Abstract
Background
Epidemiology and imaging studies showed that cognitively normal (NL) individuals with a maternal history (MH) of late-onset Alzheimer’s disease (LOAD) may be at increased risk for AD compared to NL with a paternal history (PH) and NL with a negative family history of LOAD (NH). Using a panel of cerebrospinal fluid (CSF) markers, this study examined whether NL MH showed evidence for AD pathology compared to PH and NH.
Methods
Fifty-nine 40–80 year old NL subjects were examined, including 23 MH and 14 PH whose parents had a clinician-certified diagnosis of LOAD, and 22 NH. All subjects completed clinical, neuropsychological examinations and a lumbar puncture to measure CSF levels of amyloid-beta (Aβ40, Aβ42, Aβ42/40), total and hyperphosphorylated tau (T-Tau and P-Tau231; markers of axonal degeneration and neurofibrillary tangles, respectively), and F2-isoprostanes (IsoP; a marker of oxidative stress).
Results
Groups were comparable for demographic and neuropsychological measures. MH subjects showed higher IsoP and reduced Aβ42/40 CSF levels as compared to NH and to PH (P’s≤0.05), while no differences were found between NH and PH. No group differences were found for P-Tau231 and T-Tau. IsoP and Aβ42/40 levels were correlated only within the MH group (R2=0.32, P=0.005), and discriminated MH from the other subjects with 70% accuracy (relative risk=3.7, 95% confidence interval=1.6–9.7, P<0.001). Results remained significant controlling for age, gender, education, and ApoE genotype.
Conclusions
Adult children of AD-affected mothers express a pathobiological phenotype characterized by Aβ-associated oxidative stress consistent with AD, which may reflect increased risk for developing the disease.
Keywords: Alzheimer’s disease, presymptomatic individuals, family history, amyloid-beta, oxidative stress, CSF biomarkers
INTRODUCTION
Alzheimer’s disease (AD) is the most common form of dementia in late-life, affecting over 5 million elderly nationwide (1). In order to develop prevention treatments for AD, it is necessary to identify persons who are at high risk for developing AD, who are most likely to benefit from early therapeutic intervention.
After advanced age, having a 1st degree family history of late-onset AD (LOAD) is the most significant risk factor among cognitively normal (NL) individuals (2,3). While the genetics involved in LOAD have been elusive, genetically mediated risk is evident from the familial aggregation of many LOAD cases. Risk appears to be particularly high in presence of a maternal history of LOAD (4). While there is evidence for maternal and paternal transmission in LOAD (5,6), maternally inherited LOAD accounts for a greater proportion of cases than paternally inherited disease, and having a LOAD-affected mother confers greater risk to the offspring than having an affected father (6–8). Thus far, these epidemiological findings have been scarcely characterized by the use of biomarkers for AD, and the molecular events involved at the preclinical stages of familial LOAD are largely unexplored (4).
2-[18F]fluoro-2-Deoxy-D-glucose Positron Emission Tomography studies (FDG-PET) have shown that NL with maternal history of LOAD (MH. i.e., only the mother was affected) have progressive reductions in cerebral glucose metabolism compared to NL with paternal history (PH, i.e., only the father was affected) and NL with negative family history (NH) (9,10). In contrast, NL PH had no metabolic deficits (9,10), suggesting that maternal transmission of LOAD may be distinctively associated with early hypometabolism. Additionally, on N-methyl-[11C]2-(4’-methylaminophenyl)-6-hydroxybenzothiazole (Pittsburgh Compound-B, PIB)-PET imaging, NL MH showed increased amyloid-beta (Aβ) burden, a hallmark of AD pathology, compared to PH and controls (11). While these studies suggest a connection between hypometabolism and Aβ production in NL MH, FDG- and PiB-PET studies were performed in different individuals, and the relationship between markers of AD pathology at the presymptomatic stages of LOAD remains unclear.
Many in vivo studies have examined the cerebrospinal fluid (CSF) as a source for biomarkers of AD pathology. The CSF is in direct contact with the brain and its molecular composition reflects biochemical changes in the brain (12). Currently, available CSF analytes target most aspects of AD pathology, including markers for Aβ (i.e., Aβ42 and Aβ40 amino-acid residues) and tau pathology (i.e., total tau, T-Tau, a marker of neuronal degeneration, and hyperphosphorylated tau, P-Tau231, a marker of neurofibrillary tangles, NFT), as well as oxidative stress (F2-isoprostane, IsoP). These CSF markers accurately discriminate AD from controls and other dementias (13–15) and predict decline to AD in non-demented individuals (16–20).
The present study examined all the above CSF AD markers to test whether NL MH show higher levels of AD pathology compared to PH and controls, and to examine the relationships across different markers in relation to the subjects’ family history.
MATERIAL AND METHODS
Subjects
This study retrospectively examined a cohort of 110 NL individuals recruited at the Center for Brain Health at New York University (NYU) between 2002–2008 as part of ongoing longitudinal studies in AD. Study subjects were individuals interested in research participation, memory evaluation, and risk consultation, and were family members or caregivers of impaired patients. These subjects were not previously examined for family history effects on CSF biomarkers. All participants received clinical, neuropsychological, MRI exams and a lumbar puncture (LP) within 3 months, and gave their informed consent to this NYU-IRB approved study.
Subjects were 40–80 years of age, had education≥12 yrs, MMSE 28–30, CDR=0, and normal cognitive test performance relative to normative values on an extensive neuropsychological testing battery (21). Subjective reports of memory and other cognitive changes were evaluated during a structured informant-corroborated interview with the study physician using the Global Deterioration Scale (GDS) (22). All subjects had GDS scores of 1 or 2, which indicate normal functioning subjects differentiated only by the absence (GDS=1) or presence (GDS=2) of subjective memory complaints in the absence of clinically recognizable impairment (22,23).
All subjects received a standardized whole-brain MRI protocol on a 1.5 T GE Signa imager, including a T2-weighted and a T1-weighted Fast-Gradient-Echo image, which were used to rule out hydrocephalus, intracranial mass, and strokes (24). Individuals with medical conditions or history of conditions that may affect brain structure or function, i.e. stroke, diabetes, head trauma, any neurodegenerative diseases, depression, or using psychoactive medications were excluded. Subjects had normal cholesterol levels and blood pressure, and Modified Hachinski Ischemia Scale<4 (25). ApoE genotype was determined using standard PCR procedures.
A family history (FH) of AD that included at least one 1st degree relative whose AD onset was between 60–80 years was elicited by using the NYU Brain Aging Family History questionnaire (10). Participants filled in names, dates of birth, age at death, cause of death, and clinical information of all affected family members over 3 generations. The information was confirmed with other family members by interview with the examining neurologist, discussing the parents’ symptomatology and progression of disease. All subjects were asked to provide as detailed as possible information about their parents’ diagnosis, including the name of the physician who made the diagnosis and/or where the diagnosis was made (hospital, family neurologist, etc), available medical records and medication list. Most volunteers were children or caregivers of AD patients at our ADCC, for whom full medical records and/or autopsy reports were readily available for inspection. For the remaining subjects, when possible, we followed up with the clinician who made the diagnosis to confirm the reports. Among the larger pool of possible recruits, only NL subjects whose parents’ diagnosis of AD was reportedly clinician certified were included in this study. Subjects were not included if their parents had not lived to at least age 65. Only NL with either maternal (MH, i.e., only the mother was affected) or paternal history of LOAD (PH, i.e., only the father was affected) were examined in this study and compared to NL with negative FH of any dementia (NH).
CSF measures
After an overnight fast, 15cc of clear CSF were collected using fluoroscopy to guide a 24 gauge beveled LP needle. Samples were centrifuged for 10 minutes at 1500 rpm at 4°C, aliquoted to 0.25cc polypropylene tubes, and stored at −80°C in the presence of 0.01% BHT. Assays were blinded to clinical data and were batch processed.
Aβ levels were measured using a monoclonal antibody 6E10 (specific to an epitope present on Aβ-16) and to rabbit antisera to Aβ1-40 and Aβ1-42 respectively, in a double antibody sandwich ELISA (detection limit=10 pg/ml, 8–14% intra-assay and 10–18% inter-assay reproducibility) (26). As several studies reported that the ratio of Aβ42 to Aβ40 is superior to the concentration of either measure individually in discriminating AD from controls and from other dementias (15, 20), Aβ42/40 ratios were created and compared across groups.
For F2-Isoprostane (IsoP), CSF samples were spiked with a fixed amount of internal standard (d4-iPF2α-VI) and extracted on a C18 cartridge column. The elute was purified by thin-layer chromatography and assayed by negative ion chemical ionization gas chromatography/mass spectrometry (detection limit=1 pg/ml, 4–7% intra-assay and 4.5–6.5% inter-assay reproducibility) (14).
A sandwich ELISA assay was used to detect CSF tau phosphorlyated at threonine 231, by capturing tau with two backbone-directed antibodies, tau-1 and CP-27. The captured tau is then detected by CP9, which is specific for P-Tau231 (detection limit=9 pg/ml, 6.0–10.3% intra-assay and 11.6–14.4% inter-assay reproducibility) (27). CSF T-tau measurements were determined using the commercially available INNOTEST hTAU Antigen kit from Innogenetics (detection limit=60 pg/ml, 5.5% intra-assay and 11.6% inter-assay reproducibility) (16).
Statistical Analysis
Analyses were done with SPSS 12.0 (SPSS inc., Chicago, MI). Differences in demographical and neuropsychological measures between groups were examined with χ2 tests and analysis of covariance. As determined with Shapiro Wilks test, Aβ40, Aβ42, Aβ42/40 and IsoP measures were not normally distributed. For these variables, statistically significant results obtained from raw data were confirmed after applying a log transformation of the raw values. The General Linear Model (GLM), univariate analysis, was used to examine CSF measures across groups. Post-hoc LSD tests were used to perform pair-wise group comparisons [NH vs PH, NH vs MH, PH vs MH]. Those variables yielding statistically significant group effects in the main analysis were reexamined controlling for age, gender, education, and ApoE genotype as covariates.
Linear regressions were used to test for relationships across CSF markers, and across groups, controlling for age, gender, education and ApoE as covariates. Stepwise forward logistic regressions were used to examine CSF makers as predictors of group membership and calculate associated relative risk (RR) and 95% confidence intervals (CI). Those biomarkers showing significant group differences on GLM analysis were included separately in the logistic regression model, after entering age, gender, education and ApoE as covariates as fixed effects at the first step, and entering selected CSF markers at the second step, to be examined for group discrimination using a forward conditional method. Analyses were done using the raw data for all variables, and confirmed with the log-transformed data for non-parametrically distributed measures.
Given the smaller sample of PH subjects (n=14), we created 3 demographically matched groups of 14 subjects each, based on (in order of importance): age, gender, education. All analyses were repeated for these groups, with and without controlling for ApoE status.
For all analyses, results were considered significant at P<0.05.
RESULTS
From the larger cohort of 110 subjects with a diagnosis of normal cognition and complete CSF examinations, 31 subjects were excluded because of incomplete FH information. An additional 20 subjects were excluded: 4 subjects with both mother and father affected, 5 with only siblings affected, 2 with a parent who had not lived to age 65, 2 with a parent who was not yet 65 year old, 2 with aunts/uncles affected, and 5 who reported a FH of an unspecified dementia.
A total of 59 subjects fulfilled our study criteria and were examined in this study, including 22 NH, 14 PH and 23 MH. For 4 subjects (2 MH and 2 PH), the parents’ AD diagnosis was confirmed at autopsy. For all other subjects, the parents’ diagnosis was clinician certified. The groups did not differ for age, gender, education, frequency of ApoE-4 carriers, presence of subjective memory complaints, MMSE and neuropsychological measures (Table 1). Clinical data of demographically matched groups are found in Table 2.
Table 1.
Subject characteristics and CSF data by family history group
| NH | PH | MH | ||
|---|---|---|---|---|
| N | 22 | 14 | 23 | |
| Age (years) | 61(13) | 57(12) | 61(7) | |
| Gender (M/F) | 10/12 | 6/8 | 9/14 | |
| Education (years) | 16(2) | 16(2) | 17(1) | |
| ApoE genotype (ε4−/ε4+) | 15/7 | 9/5 | 13/10 | |
| Subjective memory complaints (no/yes) | 7/15 | 5/9 | 8/15 | |
| Neuropsychological measures | ||||
| MMSE | 29.2(1.2) | 29.5(1.0) | 29.3(1.2) | |
| Object naming | 49(10) | 58(9) | 54(9) | |
| Paired Associates Delayed Recall | 5(3) | 6(3) | 6(3) | |
| Paragraph Delayed Recall | 9(3) | 10(4) | 10(2) | |
| Visual recognition | 19(9) | 20(8) | 23(4) | |
| CSF measures (pg/ml) | ||||
| Aβ40 | 7209(2822) | 6991(1640) | 7520(2114) | |
| §Covariate-adjusted raw data | 7203(2241) | 6531(2251) | 7587(2347) | |
| Log-transformed data | 8.8(0.4) | 8.7(0.3) | 8.9(0.3) | |
| §Covariate-adjusted log-transformed data | 8.8(0.3) | 8.7(0.3) | 8.9(0.3) | |
| Aβ42 | 865(528) | 744(311) | 713(277) | |
| §Covariate-adjusted raw data | 870(402) | 732(400) | 712(410) | |
| Log-transformed data | 6.6(0.6) | 6.5(0.4) | 6.4(0.3) | |
| §Covariate-adjusted log-transformed data | 6.6(0.4) | 6.5(0.5) | 6.4(0.4) | |
| Aβ42/40 (unitless) | 0.115(0.035) | 0.128(0.032) | 0.096(0.030)* † | |
| §Covariate-adjusted raw data | 0.116(0.031) | 0.124(0.031) | 0.094(0.026)* † | |
| Log-transformed data | −2.2(0.2) | −2.0(0.2) | −2.4(0.3)* † | |
| §Covariate-adjusted log-transformed data | −2.1(0.3) | −2.1(0.3) | −2.4(0.2)* † | |
| Isoprostanes | 21.8(9.2) | 26.3(9.8) | 32.9(9.8)* † | |
| §Covariate-adjusted raw data | 23.0(10.6) | 27.5(10.5) | 31.4(2.0)* † | |
| Log-transformed data | 4.5(1.2) | 4.9(0.6) | 5.7(0.9)* † | |
| §Covariate-adjusted log-transformed data | 4.7(0.9) | 5.1(1.0) | 5.6(0.9)* † | |
| P-Tau231 | 11.4(13.5) | 8.8(13.3) | 12.3(20.2) | |
| §Covariate-adjusted data | 12.0(18.3) | 12.7(17.8) | 10.8(17.4) | |
| T-Tau | 312(297) | 288(123) | 307(199) | |
| §Covariate-adjusted data | 312(174) | 287(169) | 306(165) | |
Values are means (standard deviation).
Covariate-adjusted data controlling for age, gender, education and ApoE.
Abbreviations: MH = maternal history of LOAD, NH=negative family history of AD, PH = paternal history of LOAD.
MH ≠ NH,
MH ≠ PH, P<0.05
Table 2.
Subject characteristics and CSF data by demographically matched family history group
| NH | PH | MH | ||
|---|---|---|---|---|
| N | 14 | 14 | 14 | |
| Age (years) | 55(6) | 57(12) | 58(7) | |
| Gender (M/F) | 5/9 | 6/8 | 5/9 | |
| Education (years) | 16(2) | 16(2) | 16(2) | |
| ApoE genotype (ε4−/ε4+) | 10/4 | 9/5 | 7/7 | |
| Subjective memory complaints (no/yes) | 4/10 | 5/9 | 4/10 | |
| Neuropsychological measures | ||||
| MMSE | 28.6(1.6) | 29.5(1.0) | 29.2(1.4) | |
| Object naming | 53(10) | 58(9) | 53(10) | |
| Paired Associates Delayed Recall | 6(3) | 6(3) | 7(3) | |
| Paragraph Delayed Recall | 10(4) | 10(4) | 10(2) | |
| Visual recognition | 20(10) | 20(8) | 24(4) | |
| CSF measures (pg/ml) | ||||
| Aβ40 | 6956(2789) | 6991(1640) | 7447(1878) | |
| ApoE-adjusted data | 6400(2430) | 6622(2317) | 7211(2275) | |
| Log-transformed data | 8.7(0.5) | 8.7(0.3) | 8.8(0.2) | |
| ApoE-adjusted, log-transformed data | 8.7(0.3) | 8.7(0.3) | 8.8(0.3) | |
| Aβ42 | 827(489) | 734(311) | 728(330) | |
| ApoE-adjusted data | 836(390) | 741(379) | 713(367) | |
| Log-transformed data | 6.6(0.6) | 6.5(0.4) | 6.4(0.3) | |
| ApoE-adjusted, log-transformed data | 6.6(0.5) | 6.5(0.5) | 6.4(0.4) | |
| Aβ42/40 (raw data, unitless) | 0.117(0.027) | 0.128(0.032) | 0.093(0.024)* † | |
| ApoE-adjusted data | 0.123(0.03) | 0.126(0.028) | 0.091(0.027)* † | |
| Log-transformed data | −2.2(0.2) | −2.0(0.2) | −2.4(0.2)* † | |
| ApoE-adjusted, log-transformed data | −2.2(0.3) | −2.0(0.2) | −2.4(0.2)* † | |
| Isoprostanes | 20.4(7.4) | 26.3(9.8) | 33.2(11.7)* † | |
| ApoE-adjusted data | 20.6(9.6) | 27.0(9.5) | 32.7(9.6)* † | |
| Log-transformed data | 4.4(0.9) | 4.9(0.6) | 5.7(0.9)* † | |
| ApoE-adjusted, log-transformed data | 4.4(0.9) | 5.2(0.9) | 5.7(0.9)* † | |
| P-Tau231 | 9.1(11.8) | 8.8(13.3) | 8.5(8.0) | |
| ApoE-adjusted data | 7.0(12.3) | 10.1(11.9) | 8.4(11.5) | |
| T-Tau | 346(148) | 288(123) | 330(123) | |
| ApoE-adjusted data | 245(131) | 257(124) | 323(122) | |
Values are means (standard deviation).
Abbreviations: MH = maternal history of LOAD, NH=negative family history of AD, PH = paternal history of LOAD.
MH ≠ NH,
MH ≠ PH, P<0.05
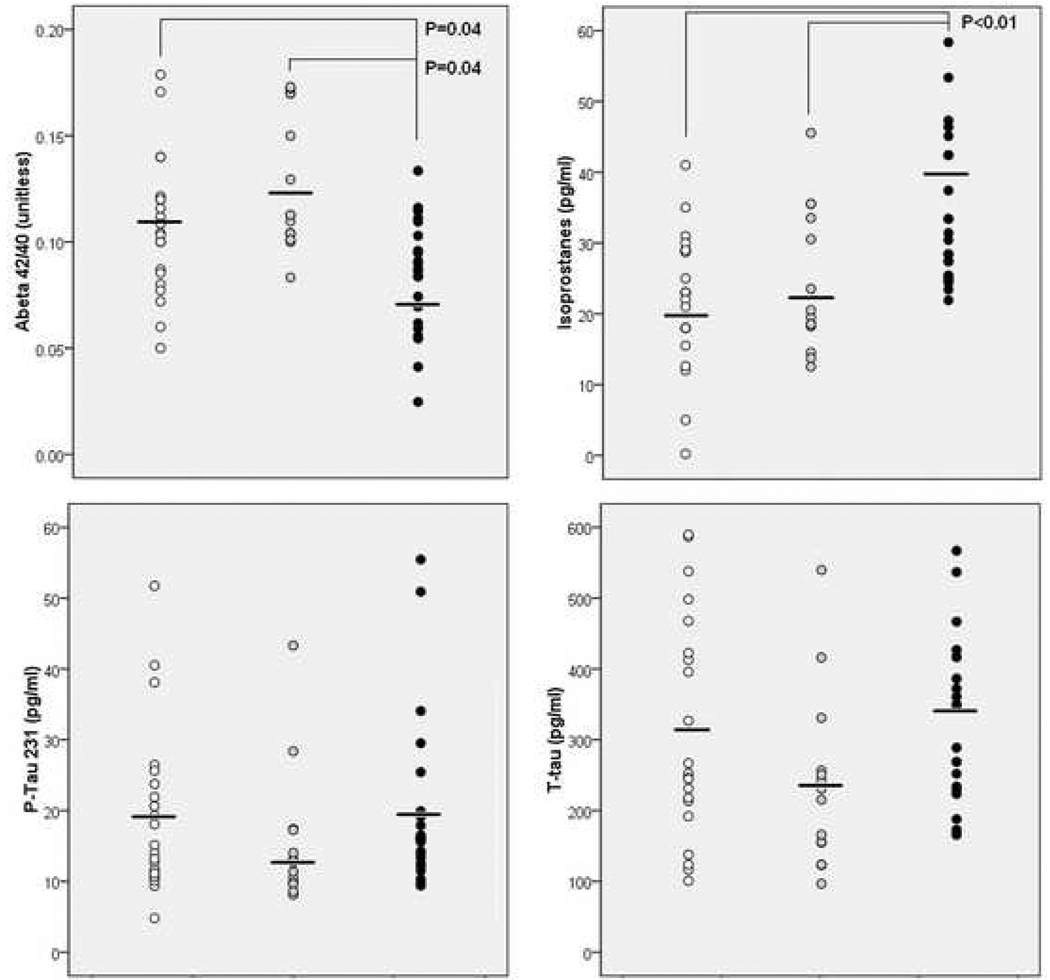
For the entire sample, significant group effects were found for Aβ42/40 (F(2,56)=4.1, P=0.02) and IsoP (F(2,56)=7.4, P=0.001). On post-hoc examination, these effects were driven by the MH group that showed lower Aβ42/40 compared to NH and to PH (16%, and 25%, respectively) and higher IsoP levels compared to NH and to PH (18% and 11%) (P’s≤0.05, Table 1). These results were confirmed with the log-transformed Aβ42/40 (F(2,56)=4.9, P=0.005) and IsoP values (F(2,56)=6.8, P=0.002). On post-hoc examination, the MH group showed lower log-transformed Aβ42/40 compared to NH and PH (9%, and 16%) and higher log-transformed IsoP levels compared to NH and PH (24% and 13%) (Table 1). All results remained significant controlling for age, gender, education, and ApoE genotype (P<0.05; Table 1 and Figure 1). Although there were no significant main group effects for Aβ42, on post-hoc examination the MH group showed a linear trend towards lower Aβ42 compared to NH (P=0.07), which was still evident on analysis of the log-transformed Aβ42 values, and accounting for age, gender, education and ApoE (P’s≥0.11) (Table 1).
Figure 1.
CSF measures by family history group. Legend: white = NH, grey = PH, black = MH. Horizontal bars indicate mean group values. Only significant P values are reported.
With and without correcting for age, gender, education and ApoE, there were no significant group differences for P-Tau231, T-Tau, raw and log-transformed Aβ40 measures across groups (Table 1). There were no differences between PH and NH for any measures (Table 1).
Significant correlations between P-Tau231 and T-Tau measures were found for all groups, as were correlations across Aβ40, Aβ42, and Aβ42/40, using both the raw and log-transformed Aβ data (P≤0.05). All correlations remained significant accounting for age, gender, education and ApoE. Accounting for these same confounds, additional significant associations between IsoP and Aβ42/40 (R2=0.32, P=0.005) and between IsoP and T-Tau measures R2=0.25, P=0.017) were found only for the MH group. These associations remained significant by using log-transformed IsoP and Aβ42/40 measures (IsoP and Aβ42/40: R2=0.29, P=0.008, IsoP and T-Tau: R2=0.20, P=0.039). There were no significant associations across CSF markers for NH and PH groups, with or without controlling for the above confounds.
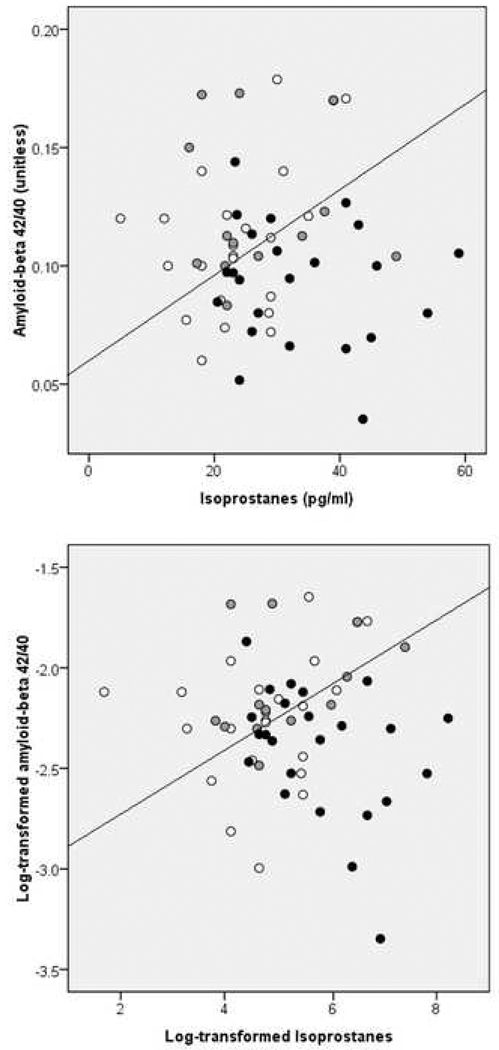
As reported in Table 3, both IsoP and Aβ42/40 discriminated MH from the other groups with accuracies of 68% and 66%, respectively (P’s≤0.007). IsoP added to the accuracy if Aβ42/40 (Pincrement=0.003), and Aβ42/40 added to that of IsoP (Pincrement=0.006), indicating that the two measures provided complementary information. When both markers were included in the model, the accuracy increased to 70% (Figure 2). Analysis of log-transformed data confirmed results from raw data, yielding 66% accuracy for log-transformed IsoP, 66% accuracy for log-transformed Aβ42/40, and 63% accuracy for the two measures combined (P’s≤0.01, Table 3 and Figure 2). Results remained unchanged controlling for age, gender, education and ApoE (Table 3). Aβ40, Aβ42, P-Tau231 and T-Tau measures were not significant predictors of group membership.
Table 3.
CSF biomarkers accuracy and relative risk in discriminating NL MH from the other subjects.
| Accuracy | Sensitivity | Specificity | P value | Relative Risk | 95% Confidence Interval |
|
|---|---|---|---|---|---|---|
| Entire sample (n=59 subjects) | ||||||
| Aβ42/40 | ||||||
| Raw data | 66 | 65 | 67 | .007 | 2.2 | 1.2–4.4 |
| Covariates-adjusted | 70 | 78 | 64 | .02 | 3.3 | 1.5–7.6 |
| Log transformed data | 66 | 65 | 67 | .01 | 2.2 | 1.2–4.3 |
| Covariates-adjusted | 70 | 78 | 64 | .02 | 3.3 | 1.5–7.6 |
| Isoprostanes | ||||||
| Raw data | 68 | 87 | 56 | .003 | 4.3 | 1.7–12.7 |
| Covariates-adjusted | 63 | 83 | 50 | .005 | 2.8 | 1.2–7.3 |
| Log transformed data | 66 | 83 | 56 | .002 | 3.3 | 1.4–8.5 |
| Covariates-adjusted | 64 | 83 | 53 | .006 | 3.0 | 1.3–7.9 |
| Aβ42/40 and Isoprostanes | ||||||
| Raw data | 70 | 83 | 61 | <.001 | 3.7 | 1.6–9.7 |
| Covariates-adjusted | 69 | 87 | 56 | .002 | 4.3 | 1.7–12.7 |
| Log transformed data | 63 | 74 | 56 | <.001 | 3.2 | 1.7–6.1 |
| Covariates-adjusted | 64 | 74 | 58 | <.001 | 2.4 | 1.3–5.2 |
| Matched groups (n=14 subjects/group) | ||||||
| Aβ42/40 | ||||||
| Raw data | 57 | 71 | 50 | .004 | 1.9 | 0.8–5.1 |
| ApoE-adjusted | 57 | 71 | 50 | .005 | 1.9 | 0.8–5.1 |
| Log transformed data | 60 | 71 | 54 | .004 | 4.1 | 1.3–15.5 |
| ApoE-adjusted | 62 | 71 | 57 | .004 | 2.2 | 0.9–6.2 |
| Isoprostanes | ||||||
| Raw data | 69 | 86 | 61 | .009 | 4.9 | 1.5–18.8 |
| ApoE-adjusted | 64 | 79 | 57 | .01 | 3.7 | 1.4–11.1 |
| Log transformed data | 69 | 86 | 61 | .007 | 5.0 | 1.5–18.8 |
| ApoE-adjusted | 64 | 79 | 57 | .01 | 3.0 | 1.1–9.3 |
| Aβ42/40 and Isoprostanes | ||||||
| Raw data | 69 | 79 | 64 | .001 | 3.7 | 1.4–11.1 |
| ApoE-adjusted | 71 | 86 | 64 | .001 | 5.5 | 1.7–20.6 |
| Log transformed data | 71 | 71 | 71 | <.001 | 3.3 | 1.4–8.7 |
| ApoE adjusted | 76 | 79 | 75 | <.001 | 4.9 | 1.8–14.5 |
Figure 2.
CSF IsoP and Aβ42/40 levels as discriminators of NL MH (black) from NH (white) and PH (grey). CSF measures are raw (top panel) and log-transformed data (bottom panel).
Comparison of demographically matched groups confirmed results with the entire group. The MH group showed lower Aβ42/40 and higher IsoP levels compared to NH and PH (P’s≤0.05), and a trend towards lower Aβ42 levels compared to NH (P’s≥0.09), using both raw and log-transformed measures. Results remained significant controlling for ApoE (Table 2). With and without accounting for ApoE, there were no group differences for P-Tau231, T-tau, raw and log-transformed Aβ40, and there were no differences between PH and NH (Table 2).
With and without controlling for ApoE, correlations between P-Tau231 and T-Tau measures were found for all groups, as were correlations across Aβ40, Aβ42, and Aβ42/40 (P≤0.05). Within the MH group, there was a significant association between IsoP and Aβ42/40 (raw data: R2=0.29, P=0.04, log-transformed data: R2=0.32, P=0.03), and a trend between IsoP and T-Tau, using either raw or log-transformed IsoP (R2=0.16, P’s=0.15). There were no significant associations across the other CSF markers for NH and PH groups.
On logistic regression, both IsoP and Aβ42/40 discriminated MH from the other groups with accuracies of 69% and 57%, respectively (P’s≤0.004, Table 3). IsoP added to the accuracy if Aβ42/40 (Pincrement=0.007), and Aβ42/40 added to that of IsoP (Pincrement=0.004), for a combined overall accuracy of 69% (P=0.001). Log-transformed data yielded similar estimates, with 69% accuracy for IsoP and 60% accuracy for Aβ42/40, for a combined accuracy of 71% (P’s≤0.004, Table 3). Results remained unchanged after controlling for ApoE status (Table 3).
DISCUSSION
The present study examined a panel of CSF markers for AD pathology, and showed that NL MH had higher levels of CSF oxidative stress and Aβ pathology compared to PH and to controls, whereas NL PH did not show biomarkers abnormalities. NL MH appear to be a high-risk subgroup of individuals presenting with preclinical, maternally inherited deficits in oxidative metabolism and increased Aβ accumulation, a characteristic finding of AD.
AD pathology develops many years before the clinical manifestations of the disease become evident and is strongly associated with oxidative stress (28). Aβ42 itself can act as a neurotoxin in AD, inducing oxidative stress, which impairs mitochondrial function and initiates a neurotoxic cascade with parallel overproduction of free radicals and peroxidation of neuronal membrane lipids (29–31). In turn, oxidative stress promotes Aβ deposition (32). Although the causal relationship between Aβ, oxidative stress and tau pathology remains to be clarified, Aβ may lead to NFT formation within cells (30,31), and NFT-containing neurons also show extensive evidence for oxidative stress (28). Our data in NL MH is consistent with the view that Aβassociated oxidative damage is an early event in AD, at least in maternally inherited disease, and that these changes may precede NFT pathology and neuronal degeneration (28,33), as reflected in the lack of abnormalities in P-Tau231 and T-tau of our NL MH.
While P-Tau and T-Tau levels were inter-correlated for all groups, consistent with the fact that neurodegeneration and NFT deposition are both neuronal processes associated with the aging process (28), Aβ and IsoP levels correlated with each other and with T-Tau only in NL MH, suggesting an ongoing pathological process involving Aβ deposition, lipid peroxidation and axonal degeneration. Population-based CSF studies have shown that reduced Aβ levels predict decline to AD in NL elderly, while there was no significant preclinical change in CSF T-tau or P-tau (34). CSF IsoP was also shown to predict decline to AD in non-demented subjects (14,20). The fact that the CSF composition of our NL MH was significantly more “AD-like” than in the other groups indicates that MH individuals may be at higher risk for developing AD by virtue of developing early, co-occurring amyloidosis and oxidative stress. These findings are consistent with previous PET imaging studies showing metabolic reductions and increased fibrillar Aβ pathology in NL MH compared to PH and NH, which involved the same brain regions that are typically affected in AD patients compared to controls (9–11). Similar abnormalities are known to precede the onset of dementia in individuals carrying autosomal dominant mutations responsible for early-onset AD (35,36), and in NL elderly before the onset of AD (37–39). Longitudinal studies are needed to clarify the temporal dynamics of change in these AD biomarkers and to determine whether the observed preclinical CSF abnormalities in NL MH are predictive of future cognitive impairment.
Studies have shown that reduced Aβ42 is predictive of AD, and reflects the early stages of brain Aβ aggregation (40). Mutations in the amyloid precursor protein (APP) and presenilin genes causative of early-onset AD are known to shift the processing of APP to Aβ42, leading to an increased ratio of Aβ42 to Aβ40 in brain (41), and a correspondingly reduced ratio in CSF. In our study, although not reaching statistical significance, NL MH showed a trend towards lower Aβ42 compared to NH and, to a lesser extent, to PH, while Aβ40 levels were comparable across groups. The resulting Aβ42/40 ratios were significantly reduced in MH compared to NH and PH, indicating that these reductions may be more closely related to Aβ42 alterations.
Evidence for increased biological risk in NL MH is in agreement with epidemiology data showing that maternal transmission of LOAD, besides being more frequent than paternal transmission (4), is associated with higher risk of developing the disease, poorer cognitive performance and a more predictable age at onset in the offspring (6–8), including findings from a recent large-scale study (42). While more replication studies are needed, our data provides a possible pathophysiological mechanism for the clinical findings. Findings of a maternally inherited form of LOAD whose phenotypic expression involves increased oxidative stress, Aβ production, and glucose hypometabolism suggest the possibility of transmission of the disease via mitochondrial genes. Mitochondrial DNA is exclusively maternally inherited in humans, and defective mitochondrial function leading to increased oxidative damage is known to affect metabolism in AD brain tissue (43). A specific mitochondrial defect in AD is reduced cytochrome oxidase (COX, mitochondrial electron transport chain Complex IV) activity in brain tissue, blood platelets and fibroblasts (44–51). COX is critically tied to ATP production in mitochondria (52). An inherited, deficient energy metabolism may render synapses more vulnerable to Aβ pathology and associated neurodegeneration during the aging process. Cell biology studies have shown that mtDNA at least partly accounts for impaired metabolism and increased oxidative stress in AD, as mitochondrial respiratory enzymes defects increased changes in calcium homeostasis, decreases ATP production and enhanced Aβ toxicity (32,42,43,53,54). If mtDNA is involved, IsoP alterations should be detectable also systemically. Examination of peripheral IsoP would be useful to determine whether oxidative stress is a systemic event in NL MH, or rather occurring downstream to Aβ pathology, as reported in early-onset AD (55). Since the present study focused on CSF abnormalities, it was beyond the scope of the study to measure plasma IsoP. Future studies are warranted to examine this marker in NL MH.
However, while some genetic studies have shown that maternally inherited mutations of mtDNA might play a pathogenic role in LOAD (56), others failed to report evidence for mtDNA mutations (4). The role of mitochondrial genome changes in the pathogenesis of LOAD needs further clarification. Alternatively, epigenetic mechanisms such as metabolic imprinting (e.g., the early programming of metabolism during the early stages of development) may play a role. Although little is known about imprinting in the brain, a recent study has shown that Aβ production may begin as early as at birth as a consequence of lack of trophic support to the embryo, triggering caspase-mediated neurodegeneration (57). Overall, our study shows that having a mother affected by LOAD predisposes the offspring to increased Aβ-related oxidative stress, supporting observations that genetic variability in Aβ catabolism may contribute to the risk of LOAD (58).
Present results were independent of the ApoE genotype, and less than 40% of our subjects were ε4 carriers, indicating that other factors contribute to the etiology and phenotypic expression of disease. As previous reports showed altered CSF Aβ and IsoP in non-demented ApoE ε4 carriers (7,23,59), other studies with larger samples are needed to examine ApoE status an as effect modifier by testing whether MH ApoE ε4 carriers would show more severe CSF biomarkers abnormalities than the other groups, and to determine whether stratifying for FH and ApoE would show different associations between biomarkers. Additionally, while CSF abnormalities in MH remained significant controlling for other potential risk factors for LOAD, such as age, gender, and education, interaction effects between these variables and FH status remain to be investigated.
Steps were taken to ensure that the AD diagnosis in the subjects’ parents was accurate. We only included subjects whose parents’ AD diagnosis was certified by an expert clinician according to established criteria for AD (60). These diagnoses and all available medical and autopsy reports of the parents were further reviewed by our study physician. Questionnaires used to elicit FH information are known to have good agreement with clinical and neuropathological findings (61), which reduces potential for misclassification. As a result of our stringent inclusion criteria, all affected parents of the 59 subjects in this study had a documented, clinician certified diagnosis of AD, including 4 cases confirmed at post-mortem. Nonetheless, in absence of postmortem confirmation, our cohort may have included subjects whose parents did not have AD but another dementia. This would lead to inclusion of subjects with decreased risk for AD in the MH and PH groups, conservatively reducing power in detecting differences.
This is a cross-sectional study and the subjects’ diagnosis of normal cognition was based on current standardized clinical assessments and neuropsychological testing. Nonetheless, Aβ and IsoP abnormalities were observed in NL MH with normal cognition, supporting the view that biochemical abnormalities precede future clinical change, and that the diagnostic process would benefit from inclusion of biomarker measures in clinical assessments (62).
In the present study, we only had the ability to assay CSF F2-α-isoprostanes. More specific markers of brain lipid peroxidation such as F4-α-isoprostanes (63) would be of interest, as well as measurements of other F2-α-isoprostanes species, like F2-α-III and IV (64). Future studies are needed to examine these markers in NL at risk for LOAD.
Overall, NL MH expressed an AD-predisposing biological phenotype, which may confer increased risk for LOAD. Larger samples, longitudinal follow-up examinations, and population-based comparisons are necessary to testing the usefulness of these CSF measures for predictive purposes and for investigations of potential susceptibility genes for LOAD.
Acknowledgements
This study was supported by: NIH-NIA AG13616, AG12101, AG08051, AG022374, AG032554, and NIH-NCRR MO1RR0096, and the Alzheimer’s Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, Schellenberg GD, et al. Dementia and Alzheimer disease incidence: A prospective cohort study. Arch Neurol. 2002;59:1737–1746. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 2.Farrer LA, O'Sullivan DM, Cupples AC, Growdon JH, Myers RH. Assessment of genetic risk for Alzheimer's disease among first-degree relatives. Ann Neurol. 1989;25:485–493. doi: 10.1002/ana.410250511. [DOI] [PubMed] [Google Scholar]
- 3.Green RC, Cupples LA, Go R, Benke KS, Edeki T, Griffith PA, et al. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002;287:329–336. doi: 10.1001/jama.287.3.329. [DOI] [PubMed] [Google Scholar]
- 4.Mosconi L, Berti V, Swerdlow RH, Mistur R, Pupi A, Duara R, et al. Maternal transmission of Alzheimer's disease: Prodromal metabolic phenotype and the search for genes. Human Genomics. 2010;4:24. doi: 10.1186/1479-7364-4-3-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehrenkrantz D, Silverman JM, Smith CJ, Birstein S, Marin D, Mohs RC, et al. Genetic epidemiological study of maternal and paternal transmission of Alzheimer's disease. Am J Med Genet. 1999;88:378–382. doi: 10.1002/(sici)1096-8628(19990820)88:4<378::aid-ajmg15>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 6.Edland SD, Silverman JM, Peskind ER, Tsuang D, Wijsman E, Morris JC. Increased risk of dementia in mothers of Alzheimer's disease cases: evidence for maternal inheritance. Neurology. 1996;47:254–256. doi: 10.1212/wnl.47.1.254. [DOI] [PubMed] [Google Scholar]
- 7.Gomez-Tortosa E, Barquero MS, Baron M, Sainz MJ, Manzano S, Payno M, et al. Variability of Age at Onset in Siblings with Familial Alzheimer Disease. Arch Neurol. 2007;64:1743–1748. doi: 10.1001/archneur.64.12.1743. [DOI] [PubMed] [Google Scholar]
- 8.Silverman JM, Ciresi G, Smith CJ, Marin DB, Schnaider-Beeri M. Variability of familial risk of Alzheimer disease across the late life span. Arch Gen Psychiat. 2005;62:565–573. doi: 10.1001/archpsyc.62.5.565. [DOI] [PubMed] [Google Scholar]
- 9.Mosconi L, Brys M, Switalski R, Mistur R, Glodzik-Sobanska L, Pirraglia E, et al. Maternal family history of Alzheimer's disease predisposes to reduced brain glucose metabolism. Proc Natl Acad Sci USA. 2007;104:19067–19072. doi: 10.1073/pnas.0705036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosconi L, Mistur R, Glodzik L, Brys M, Switalski R, Pirraglia E, et al. Declining brain glucose metabolism in normal individuals with a maternal history of Alzheimer's. Neurology. 2009;72:513–520. doi: 10.1212/01.wnl.0000333247.51383.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosconi L, Rinne JO, Tsui W, Berti V, Li Y, Murray J, et al. Increased fibrillar amyloid-β burden in normal individuals with a family history of late-onset Alzheimer's disease. Proc Natl Acad Sci USA. 2010;107:5949–5954. doi: 10.1073/pnas.0914141107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet Neurology. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 13.Buerger K, Zinkowski R, Teipel SJ, Tapiola T, Arai H, Blennow K, et al. Differential diagnosis of Alzheimer disease with cerebrospinal fluid levels of tau protein phosphorylated at Threonine 231. Arch Neurol. 2002;59:1267–1272. doi: 10.1001/archneur.59.8.1267. [DOI] [PubMed] [Google Scholar]
- 14.Pratico D, Clark CM, Lee VM, Trojanowski JQ, Rokach J, Fitzgerald GA. Increased 8,12-iso-iPF2alpha-VI in Alzheimer's disease: correlation of a noninvasive index of lipid peroxidation with disease severity. Ann Neurol. 2000;48:809–812. [PubMed] [Google Scholar]
- 15.Lewczuk P, Esselmann H, Otto M, Maler JM, Henkel AW, Henkel MK, et al. Neurochemical diagnosis of Alzheimer's dementia by CSF A[beta]42, A[beta]42/A[beta]40 ratio and total tau. Neurobiol Aging. 2004;25:273–281. doi: 10.1016/S0197-4580(03)00086-1. [DOI] [PubMed] [Google Scholar]
- 16.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 17.Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M, et al. CSF Biomarkers and Incipient Alzheimer Disease in Patients With Mild Cognitive Impairment. JAMA. 2009;302:385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- 18.Hansson O, Zetterberg H, Buchhave P, Andreasson U, Londos E, Minthon L, et al. Prediction of Alzheimer's disease using the CSF Abeta42/Abeta40 ratio in patients with mild cognitive impairment. Dement Geriatr Cogn Disord. 2007;23:316–320. doi: 10.1159/000100926. [DOI] [PubMed] [Google Scholar]
- 19.Fagan AM, Roe CM, Xiong C, Mintun M, Morris JC, Holtzman DM. Cerebrospinal Fluid tau/beta-Amyloid42 Ratio as a Prediction of Cognitive Decline in Nondemented Older Adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 20.Brys M, Pirraglia E, Rich K, Rolstad S, Mosconi L, Switalski R, et al. Prediction and longitudinal study of CSF biomarkers in mild cognitive impairment. Neurobiol Aging. 2009;30:682–690. doi: 10.1016/j.neurobiolaging.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Santi S, Pirraglia E, Barr WB, Babb J, Williams S, Rogers K, et al. Robust and conventional neuropsychological norms: Diagnosis and prediction of age-related cognitive decline. Neuropsychology. 2008;22:469–484. doi: 10.1037/0894-4105.22.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reisberg B, Ferris SH, de Leon MJ, Crook T. The global deterioration scale for assessment of primary degenerative dementia. Am J Psychiat. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 23.Mosconi L, De Santi S, Brys M, Tsui WH, Pirraglia E, Glodzik-Sobanska L, et al. Hypometabolism and altered CSF markers in normal ApoE E4 carriers with subjective memory complaints. Biol Psychiatry. 2008;63:609–618. doi: 10.1016/j.biopsych.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.George AE, de Leon MJ, Kalnin A, Rosner L, Goodgold A, Chase N. Leukoencephalopathy in normal and pathologic aging: 2. MRI and brain lucencies. Am J Neuroradiol. 1986;7:567–570. [PMC free article] [PubMed] [Google Scholar]
- 25.Hachinski VC, Lassen NA, Marshall J. Multi-infarct dementia, a cause of mental deterioration in the elderly. Lancet. 1974;2:207–210. doi: 10.1016/s0140-6736(74)91496-2. [DOI] [PubMed] [Google Scholar]
- 26.Mehta PD, Pirttila T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid beta proteins 1–40 and 1–42 in Alzheimer disease. Arch Neurol. 2000;57:100–105. doi: 10.1001/archneur.57.1.100. [DOI] [PubMed] [Google Scholar]
- 27.Kohnken R, Buerger K, Zinkowski R, Miller C, Kerkman D, DeBernardis J, et al. Detection of tau phosphorylated at threonine 231 in cerebrospinal fluid of Alzheimer's disease patients. Neurosci Lett. 2000;287:187–190. doi: 10.1016/s0304-3940(00)01178-2. [DOI] [PubMed] [Google Scholar]
- 28.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 29.Yankner BA, Duffy L, Kirschner D. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990;250:279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- 30.Behl C, Davis J, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid [beta]protein toxicity. Cell. 1994;77:817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 31.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:1424–1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 32.Cardoso SM, Santana I, Swerdlow RH, Oliveira CR. Mitochondria dysfunction of Alzheimer's disease cybrids enhances Abeta toxicity. J Neurochem. 2004;89:1417–1426. doi: 10.1111/j.1471-4159.2004.02438.x. [DOI] [PubMed] [Google Scholar]
- 33.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;8:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gustafson DR, Skoog I, Rosengren L, Zetterberg H, Blennow K. Cerebrospinal fluid beta-amyloid 1–42 concentration may predict cognitive decline in older women. J Neurol Neurosurgery Psych. 2006;78:461–464. doi: 10.1136/jnnp.2006.100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosconi L, Sorbi S, de Leon MJ, Li Y, Nacmias B, Bessi V, et al. Hypometabolism exceeds atrophy in presymptomatic early-onset Familial Alzheimer's disease. J Nucl Med. 2006;47:1778–1786. [PubMed] [Google Scholar]
- 36.Klunk WE, Price JC, Mathis CA, Tsopelas ND, Lopresti BJ, Ziolko SK, et al. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J Neurosci. 2007;27:6174–6184. doi: 10.1523/JNEUROSCI.0730-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Leon MJ, Convit A, Wolf OT, Tarshish CY, De Santi S, Rusinek H, et al. Prediction of cognitive decline in normal elderly subjects with 2-[18F]fluoro-2-deoxy-D-glucose/positron-emission tomography (FDG/PET) Proc Natl Acad Sci USA. 2001;98:10966–10971. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mintun M, LaRossa GN, Sheline YIM, Dence CSM, Lee SYP, Mach RHP, et al. [11C]PIB in a nondemented population: Potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 39.Mosconi L, De Santi S, Li J, Tsui WH, Li Y, Boppana M, et al. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiol Aging. 2008;29:676–692. doi: 10.1016/j.neurobiolaging.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 41.St.George-Hyslop P. Molecular genetics of Alzheimer's disease. Biol Psychiatry. 2000;47:183–199. doi: 10.1016/s0006-3223(99)00301-7. [DOI] [PubMed] [Google Scholar]
- 42.Debette S, Wolf PA, Beiser A, Au R, Himali JJ, Pikula A, et al. Association of parental dementia with cognitive and brain MRI measures in middle-aged adults. Neurology. 2009;73:2071–2078. doi: 10.1212/WNL.0b013e3181c67833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 44.Mutisya EM, Bowling AC, Beal MF. Cortical cytochrome oxidase activity is reduced in Alzheimer's disease. J Neurochem. 1994;63:2179–2184. doi: 10.1046/j.1471-4159.1994.63062179.x. [DOI] [PubMed] [Google Scholar]
- 45.Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, et al. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valla J, Berndt JD, Gonzales-Lima F. Energy hypometabolism in posterior cingulate cortex of Alzheimer's patients: superficial laminar cytochrome oxidase associated with disease duration. J Neurosci. 2001;21:4923–4930. doi: 10.1523/JNEUROSCI.21-13-04923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parker WD, Jr, Mahr NJ, Filley CM, Parks JK, Hughes D, Young DA, et al. Reduced platelet cytochrome c oxidase activity in Alzheimer's disease. Neurology. 1994;44:1086–1090. doi: 10.1212/wnl.44.6.1086. [DOI] [PubMed] [Google Scholar]
- 48.Swerdlow RH, Parks JK, Cassarino DS, Maguire DJ, Maguire RS, Bennett JP, Jr, et al. Cybrids in Alzheimer's disease: a cellular model of the disease? Neurology. 1997;49:918–925. doi: 10.1212/wnl.49.4.918. [DOI] [PubMed] [Google Scholar]
- 49.Bosetti F, Brizzi F, Barogi S, Mancuso M, Siciliano G, Tendi EA, et al. Cytochrome c oxidase and mitochondrial F1F0-ATPase (ATP synthase) activities in platelets and brain from patients with Alzheimer's disease. Neurobiol Aging. 2002;23:371–376. doi: 10.1016/s0197-4580(01)00314-1. [DOI] [PubMed] [Google Scholar]
- 50.Cardoso SM, Proenca MT, Santos S, de Oliveira CR. Cytochrome c oxidase is decreased in Alzheimer's disease platelets. Neurobiol Aging. 2004;25:105–110. doi: 10.1016/s0197-4580(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 51.Valla J, Schneider L, Niedzielko T, Coon KD, Caselli RJ, Sabbagh MN, et al. Impaired platelet mitochondrial activity in Alzheimer's disease and mild cognitive impairment. Mitochondrion. 2006;6:323–330. doi: 10.1016/j.mito.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong-Riley MTT. Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends Neurosci. 1898;12:94–101. doi: 10.1016/0166-2236(89)90165-3. [DOI] [PubMed] [Google Scholar]
- 53.Khan SM, Cassarino DS, Abramova NN, Keeney PM, Borland MK, Trimmer PA, et al. Alzheimer's disease cybrids replicate beta-amyloid abnormalities through cell death pathways. Ann Neurol. 2000;48:148–155. [PubMed] [Google Scholar]
- 54.Trimmer PA, Swerdlow RH, Parks JK, Keeney PM, Bennett JP, Jr, Miller SW, et al. Abnormal mitochondrial morphology in sporadic Parkinson's and Alzheimer's disease cybrid cell lines. Experimental Neurology. 2000;162:37–50. doi: 10.1006/exnr.2000.7333. [DOI] [PubMed] [Google Scholar]
- 55.Ringman JM, Younkin SG, Pratico D, Seltzer W, Cole GM, Geschwind DH, et al. Biochemical markers in persons with preclinical familial Alzheimer disease. Neurology. 2008;71:85–92. doi: 10.1212/01.wnl.0000303973.71803.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coskun PE, Beal MF, Wallace DC. Alzheimer's brains harbor somatic mtDNA controlregion mutations that suppress mitochondrial transcription and replication. Proc Natl Acad Sci USA. 2004;101:10726–10731. doi: 10.1073/pnas.0403649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nikolaev A, McLaughlin T, O'Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Hardy J, Selkoe DJ. The Amyloid Hypothesis of Alzheimer's disease: Progress and Problems on the road to Therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 59.Morris JC, Roe CM, Xiong C, Fagan AM, Goate A, Holtzman DM, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67:122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 61.Kawas C, Segal J, Stewart WF, Corrada M, Thal LJ. A validation study of the Dementia Questionnaire. Arch Neurol. 1994;51:901–906. doi: 10.1001/archneur.1994.00540210073015. [DOI] [PubMed] [Google Scholar]
- 62.Gauthier S, Dubois B, Feldman H, Scheltens P. Revised research diagnostic criteria for Alzheimer's disease. Lancet Neurol. 2008;7:668–670. doi: 10.1016/S1474-4422(08)70146-7. [DOI] [PubMed] [Google Scholar]
- 63.Reich EE, Markesbery WR, Roberts LJ, II, Swift LL, Morrow JD, Montine TJ. Brain Regional Quantification of F-Ring and D-/E-Ring Isoprostanes and Neuroprostanes in Alzheimer's Disease. Am J Pathol. 2001;158:293–297. doi: 10.1016/S0002-9440(10)63968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li H, Lawson JA, Reilly M, Adiyaman M, Hwang SW, Rokach J, et al. Quantitative high performance liquid chromatography/tandem mass spectrometric analysis of the four classes of F2-isoprostanes in human urine. Proc Natl Acad Sci USA. 1999;96:13381–13386. doi: 10.1073/pnas.96.23.13381. [DOI] [PMC free article] [PubMed] [Google Scholar]