linked to metabolic dysregulation and hepatic steatosis. Methionine-choline-deficient (MCD) diets cause severe fatty liver disease and have the potential to cause many types of cellular stress. The purpose of this study was to characterize hepatic stress in MCD-fed mice and explore the relationship between MCD-mediated stress and liver injury.
Methods
Stress signaling was examined in mice fed MCD formulas for 4–21 days. Signaling was also evaluated in mice fed MCD formulas supplemented with clofibrate, which inhibits hepatic triglyceride accumulation. The role of the pro-apoptotic stress protein C/EBP homologous protein (CHOP) in MCD-mediated liver injury was assessed by comparing the responses of wild-type and CHOP-deficient mice to an MCD diet.
Results
MCD feeding caused steatohepatitis coincident with the activation of cJun N-terminal kinase and caspase-12. In contrast, MCD feeding did not activate inositol-requiring protein-1 and actually suppressed the expression of X-box protein-1s. MCD feeding caused weak stimulation of PKR-like ER-resident kinase, but robust activation of general control non-derepressible-2, followed by the phosphorylation of eukaryotic initiating factor-2α and induction of CHOP. Clofibrate eliminated MCD-mediated hepatic steatosis but did not inhibit diet-induced stress. CHOP deficiency did not alleviate, and in fact worsened, MCD-mediated liver disease.
Conclusions
MCD feeding causes an integrated stress response in the liver rather than a classical unfolded protein response. This stress response does not by itself lead to liver injury. CHOP, despite its identity as a mediator of stress-related cell death, does not play a central role in the pathogenesis of MCD-mediated liver disease.
Keywords: steatosis, steatohepatitis, endoplasmic reticulum stress, integrated stress, unfolded protein response
Introduction
Endoplasmic reticulum (ER) stress is the term used to describe a situation in which the amount of unfolded protein entering the ER exceeds the processing capacity of the organelle. This imbalance is recognized by three signal transducers in the ER membrane: inositol-requiring protein-1 (IRE1), activating transcription factor-6 (ATF6) and PKR-like ER-resident kinase (PERK). IRE1, ATF6 and PERK work together to provide a comprehensive response to ER stress that includes the suppression of further protein synthesis, the enhancement of protein folding capacity and the degradation of unfolded or misfolded proteins. Collectively, this series of events is designated the unfolded protein response (UPR).1
Interestingly, ER stress is often observed in fatty livers, even though steatosis does not represent the type of abnormality that should induce a UPR. The connection lies in the ability of triglyceride to induce the synthesis of apolipoprotein B100 (Apo B100) within hepatocytes.2, 3 If excessive, ApoB 100 synthesis can initiate a UPR; importantly, once activated in a fatty liver, the UPR can reduce Apo B100 synthesis and up-regulate the expression of enzymes involved in hepatic lipogenesis, which can result in the exaggeration of hepatic steatosis.2, 4, 5 This scenario predicts a vicious cycle in which steatosis and ER stress fuel each other to promote the progressive accumulation of hepatic triglyceride. Indeed, such cross-talk between ER stress and hepatic steatosis has been documented experimentally: manipulations that affect ER stress influence hepatic steatosis,4–8 and maneuvers that affect steatosis influence ER stress. 2, 9
Whether ER stress contributes specifically to hepatocellular injury in a fatty liver is not completely understood. Such a question could be directly addressed in an animal model of severe steatohepatitis such as that induced by a methionine-choline-deficient (MCD) diet. The MCD model is attractive for this purpose because it progresses beyond hepatic steatosis to include significant hepatocellular injury and inflammation.10, 11 When investigating the impact of ER stress on the pathogenesis of fatty liver disease in MCD-fed mice, however, it is important to consider that hepatic triglyceride accumulation may not be the principal event that perturbs the ER. For example, MCD feeding depletes cellular phospholipids, which can activate IRE1 in an effort to restore normal phospholipid levels.12, 13 In addition, MCD formulas are by definition amino acid-deficient; amino acid deprivation can activate general control non-derepressible-2 (GCN2), a stress transducer similar to PERK.14 GCN2 is one of a family of PERK-like signal transducers that includes heme-regulated inhibitor kinase (HRI) and double-stranded RNA-activated protein kinase (PKR). GCN2, HRI and PKR are distinct from PERK in that they reside in the cytoplasm rather than the ER.1 All three, however, are functionally similar to PERK in that they activate a common signaling pathway beginning with eukaryotic initiating factor-2α (eIF2α). The ability of these diverse stress transducers to converge on eIF2α has led to the designation of eIF2α and its downstream targets as components of an “integrated stress response” (ISR).15
Whatever the origin of cellular stress in the livers of MCD-fed mice, the model offers an opportunity to investigate the role of stress signaling in the pathogenesis of steatohepatitis. In this context it is important to note that although the primary purpose of a UPR or ISR is to alleviate stress,1 the UPR and ISR signaling cascades may commit cells to apoptosis if stress is particularly severe or prolonged. Specifically, stress signaling results in the activation of pro-apoptotic proteins such as c-Jun N-terminal kinase (JNK), C/EBP homologous protein (CHOP) and caspase-12.16–18 In many experimental systems, ER stress-induced cell death is discernable by a robust or protracted increase in CHOP expression.8, 19 While CHOP has been linked to fatty liver injury in response to excess alcohol consumption,20 its role in nonalcoholic steatohepatitis is unknown.
The objective of this study was two-fold. First, we wished to determine the extent to which MCD feeding causes activation of a UPR, ISR or both, and to assess whether the stress that leads to these responses is related to hepatic steatosis or some other diet-induced abnormality. Second, we wanted to explore whether the induction of CHOP in MCD-fed mice plays a causative role in MCD-mediated liver disease. The results indicate that MCD feeding activates a unique pattern of stress in the liver dominated by features of an ISR rather than a UPR. CHOP is induced in MCD-fed livers as part of this diet-related ISR, but its up-regulation is not central to the pathogenesis of MCD-mediated liver injury.
Methods
Animals and diets
Adult male C3H mice were fed methionine-choline-sufficient (MCS) or methionine-choline-deficient (MCD) formulas (Dyets, Inc., Bethlehem, PA) for 4–21 days. The diets were matched in all nutrients except L-methionine (2 g/kg) and choline chloride (2 g/kg), which were present in the MCS formula only. Both diets provided 17% kcal as protein, 62% kcal as carbohydrate (70:30 sucrose:starch) and 19% kcal as fat. All animals had free access to diet and drinking water for the duration of study. In some experiments, mice were fed MCS and MCD formulas containing 0.5% (w/w) clofibrate (Sigma-Aldrich, St. Louis, MO). In other experiments, MCD formulas were fed to CHOP-deficient mice (B6.129S-Ddit3tm1Dron/J) and wild-type controls (C57Bl/6J) (The Jackson Laboratory, Bar Harbor, ME). In all dietary studies, mice were fasted for 4 h prior to killing.
All animals received humane care according the guidelines of the US Public Health Service. All experimental procedures performed on live animals were approved by the Institutional Animal Care and Use Committee at the University of California, San Francisco.
Serum chemistries
Alanine aminotransferase (ALT), glucose, cholesterol, and triglycerides were measured in mouse serum using an ADVIA 1800 autoanalyzer (Siemens Healthcare Diagnostics, Deerfield, IL) in the clinical chemistry laboratory at San Francisco General Hospital.
Liver histology
Paraffin sections of liver tissue were stained with hematoxylin and eosin. Slides were blindly evaluated and scored for steatosis (0–3), ballooning (0–2) and inflammation (0–3).21 Cell death was evaluated in tissue sections by terminal deoxynucleotide transferase-mediated deoxyuridine triphosphate nick end-labeling (TUNEL) (ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit, Millipore, Billerica, MA). Sections were counterstained with hematoxylin for viewing and photography.
Measurement of hepatic triglyceride
Lipids were extracted from fresh liver tissue. Triglyceride was quantitated as described previously.10 Results were reported as mg triglyceride per gram liver.
Evaluation of stress responses in liver homogenates
The expression or activation of proteins involved in the unfolded protein response was assessed in mouse liver homogenates by immunoblotting. Antibodies against β-actin, Bcl-xL, c/EBPα, C/EBPβ, BiP, caspase-12, c-Jun/P-cJun, eIF2α/P-eIF2α, IRE1, JNK/P-JNK, PERK and P-GCN2 and were from Cell Signaling Technology (Danvers, MA). Antibodies against CHOP and XBP-1 were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-IRE1-P was from Novus Biologicals (Littleton, CO). Proteins of interest were identified by chemiluminescence (Super Signal West Dura, Thermo Fisher Scientific, Rockford, IL).
Quantitation of hepatic gene expression
The expression of stress-related genes in mouse liver was assessed by quantitative real-time PCR as described previously.22 Assays-on-Demand® primer and probe sets (Applied Biosystems) were used for all the genes of interest. The expression of each test gene was normalized to that of mouse β-glucuronidase.
Statistical analysis
All studies, including Western blots, were performed on 5 or more mice per group. Comparisons between two groups were analyzed by unpaired t-test. Comparisons among 3 or more groups were analyzed by ANOVA with Bonferroni correction. P values < 0.05 were considered significant.
Results
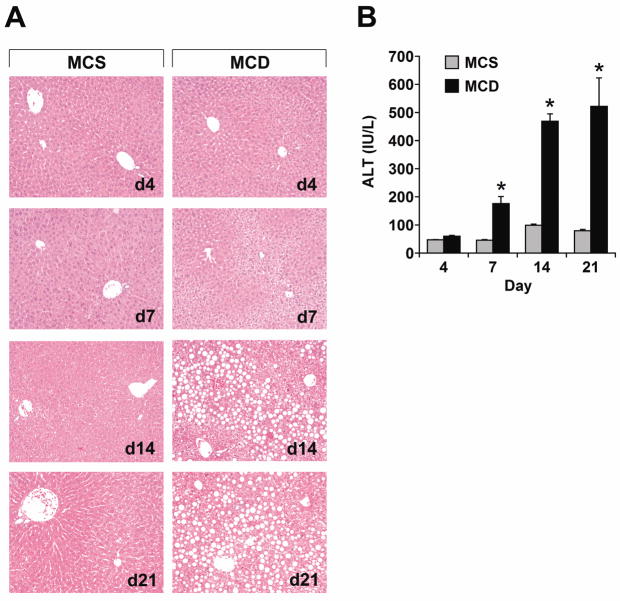
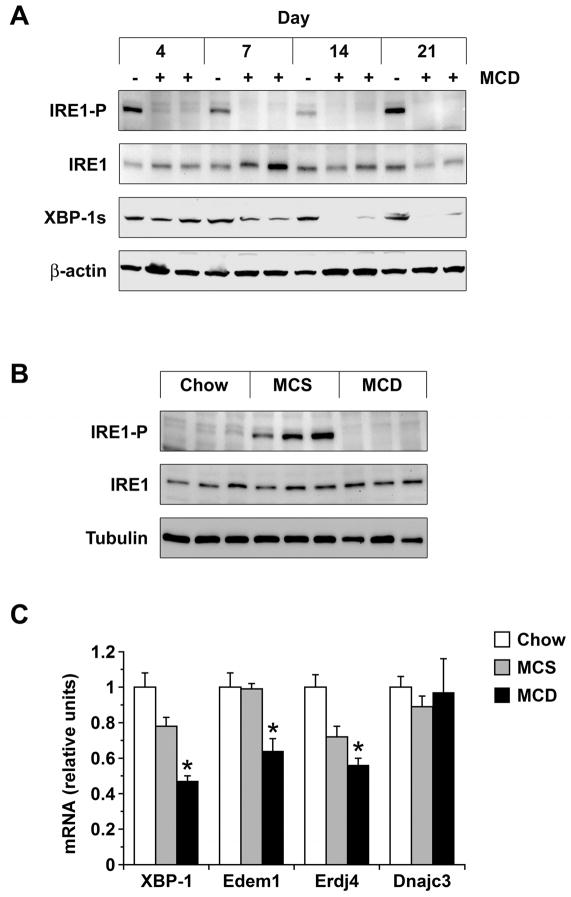
Mice were fed MCD formulas for 4–21 days to assess the time course of ER stress in the liver as they developed steatohepatitis. Over this interval there was a progressive increase in hepatic steatosis (Figure 1A) as well as a significant rise in the serum ALT level (Figure 1B). Specific UPR/ISR pathways were interrogated by looking for induction or activation of stress-related genes and proteins; the first to be analyzed was IRE1. MCD feeding did not activate IRE1 at any time during the 21-day experiment (Figure 2A). Nor did MCD feeding induce hepatic expression of XBP-1s, the protein product that results from IRE1-mediated splicing of XBP-1 mRNA.23 In fact, XBP-1s levels decreased in the livers of MCD-fed mice over 21 days, consistent with a prolonged absence of IRE1 activity. In mice fed the MCS control diet, IRE1 was activated in the liver and XBP-1s levels remained constant over 21 days. This was likely due to the nutrient composition of the MCS formula, as diets enriched in sugar or fat can provoke sustained IRE1 activation.24 Mice fed a chow diet for 21 days did not display IRE1 activation (Figure 2B). Analysis of hepatic XBP-1 mRNA levels demonstrated that MCD feeding suppressed XBP-1 at a pre-translational level (Figure 2C). MCD feeding also down-regulated the expression of several XBP-1 target genes, including those encoding the ER-associated degradation (ERAD) proteins Edem1 and Erdj4 (Figure 2C). Taken together, these findings indicate that MCD feeding does not induce a classical UPR in the liver.
Figure 1. Time course of steatosis and steatohepatitis in MCD-fed mice.
(A) Mice were fed control (MCS) or methionine-choline-deficient (MCD) diets for 4–21 days. Liver sections were stained with hematoxylin and eosin. (B) ALT was quantitated in the serum of mice fed MCS or MCD formulas (n = 5) for 4–21 days. * P < 0.05 vs. MCS.
Figure 2. IRE1 activation and expression of downstream targets in MCD-fed mice.
(A) IRE1 activation and XBP-1s expression were assessed by immunoblotting in liver homogenates from mice fed MCS (−) or MCD (+) formulas for 4–21 days. (B) IRE1 activation was compared in liver homogenates from mice fed chow, MCS, or MCD formulas for 21 days. (C) RNA was extracted from the livers of mice fed chow, MCS or MCD diets for 21 days. mRNA encoding XBP-1 and target genes Edem1, Erdj4 and Dnajc3 was measured by quantitative PCR (n = 5). *P < 0.05 vs. chow.
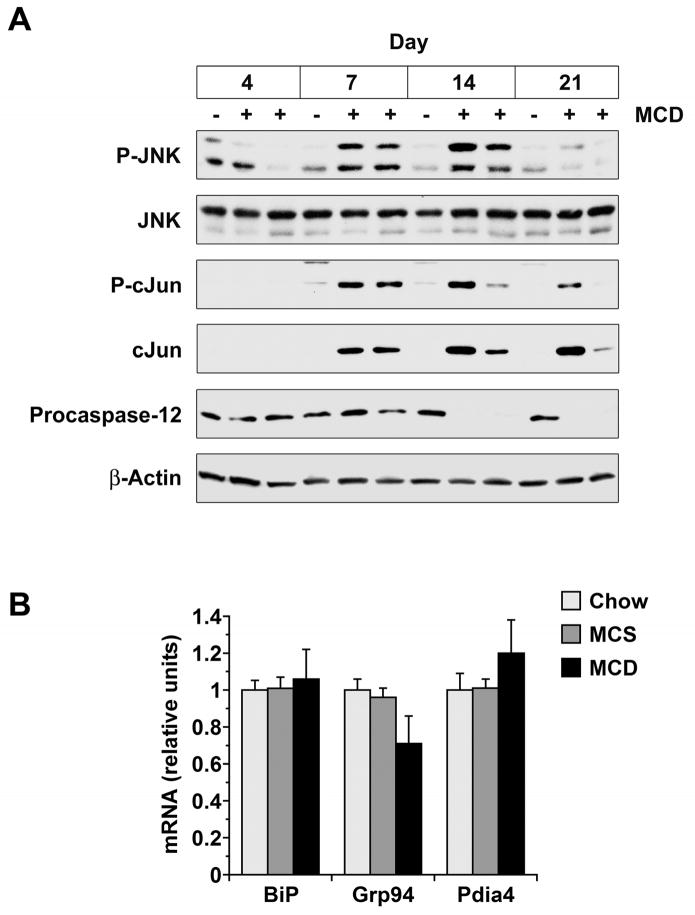
Although MCD feeding did not activate IRE1, it did activate JNK and promote the cleavage of procaspase-12 in the liver (Figure 3A). JNK and caspase-12 can be activated downstream of IRE1 during ER stress,16, 18 but in MCD-fed mice these events occurred independent of IRE1. JNK activation was greatest at 7–14 days of MCD feeding, coincident with the rise serum ALT activity. JNK activity declined as ALT levels reached a plateau, while activation of its target, cJun, displayed a more protracted time course.
Figure 3. Effect of MCD feeding on JNK, caspase-12 and ATF6 signaling in liver.
(A) Liver homogenates were prepared from mice fed MCS (−) or MCD (+) diets for 4–21 days. JNK activation, cJun induction and procaspase-12 cleavage were assessed by Western blotting. (B) mRNA encoding three ATF6 target genes was measured by quantitative PCR in the livers of mice fed chow, MCS or MCD formulas for 21 days (n = 5).
Activating transcription factor-6 (ATF6) cooperates with XBP-1 to regulate the transcription of ERAD proteins and also functions independently to stimulate the transcription of genes encoding ER chaperones.25 To determine whether MCD feeding activates ATF6, we looked for evidence that it increased the hepatic expression of mRNAs encoding the chaperone proteins immunoglobulin-binding protein (BiP), glucose-regulated protein 94 (Grp94) and protein disulfide isomerase-associated-4 (Pdia4). ER chaperone genes were expressed no differently in MCD-fed mice than MCS or chow controls (Figure 3B); MCD feeding, therefore, did not affect ATF6.
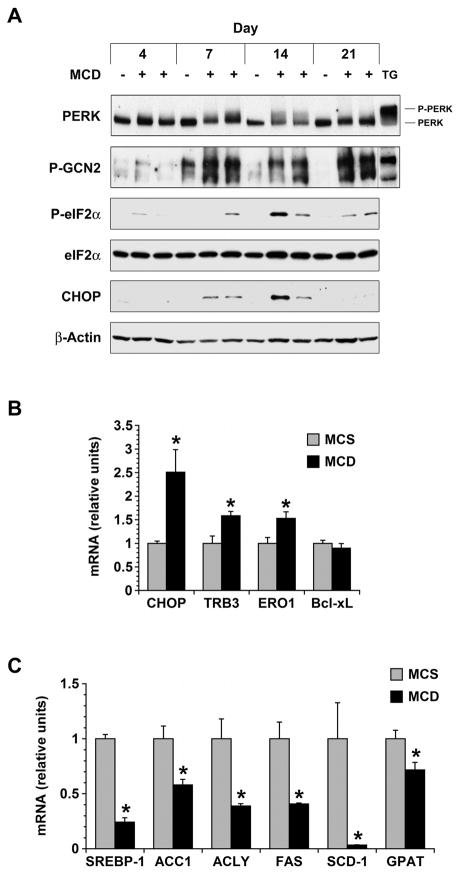
PERK was activated by MCD feeding, although diet-induced PERK phosphorylation was very weak in comparison to that stimulated by thapsigargin (Figure 4A). By contrast, eIF2α was clearly phosphorylated in response to MCD feeding (Figure 4A), and several molecules downstream of eIF2α were also up-regulated including CHOP and its targets tribbles homolog 3 (TRB3) and endoplasmic oxidoreductase protein-1 (ERO1) (Figure 4A, B).26, 27 This raised the possibility that eIF2α was being activated by a kinase other than PERK as part of a diet-induced ISR. Because the MCD formula is deficient in methionine, we suspected it would activate the amino acid-sensitive eIF2α kinase GCN2 as has been reported in the setting of leucine deprivation.28 Indeed, MCD feeding caused robust activation of GCN2 from day 7 through day 21 (Figure 4A). To distinguish whether PERK or GCN2 is the predominant eIF2α kinase in the MCD-fed liver, we evaluated lipogenic gene expression. PERK and GCN2 activate many of the same stress responses29 but they have opposing effects on lipogenesis, with PERK stimulating and GCN2 suppressing the expression of genes involved in fatty acid and triglyceride synthesis.28, 30 In MCD-fed mice, lipogenic gene expression was markedly reduced in comparison to MCS controls (Figure 4C), implicating GCN2 as the predominant eIF2α kinase activated by MCD feeding. The activation of GCN2 in MCD-fed livers, together with the suppression of lipogenic gene expression, supports the notion that amino acid deprivation is a pivotal derangement driving the integrated stress response in the MCD model of liver disease.
Figure 4. Effect of MCD feeding on molecules involved in the ISR.
(A) The phosphorylation of PERK and GCN2 was monitored in the livers of mice fed MCS (−) or MCD (+) diets for 4–21 days. Thapsigargin treatment (TG) was used as a positive control. The activation of eIF2α and expression of CHOP were also followed over the 21-day interval by Western blotting. (B) mRNA encoding CHOP and its downstream targets TRB3, ERO1 and Bcl-xL were measured by quantitative PCR in the livers of mice fed MCS or MCD formulas for 21 days (n = 5). (C) mRNA encoding several lipogenic genes was measured by quantitative PCR in the livers of mice fed MCS or MCD formulas for 21 days (n = 5). * P < 0.05 vs. MCS.
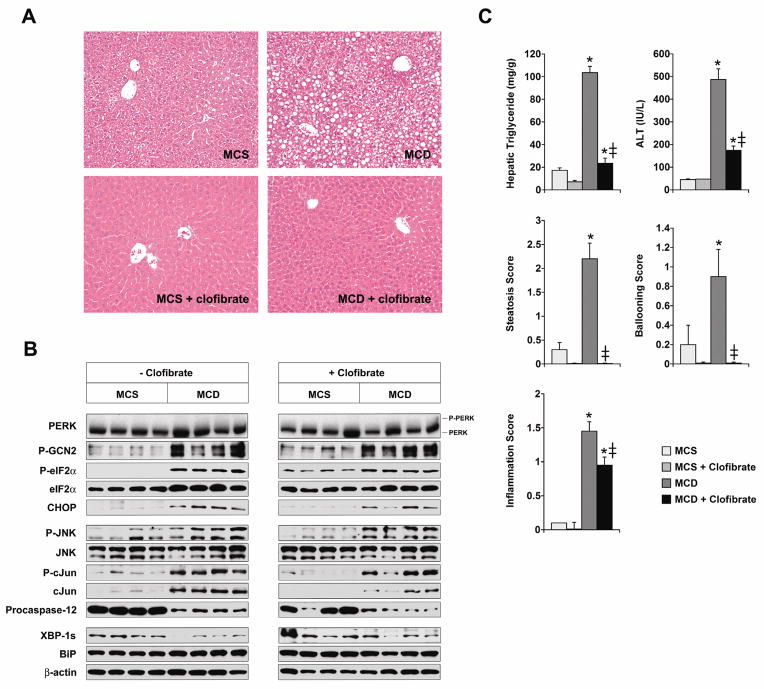
Although our results pointed to methionine deficiency as a key inducer of hepatic stress in MCD-fed mice, MCD feeding also provoked hepatic steatosis, which by itself can generate ER stress.2, 9 To determine whether steatosis contributes independently to ER stress in the livers of MCD-fed mice, we elected to compare two groups of MCD-fed mice that differed in their degree of hepatic lipid accumulation. Hepatic steatosis can be averted in MCD-fed mice by treating them with a PPARα agonist to stimulate fatty acid oxidation.31 We therefore added 0.5% (w/w) clofibrate to the MCS and MCD formulas, and confirmed that the addition of clofibrate caused marked induction of enzymes involved in peroxisomal and mitochondrial fatty acid oxidation (Supplemental Figure 1). Mice fed MCD diets with clofibrate behaved in some respects like mice fed MCD diets without clofibrate in that they developed typical MCD-mediated hypolipidemia and weight loss (Supplemental Table 1).11, 22 Mice fed MCD with clofibrate, however, accumulated much less hepatic lipid than those fed MCD without clofibrate (Figure 5A, C). Despite their reduced hepatic steatosis, mice fed MCD with clofibrate exhibited the same pattern of hepatic stress as mice fed MCD without clofibrate (Figure 5B). Specifically, GCN2 was activated in both groups, which was predictable since both were similarly deprived of amino acids, and eIF2α was also activated in both groups along with the induction of CHOP. Some phosphorylation of eIF2α was observed in mice fed the MCS control formula with clofibrate, without any other accompanying features of a hepatic stress response. Activation of eIF2α has been reported to occur in cultured hepatoma cells treated with clofibrate, which could explain this induction.32 Notably, JNK, which is characteristically activated in fatty livers,9, 33–35 was not suppressed in mice fed MCD with clofibrate even though steatosis was alleviated. The elimination of steatosis also had no effect on the MCD-mediated cleavage of procaspase-12. Noteworthy from a disease perspective, stress signaling remained robust in the liver despite the fact that clofibrate significantly reduced MCD-mediated hepatocellular injury and inflammation (Figure 5C). This suggests that in the MCD model of fatty liver disease, the diet-induced ISR per se is not the dominant factor in the pathogenesis of organ damage.
Figure 5. Impact of clofibrate treatment on stress signaling in MCD-fed mice.
(A) Mice were fed MCS or MCD formulas with or without 0.5% (w/w) clofibrate for 14 days. Liver sections were stained with hematoxylin and eosin. (B) Stress signaling was assessed by Western blotting in the livers of mice fed MCS and MCD formulas with or without clofibrate for 14 days. (C) The hepatic triglyceride content of mice fed diets with or without clofibrate was assayed as outlined in Methods. Biochemical and histologic parameters of liver injury were also measured at 14 days (n = 10). * P < 0.05 for MCD vs. MCS; ‡ P < 0.05 for MCD vs. MCD + clofibrate.
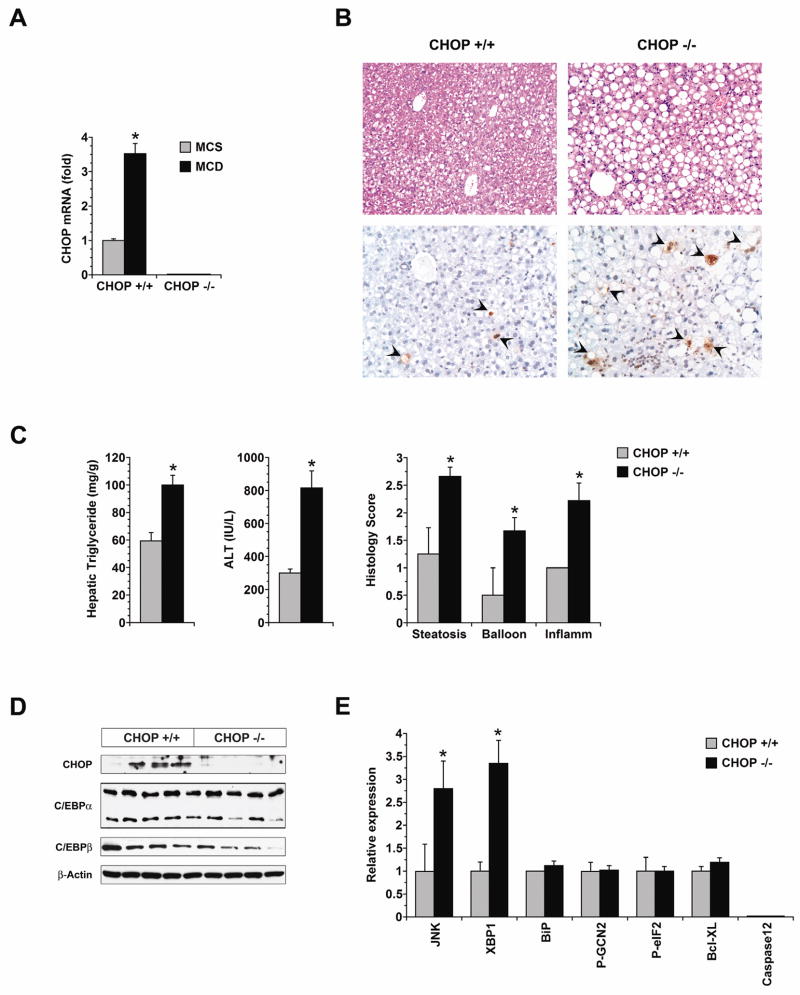
Because mice fed MCD with clofibrate still developed some degree of liver disease (Figure 5C), the diet-induced ISR could still be playing a contributory role in the development of hepatic injury in mice deprived of methionine and choline. In the setting of an ISR, an important stimulus to cell death and tissue injury is the induction of CHOP.19, 20, 36–38 To test whether the CHOP induction that accompanies MCD feeding contributes to liver injury, we compared hepatic outcomes in wild-type (WT) and CHOP-deficient (CHOP−/−) mice fed MCD formulas for 21 days. These WT and CHOP−/− mice were on a C57Bl/6 background, which develops milder hepatic steatosis after MCD feeding than the C3H strain.39 As anticipated, MCD feeding induced a 3.5-fold increase in CHOP mRNA in WT mice (P = 0.00005 vs. MCS), whereas in CHOP−/− mice, CHOP mRNA remained undetectable (Figure 6A). CHOP deficiency did not, however, make mice resistant to MCD-mediated liver injury. On the contrary, CHOP−/− mice developed more hepatic steatosis and significantly worse liver disease than WT mice (Supplemental Table 2 and Figure 6B, C). In cell culture studies, primary hepatocytes from CHOP−/− and WT mice were equally sensitive to fatty acid toxicity (not shown). This suggested that the enhanced sensitivity of CHOP−/− mice to MCD-mediated steatohepatitis was due to factors outside the liver. CHOP−/− mice have more have adipose tissue than weight-matched WT mice,40 presumably because of reciprocal up-regulation of the adipogenic transcription factors C/EBPα and C/EBPβ that are normally suppressed by CHOP.40–42 Notably, C/EBPα and C/EBPβ were not up-regulated in the CHOP-deficient liver (Figure 6D and Supplemental Figure 2). It is possible, therefore, that under the lipolytic stimulus of MCD feeding, CHOP−/− mice released an excess of fatty acids from adipose tissue into the circulation, which were then taken up by the liver where they accentuated fatty liver disease. CHOP−/− mice exhibited increased JNK activation in the liver coincident with their increased hepatic steatosis and liver injury. CHOP−/− mice also exhibited increased expression of XBP-1s, but the significance of this finding is uncertain because there was no accompanying induction of XBP-1 target genes (Supplemental Figure 2). No other markers of hepatic stress were different between CHOP−/− mice and WT mice.
Figure 6. Influence of CHOP on the development of MCD-mediated liver disease.
(A) CHOP mRNA expression was measured by quantitative PCR in the livers of wild-type (CHOP+/+) and CHOP-deficient (CHOP−/−) mice after 21 days of MCS or MCD feeding (n = 8). (B) Liver histology in wild-type and CHOP−/− mice was assessed after 21 days of MCD feeding. Top panels demonstrate hematoxylin and eosin stains; bottom panels illustrate TUNEL stains. TUNEL-positive cells are marked with arrowheads. (C) Hepatic triglyceride content, serum ALT and histologic features of liver injury were measured in CHOP+/+ and CHOP−/− mice fed MCD formulas for 21 days (n = 8). (D) The expression of CHOP, C/EBPα and C/EBPβ was measured by Western blotting in the livers of CHOP+/+ and CHOP−/− mice after 21 days of MCD feeding. (E) The expression of stress signaling molecules in CHOP−/− mice was assessed by Western blotting after 21 days of MCD feeding and expressed as a relative value in comparison to CHOP+/+ controls treated in the same fashion (n = 5). * P < 0.05 vs. CHOP+/+.
Discussion
The purpose of this study was to investigate how MCD feeding provokes ER stress in the liver and explore the degree to which stress signaling contributes to liver injury. One of the most notable findings was that MCD feeding did not trigger a classical UPR in the liver. Specifically, MCD feeding did not activate IRE1, an ER resident signal transducer whose phosphorylation is a central event in the response to an unfolded protein load.1 In accordance with its failure to activate IRE1, MCD feeding also failed to induce the expression of XBP-1s, which typically results from IRE1-mediated splicing of XBP-1 mRNA. MCD feeding activated JNK and caspase-12 in the liver, but these events occurred independently of IRE1. Furthermore, MCD-fed mice displayed no evidence of ER chaperone induction in the liver, signifying that the diet induced steatohepatitis without the contribution of a second ER stress inducer, ATF6. These data demonstrate that in the MCD model of steatohepatitis, liver injury is not dependent upon the activation of a UPR.
The lack of a UPR in mice with MCD-mediated steatohepatitis is of particular interest because this same phenomenon has been observed in humans with nonalcoholic steatohepatitis (NASH).43 In fact, the absence of detectable XBP-1s in the liver is one of the few characteristics that distinguishes NASH from benign hepatic steatosis in human beings. In humans, low XBP-1s expression has been theorized to result from a block in XBP-1s translation. In MCD-fed mice, XBP-1 mRNA and protein were both down-regulated, perhaps because XBP-1 activates its own transcription.44 The suppression of XBP-1s that occurred in response to MCD feeding contrasted sharply with the activation of IRE1 and expression of XBP-1s that occurred in MCS controls. The response of the control mice was consistent with published data indicating that an energy-rich diet can chronically activate IRE1 and XBP-1.24 If true, then the MCD diet, which is nutritionally matched to MCS, should have induced a similar outcome. MCD feeding may have activated IRE1 initially and then stopped, based upon evidence that XBP1s was expressed transiently in MCD-fed livers during the time-course study (Figure 2A). If MCD feeding did initially stimulate XBP-1s expression, then the data imply a chronological association between the loss of XBP-1 from the liver and the onset of steatohepatitis. When the findings in MCS and MCD mice are viewed together, they indicate that the lack of a UPR portends steatohepatitis whereas the sustained activation of a UPR does not result in hepatic steatosis or liver injury.
Although MCD-fed mice did not exhibit signs of a classical UPR, they did display robust phosphorylation of eIF2α, a stress signal associated with hepatic steatosis in experimental animals3, 7 and human beings.43 In some models of fatty liver, eIF2α activation is a consequence of UPR signaling through PERK.6, 33 In our experiments, eIF2α phosphorylation was accompanied by only weak evidence of PERK activation; this was in keeping with our other results that argued against MCD-mediated induction of a UPR. Instead, MCD feeding activated GCN2, an amino acid-sensitive eIF2α kinase that activates many of the same stress-related target genes as PERK.45 Why MCD feeding caused relatively weak activation of PERK despite inducing hepatic steatosis is uncertain. The answer may lie in the fact that fatty acids activate PERK in hepatocytes by stimulating the synthesis of Apo B100,3 whereas MCD feeding interferes with normal Apo B100 production, perhaps due to amino acid deficiency.46 Regardless of the stimulus, eIF2α phosphorylation is pertinent to fatty liver disease because it is one of the features displayed by human beings with NASH.43
eIF2α activation occurs early in the course of human fatty liver disease and eIF2α remains phosphorylated in patients with full-blown NASH.43 This begs the question whether sustained activation of an eIF2α signaling cascade contributes to the pathogenesis of steatohepatitis. Our experiments argue against this; they show that in mice fed an MCD diet supplemented with clofibrate, eIF2α was activated in the liver for 21 days but did not lead to serious liver disease. Mice fed the MCD formula with clofibrate did not develop hepatic steatosis, and thus we cannot exclude the possibility that prolonged integrated stress could provoke liver disease if steatosis were present. Indeed, integrated stress and hepatic steatosis often coexist in the liver.8, 9 Still, our experiments lead to the conclusion that prolonged integrated stress by itself is insufficient to provoke liver disease.
In keeping with our finding that a prolonged ISR did not induce serious liver disease, we identified a disconnection between steatohepatitis and the ISR-related death effector CHOP. Stress signaling through eIF2α commonly leads to the induction of CHOP, a protein that promotes cell death and tissue damage by regulating the expression of survival proteins such as Bcl-2.47, 48 In our experiments, MCD feeding up-regulated CHOP in the liver as it induced steatohepatitis, but CHOP did not contribute to MCD-mediated liver disease. This suggests an element of tissue specificity in CHOP’s ability to induce organ injury, with the molecule promoting disease in organs such as the kidney,49 pancreas37, 50 and vascular system38 but not in the liver. We found no evidence of a reciprocal relationship between CHOP and Bcl-xL in the livers of MCD-fed mice (Figures 4B and 6E). We did find a reciprocal relationship between CHOP expression and JNK activation in the liver, which may have contributed to the severe steatohepatitis in CHOP−/− mice.
JNK activation, which occurred in the livers of MCD-fed mice, has been documented previously by others and shown to be of major importance to the development of experimental steatohepatitis.34, 35 In our study, mice fed the MCD formula with clofibrate developed no hepatic steatosis and very little liver injury but had persistent JNK activity in the liver. This indicates that JNK activation, like chronic integrated stress, is not by itself hepatotoxic in MCD-fed mice; moreover, it underscores that hepatic lipid accumulation is essential to the development of MCD-mediated liver injury. Notably, JNK appears pivotal to the process of hepatic lipid accumulation, for when it is inhibited, steatosis is averted.34, 35 Our study supports the notion that JNK causes fatty liver injury through its ability to provoke steatosis, because the elimination of hepatic steatosis renders JNK non-toxic.
In summary, our results indicate that MCD feeding causes a stress response in the liver characterized by the lack of a UPR and the presence of an ISR. These features are noteworthy because they recapitulate the hepatic stress profile present in patients with NASH. In MCD-fed mice, the absence of a UPR coincides with steatohepatitis; the presence of an ISR, however, does not guarantee liver injury. In the MCD model, hepatic lipid accumulation is paramount to the pathogenesis of steatohepatitis, for in its absence liver injury does not occur. This is true even if integrated stress and JNK activation are sustained. Further study is required to determine whether integrated stress plays a cooperative role with hepatic steatosis resulting in the progression to steatohepatitis.
Supplementary Material
Acknowledgments
Grant support: Supported by R01 DK068450 (to JJM), the Pathology & Imaging Core of the UCSF Liver Center (P30 DK026743), the Tool and Technology Program within the UCSF Liver Center (P30 DK026743) and the Genome Analysis Core of the Hellen Diller Family Comprehensive Cancer Center (P30 CA082103).
The authors are grateful to Drs. Ray Ng and Feroz Papa for helpful discussions.
Abbreviations
- ALT
Alanine aminotransferase
- Apo B100
apolipoprotein B100
- ATF6
activating transcription factor-6
- BCL-xL
B-cell lymphoma extra-large
- BiP
immunoglobulin-binding protein
- CHOP
C/EBP homologous protein
- eIF2α
eukaryotic initiation factor-2α
- ER
endoplasmic reticulum
- ERAD
ER-associated degradation
- ERO1
endoplasmic oxidoreductase protein-1
- GRP94
glucose-regulated protein 94
- HRI
heme-regulated inhibitor kinase
- IRE1
inositol-requiring protein-1
- ISR
integrated stress response
- JNK
c-Jun N-terminal kinase
- MCD
methionine-choline-deficient
- MCS
methionine-choline-sufficient
- NASH
nonalcoholic steatohepatitis
- Pdia4
protein disulfide isomerase-associated-4
- PERK
PKR-like ER-resident kinase; PKR double-stranded RNA-activated protein kinase
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- TRB3
tribbles homolog 3
- UPR
unfolded protein response
- WT
wild-type
- XBP-1
X-box protein-1
- VLDL
very low density lipoprotein
Footnotes
Disclosures: Russell K. Soon nothing to disclose
Jim S. Yan nothing to disclose
James P. Grenert nothing to disclose
Jacquelyn J. Maher nothing to disclose
Co-author contributions: Russell K. Soon: acquired, analyzed, interpreted data; wrote manuscript
Jim S. Yan: acquired, analyzed, interpreted data
James P. Grenert: acquired and analyzed data
Jacquelyn J. Maher: analyzed and interpreted data; obtained funding; wrote manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 2.Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest. 2008;118:316–32. doi: 10.1172/JCI32752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su Q, Tsai J, Xu E, et al. Apolipoprotein B100 acts as a molecular link between lipid-induced endoplasmic reticulum stress and hepatic insulin resistance. Hepatology. 2009;50:77–84. doi: 10.1002/hep.22960. [DOI] [PubMed] [Google Scholar]
- 4.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–6. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kammoun HL, Chabanon H, Hainault I, et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–15. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–40. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–32. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutkowski DT, Wu J, Back SH, et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15:829–40. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monetti M, Levin MC, Watt MJ, et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007;6:69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Lee GS, Yan JS, Ng RK, Kakar S, Maher JJ. Polyunsaturated fat in the methionine-choline-deficient diet influences hepatic inflammation but not hepatocellular injury. J Lipid Res. 2007;48:1885–96. doi: 10.1194/jlr.M700181-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Pickens MK, Yan JS, Ng RK, et al. Dietary sucrose is essential to the development of liver injury in the MCD model of steatohepatitis. J Lipid Res. 2009;50:2072–82. doi: 10.1194/jlr.M900022-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox JS, Chapman RE, Walter P. The unfolded protein response coordinates the production of endoplasmic reticulum protein and endoplasmic reticulum membrane. Mol Biol Cell. 1997;8:1805–14. doi: 10.1091/mbc.8.9.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng B, Yao PM, Li Y, et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781–92. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 14.Berlanga JJ, Santoyo J, De Haro C. Characterization of a mammalian homolog of the GCN2 eukaryotic initiation factor 2alpha kinase. Eur J Biochem. 1999;265:754–62. doi: 10.1046/j.1432-1327.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 15.Harding HP, Zhang Y, Zeng H, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–33. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 16.Urano F, Wang X, Bertolotti A, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–6. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 17.Wang XZ, Lawson B, Brewer JW, et al. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153) Mol Cell Biol. 1996;16:4273–80. doi: 10.1128/mcb.16.8.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoneda T, Imaizumi K, Oono K, et al. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J Biol Chem. 2001;276:13935–40. doi: 10.1074/jbc.M010677200. [DOI] [PubMed] [Google Scholar]
- 19.Rutkowski DT, Arnold SM, Miller CN, et al. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4:e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji C, Mehrian-Shai R, Chan C, Hsu YH, Kaplowitz N. Role of CHOP in hepatic apoptosis in the murine model of intragastric ethanol feeding. Alcohol Clin Exp Res. 2005;29:1496–503. doi: 10.1097/01.alc.0000174691.03751.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 22.Rizki G, Arnaboldi L, Gabrielli B, et al. Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD-1. J Lipid Res. 2006;47:2280–90. doi: 10.1194/jlr.M600198-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Calfon M, Zeng H, Urano F, et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–6. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 24.Cretenet G, Le Clech M, Gachon F. Circadian clock-coordinated 12 Hr period rhythmic activation of the IRE1alpha pathway controls lipid metabolism in mouse liver. Cell Metab. 2010;11:47–57. doi: 10.1016/j.cmet.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto K, Sato T, Matsui T, et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell. 2007;13:365–76. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 26.Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. Embo J. 2005;24:1243–55. doi: 10.1038/sj.emboj.7600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marciniak SJ, Yun CY, Oyadomari S, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–77. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo F, Cavener DR. The GCN2 eIF2alpha kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab. 2007;5:103–14. doi: 10.1016/j.cmet.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Harding HP, Novoa I, Zhang Y, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 30.Bobrovnikova-Marjon E, Hatzivassiliou G, Grigoriadou C, et al. PERK-dependent regulation of lipogenesis during mouse mammary gland development and adipocyte differentiation. Proc Natl Acad Sci U S A. 2008;105:16314–9. doi: 10.1073/pnas.0808517105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ip E, Farrell GC, Robertson GR, Hall P, Kirsch R, Leclercq IA. Central role of PPAR-a-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology. 2003;38:123–32. doi: 10.1053/jhep.2003.50307. [DOI] [PubMed] [Google Scholar]
- 32.Penna F, Reffo P, Muzio G, et al. Mechanisms of clofibrate-induced apoptosis in Yoshida AH-130 hepatoma cells. Biochem Pharmacol. 2009;77:169–76. doi: 10.1016/j.bcp.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 34.Schattenberg JM, Singh R, Wang Y, et al. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 2006;43:163–72. doi: 10.1002/hep.20999. [DOI] [PubMed] [Google Scholar]
- 35.Singh R, Wang Y, Xiang Y, Tanaka KE, Gaarde WA, Czaja MJ. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology. 2009;49:87–96. doi: 10.1002/hep.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ariyama Y, Tanaka Y, Shimizu H, et al. The role of CHOP messenger RNA expression in the link between oxidative stress and apoptosis. Metabolism. 2008;57:1625–35. doi: 10.1016/j.metabol.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 37.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118:3378–89. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thorp E, Li G, Seimon TA, Kuriakose G, Ron D, Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab. 2009;9:474–81. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rangnekar AS, Lammert F, Igolnikov A, Green RM. Quantitative trait loci analysis of mice administered the methionine-choline deficient dietary model of experimental steatohepatitis. Liver Int. 2006;26:1000–5. doi: 10.1111/j.1478-3231.2006.01314.x. [DOI] [PubMed] [Google Scholar]
- 40.Ariyama Y, Shimizu H, Satoh T, et al. Chop-deficient mice showed increased adiposity but no glucose intolerance. Obesity (Silver Spring) 2007;15:1647–56. doi: 10.1038/oby.2007.197. [DOI] [PubMed] [Google Scholar]
- 41.Ron D, Habener JF. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev. 1992;6:439–53. doi: 10.1101/gad.6.3.439. [DOI] [PubMed] [Google Scholar]
- 42.Batchvarova N, Wang XZ, Ron D. Inhibition of adipogenesis by the stress-induced protein CHOP (Gadd153) Embo J. 1995;14:4654–61. doi: 10.1002/j.1460-2075.1995.tb00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puri P, Mirshahi F, Cheung O, et al. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134:568–76. doi: 10.1053/j.gastro.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 44.Lee K, Tirasophon W, Shen X, et al. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 2002;16:452–66. doi: 10.1101/gad.964702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dang Do AN, Kimball SR, Cavener DR, Jefferson LS. eIF2alpha kinases GCN2 and PERK modulate transcription and translation of distinct sets of mRNAs in mouse liver. Physiol Genomics. 2009;38:328–41. doi: 10.1152/physiolgenomics.90396.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rinella ME, Elias MS, Smolak RR, Fu T, Borensztajn J, Green RM. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J Lipid Res. 2008;49:1068–76. doi: 10.1194/jlr.M800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–5. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–59. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zinszner H, Kuroda M, Wang X, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–95. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oyadomari S, Koizumi A, Takeda K, et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002;109:525–32. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.