Abstract
The melanocortin system has been implicated in a multitude of physiological pathways including obesity, satiety, energy homeostasis, sexual behavior, pigmentation, sodium regulation, hypertension, and many others. Based upon studies of the endogenous melanocortin receptor agonists at the cloned human melanocortin receptor proteins, it was concluded that the γ-MSH related agonist ligands are selective for the MC3 versus the MC4 and MC5 receptors. In attempts to understand and identify the specific amino acids of γ2-MSH important for MC3R selectivity, we have performed N- and C-terminal truncation studies and pharmacologically characterized twenty-eight ligands at the mouse MC1 and MC3-5 melanocortin receptors. The C-terminal Trp-Asp9-Arg10-Phe11 residues are important for nM potency at the mMC3R and the Arg7-Trp8 residues are important for mMC5R nM potency. We observed the unanticipated results that several of the C-terminal truncated analogues possessed nM agonist potency at the mMC3 and mMC5Rs which lead us to performed a comparative side-by-side study of the mouse and human MC5R. These data resulted in μM γ2-MSH analogue potency at the hMC5R, consistent with previous reports, however at the mMC5R, nM γ2-MSH analogue potency was observed. Thus, these data support the hypothesis of important species specific differences in γ-MSH related ligand potency at the rodent versus human MC5R subtype that is critical for the interpretation of in vivo rodent physiological studies. These results prompted us to examine the affects of a peripherally administered melanocortin agonist on hypothalamic gene expression levels of the MC3R, MC4R, and MC5R. The super potent non-selective NDP-MSH agonist was administered i.p. and resulted in significantly decreased levels of mMC3R and mMC5R hypothalamic mRNA versus saline control. These data provide for the first time data demonstrating peripherally administered NDP-MSH can modify hypothalamic melanocortin receptor expression levels.
Keywords: Melanotropin, heart, blood pressure, obesity, receptor brain expression, GPCR
1.0 Introduction
The melanocortin receptor family belongs to the super family of proteins that span the cell membrane seven times and are referred to as G protein-coupled receptors (GPCRs) that activate the adenylate cyclase signal transduction pathway. The melanocortin system includes the endogenous agonists α-, β-, γ-MSH and ACTH and the only two identified naturally occurring antagonists of GPCRs [Agouti-related protein (AGRP) and Agouti/ASP] [53,61]. The melanocortin endogenous peptide agonists are derived by posttranslational processing of the proopiomelanocortin (POMC) gene transcript [20,67]. The melanocortin receptor family consists of five receptor subtypes (MC1-5R) identified and characterized to date [15,24-26,57,58,64]. The MC1R is expressed in melanocytes and is involved in pigmentation[15,58]. The MC2R is expressed in the adrenal cortex and is involved in steroidogenesis [57]. The MC3R is expressed in the brain, placenta and gut [24,57,64] and is involved in metabolism and energy homeostasis [3,10]. The MC4R is expressed in the brain [25,49,57] and is involved in feeding behavior, obesity, energy homeostasis, and sexual function [21,42,74,76]. The MC5R is expressed in muscle, liver, spleen, lung, brain and a number of other tissues and is involved in exocrine gland function [14,26].
The MC3R and MC4R subtypes found in the brain are 60% homologous in amino acid sequence but possess distinct pharmacological properties and expression patterns. Pharmacologically, these receptors respond differently to the endogenous melanocortin agonist ligands [19] and synthetic peptide agonists [32,33]. However, both are antagonized by the synthetic ligand, SHU9119 [37] and SHU9005 [31]. AGRP has been shown to antagonize only the brain melanocortin receptors, MC3R and MC4R [45,48,61,77]. During the past decade, highly selective molecules (peptide and small molecule) have been reported for the MC4R. However, highly selective and potent agonists and/or antagonists for the MC3R are lacking in the field.
γ2-MSH is a twelve amino acid peptide that is derived from the N-terminal fragment of POMC and contains the His-Phe-Arg-Trp motif common to all melanocortin endogenous agonist ligands (Table 1). γ2-MSH has been reported to be ca 44-fold hMC3R versus hMC4R selective and 83-fold hMC3R versus hMC5R selective [29]. Modification of the Trp to the D-isomer was reported to result in ca 100-fold hMC3R versus hMC4R selectivity and ca 250-fold hMC3R versus hMC5R selectivity at the human isoforms [29]. Use of γ2-MSH has resulted in the hypothesis that the MC3R is involved in the cardiovascular role of melanocortin receptor ligands [51,60,71-73]. This study was initiated to identify the γ2-MSH domains and specific amino acids that resulted in the purported ligand selectivity for the MC3R versus MC4R. The strategy included single N- and C-terminal truncations to probe the specific role of amino acids at the termini as well as simultaneous N- and C-terminal truncation approaches to determine the importance of the central peptide core domain, as Ala and D-amino acid scans have been previously reported [29,30].
Table 1.
Functional activity of the modified peptides at the mouse melanocortin receptors
| mMC1R | mMC3R | mMC4R | mMC5R | hMC5R | ||
|---|---|---|---|---|---|---|
| Peptide | Structure | EC50 (nM) | EC50 (nM) | EC50 (nM) | EC50 (nM) | EC50 (nM) |
| α-MSH | Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 0.70±0.23 | 2.10±0.46 | 2.6±0.29 | 2.00±0.20 | |
| NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 1.0±0.78 | 0.09±0.02 | 0.10±0.01 | 0.22±0.03 | 0.53±0.10 |
| γ2-MSH (Bachem control) |
Tyr-Val-Met-Gly-His-Phe-Arg-Trp-Asp-Arg-Phe-Gly | 680±190 | 38±6.5 | 420±68 | 42±14 | 2700±900 |
| 1 γ2-MSH | Tyr-Val-Met-Gly-His-Phe-Arg-Trp-Asp-Arg-Phe-Gly | 690±260 | 51±5.3 | 580±160 | 73±14 | 2600±620 |
| 2 | Tyr-Val-Met-Gly-His-Phe-Arg-DTrp8-Asp-Arg-Phe-Gly | 120±44 | 90±46 | 5580±2300 | 48±20 | 580±280 |
| 3 | Tyr-Val-Nle3-Gly-His-Phe-Arg-Trp-Asp-Arg-Phe-Gly | 390±90 | 68±30 | 270±82 | 140±80 | 1900±620 |
| 4 | Tyr-Val-Nle3-Gly-His-Phe-Arg-DTrp8-Asp-Arg-Phe-Gly | 31±11 | 40±20 | 94±33 | 10±1.7 | 210±58 |
| 5 | Tyr-Val-Met-Gly-His-Phe-Arg-Trp-Asp-Arg-Phe (1-11) | 1500±90 | 160±10 | 1100±78 | 130±52 | 2010±460 |
| 6 | Tyr-Val-Met-Gly-His-Phe-Arg-Trp-Asp-Arg (1-10) | 250±60 | 36±9.2 | 660±190 | 62±23 | 7360±2300 |
| 7 | Tyr-Val-Met-Gly-His-Phe-Arg-Trp-Asp (1-9) | 430±90 | 800±96 | 1380±470 | 73±17 | 4455±1510 |
| 8 | Tyr-Val-Met-Gly-His-Phe-Arg-Trp (1-8) | 2900±960 | 2800±950 | 4200±590 | 710±160 | 23900±8600 |
| 9 | Tyr-Val-Met-Gly-His-Phe-Arg- (1-7) | 28100±6700 | 31600±2100 | >100000 | 2360±13600 | >100000 |
| 10 | Tyr-Val-Met-Gly-His-Phe (1-6) | >100000 | >100000 | >100000 | >100000 | >100000 |
| 11 | Tyr-Val-Met-Gly-His (1-5) | 60%@100μM | >100000 | 25%@100μM | 26800±3400 | |
| 12 | Tyr-Val-Met-Gly (1-4) | >100000 | >100000 | >100000 | >100000 | |
| 13 | Tyr-Val-Met-OH (1-3) | >100000 | >100000 | >100000 | >100000 | |
| 14 | Val-Met-Gly-His-Phe-Arg-Trp-Asp-Arg-Phe-Gly (2-12) | 1260±480 | 400±250 | 830±350 | 81±32 | |
| 15 | Met-Gly-His-Phe-Arg-Trp-Asp-Arg-Phe-Gly (3-12) | 1590±520 | 260±67 | 1200±460 | 94±14 | |
| 16 | Gly-His-Phe-Arg-Trp-Asp-Arg-Phe-Gly (4-12) | 7860±2650 | 760±240 | 2700±550 | 290±150 | |
| 17 | His-Phe-Arg-Trp-Asp-Arg-Phe-Gly (5-12) | 20100±5900 | 2670±100 | 8380±2700 | 760±290 | |
| 18 | Phe-Arg-Trp-Asp-Arg-Phe-Gly (6-12) | 30%@100μM | >100000 | 47400±17700 | 13500±7200 90%@100μM |
|
| 19 | Arg-Trp-Asp-Arg-Phe-Gly (7-12) | >100000 | >100000 | >100000 | >100000 | |
| 20 | Trp-Asp-Arg-Phe-Gly (8-12) | >100000 | >100000 | >100000 | >100000 | |
| 21 | Asp-Arg-Phe-Gly (9-12) | >100000 | >100000 | >100000 | >100000 | |
| 22 | Arg-Phe-Gly (10-12) | >100000 | >100000 | >100000 | >100000 | |
| 23 | Tyr-Met-His-Phe-Arg-Trp-Phe (1,3,5-8,11) | 67100±1110 | 10400±2250 | 14010±3700 | 1100±280 | |
| 24 | Met-Gly-His-Phe-Arg-Trp (3-8) | 14500±2100 | 22400±5200 | 41900±11800 | 4430±3800 | |
| 25 | His-Phe-Arg-Trp-Asp-Arg-Phe (5-11) | 44800±6100 | 6600±700 | 64400±19800 | 1870±620 | |
| 26 | Phe-Arg-Trp-Asp-Arg-Phe (6-11) | 3170±750 | 20400±3300 | 34040±9500 | 6730±1490 | |
| 27 | Met-Gly-His-Phe-Arg-Trp-Asp-Arg-Phe (3-11) | 1770±270 | 365±90 | 2410±860 | 100±23 | |
| 28 | Asp-Arg-Phe (9-11) | >100000 | >100000 | >100000 | >100000 | |
| 29 | His-Phe-Arg-Trp (5-8) | >100000 | >100000 | >100000 | 10100±330 85%@100μM |
>100000 |
The indicated errors represent the standard error of the mean determined from at least three independent experiments. >100,000 indicates that agonist or antagonist activity was not observed for these compounds at up to 100μM concentrations. A percentage value indicates that some stimulatory agonist pharmacology resulted at up to 100 μM concentrations, but the maximal stimulation levels were less then the 100% control level of NDP-MSH.
2.0 Material and Methods
2.1 Peptide synthesis
Synthesis was performed using standard 9-Fluorenylmethoxycarbonyl (Fmoc) methodology [5,6] by automation on an Advanced ChemTech 440MOS automated synthesizer (Louisville, KY, USA). The amino acids Fmoc-Tyr(tBu), Fmoc-Val, Fmoc-Met, Fmoc-Gly, Fmoc-His(Trt), Fmoc-Phe, Fmoc-Arg(Pbf), Fmoc-Trp(Boc), and Fmoc-Asp(OtBu) were purchased from Peptides International (Louisville, KY, USA). The peptides were assembled on 9-fluorenylmethoxycarbonyl-L-amino acid-p-alkoxybenzyl alcohol (Fmoc-amino acid Wang) resin (0.40 -0.62 meq/g substitution), purchased from Peptides International (Louisville, KY, USA). All reagents were ACS grade or better and were used without further purification. The Fmoc protecting groups were removed using 20% piperidine (Sigma Aldrich) in N,N-Dimethylformamide (DMF), amino acid coupling (3-fold excess) was accomplished using 2-(1H-Benzotriazol-1-yl)-1,1,3,3-Tetramethyluronium Hexafluorophosphate (HBTU, 3-fold excess), 1-Hydroxybenzotriazole Anhydrous (HOBt, 3-fold excess) and Diisopropylethylamine (DIEA, 5.1-fold excess) manually. On the automated synthesizer the synthesis was performed using a 16 well Teflon reaction block with a course frit. Approximately 250 mg resin (0.2 mmole) was added to each reaction block well. The resin was allowed to swell for 2 hrs in methylene chloride (DCM) and deprotected using 25% piperidine in DMF twice for 5 min then 20 min at 450 rpm. A positive ninhydrin test [46] result indicates free amine groups on the resin. The growing peptide chain was added to the Wang resin using the following general amino acid cycle: 500 μL DMF is added to each reaction well to wet the frit, 3-fold excess amino acid starting from the C-terminus is added (900 μL of 0.5M amino acid solution containing 0.5M HOBt in DMF) followed by the addition of 900 μL 0.5M diisopropylcarbodiimide (DIC) in DMF and the reaction well volume is brought up to 6 mL using DMF. The coupling reaction is mixed for 1 hr at 450 rpm, followed by emptying of the reaction block by positive nitrogen gas pressure. A second coupling reaction is performed by addition of 500 μL of DMF to each reaction vessel, followed by addition of 900 μL of 0.5 M of the respective amino acid, 900 μL 0.5 M HBTU, 765 μL 1M DIEA, the reaction well volume is brought up to 6 mL with DMF and mixed at 450 rpm for 1 hr. After the second coupling cycle, the reaction block is emptied and the Fmoc protected resin is washed with DMF (6 mL 4 times). Fmoc deprotection is performed by addition of 6 mL 25% piperidine in DMF and for 5 min at 450 rpm followed by a 20 min deprotection at 450 rpm. The reaction well was washed with 6 mL DMF 4 times and the next coupling cycle is performed as above. Completion of amino acid coupling and Fmoc deprotection were monitored using the ninhydrin test [46]. Final peptide cleavage from the resin and amino acid side chain protecting group removal was performed using 5 mL of (89.9:5:5:0.1) trifluoroacetic acid (TFA), triethylsilane, water, and p-thiocresol/p-cresol (1:1) for 2-3 hours. The cleavage product was emptied from the reaction block into a cleavage block containing 15 mL collection vials under nitrogen gas pressure. The resin was washed with 3 mL cleavage cocktail for 5 min at 450 rpm and emptied into the previous cleavage solution. The crude peptides was transferred to pre-weighed 50 mL conical tubes and precipitated with cold (4 °C) anhydrous ethyl ether (up to 50 mL). The crude peptides was centrifuged (Sorval Super T21 high speed centrifuge using the swing bucket rotor) at 4000 rpm for 5 min and 4 °C. The ether was decanted off and the peptide was washed one more time with cold anhydrous ethyl ether and pelleted as before. The crude peptides were dried in vacuo for 48 hrs. The crude peptide yields ranged from 75% to 95% of the theoretical yields based on resin loading. A 30 to 40 mg sample of crude peptide was purified by Reversed-Phase High Performance Liquid Chromatography (RP-HPLC) using a Shimadzu chromatography system with a photodiode array detector. Final peptide purification was achieved using a semi-preparative RP-HPLC C18 bonded silica column (Vydac 218TP1010, 1.0 × 25 cm). The purified peptides were >96% pure as determined by analytical RP-HPLC in two diverse solvent systems and had the correct molecular mass (University of Florida protein core facility), Table 2.
Table 2.
Analytical Data of the γ2-MSH peptide analogues
| Peptide | Sequence | HPLC k’ (system 1) |
HPLC k’ (system 2) |
% purity | m/z (M, calcd) |
m/z (M + 1, expt) |
|---|---|---|---|---|---|---|
| 1 (γ2-MSH) | Tyr-Val-Met-Gly-His-Phe-Arg-Trp-Asp-Arg-Phe-Gly | 5.3 | 9.9 | >99 | 1570.8 | 1571.4 |
| 2 | Tyr-Val-Met-Gly-His-Phe-Arg-DTrp8-Asp-Arg-Phe-Gly | 5.0 | 9.6 | >96 | 1570.8 | 1570.9 |
| 3 | Tyr-Val-Nle3-Gly-His-Phe-Arg-Trp-Asp-Arg-Phe-Gly | 5.5 | 10.4 | >97 | 1552.7 | 1552.2 |
| 4 | Tyr-Val-Nle3-Gly-His-Phe-Arg-DTrp8-Asp-Arg-Phe-Gly | 5.3 | 10.1 | >97 | 1552.7 | 1554.4 |
| 5 | Tyr-Val-Met-Gly-His-Phe-Arg-Trp-Asp-Arg-Phe (1-11) | 6.0 | 10.3 | >98 | 1513.7 | 1513.7 |
| 6 | Tyr-Val-Met-Gly-His-Phe-Arg-Trp-Asp-Arg (1-10) | 4.5 | 8.5 | >98 | 1366.6 | 1366.0 |
| 7 | Tyr-Val-Met-Gly-His-Phe-Arg-Trp-Asp (1-9) | 5.4 | 9.9 | >99 | 1210.0 | 1210.2 |
| 8 | Tyr-Val-Met-Gly-His-Phe-Arg-Trp (1-8) | 6.0 | 10.0 | >99 | 1095.3 | 1095.0 |
| 9 | Tyr-Val-Met-Gly-His-Phe-Arg (1-7) | 3.5 | 7.0 | >99 | 909.1 | 908.7 |
| 10 | Tyr-Val-Met-Gly-His-Phe (1-6) | 4.4 | 8.5 | >98 | 752.9 | 753.1 |
| 11 | Tyr-Val-Met-Gly-His (1-5) | 2.3 | 4.3 | >96 | 605.7 | 606.2 |
| 12 | Tyr-Val-Met-Gly (1-4) | 2.7 | 4.9 | >98 | 468.6 | 468.9 |
| 13 | Tyr-Val-Met (1-3) | 2.9 | 5.5 | >99 | 411.5 | 413.3 |
| 14 | Val-Met-Gly-His-Phe-Arg-Trp-Asp-Arg-Phe-Gly (2-12) | 5.1 | 10.6 | >97 | 1407.6 | 1408.0 |
| 15 | Met-Gly-His-Phe-Arg-Trp-Asp-Arg-Phe-Gly (3-12) | 5.0 | 9.3 | >98 | 1308.5 | 1308.3 |
| 16 | Gly-His-Phe-Arg-Trp-Asp-Arg-Phe-Gly (4-12) | 4.8 | 8.8 | >98 | 1177.1 | 1177.4 |
| 17 | His-Phe-Arg-Trp-Asp-Arg-Phe-Gly (5-12) | 4.8 | 9.0 | >98 | 1120.2 | 1120.2 |
| 18 | Phe-Arg-Trp-Asp-Arg-Phe-Gly (6-12) | 4.9 | 9.0 | >98 | 983.1 | 983.7 |
| 19 | Arg-Trp-Asp-Arg-Phe-Gly (7-12) | 3.9 | 7.2 | >98 | 835.9 | 836.5 |
| 20 | Trp-Asp-Arg-Phe-Gly (8-12) | 3.7 | 6.7 | >99 | 679.7 | 680.5 |
| 21 | Asp-Arg-Phe-Gly (9-12) | 2.3 | 3.9 | >99 | 493.5 | 494.4 |
| 22 | Arg-Phe-Gly (10-12) | 2.8 | 3.4 | >99 | 378.4 | 379.2 |
| 23 | Tyr-Met-His-Phe-Arg-Trp-Phe (1,3,5-8,11) | 6.4 | 10.7 | >98 | 1086.2 | 1086.9 |
| 24 | Met-Gly-His-Phe-Arg-Trp (3-8) | 5.5 | 9.0 | >99 | 833.0 | 832.9 |
| 25 | His-Phe-Arg-Trp-Asp-Arg-Phe (5-11) | 4.9 | 9.4 | >98 | 1063.2 | 1063.4 |
| 26 | Phe-Arg-Trp-Asp-Arg-Phe (6-11) | 5.0 | 9.4 | >99 | 926.0 | 926.5 |
| 27 | Met-Gly-His-Phe-Arg-Trp-Asp-Arg-Phe (3-11) | 5.7 | 9.7 | >99 | 1251.4 | 1251.2 |
| 28 | Asp-Arg-Phe (9-11) | 3.3 | 4.5 | >99 | 436.4 | 437.1 |
| 29 | His-Phe-Arg-Trp (5-8) | 4.5 | 8.8 | >97 | 644.7 | 645.0 |
HPLC k’ = [(peptide retention time – solvent retention time)/(solvent retention time)] in solvent system 1 (10 % acetonitrile in 0.1 % trifluoroacetic acid/water and a gradient to 90 % acetonitrile over 35 min) or solvent system 2 (10 % methanol in 0.1 % trifluoroacetic Acid/water and a gradient to 90 % methanol over 35 min). An analytical Vydac C18 column (Vydac 218TP104) was used with a flow rate of 1.5 ml/min. The peptide purity was determined by HPLC at a wavelength of 214 nm.
2.2 Generation of Stable Cell Lines
HEK-293 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal calf serum (FCS) and seeded 1 day prior to transfection at (1-2) ×106 cell/100-mm dish. Melanocortin receptor DNA in pCDNA3 expression vector (20 μg) were transfected using the calcium phosphate method [11]. Stable receptor populations were generated using G418 selection (0.7-1 mg/mL) for subsequent bioassay analysis. The human MC5R stably expressing HEK-293 cells were generated in the laboratory of Dr. Kathleen G. Mountjoy. The stably expressed mouse MCRs were generated in the laboratory of Dr. Carrie Haskell-Luevano.
2.3 cAMP Based Functional Bioassay
HEK-293 cells stably expressing the wild type melanocortin receptors were transfected with 4 μg CRE/β-galactosidase reporter gene as previously described [13]. Briefly, 5,000 to 15,000 post transfection cells were plated into 96 well Primera plates (Falcon) and incubated overnight. Forty-eight hours post-transfection the cells were stimulated with 100 μL peptide (10−4 - 10−12 M) or forskolin (10−4 M) control in assay medium (DMEM containing 0.1 mg/mL BSA and 0.1 mM isobutylmethylxanthine) for 6 hrs. The assay media was aspirated and 50 μL of lysis buffer (250 mM Tris-HCl pH=8.0 and 0.1% Triton X-100) was added. The plates were stored at −80°C overnight. The plates containing the cell lysates were thawed the following day. Aliquots of 10 μL were taken from each well and transferred to another 96-well plate for relative protein determination. To the cell lysate plates, 40 μL phosphate-buffered saline with 0.5% BSA was added to each well. Subsequently, 150 μL substrate buffer (60 mM sodium phosphate, 1 mM MgCl2, 10 mM KCl, 5 mM β-mercaptoethanol, 2 mg/mL ONPG) was added to each well and the plates were incubated at 37°C. The sample absorbance, OD405, was measured using a 96 well plate reader (Molecular Devices). The relative protein was determined by adding 200 μL 1:5 dilution Bio Rad G250 protein dye:water to the 10 μL cell lysate sample taken previously, and the OD595 was measured on a 96 well plate reader (Molecular Devices). Data points were normalized both to the relative protein content and non-receptor dependent forskolin stimulation.
Protease inhibitors were not utilized in this functional bioassay. The POMC pro-hormone, from which the melanocortin agonists are derived, is post-translationally processed by several enzymes, including the prohormone convertases (PCs), resulting in the different forms of the α-MSH, β-MSH, γ-MSH, and ACTH melanocortin agonists [62,63]. The peptide γ3-MSH is processed from the N-POC POMC fragment by the PC2 enzyme which further cleaves the γ3-MSH fragment to the γ1-MSH and γ2-MSH fragments. The γ2-MSH peptide, which is the sequence of focus in this study, has not been reported to be further enzymatically processed to smaller fragments.
2.4 Data Analysis
Agonist EC50 values represent the mean of duplicate experiments performed in triplicate or more independent experiments. EC50 estimates and their associated standard errors of the mean were determined by fitting the data to a nonlinear least-squares analysis using the PRISM program (GraphPad Inc.).
2.5 Experimental Animals
Male wild type mice on a mixed 129/C57Bl6 mixed background, derived from our in house breeding colony [1,34,44] 3-4 months of age (average body weights 22.1 ± 0.69g), were housed individually in a 12-h light/dark cycle (lights on at 11PM and off at 11AM) and had ad libitum access to rodent chow and water for the duration of this study. The mice were divided into two groups of n=3 and treated by intraperitoneal (i.p.) injection. One group received 100uL of 4.5 μmol/kg NDP-MSH peptide dissolved in saline and a second group received 100μL saline control. The mice were sacrificed 24hr post treatment. All studies performed were conducted in accord with accepted standards of humane animal care and were approved by the Institutional Animal Care and Use Committee at the University of Florida.
2.6 Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
At the time of sacrifice, the hypothalamus of the brain was dissected (excised with a razor blade by trimming out all of the brain structures including the midbrain, cerebellum, and cortex) from each mouse and placed immediately in RNALater (Ambion) and held at 4°C for 24 hrs, then transferred to −20°C until RNA extraction. The RNA was extracted using the Trizol reagent. RNA was quantified and integrity was confirmed by gel electrophoresis. cDNA was generated from 2 μg of total RNA per the manufactures instruction (Applied Biosystems, Foster City, CA). Quantitative real-time PCR reactions were performed using 50 ng cDNA as template with Taqman primers and reagents in a ABI 7300 System (Applied Biosystems). Samples were run in duplicate reactions on a single 96-well plate for each gene probe to confirm consistency in the amount of PCR products. The hypoxanthine guanine phosphoribosyl transferase 1 (Hprt1) housekeeping gene was used to correct for the amount of input mRNA and for data normalization. Levels of mRNA are expressed as fold differences compared to the saline treated group. The fold difference over the control Hprt1 gene is calculated a 2−ΔCt, where ΔCt = Ct(gene)-Ct(Hprt1 gene). Ct is the PCR cycle where fluorescent signal associated with the exponential growth exceeds the threshold (10x noise level). Data is expressed as the mean ± SEM. Statistical differences between treatment groups of mice were determined using a two-way analysis of variance (ANOVA) with a Bonferroni post hoc test using the delta Ct values for treatment effects. Statistical significance is considered if p<0.05.
3.0 RESULTS
3.1 Chemical Design, Synthesis, and Characterization
The peptides reported herein were synthesized using standard fluorenylmethyloxycarbonyl (Fmoc) [5,6] chemistry and a parallel synthesis strategy on an automated synthesizer (Advanced ChemTech 440MOS, Louisville, KY). The peptides were purified to homogeneity using semi-preparative reversed-phase high pressure liquid chromatography (RP-HPLC). The peptides possessed the correct molecular weights as determined by mass spectrometry. The purity of these peptides (>95%) were assessed by analytical RP-HPLC in two diverse solvent systems.
3.2 Biological Evaluation
Table 1 summarizes the γ2-MSH based ligand agonist pharmacology at the mouse melanocortin receptors MC1R, MC3R, MC4R, and MC5R. The γ2-MSH control peptide listed in the table first was purchased from Bachem (Torrence CA) which is a common commercial source of this ligand that has been used to previously characterize the human melanocortin receptors as well as for rodent in vivo physiological studies. The γ2-MSH control peptide 1 was synthesized in parallel with the other analogues prepared in this study as an internal synthetic and pharmacological control and has been used herein as the reference control in which fold changes in agonist potency are compared to.
3.3 Reference Compounds and DTrp8 Containing Peptides
A previous report performing a D-amino acid scan of the γ2-MSH peptide resulted in the discovery that incorporation of DTrp at the eight position (γ2-MSH numbering) resulted in a compound that was 300-fold selective for the human MC3R verses the MC4R [29]. This compound has been utilized in rodent studies in attempts to understand the physiological role of the MC3R [27,54], based upon the assumption that when administered at nM concentrations that the MC3R would be preferentially stimulated versus the other melanocortin receptor subtypes. As this ligand was being utilized in rodent studies and we were interested in this compound as a lead for MC3R versus MC4R selective ligand design, we synthesized this compound and pharmacologically characterized it (peptide 2, Table 1) at the mouse melanocortin receptors. We did observe 60-fold selectivity for the mMC3R versus the mMC4R, however we also observed equipotency (within the 3-fold inherent experimental error) of this ligand at the mMC3R and mMC5R. These results were unanticipated, but the equipotency trend between the mMC3R and mMC5Rs was evident throughout this study for γ2-MSH purchased from a commercial source, the peptide 1 γ2-MSH which was synthesized as an internal control for this study, as well as other potent γ2-MSH derivatives. These observations resulted in the comparison of selected analogues prepared in this study at the mouse and human MC5R isoforms, discussed below.
Cai et al. performed a study examining the pharmacology effects of α-MSH/γ-MSH peptide ligands at the human melanocortin receptors that resulted in the development of a novel pharmacophore model as well as incorporation of Nle3 into the γ2-MSH template which resulted in a full nM agonist at the hMC1 and hMC3-5Rs [4]. We also wanted to study the effect at the mouse melanocortin receptors of replacing the Met3 of γ2-MSH with the Norleucine (Nle) amino acid as that had been previously been demonstrated to increase ligand potency in Nle4, DPhe7-αMSH (NDP-MSH) [38,65]. In this study, Peptide 3, Nle3-γ2-MSH possessed 68 nM mMC3R agonist potency, and possessed between 140 and 390 nM agonist potency at the mMC1R, mMC4R, and mMC5R, but was only 4-fold selective for the mMC3R versus the mMC4R. Combination of the Nle3 and DTrp8 substitutions in γ2-MSH resulted in peptide 4 possessed 10 to 90 nM agonist potency at the mouse melanocortin receptors examined in this study but was not selective for the mMC3R versus mMC4R, within our experimental error.
3.4 Mouse melanocortin-1 receptor
The peripherally expressed MC1R [15,58] is considered the “classic” skin melanocortin receptor and is involved in human skin pigmentation [50], and animal coat coloration [52]. The endogenous melanocortin agonist γ2-MSH peptide, Tyr-Val Met-Gly-His-Phe-Arg-Trp-Asp-Arg-Phe-Gly has been previously reported to possess high nM to μM human MC1R agonist potency [4,15], and possessed ca 680 nM agonist potency at the mouse MC1R herein, demonstrating consistency between the human and mouse MC1R isoforms. Incorporation of DTrp8 (2) resulted in 6-fold increased agonist potency, incorporation of the Nle3 (3) resulted in ca equipotency, and combining the Nle3 and DTrp8 γ2-MSH substitutions resulted in 20-fold increased mMC1R agonist potency as compared to γ2-MSH 1. Thus, there appears to be a synergistic additive effect for these two residues in γ2-MSH.
Deletion of the C-terminal Arg-Phe-Gly (10-12) γ2-MSH residues, peptides 5-7, resulted in nM full agonist potency at the mMC1R essentially equipotent to γ2-MSH. Deletion of the Asp9 γ2-MSH residue resulted in 7-fold decreased mMC1R potency, comparing peptides 7 and 8. Deletion of the Trp8 γ2-MSH residue of peptide 9 resulted in ca 10-fold decreased potency as compared to peptide 8. Deletion of the Arg7 γ2-MSH (peptide 10) resulted in the inability to stimulate the mMC1R at up to 100 μM concentrations. These data support the hypothesis that the C-terminal γ2-MSH Arg7, Trp8, and Asp9 residues are important for γ2-MSH based MC1R ligand potency.
Deletion of the N-terminal Tyr1 and Val2 γ2-MSH residues (peptides 14 and 15) resulted in ca equipotent mMC1R activity as γ2-MSH. Removal of the Met3 in peptide 16, resulted in 10-fold decreased potency as compared to γ2-MSH (1), yet resulted in only 5-fold decreased potency as compared to 15. Deletion of Gly4 in peptide 17, resulted in 29-fold decreased mMC1R potency as compared to γ2-MSH but only 3-fold as compared with peptide 16. These data suggest that the γ2-MSH Met3 and Gly4 residues may be participating in intra-molecular ligand tertiary structure versus key ligand-mMC1R receptor interactions important for molecular recognition of receptor stimulatory events.
3.5 Mouse melanocortin-3 receptor
The MC3R is believed to be involved in metabolism and energy homeostasis and is expressed both centrally and peripherally [3,10,24,64]. The lead peptide γ2-MSH, Tyr-Val Met-Gly-His-Phe-Arg-Trp-Asp-Arg-Phe-Gly-OH (1), has been previously reported to possess a 5.9 and 1.0 nM agonist EC50 potency at the hMC3R [29,30] and possessed ca a 45 nM agonist EC50 at the mMC3R herein (average of the purchased and control peptide 1). Agonist activity was maintained at the mMC3R when the first five residues (Gly-Phe-Arg-Asp-Trp) were removed from the C-terminal (peptides 5-9). Peptide 5, with Gly removed resulted in a 4-fold decreased in potency. Interestingly, peptide 6, with both Gly and Phe removed from the C-terminal is an equipotent agonist (EC50 = 36 nM) compared to 1. Peptide 8 was 70-fold less potent than 1 and peptide 9 was a high μM agonist with 915-fold decreased potency at the mMC3R compared to 1. Further amino acid removal from the C-terminal (peptides 10-13) resulted in loss of stimulatory activity at up to 100 μM at the mMC3R. The first four amino acid residues (Tyr-Val-Met-Gly) can be removed from the N-terminal without loss of agonist activity but with reduced potency ranging from 6- to 68-fold (peptides 14-17). Peptides 18-22 resulted in loss of stimulatory activity at up to 100 μM. Peptide 23 containing all the residues that were shown to be important for binding and functional activity at the human melanocortin receptors [29,30], resulted in μM agonist activity and 260-fold decreased potency at the mMC3R. When both C- and N-terminal truncation was performed at the same time (peptides 24-29) there was generally a reduction in potency ranging from 9- to 560-fold. Interestingly, peptide 29 containing the His-Phe-Arg-Trp pharmacophore common to all the endogenous melanocortin agonists resulted in complete loss of agonist activity at up to 100 μM at the mMC3R.
3.6 Mouse melanocortin-4 receptor
The MC4R expressed in the brain has been identified as participating in food intake [21] and disruption of this gene leads to obesity in mice [42]. Obesity has also been observed in humans possessing single nucleotide polymorphisms in the MC4R [22,35,66,69,70]. The lead peptide γ2-MSH, Tyr-Val Met-Gly-His-Phe-Arg-Trp-Asp-Arg-Phe-Gly-OH (1), has been previously reported to possess a 260 and 55 nM agonist EC50 at the hMC4R [29,30] and possess ca a 500 nM EC50 at the mMC4R herein (average of the purchased and control peptide 1). Agonist activity was maintained at the mMC4R when the first four residues (Gly-Phe-Arg-Asp) were removed from the C-terminal (peptides 5-8). Peptide 5 (Gly removed) and 6 (Gly-Phe removed, both had equipotent (within experimental error) agonist activity compared to 1 at the mMC4R. Further amino acid removal from the C-terminal (peptides 9-13) resulted in loss of stimulatory activity up to 100 μM at the mMC4R. The first five amino acid residues (Tyr-Val-Met-Gly-His) can be removed from the N-terminal without loss of agonist activity at the mMC4R (14-18). Peptides 14 and 15 had equipotent agonist activity whereas, peptides 16-18 resulted in reduced potency ranging from 6- to 114-fold. Peptide 19-22 with further N-terminal residue removal resulted in loss of stimulatory activity at up to 100 μM. Peptide 23 containing all the residues that were shown to be important for binding and functional activity at the human melanocortin receptors [29,30], resulted in μM agonist activity and 34-fold decreased potency at the mMC4R. When both C- and N-terminal truncation was performed at the same time (peptides 24-29) there was generally reduced potency ranging from 6- to 155-fold. Interestingly, peptide 29 containing the His-Phe-Arg-Trp pharmacophore common to all the endogenous melanocortin agonists resulted in complete loss of agonist activity at up to 100 μM at the mMC4R.
3.7 Mouse melanocortin-5 receptor
The MC5R expressed in a variety of tissues and has been implicated as participating in exocrine gland function in mice [12]. The lead peptide γ2-MSH, Tyr-Val Met-Gly-His-Phe-Arg-Trp-Asp-Arg-Phe-Gly-OH (1), has been previously reported to possess a 490 and 200 nM agonist EC50 at the hMC5R [29,30] and possessed ca a 60 nM EC50 at the mMC5R herein (average of the purchased and control peptide 1). Agonist activity was maintained at the mMC5R when the first five residues (Gly-Phe-Arg-Asp-Trp) were removed from the C-terminal (peptides 5-9). Peptide 5 (Gly removed) and 6 (Gly-Phe removed), both had equipotent (within experimental error) agonist activity compared to 1 at the mMC5R. Further amino acid removal from the C-terminal (peptides 10-13) resulted in loss of stimulatory activity at up to 100 μM at the mMC5R with the exception of 11. Peptide 11, (Tyr-Val-Met-Gly-His-OH) retained full μM agonist activity at the mMC5R, albeit with 680-fold reduced potency. The first five amino acid residues (Tyr-Val-Met-Gly-His) can be removed from the N-terminal without loss of agonist activity at the mMC5R (14-18). Peptides 14 and 15 had equipotent agonist activity. Peptides 16-18 resulted in reduced potency ranging from 8- to 340-fold with 18 possessing only 90% maximal stimulation at 100 μM concentrations relative to the NDP-MSH control. Peptide 19-22 with further N-terminal residue removal resulted in loss of stimulatory activity at up to 100 μM. Peptide 23 containing all the residues which were shown to be important for binding and functional activity at the human melanocortin receptors [29,30], resulted in μM full agonist activity and 28-fold decreased potency at the mMC5R. When both C- and N-terminal truncation was performed at the same time (peptides 24-29) there was generally reduced potency ranging from 3- to 1240-fold. Interestingly, peptide 29 containing the His-Phe-Arg-Trp pharmacophore common to all the endogenous melanocortin agonists resulted in an 85% maximal NDP-MSH relative response at the mMC5R.
3.8 Human Melanocorin-5 Receptor
Ten of the γ2-MSH analogues were selected and tested at the human MC5 receptor to determine whether the observed equipotent activity of γ2-MSH at the mouse MC3 and MC5 receptors were associated with species specific receptor isoform differences (Table 1). Based on the results obtained, there appears to be a significant species difference resulting in a 68-fold decrease in potency at the hMC5R when compared at the mMC5R with peptide 1. Generally, all ten analogues resulted in decreased potency ranging from 10- to 80-fold at the hMC5R compared to the mMC5R (Table 1).
3.9 Peripheral intraperitoneal (i.p.) treatment with NDP-MSH and changes in melanocortin receptor mRNA gene expression in the brain
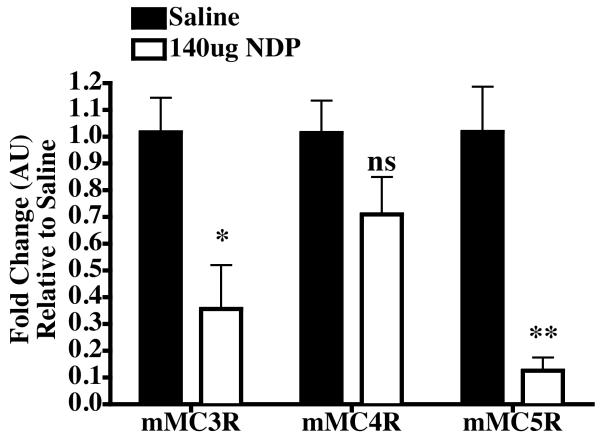
The non-selective, super potent, and enzymatically stable NDP-MSH melanocortin agonist was peripherally administered (i.p. 4.5 μmol/kg) into satiated male wild type mice. Food intake was measured during the normal nocturnal feeding cycle at 2, 4, 6, and 24 hrs post treatment and was not found to be significantly different from the saline treated control mice (data not shown). Twenty-four hours post treatment, the mice were sacrificed and the brain removed. Dissection of the hypothalamus from the brain and conversion of the mRNA into cDNA for analysis by real-time polymerase chain reaction (RT-PCR) resulted in a 2.8-fold reduction in mMC3R expression and 7.9-fold reduction of mMC5R expression levels that were statistically different (p<0.05) compared to the saline treated control group (Figure 1). No statistical significant difference was observed for the mMC4R expression between NDP-MSH and saline treated groups.
Figure 1.
Hypothalamic melanocortin receptor mRNA expression levels in mice 24hrs post peripheral (intraperitoneal i.p.) treatment with either NDP-MSH or saline control. Statically significant decreased expression is observed for the MC3 and MC5 receptors *p<0.05, **p<0.01.
4.0 DISCUSSION
The γ2-MSH melanocortin receptor agonist has been presumed for decades to be selective for the MC3R versus the MC4 and MC5 receptors. While this assumption holds true for pharmacological profiles at the human melanocortin receptor isoforms, it does not appear to be the case for the mouse receptor isoforms. In the study presented herein, while trying to use the γ2-MSH peptide template for the determination of amino acid residues and ligand structural domains that result in the MC3R selectivity it became evident that significant species specific differences and pharmacological profiles exist between the mouse and human MC5 receptor (Table 1).
4.1 Mouse MC3R versus MC4R Ligand Selectivity
Based upon the previously reported γ2-MSH receptor pharmacology at the human melanocortin receptors and the assumption that MC3R is selective versus the MC4R, we initiated the studies reported herein. Functionally, the γ2-MSH agonist has been reported to be between 44-fold [29], 55-fold [30], or equipotent [4] at the hMC3R versus the hMC4R. The DTrp8 γ2-MSH has been reported to be ca 300-fold selective for the human MC3R versus MC4R [4,29]. The Nle3 γ2-MSH ligand was reported to be 14-fold selective for the hMC4R versus the hMC3R [4]. At the mouse receptors, it appears that γ2-MSH is only ca 10-fold selective, the DTrp8 peptide 2 is 62-fold selective, the Nle3 γ2-MSH peptide 3 is 4-fold selective, and the combination Nle3 and DTrp8 peptide 4 is only 2-fold selective for the mMC3R versus mMC4R (Table 1). From the study presented herein, the most mMC3R versus mMC4R selective peptide is the γ2-MSH(1-10) compound 6 that possessed a modest 18-fold selectivity.
4.2 C-Terminal Truncation of γ2-MSH
C-terminal deletion of the Gly and Phe residues in peptides 5 and 6, resulted in ca equipotent agonist activity at the mMC1R and mMC3-5Rs, as compared to the γ2-MSH control 1. Removal of the Arg10 γ2-MSH residue (7) resulted in equipotent activity at the mMC1R, mMC4R, and mMC5R, yet resulted in 16-fold decreased mMC3R agonist potency as compared to 1. Deletion of the terminal Asp-Arg-Phe-Gly γ2-MSH residues in analogue 8 resulted in 4- to 10-fold decreased potency at the mMC1R, mMC4R, and mMC5R, and 54-fold decreased mMC3R potency, as compared to 1. The γ2-MSH 1-7 peptide 9 possessed 40-fold decreased mMC1R potency, 610-fold decreased mMC3R potency, 320-fold decreased mMC5R potency, as compared to 1, and was unable to stimulate the mMC4R at up to 100 μM concentrations. Further truncation of γ2-MSH of the fourth to sixth residues resulted in the respective ligands inability to stimulate any of the mouse melanocortin receptors examined in this study at up to 100 μM concentrations (peptides 10-13).
4.3 N-Terminal Truncation of γ2-MSH
Deletion of the N-terminal γ2-MSH Tyr and Val amino acids, analogues 14 and 15, respectively, resulted in essential equipotent activity as the control peptide 1 at the mMC1R, mMC4R, and mMC5R. However, at the mMC3R, 5- to 8-fold decreased agonist potency resulted as compared to 1 for analogues 14 and 15. Relative to γ2-MSH (1), removal of the Tyr-Val-Met residues in peptide 16, 4- to 15-fold decreased agonist potency resulted at the mMC1R and mMC3-5Rs. Peptide 17, consisting of the γ2-MSH 5-12 sequence resulted in 10- to 29-fold decreased mMC1R, mMC4R, and mMC5R potency and 52-fold decreased mMC3R potency. The γ2-MSH 6-12 sequence at 100 μM concentrations was only able to stimulate the mMC1R and mMC5R at 30% and 90% the full agonists levels observed for the NDP-MSH (100%) control respectively. This peptide 18 was unable to stimulate the mMC3R at up to 100 μM concentrations, but was able to maximally stimulate the mMC4R albeit with 82-fold decreased potency as compared to 1. Further truncation of the γ2-MSH N-terminal sequence from the 7 to 9 Arg-Trp-Asp sequence residues resulted in analogues that were unable to stimulate any of the mouse melanocortin receptors examined at up to 100 μM concentrations.
4.4 N- and C-Terminal Truncation of γ2-MSH
Based upon previous studies of γ2-MSH including an Ala and D-amino acid scan [29,30], it was concluded that the Tyr1, Met3, His-Phe-Arg-Trp (5-8 residues) as well as the Phe11 residues were important for γ2-MSH ligand binding and functional activity at the human melanocortin receptors. Thus, we wanted to evaluate this string of residues and synthesized peptide 23. This peptide possessed μM full agonist activity at the mouse melanocortin receptors, albeit with 15- to 200-fold decreased ligand potency as compared to control 1 at the respective receptor isoforms. Peptide 24, possessing the γ2-MSH 3-8 residue sequence, peptide 25, possessing the γ2-MSH 5-11 sequence, and peptide 26, possessing the 6-11 γ2-MSH sequence all resulted in full agonist μM potency at the melanocortin receptors examined in this study. The 3-11 γ2-MSH sequence (peptide 27) resulted in 100 and 365 nM agonist potency at the mMC3 and mMC5 receptors and possessed μM agonist potency at the mMC1 and mMC4 receptors. Interestingly, at the mMC5R it is essentially equipotent with the commercially purchased γ2-MSH as well as the synthesized γ2-MSH control peptide 1, within the 3-fold inherent experimental error (Table 1). The γ2-MSH Asp-Arg-Phe (9-11) residues were unable to generate any stimulatory response at the melanocortin receptors at up to 100 μM concentrations. Similarly, the core melanocortin His-Phe-Arg-Trp (γ2-MSH 5-8) peptide was unable to stimulate the mMC1R, mMC3R, and mMC4R at up to 100 μM concentrations, but was able to generate a slight stimulatory response at the mMC5R, but not enough to determine an EC50 value. Of note is the fact that the N-terminal acetylated and C-terminal amidated Ac-His-Phe-Arg-Trp-NH2 tetrapeptide was able to fully stimulate melanocortin receptors [7,36,39,56,68].
4.5 Mouse Versus Human MC5R
Based upon or observation that the commercially purchased γ2-MSH as well as the synthesized γ2-MSH (peptide 1) and the DTrp8- γ2-MSH (peptide 2) possessed essentially equipotent mMC3R and mMC5R agonist activity, we became concerned. It has been well documented since the original cloning of the melanocortin receptors that γ-MSH ligands possessed high nM to μM agonist potency at the MC1, MC4, and MC5 receptors [15,24-26,57,58,64]. Interestingly however, all these studies were performed on the human melanocortin receptors, which posed the question if there is a species specific difference between the human and mouse MC5R towards γ2-MSH related ligands. Examination of the amino acid sequence between the mouse and human isoforms are different enough, that this hypothesis might be feasible. To test this hypothesis, we selected twelve compounds to assay in parallel at the human and mouse MC5Rs. Thus, we were utilizing the exact same compounds, dilutions, assay, etc. in the same experiment and cell line, to minimize any differences attributed to inherent bioassay variation which is a frequent explanation regarding different assay results obtained in different laboratories. These data are summarized in Table 1. The agonist potency obtained for our commercially purchased γ2-MSH and control γ2-MSH peptide 1 resulted in μM agonist potency at the human MC5R, consistent with previous literature. Additionally, the DTrp8 γ2-MSH possessed ca 580 nM agonist potency at the hMC5R which is consistent with the results published for this analogue at the hMC5R [4,29]. Additionally, the other γ2-MSH peptides 3-8 also resulted in full μM agonist potency at the hMC5R, and interestingly were equipotent as compared to 1, with the exception of peptide 8 (γ2-MSH 1-8) that possessed 9-fold decreased hMC5R agonist potency. A previous report by Bednarek et al. [2] found from a SAR study of cyclic melanocortin peptides, that for the compound referred to in the literature as HS024, first characterized as a hMC5R antagonist [47], was a full hMC5R agonist in their study. They further examined the rat MC5R in CHO cells and found the HS024 to be a full agonist as well. The authors propose that the expression systems, receptor density, and cell assays may explain the differences between the reported antagonist versus agonist MC5R pharmacology [2]. This latter study found differences between functional activity at the hMC5R, however, in this study only consistent differences between potency are observed in assay run under parallel conditions herein. While confounding experimental conditions could be attributed to differences in melanocortin receptor pharmacology’s reported by different groups, the observation that for the γ2-MSH peptides reported in this study are consistent with previous reports at the human MC1, MC3, and MC4 receptors but not the MC5Rs when run under parallel assay conditions, support the hypothesis that there appears to be an important species specific difference between the human and mouse MC5Rs in terms of γ-MSH related ligand potency.
4.6 Changes in hypothalamic melanocortin receptor gene expression levels upon intraperitoneal (i.p.) treatment with NDP-MSH
The physiological relevance of peripherally administered melanocortin receptor agonists has resulted in linking the MC3R to cardiovascular effects using γ-MSH and derivatives thereof, as reviewed [23,40,41,43,59,75]. More recent studies however, have clearly associated the MC4R’s involvement in the regulation of blood pressure using human patients with dysfunctional MC4R polymorphisms as well treatment with a melanocortin receptor agonist [28]. Little is know about how peripheral treatment with a melanocortin agonist could effect expression of the melanocortin receptors in the brain. These data are relevant to the current study as the link between peripherally administered γ-MSH ligands and cardiovascular effects may be via a mechanism involving the central regulation of melanocortin receptor expression. It has been demonstrated in this study that γ2-MSH is not greater then ca 10-fold selective for the mouse MC3R, MC4R, and MC5R (Table 1), however very little is known about the in vivo pharmacokinetic profiles and stability of this peptide. For this study, the super potent NDP-MSH non-specific melanocortin receptor agonist [65] was selected as a general probe to evaluate changes in hypothalamic mRNA expression of the MC3R, MC4R, and MC5R. NDP-MSH has been used in human clinical studies and shown to be resistant to enzymatic degradation [8,9]. While it has been clearly established that the MC3R and MC4R are expressed in the brain, controversy remains about expression of the MC5R [12,16-19,26]. A concentration of 4.5 μmol/kg NDP-MSH was selected based upon previous studies using 0.2 μmol/kg i.p. administration into 24hr fasted wild type mice resulting in an acute decrease in food intake 0-2hr post treatment that was not different than control treatment at 24hrs [55]. In this study, no statistically significant changes in food intake were observed (data not shown), however, a satiated feeding experimental paradigm was used and could easily explain the differences. Significantly reduced mRNA expression of hypothalamic MC3 and MC5 receptors were observed (p<0.05) upon i.p. NDP-MSH treatment versus saline control (Figure 1). The MC4R mRNA expression was not statistically different between the saline control and NDP-MSH treatment groups. These data demonstrate that peripherally administered NDP-MSH may or may not affect mouse food intake, depending upon the use of a fasted [55] or satiated (herein) feeding paradigms, but have an affect on melanocortin receptor expression in the hypothalamus of the brain. These data demonstrate that a peripherally administered non-selective melanocortin receptor agonist can modify both MC3 and MC5 receptor mRNA expression in the hypothalamus of the brain, making the interpretation of rodent in vivo physiological studies using γ-MSH as a purported MC3R selective ligand to be interpreted with caution and taking into account the findings reported herein.
5.0 CONCLUSIONS
This study examining truncation studies of the γ2-MSH peptide have resulted in new information regarding the importance of specific amino acids and peptide domains important for γ2-MSH functional potency at the different melanocortin receptor isoforms. Another important discovery from this study is an apparent species specific differences between the mouse and human MC5R as related to the γ2-MSH ligand potency. These data could have far reaching physiological importance as a multitude of studies using the γ2-MSH ligand and derivatives thereof, as MC3R selective ligands based upon the human melanocortin receptor pharmacology. However, as peripheral expression of the MC5R overlaps extensively with the MC3R, and γ2-MSH possess essentially equipotent agonist activity at the mouse MC3R and MC5R, some of the observed physiological results may be a function of the MC5R and not just the MC3R as previously assumed. Albeit additional studies using selective ligands would need to be performed to clarify one way or the other. Peripheral administration of the NDP-MSH non-selective agonist has also been shown to decrease MC3R and MC5R mRNA (but not MC4R) expression levels in the hypothalamus of the brain, implicating associated CNS mediated physiological responses associated to the MC3R and MC5R that could result by the use of non-selective ligands in rodent models.
Research Highlights.
Identification of γ2-MSH peptide amino acids important for melanocortin receptor potency
The γ2-MSH ligands have different mouse versus human species specific potencies
Intraperitoneal (i.p.) injection of NDP-MSH into mice reduces hypothalamic MC3R and MC5R mRNA gene expression levels.
6.0 ACKNOWLEDGEMENTS
This work has been supported by NIH Grant R01DK57080 (CHL) and a NIH T32HL083810 training grant (HY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7.0 REFERENCES
- [1].Atalayer D, Robertson KL, Haskell-Luevano C, Andreasen A, Rowland NE. Food Demand and Meal Size in Mice with Single or Combined Disruption of Melanocortin Type 3 and 4 Receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298:R1667–1674. doi: 10.1152/ajpregu.00562.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bednarek MA, MacNeil T, Kalyani RN, Tang R, Van Der Ploeg LH, Weinberg DH. Selective, High Affinity Peptide Antagonists of α-Melanotropin Action at Human Melanocortin Receptor 4: Their Synthesis and Biological Evaluation in Vitro. J. Med. Chem. 2001;44:3665–3672. doi: 10.1021/jm010165y. [DOI] [PubMed] [Google Scholar]
- [3].Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD. A Unique Metabolic Syndrome Causes Obesity in the Melanocortin-3 Receptor-deficient Mouse. Endocrinology. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- [4].Cai M, Mayorov AV, Cabello C, Stankova M, Trivedi D, Hruby VJ. Novel 3D pharmacophore of alpha-MSH/gamma-MSH hybrids leads to selective human MC1R and MC3R analogues. J. Med. Chem. 2005;48:1839–1848. doi: 10.1021/jm049579s. [DOI] [PubMed] [Google Scholar]
- [5].Carpino LA, Han GY. The 9-Fluorenylmethoxycarbonyl Function, a New Base-Sensitive Amino-Protecting Group. J. Am. Chem. Soc. 1970;92:5748–5749. [Google Scholar]
- [6].Carpino LA, Han GY. The 9-Fluorenylmethyloxycarbonyl Amino-Protecting Group. J. Org. Chem. 1972;37:3404–3409. [Google Scholar]
- [7].Castrucci AML, Hadley ME, Sawyer TK, Wilkes BC, Al-Obeidi F, Staples DJ, DeVaux AE, Dym O, Hintz MF, Riehm J, Rao KR, Hruby VJ. α-Melanotropin: The Minimal Active Sequence in the Lizard Skin Bioassay. Gen. Comp. Endocrinol. 1989;73:157–163. doi: 10.1016/0016-6480(89)90066-x. [DOI] [PubMed] [Google Scholar]
- [8].Castrucci AML, Hadley ME, Yorulmazoglu EI, Wilkes BC, Sawyer TK, Hruby VJ. Synthesis and Studies of Melanotropins Resistant to Enzyme Degradation. Yale J. of Biology and Medicine. 1984;57:345–346. [Google Scholar]
- [9].Castrucci AML, Hadley ME, Yorulmazoglu EI, Wilkes BC, Sawyer TK, Hruby VJ. Synthesis and Studies of Superpotent Melanotropins Resistant to Enzyme Degradation. In: Bagnara J, Klaus SN, Paul E, Schartl M, editors. Pigment Cell 1985, Biological, Molecular and Clinical Aspects of Pigmentation. University of Tokyo Press; 1985. pp. 145–151. [Google Scholar]
- [10].Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, Metzger JM, Strack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, MacIntyre DE, Chen HY, Van Der Ploeg LH. Inactivation of the Mouse Melanocortin-3 Receptor Results in Increased Fat Mass and Reduced Lean Body Mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- [11].Chen CA, Okayama H. Calcium Phosphate-Medicated Gener Transfer: A Highly Efficient Transfections System for Stably Transforming Cells with Plasmid DNA. Biotechniques. 1988;6:632–638. [PubMed] [Google Scholar]
- [12].Chen W, Kelly MA, Opitz-Araya X, Thomas RE, Low MJ, Cone RD. Exocrine Gland Dysfunction In MC5-R Deficient Mice: Evidence For Coordinated Regulation Of Exocrine Gland Functions By Melanocortin Peptides. Cell. 1997;91:789–798. doi: 10.1016/s0092-8674(00)80467-5. [DOI] [PubMed] [Google Scholar]
- [13].Chen W, Shields TS, Stork PJS, Cone RD. A Colorimetric Assay for Measuring Activation of Gs- and Gq-Coupled Signaling Pathways. Anal. Biochem. 1995;226:349–354. doi: 10.1006/abio.1995.1235. [DOI] [PubMed] [Google Scholar]
- [14].Chhajlani V, Muceniece R, Wikberg JES. Molecular Cloning of a Novel Human Melanocortin Receptor. Biochem. Biophys. Res. Commun. 1993;195:866–873. doi: 10.1006/bbrc.1993.2125. [DOI] [PubMed] [Google Scholar]
- [15].Chhajlani V, Wikberg JES. Molecular Cloning and Expression of the Human Melanocyte Stimulating Hormone Receptor cDNA. FEBS Lett. 1992;309:417–420. doi: 10.1016/0014-5793(92)80820-7. [DOI] [PubMed] [Google Scholar]
- [16].Chowdhary BP, Gustavsson I, Wikberg JES, Chhajlani V. Localizaton of the Human Melanocortin-5 Recpetor Gene (MC5R) to Chromosome Band 18p11.2 by Fluorescence in Situ Hybridizaiton. Cytogenet Cell Genet. 1995;68:79–81. doi: 10.1159/000133895. [DOI] [PubMed] [Google Scholar]
- [17].Author . In: The Melanocortin Receptors. Cone RD, editor. The Humana Press Inc.; New Jersey: 2000. [Google Scholar]
- [18].Author . In: The Melanocortin System. Cone RD, editor. Vol. 994. Annals of the New York Academy of Sciences; New York: 2003. [Google Scholar]
- [19].Cone RD, Lu D, Kopula S, Vage DI, Klungland H, Boston B, Chen W, Orth DN, Pouton C, Kesterson RA. The Melanocortin Receptors: Agonists, Antagonists, and the Hormonal Control of Pigmentation. Recent Progress in Hormone Research. 1996;51:287–318. [PubMed] [Google Scholar]
- [20].Eipper BA, Mains RE. Structure and Biosynthesis of Pro-ACTH/Endorphin and Related Peptides. Endocrin. Rev. 1980;1:1–26. doi: 10.1210/edrv-1-1-1. [DOI] [PubMed] [Google Scholar]
- [21].Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of Melanocortinergic Neurons in Feeding and the Agouti Obesity Syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- [22].Farooqi IS, Yeo GS, Keogh JM, Aminian S, Jebb SA, Butler G, Cheetham T, O’Rahilly S. Dominant and Recessive Inheritance of Morbid Obesity Associated with Melanocortin 4 Receptor Deficiency. J. Clin. Invest. 2000;106:271–279. doi: 10.1172/JCI9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gantz I, Fong TM. The Melanocortin System. Am. J. Physiol. Endocrinol. Metab. 2003;284:E468–E474. doi: 10.1152/ajpendo.00434.2002. [DOI] [PubMed] [Google Scholar]
- [24].Gantz I, Konda Y, Tashiro T, Shimoto Y, Miwa H, Munzert G, Watson SJ, DelValle J, Yamada T. Molecular Cloning of a Novel Melanocortin Receptor. J. Biol. Chem. 1993;268:8246–8250. [PubMed] [Google Scholar]
- [25].Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, DelValle J, Yamada T. Molecular Cloning, Expression, and Gene Localization of a Fourth Melanocortin Receptor. J. Biol. Chem. 1993;268:15174–15179. [PubMed] [Google Scholar]
- [26].Gantz I, Shimoto Y, Konda Y, Miwa H, Dickinson CJ, Yamada T. Molecular Cloning, Expression, and Characterization of a Fifth Melanocortin Receptor. Biochem. Biophys. Res. Commun. 1994;200:1214–1220. doi: 10.1006/bbrc.1994.1580. [DOI] [PubMed] [Google Scholar]
- [27].Getting SJ, Lam CW, Leoni G, Gavins FN, Grieco P, Perretti M. D-Trp8-Gamma-Melanocyte-Stimulating Hormone Exhibits Anti-Inflammatory Efficacy in Mice Bearing a Nonfunctional MC1R (Recessive Yellow e/e Mouse) Mol. Pharmacol. 2006;70:1850–1855. doi: 10.1124/mol.106.028878. [DOI] [PubMed] [Google Scholar]
- [28].Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, Lomas DJ, O’Rahilly S, Farooqi IS. Modulation of Blood Pressure by Central Melanocortinergic Pathways. N. Engl. J Med. 2009;360:44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- [29].Grieco P, Balse PM, Weinberg D, MacNeil T, Hruby VJ. D-Amino Acid Scan of γ-Melanocyte-Stimulating Hormone: Importance of Trp(8) on Human MC3 Receptor Selectivity. J. Med. Chem. 2000;43:4998–5002. doi: 10.1021/jm000211e. [DOI] [PubMed] [Google Scholar]
- [30].Grieco P, Balse-Srinivasan P, Han G, Hruby VJ, Weinberg D, MacNeil T, Van Der Ploeg LH. Synthesis and Biological Evaluation on hMC3, hMC4 and hMC5 Receptors of γ-MSH Analogs Substituted with L-Alanine. J. Pept. Res. 2002;59:203–210. doi: 10.1034/j.1399-3011.2002.01966.x. [DOI] [PubMed] [Google Scholar]
- [31].Haskell-Luevano C, Cone RD, Monck EK, Wan Y-P. Structure Activity Studies of the Melanocortin-4 Receptor by In Vitro Mutagenesis: Identification of Agouti-Related Protein (AGRP), Melanocortin Agonist and Synthetic Peptide Antagonist Interaction Determinants. Biochemistry. 2001;40:6164–6179. doi: 10.1021/bi010025q. [DOI] [PubMed] [Google Scholar]
- [32].Haskell-Luevano C, Hendrata S, North C, Sawyer TK, Hadley ME, Hruby VJ, Dickinson C, Gantz I. Discovery of Prototype Peptidomimetic Agonists at the Human Melanocortin Receptors MC1R and MC4R. J. Med. Chem. 1997;40:2133–2139. doi: 10.1021/jm960840h. [DOI] [PubMed] [Google Scholar]
- [33].Haskell-Luevano C, Nikiforovich GV, Sharma SD, Yang Y-K, Dickinson C, Hruby VJ, Gantz I. Biological and Conformational Evaluation of Stereochemical Modifications Using the Template Melanotropin Peptide, Ac-Nle-c[Asp-His-Phe-Arg-Trp-Ala-Lys]-NH2, on Human Melanocortin Receptors. J. Med. Chem. 1997;40:1738–1748. doi: 10.1021/jm960845e. [DOI] [PubMed] [Google Scholar]
- [34].Haskell-Luevano C, Schaub JW, Andreasen A, Haskell KR, Moore MC, Koerper LM, Rouzaud F, Baker HV, Millard WJ, Walter G, Litherland SA, Xiang Z. Voluntary Exercise Prevents the Obese and Diabetic Metabolic Syndrome of the Melanocortin-4 Receptor Knockout Mouse. FASEB J. 2009;23:642–655. doi: 10.1096/fj.08-109686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hinney A, Schmidt A, Nottebom K, Heibult O, Becker I, Ziegler A, Gerber G, Sina M, Gorg T, Mayer H, Siegfried W, Fichter M, Remschmidt H, Hebebrand J. Several Mutations in the Melanocortin-4 Receptor Gene Including a Nonsense and a Frameshift Mutation Associated with Dominantly Inherited Obesity in Humans. J. Clin. Endocrinol. Metab. 1999;84:1483–1486. doi: 10.1210/jcem.84.4.5728. [DOI] [PubMed] [Google Scholar]
- [36].Holder JR, Bauzo RM, Xiang Z, Haskell-Luevano C. Structure-Activity Relationships of the Melanocortin Tetrapeptide Ac-His-DPhe-Arg-Trp-NH2 at the Mouse Melanocortin Receptors: Part 2 Modifications at the Phe Position. J. Med. Chem. 2002;45:3073–3081. doi: 10.1021/jm010524p. [DOI] [PubMed] [Google Scholar]
- [37].Hruby VJ, Lu D, Sharma SD, Castrucci AML, Kesterson RA, Al-Obeidi FA, Hadley ME, Cone RD. Cyclic Lactam α-Melanotropin Analogues of Ac-Nle4-c[Asp5, DPhe7, Lys10]-α-MSH(4-10)-NH2 With Bulky Aromatic Amino Acids at Position 7 Show High Antagonist Potency and Selectivity at Specific Melanocortin Receptors. J. Med. Chem. 1995;38:3454–3461. doi: 10.1021/jm00018a005. [DOI] [PubMed] [Google Scholar]
- [38].Hruby VJ, Wilkes BC, Cody WL, Sawyer TK, Hadley ME. Melanotropins: Structural, Conformational and Biological Considerations in the Development of Superpotent and Superprolonged Analogs. Peptide Protein Rev. 1984;3:1–64. [Google Scholar]
- [39].Hruby VJ, Wilkes BC, Hadley ME, Al-Obeidi F, Sawyer TK, Staples DJ, DeVaux A, Dym O, Castrucci AM, Hintz MF, Riehm JP, Rao KR. α-Melanotropin: The Minimal Active Sequence in the Frog Skin Bioassay. J. Med. Chem. 1987;30:2126–2130. doi: 10.1021/jm00394a033. [DOI] [PubMed] [Google Scholar]
- [40].Humphreys MH. γ-MSH, Sodium Metabolism, and Salt-Sensitive Hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R417–430. doi: 10.1152/ajpregu.00365.2003. [DOI] [PubMed] [Google Scholar]
- [41].Humphreys MH. Cardiovascular and renal actions of melanocyte-stimulating hormone peptides. Curr Opin Nephrol Hypertens. 2007;16:32–38. doi: 10.1097/MNH.0b013e3280117fb5. [DOI] [PubMed] [Google Scholar]
- [42].Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Smith FJ, Kesterson RA, Boston BA, Fang Q, Berkemeir LR, Gu W, Cone RD, Campfield LA, Lee F. Targeted Disruption of the Melanocortin-4 Receptor Results in Obesity in Mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- [43].Irani BG, Holder JR, Todorovic A, Wilczynski AM, Joseph CG, Wilson KR, Haskell-Luevano C. Progress in the Development of Melanocortin Receptor Selective Ligands. Curr. Pharm. Des. 2004;10:3443–3479. doi: 10.2174/1381612043382891. [DOI] [PubMed] [Google Scholar]
- [44].Irani BG, Xiang Z, Moore MC, Mandel RJ, Haskell-Luevano C. Voluntary Exercise Delays Monogenetic Obesity and Overcomes Reproductive Dysfunction of the Melanocortin-4 Receptor Knockout Mouse. Biochem. Biophys. Res. Commun. 2005;326:638–644. doi: 10.1016/j.bbrc.2004.11.084. [DOI] [PubMed] [Google Scholar]
- [45].Joseph CG, Bauzo RM, Xiang Z, Shaw AM, Millard WJ, Haskell-Luevano C. Elongation Studies of the Human Agouti-Related Protein (AGRP) Core Decapeptide (Yc[CRFFNAFC]Y) Results in Antagonism at the Mouse Melanocortin-3 Receptor. Peptides. 2003;27:263–270. doi: 10.1016/s0196-9781(03)00030-5. [DOI] [PubMed] [Google Scholar]
- [46].Kaiser E, Colescott RL, Bossinger CD, Cook PI. Color Test for Detection of Free Terminal Amino Groups in the Solid-Phase Synthesis of Peptides. Anal. Biochem. 1970;34:595–598. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- [47].Kask A, Mutulis F, Muceniece R, Pahkla R, Mutule I, Wikberg JE, Rago L, Schioth HB. Discovery of a Novel Superpotent and Selective Melanocortin-4 Receptor Antagonist (HS024): Evaluation in vitro and in vivo. Endocrinology. 1998;139:5006–5014. doi: 10.1210/endo.139.12.6352. [DOI] [PubMed] [Google Scholar]
- [48].Kiefer LL, Ittoop OR, Bunce K, Truesdale AT, Willard DH, Nichols JS, Blanchard SG, Mountjoy K, Chen WJ, Wilkison WO. Mutations In The Carboxyl Terminus Of The Agouti Protein Decrease Agouti Inhibition Of Ligand Binding To The Melanocortin Receptors. Biochemistry. 1997;36:2084–2090. doi: 10.1021/bi962647v. [DOI] [PubMed] [Google Scholar]
- [49].Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of Melanocortin 4 Receptor mRNA in the Central Nervous System of the Rat. J. Comp. Neurol. 2003;457:213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- [50].Lerner AB, McGuire JS. Effect of Alpha- and Beta-Melanocyte Stimulating Hormones on the Skin Colour of Man. Nature. 1961;189:176–179. doi: 10.1038/189176a0. [DOI] [PubMed] [Google Scholar]
- [51].Li S-J, Varga K, Archer P, Hruby VJ, Sharma SD, Kesterson RA, Cone RD, Kunos G. Melanocrotin Antagonists Define Two Distinct Pathways of Cardiovascular Control by α- and γ-Melanocyte-Stimulating Hormones. J. Neuroscience. 1996;16:5182–5188. doi: 10.1523/JNEUROSCI.16-16-05182.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lu D, Väge DI, Cone RD. A Ligand-Mimetic Model for Constitutive Activation of the Melanocortin-1 Receptor. Mol. Endo. 1998;12:592–604. doi: 10.1210/mend.12.4.0091. [DOI] [PubMed] [Google Scholar]
- [53].Lu D, Willard D, Patel IR, Kadwell S, Overton L, Kost T, Luther M, Chen W, Yowchik RP, Wilkison WO, Cone RD. Agouti Protein is an Antagonist of the Melanocyte-Stimulating-Hormone Receptor. Nature. 1994;371:799–802. doi: 10.1038/371799a0. [DOI] [PubMed] [Google Scholar]
- [54].Marks DL, Hruby V, Brookhart G, Cone RD. The Regulation of Food Intake by Selective Stimulation of the Type 3 Melanocortin Receptor (MC3R) Peptides. 2006;27:259–264. doi: 10.1016/j.peptides.2005.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Martin NM, Small CJ, Sajedi A, Patterson M, Ghatei MA, Bloom SR. Pre-Obese and Obese Agouti Mice are Sensitive to the Anorectic Effects of Peptide YY(3-36) but Resistant to Ghrelin. Int. J. Obes. Relat. Metab. Disord. 2004;28:886–893. doi: 10.1038/sj.ijo.0802646. [DOI] [PubMed] [Google Scholar]
- [56].Medzihradszky K. The Bio-Organic Chemistry of α-Melanotropin. Medicinal Res. Rev. 1982;2:247–270. doi: 10.1002/med.2610020303. [DOI] [PubMed] [Google Scholar]
- [57].Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the Melanocortin-4 Receptor (MC4-R) in Neuroendocrine and Autonomic Control Circuits in the Brain. Mol. Endo. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- [58].Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The Cloning of a Family of Genes that Encode the Melanocortin Receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- [59].Ni XP, Butler AA, Cone RD, Humphreys MH. Central Receptors Mediating the Cardiovascular Actions of Melanocyte Stimulating Hormones. J. Hypertens. 2006;24:2239–22346. doi: 10.1097/01.hjh.0000249702.49854.fa. [DOI] [PubMed] [Google Scholar]
- [60].Nijsen MJ, de Ruiter GJ, Kasbergen CM, Hoogerhout P, de Wildt DJ. Relevance of the C-terminal Arg-Phe Sequence in Gamma(2)-Melanocyte-Stimulating Hormone (Gamma(2)-MSH) for Inducing Cardiovascular Effects in Conscious Rats. Br. J. Pharmacol. 2000;131:1468–1474. doi: 10.1038/sj.bjp.0703709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ollmann MM, Wilson BD, Yang Y-K, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of Central Melanocortin Receptors in Vitro and in Vivo by Agouti-Related Protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- [62].Pritchard LE, Turnbull AV, White A. Pro-opiomelanocortin Processing in the Hypothalamus: Impact on Melanocortin Signalling and Pbesity. J Endocrinol. 2002;172:411–421. doi: 10.1677/joe.0.1720411. [DOI] [PubMed] [Google Scholar]
- [63].Pritchard LE, White A. Neuropeptide Processing and its Impact on Melanocortin Pathways. Endocrinology. 2007;148:4201–4207. doi: 10.1210/en.2006-1686. [DOI] [PubMed] [Google Scholar]
- [64].Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, Entwistle ML, Simerly RB, Cone RD. Identification of a Receptor for γ Melanotropin and Other Proopiomelanocortin Peptides in the Hypothalamus and Limbic System. Proc. Natl. Acad. Sci. USA. 1993;90:8856–8860. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Sawyer TK, Sanfillippo PJ, Hruby VJ, Engel MH, Heward CB, Burnett JB, Hadley ME. 4-Norleucine, 7-D-Phenylalanine-α-Melanocyte-Stimulating Hormone: A Highly Potent α-Melanotropin with Ultra Long Biological Activity. Proc. Natl. Acad. Sci. USA. 1980;77:5754–5758. doi: 10.1073/pnas.77.10.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sina M, Hinney A, Ziegler A, Neupert T, Mayer H, Siegfried W, Blum WF, Remschmidt H, Hebebrand J. Phenotypes in Three Pedigrees with Autosomal Dominant Obesity Caused by Haploinsufficiency Mutations in the Melanocortin-4 Receptor Gene. Am. J. Hum. Genet. 1999;65:1501–1507. doi: 10.1086/302660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Smith AI, Funder JW. Proopiomelanocortin Processing in the Pituitary, Central Nervous System and Peripheral Tissues. Endocrin. Rev. 1988;9:159–179. doi: 10.1210/edrv-9-1-159. [DOI] [PubMed] [Google Scholar]
- [68].Todorovic A, Joseph CG, Sorensen NB, Wood MS, Haskell-Luevano C. Structure-Activity Relationships of Melanocortin Agonists Containing the Benzimidazole Scaffold. Chem Biol Drug Des. 2007;69:338–349. doi: 10.1111/j.1747-0285.2007.00511.x. [DOI] [PubMed] [Google Scholar]
- [69].Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B, Froguel P. Melanocortin-4 Receptor Mutations are a Frequent and Heterogeneous Cause of Morbid Obesity. J. Clin. Invest. 2000;106:253–262. doi: 10.1172/JCI9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Vaisse C, Clement K, Guy-Grand B, Froguel P. A Frameshift Mutation In Human MC4R Is Associated With A Dominant Form of Obesity. Nat. Genet. 1998;20:113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- [71].Van Bergen P, Janssen PM, Hoogerhout P, De Wildt DJ, Versteeg DH. Cardiovascular Effects of Gamma-MSH/ACTH-like Peptides: Structure-Activity Relationship. Eur. J. Pharmacol. 1995;294:795–803. doi: 10.1016/0014-2999(95)00657-5. [DOI] [PubMed] [Google Scholar]
- [72].Van Bergen P, Kleijne JA, De Wildt DJ, Versteeg DH. Different Cardiovascular Profiles of Three Melanocortins in Conscious Rats; Evidence for Antagonism between Gamma 2-MSH and ACTH-(1-24) Br. J. Pharmacol. 1997;120:1561–1567. doi: 10.1038/sj.bjp.0701065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Van Bergen P, Van Der Vaart JG, Kasbergen CM, Versteeg DH, De Wildt DJ. Structure-Activity Analysis for the Effects of Gamma-MSH/ACTH-like Peptides on Cerebral Hemodynamics in Rats. Eur. J. Pharmacol. 1996;318:357–368. doi: 10.1016/s0014-2999(96)00806-0. [DOI] [PubMed] [Google Scholar]
- [74].Van Der Ploeg LH, Martin WJ, Howard AD, Nargund RP, Austin CP, Guan X, Drisko J, Cashen D, Sebhat I, Patchett AA, Figueroa DJ, DiLella AG, Connolly BM, Weinberg DH, Tan CP, Palyha OC, Pong SS, MacNeil T, Rosenblum C, Vongs A, Tang R, Yu H, Sailer AW, Fong TM, Huang C, Tota MR, Chang RS, Stearns R, Tamvakopoulos C, Christ G, Drazen DL, Spar BD, Nelson RJ, MacIntyre DE. A Role for the Melanocortin 4 Receptor in Sexual Function. Proc. Natl. Acad. Sci. U S A. 2002;99:11381–11386. doi: 10.1073/pnas.172378699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Versteeg DHG, Van Bergen P, Adan RAH, De Wildt DJ. Melanocortins and Cardiovascular Regulation. Eur. J. Pharmacol. 1998;360:1–14. doi: 10.1016/s0014-2999(98)00615-3. [DOI] [PubMed] [Google Scholar]
- [76].Wessells H, Fuciarelli K, Hansen J, Hadley ME, Hruby VJ, Dorr R, Levine N. Synthetic Melanotropic Peptide Initiates Erections in Men with Psychogenic Erectile Dysfunction: Double-blind, Placebo Controlled Crossover Study. J. Urol. 1998;160:389–393. [PubMed] [Google Scholar]
- [77].Yang Y-K, Ollmann MM, Wilson BD, Dickinson C, Yamada T, Barsh GS, Gantz I. Effects of Recombinant Agouti Signaling Protein on Melanocortin Action. Mol. Endo. 1997;11:274–280. doi: 10.1210/mend.11.3.9898. [DOI] [PubMed] [Google Scholar]