Abstract
The human visual system is extremely sensitive to biological signals around us. In the current study, we demonstrate that biological motion walking direction can induce robust reflexive attentional orienting. Following a brief presentation of a central point-light walker walking towards either the left or right direction, observers’ performance was significantly better on a target in the walking direction compared with that in the opposite direction even when participants were explicitly told that walking direction was not predictive of target location. Interestingly, the effect disappeared when the walker was shown upside-down. Moreover, the reflexive attentional orienting could be extended to motions of other biological entities but not inanimate objects, and was not due to the viewpoint effect of the point-light figure. Our findings provide strong evidence that biological motion cues can trigger reflexive attentional orienting, and highlight the intrinsic sensitivity of the human visual attention system to biological signals.
Keywords: biological motion, attention, reflexive orienting, walking direction
INTRODUCTION
Humans are social creatures. Biological signals (e.g., human faces) are arguably the most important sources of social information in human interactions, and the human visual system is extremely sensitive and highly adaptive to the social cues in the environment. Among them, biological motion represents a special type of biological signal that is of prime importance for species’ survival (e.g., predator and prey). Indeed, numerous studies have demonstrated that observers are remarkably adept at recognizing the characteristics of biological motion signals in complex visual scenes, even when they are portrayed by just a handful of point-lights attached to the head and major joints (Johansson, 1973). Observers can readily recognize the action (Dittrich, 1993; Norman, Payton, Long, & Hawkes, 2004), gender (Kozlowski & Cutting, 1977; Mather & Murdoch, 1994; Troje, 2002), and identity information (Cutting & Kozlowski, 1977; Fani, Prasad, Harber, & Shiffrar, 2005; Troje, Westhoff, & Lavrov, 2005) from point-light biological motion.
Walking direction is another important attribute of biological motion, which provides critical information about another living creature’s disposition and intention. Previous studies have found that the walking direction can be successfully discriminated even when the point-light displays are embedded in dynamic visual noise (Bertenthal & Pinto, 1994; Neri, Morrone, & Burr, 1998; Thurman & Grossman, 2008). Walking direction information can be fully extracted in the peripheral vision (Thompson, Hansen, Hess, & Troje, 2007) and be processed incidentally (Thornton & Vuong, 2004). Developmental studies have also shown that young infants (6-month-old) are able to discriminate the walking direction of an upright point-light walker (Kuhlmeier, Troje, & Lee, 2010). Newly hatched chicks, lacking of any visual experience, tend to align their bodies in the apparent direction of the point-light animations (Vallortigara & Regolin, 2006). These findings suggest that there might be an intrinsic sensitivity to biological motion walking direction in the primitive visual system (Simion, Regolin, & Bulf, 2008). However, what are the functional properties and brain mechanisms underlying the processing of biological motion walking direction? Intuitively, it should serve as a type of detection or alert system to help us quickly assess others’ intentions. Functionally, for such a system to be evolutionarily important, it should have real consequences. One possibility, we hypothesize, is that the walking direction of biological motion can be processed to the level sufficient to direct our spatial attention in order to help the observer to better understand others’ aims.
Recently, researchers have discovered an interesting attentional orienting effect which seems to be specific to eye gaze and head direction. Observers’ visuospatial attention can be automatically oriented to the direction signaled by eye gaze or head direction, even though they have been explicitly told that the gaze or head direction is not predictive of target location or is even counterpredictive (Driver et al., 1999; Friesen & Kingstone, 1998; Langton & Bruce, 1999). This type of reflexive attentional orienting, usually evoked by central social cues (but see Tipples, 2002), is quite different from endogenous or exogenous attention (Posner, 1980), as endogenous attention is not a reflexive response (Jonides, 1981) and exogenous attention is caused by the abrupt onset of the cue in a peripheral target location (see Frischen, Bayliss, & Tipper, 2007 for a review). Although the effect is well documented with eye gaze and head direction (Langton, Watt, & Bruce, 2000), it remains debated whether there is indeed a specialized attentional mechanism tuned to biological signals (Frischen & Tipper, 2004; Ristic, Friesen, & Kingstone, 2002; Ristic & Kingstone, 2005; Tipples, 2002).
To address these issues, the point-light walker stimuli were adopted in a central cueing paradigm. We found that the walking direction of an upright point-light walker induced a reflexive shift of observers’ spatial attention even when participants explicitly knew that walking direction was not predictive of target location, and this attentional effect disappeared when the walker was shown upside-down (Exp. 1). To further investigate whether the observed effect is essentially triggered by biological (motion) signals, we designed additional experiments in which the walking direction of a non-human animal (Exp. 2), the viewpoint information of a static figure (Exp. 3), and the motion direction of an inanimate common object (Exp. 4) were adopted as potential attentional cues.
METHODS
Participants
Thirty-two graduate students from the Chinese Academy of Sciences (who aged between 24 to 32 years; 16 female) participated in the current study with twelve in each of the experiments (Exp. 1 – 4). Eight participants completed the first three experiments. Observers had normal or corrected-to-normal vision and gave written, informed consent in accordance with procedures and protocols approved by the institutional review board of the Institute of Psychology, Chinese Academy of Sciences. They were all naïve to the purpose of the experiments.
Stimuli and Procedure
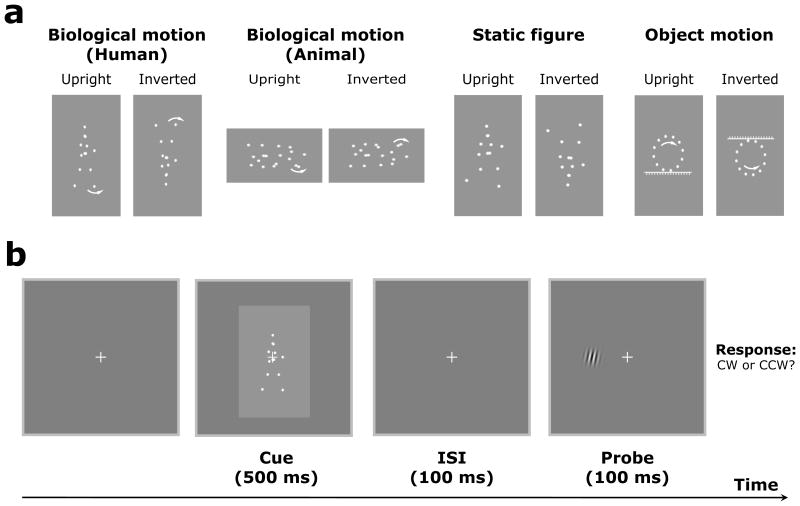
Stimuli were generated and displayed using MATLAB (Mathworks, Inc.) together with the Psychophysics Toolbox extensions (Brainard, 1997; Pelli, 1997). Point-light human and cat biological motion sequences were adopted from Vanrie & Verfaillie (2004) and Troje & Westhoff (2006), respectively, and the head and joint positions in each frame were encoded as motion vectors with initial starting positions. Each gait cycle was 1 sec and contained 60 frames. In Exp. 1, the upright and inverted point-light walkers were used as central cues, and the walking direction of the walkers could be either towards the left or right of fixation. The initial frame of the point-light display was randomized for each trial to avoid observers’ prediction. In Exp. 2, everything was exactly the same as in Exp. 1 except that the point-light walker was changed to the point-light cat. Similarly, the walking direction of the point-light cat could be either towards the left or right of fixation, and the initial frame of the point-light display was also randomized for each trial. In Exp. 3, static biological motion frames with the most extended points of a gait cycle (i.e., the most explicit facing direction) were captured from the real point-light walker stimuli (Exp. 1) and displayed as the cues. The static point-light human figures could be either facing left or right. Inverted counterparts were created by mirror flipping all of the stimuli vertically. In Exp. 4, a rotating point-light circle (rotating clockwise or counter-clockwise) with a flat floor or ceiling was created so that it looks like that the circle is moving towards the left or right on the floor or the ceiling, similar to the percept of the point-light walker.
Stimuli were presented in white on a gray background, and the viewing distance was about 50 cm. Each trial began with fixation on a central cross (0.8°×0.8°) within a frame (24. 5°×24. 5°) that extended beyond the outer border of the stimuli. Observers fixated at the central cross and viewed the human biological motion sequences (Exp. 1), cat biological motion sequences (Exp. 2), static biological motion frames (Exp. 3), and rotating motion sequences (Exp. 4) that were all presented at the center of the screen. Each point-light walker sequence (or static point-light walker frame) subtended approximately 4.0°×6.8° in visual angle and each point-light cat sequence was about 5.8°×3.4° in visual angle. Each stimulus was displayed for 500 ms. After the stimulus (cue) presentation, there was a 100 ms inter-stimulus interval (ISI) in which only the fixation was displayed, followed by a small Gabor patch (2.5°×2.5°, 4.8 cpd) that was presented briefly (100 ms) as a probe on the left or right side of the fixation, and the horizontal distance between the center of the Gabor patch and the fixation was 5.0° (see Figure 1 for a schematic experimental procedure). The Gabor patch was slightly tilted clockwise or counterclockwise, and observers were required to press one of two buttons to indicate their perceived orientation of the Gabor patch regardless of the side of presentation. In the beginning of each experiment, observers were explicitly told that none of the human (Exp. 1) and animal (Exp. 2) walking direction, the human figure facing direction (Exp. 3), or the rotating direction (Exp. 4) would predict target location, and they were asked to fixate at the central cross throughout the experiment. The upright and inverted conditions were run in separate blocks. There were a total of 80 trials for each subject and for each experiment, with 40 trials for the upright and inverted conditions, respectively. The order of the test conditions (e.g., upright vs. inverted) was counter-balanced across subjects.
Figure 1.
Static frames of sample stimuli used in the current study and schematic representation of the experimental paradigm. (a) Point-light walkers, point-light cats, static frames of point-light walkers, and point-light circles were used in the current study, including both upright and inverted versions. The arrows here indicate the motion direction and were not presented in the actual experiments. (b) After a point-light motion (or static frame) stimulus was presented for 500 ms in each trial, there was a 100 ms inter-stimulus interval (ISI) in which only the fixation was displayed, followed by a small Gabor patch that was presented briefly (100 ms) as a probe on the left or right side of the fixation. The Gabor patch was slightly tilted clockwise or counter-clockwise, and observers were required to press one of two buttons to indicate their perceived orientation of the Gabor patch regardless of the side of presentation. In the beginning of each experiment, observers were explicitly told that the cue (e.g., walking direction) was not predictive of target location.
RESULTS
Experiment 1: Reflexive attentional orienting triggered by biological motion walking direction
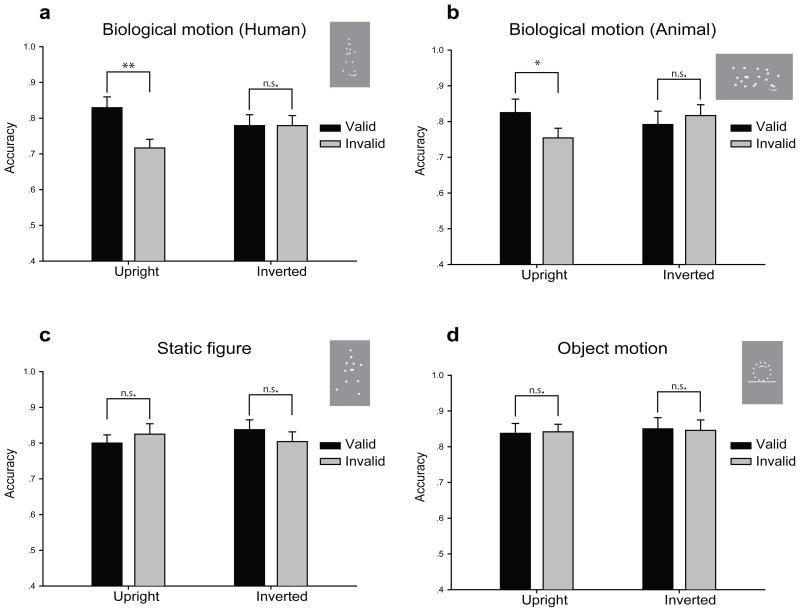
When point-light biological motion sequences were presented as potential central cues, we found a significant interaction between biological motion walking direction (congruent vs. incongruent) and biological motion orientation (upright vs. inverted) conditions (F1, 11 = 8.12, p < 0.02; Figure 2a). Specifically, observers’ performance on a subsequent Gabor probe orientation discrimination task was significantly better when the probe was presented in the walking direction of the upright point-light walker (congruent condition) compared with when the probe was presented in the opposite direction (incongruent condition) regardless of whether the probe was on the left or right side of the fixation (82.9% vs. 71.2%, t11 = 3.80, p < 0.005). This result was quite consistent across individual observers (see Supplementary Figure 1 for individual data), and suggests that observers’ spatial attention was involuntarily oriented to the walking direction of the point-light walkers. This was true even though all of the observers were explicitly told prior to the experiment that the walking direction was not predictive of target location. Interestingly, such a reflexive attentional orienting effect disappeared when the point-light walkers were shown upside-down (77.9% vs. 77.9%, t11 = 0.01, p > 0.9).
Figure 2.
Results from Exp. 1–4. When human (a) or cat (b) motion sequences were used as attentional cues, results showed that observers’ performance on a subsequent Gabor probe orientation discrimination task was significantly better when the probe was presented in the walking direction of the point-light walkers or point-light cats (congruent condition) compared with when the probe was presented in the opposite direction (incongruent condition). However, such a reflexive attentional orienting effect disappeared when the point-light walkers or point-light cats were shown upside-down. (c) There was no significant difference whether the probe was presented on the facing or opposite direction of the static point-light figures. (d) Inanimate object motion could not induce reflexive attentional orienting. * p < 0.05; ** p < 0.01; n.s., no significance.
Previous studies that reported the reflexive orienting effects of eye gaze and head direction usually measured reaction time as an index (Driver et al., 1999; Friesen & Kingstone, 1998; Langton & Bruce, 1999). In order to directly compare the effect observed here with those found with eye gaze and head direction, we carried out another experiment in which everything was exactly the same as Exp. 1 except that observers were asked to respond, as quickly as possible, to the position of the Gabor probe (left vs. right) relative to the central fixation. In addition, the cue duration was also manipulated to be 200ms, 500ms, and 1000ms. Consistently, we found significant reflexive attentional orienting effects when the cues were presented for 200ms (t11 = 2.86, p < 0.02) and 500ms (t11 = 2.21, p < 0.05) but not for 1000ms (t11 = 0.25, p > 0.8; see Supplementary Figure 2). This pattern of results is quite similar to what have been found on eye gaze and head direction. The reflexive attentional orienting effect induced by the eye gaze direction can be consistently found with a cue-target interval of 100 ms to 600 ms, but not 1000 ms at which point the cueing effect appears to vanish (see Langton et al., 2000 for a review). Taken together, our findings provide strong evidence that observers’ visuospatial attention can be automatically oriented to upright but not inverted biological motion walking direction, suggesting an intrinsic sensitivity to biological motion signals in the human brain (Johnson, 2006; Simion et al., 2008).
Experiment 2: Attentional orienting elicited by non-human animal walking direction
If the observed effect from Exp. 1 is indeed a specialized brain mechanism tuned to biological motion signals, then it should be able to generalize to motions of other biological entities including non-human animals (Mather & West, 1993). When the walking direction of a point-light cat was used as potential cues, there was also a significant interaction between biological motion walking direction (congruent vs. incongruent) and biological motion orientation (upright vs. inverted) conditions (F1, 11 = 6.15, p < 0.03; Figure 2b). Similarly, observers’ performance was significantly better when the probe was presented in the walking direction of the upright point-light cat compared with when the probe was presented in the opposite direction (82.5% vs. 75.4%, t11 = 2.75, p < 0.02). Again, this effect disappeared when the point-light cat was shown upside-down (79.2% vs. 81.7%, t11 = −1.20, p > 0.2).
Experiment 3: No reflexive orienting elicited by static figure viewpoint information
The results from Exp. 1 and Exp. 2 provide strong evidence that there exists robust reflexive attentional orienting to the direction signaled by biological motion walking direction. However, it is possible that the observed effect is not due to the walking direction of biological motion per se, but instead relies on the viewpoint information of the point-light figures (e.g., a point-light figure facing left or right). To test this possibility, we designed Exp. 3 in which static point-light human figures (captured from the real human biological motion sequences) with the most explicit facing direction were used as the central cues and all the other aspects were kept the same as Exp. 1. Results are shown in Figure 2c, and the interaction between figure facing direction (congruent vs. incongruent) and figure orientation (upright vs. inverted) was not significant (F1, 11 = 2.37, p > 0.2). There was also no significant difference whether the probe was presented on the facing direction or the opposite direction of the static point-light figure no matter the human figure was shown upright (80.0% vs. 82.5%, t11 = −1.00, p > 0.3) or inverted (83.8% vs. 80.4%, t11 = 1.20, p > 0.2).
Experiment 4: Reflexive attentional orienting specialized for biological motion signals
The results from Exp. 1 – 3 suggest that biological motion cues can trigger reflexive attentional orienting. However, a more general question remains unresolved: whether such reflexive orienting effect is specialized for biological motion signals or it is a general mechanism applied to inanimate motions as well? By tapping into this issue, the answer is not only important for this particular phenomenon, but may also be generalized to the attentional effect obtained from eye gaze and head direction (see Frischen et al., 2007 for a review). We therefore adopted point-light rotating circles that were created with the same amount of point-lights as the point-light walkers. This type of stimuli contains no biological information, but has clear moving direction. Results from this experiment showed that there was no significant interaction between motion direction (congruent vs. incongruent) and motion orientation (upright vs. inverted) conditions (F1, 11 = 0.02, p > 0.8; Figure 2d). Observers’ performance was not different when the probe was presented in the moving direction or the opposite direction of the point-light circles (upright condition: 83.8% vs. 84.2%, t11 = −0.16, p > 0.8; inverted condition: 85.0% vs. 84.6%, t11 = 0.10, p > 0.9).
To further investigate the role of biological motion signals in the observed attentional orienting effect, we carried out another control experiment in which everything was the same as in Exp. 1 except that some critical biological motion information was disrupted from the point-light walker. Specifically, each individual dot moved along a path identical to that in Exp. 1 but with a constant speed equal to the average speed, and the initial motion phase of each individual dot was also randomized (Chang & Troje, 2009). Interestingly, the attentional orienting effect disappeared no matter the point-light walker was shown upright (t11 = 0.17, p > 0.8) or inverted (t11 = 1.20, p > 0.2) and there was no significant interaction between biological motion walking direction (congruent vs. incongruent) and biological motion orientation (upright vs. inverted) conditions (F1, 11 = 1.00, p > 0.3). This result suggests that the kinematics of the intrinsic biological motion signals plays a key role in inducing the reflexive attentional orienting, which is also consistent with the findings that the brain regions engaged in biological motion processing is distinct from those for other types of motion signals (Bonda, Petrides, Ostry, & Evans, 1996; Grossman et al., 2000).
DISCUSSION
Many vertebrates, including humans, own a primitive visual system that preferentially processes biological motion. Since walking is perhaps the most common movement generated by all living creatures with feet, it makes sense that we are extremely sensitive to others’ walking direction (Bertenthal & Pinto, 1994; Neri et al., 1998; Thompson et al., 2007; Thornton & Vuong, 2004; Thurman & Grossman, 2008), and this sensitivity develops very early in life (Kuhlmeier et al., 2010; Vallortigara & Regolin, 2006). However, little is known about the functional properties and brain mechanisms underlying the processing of biological motion walking direction (Mori et al., 2006; Simion et al., 2008; Yoon & Johnson, 2009). Here we provide clear evidence that the walking direction of a point-light walker can trigger robust reflexive orienting of spatial attention. Moreover, the attentional orienting effect takes place over a relatively long time course, and can be extended to the walking direction of non-human animals. Critically, the reflexive orienting effect disappears when the point-light walker is shown upside-down or only the static point-light human figure (with clear facing direction) is shown. Moreover, an inanimate object’s motion cannot produce attentional orienting. These findings point to a specialized attentional mechanism that is highly sensitive to meaningful biological motion walking direction.
Some recent studies have shown that local biological motion, independent of global configuration, conveys walking direction information (Chang & Troje, 2009; Saunders, Suchan, & Troje, 2009; Troje & Westhoff, 2006). Indeed, when the critical local biological motion information was disrupted from the cues, no significant attentional orienting effect was found. This result suggests that such attentional orienting relies on the kinematics of the intrinsic biological motion signals (Chang & Troje, 2008; Troje & Westhoff, 2006; Wang, Zhang, He, & Jiang, 2010) and does not require a particular global configuration that matches a static template. It should also be noted that although the static point-light human figure cannot induce robust reflexive attentional orienting and the effect observed in the current study is likely due to the local biological motion processing, a real human body image with strong implied motion information might also be able to elicit the orienting effect (Gervais, Reed, Beall, & Roberts, 2010; Kourtzi & Kanwisher, 2000), as the effect could partially rely on the perceived motion direction of biological entities.
Nevertheless, the fact that the reflexive attentional orienting effects have been reliably found with biological signals (e.g., eye gaze, head direction, and walking direction) suggests that the underlying neural mechanisms are specialized and distinct from those of endogenous or exogenous attention (Driver et al., 1999; Friesen & Kingstone, 1998; Frischen et al., 2007; Langton & Bruce, 1999). Indeed, recent brain imaging studies have provided much stronger evidence that the neural circuitry subserving the reflexive orienting response triggered by gaze cues involves complex cortical connections between temporal and parietal areas (Kingstone, Friesen, & Gazzaniga, 2000; Kingstone, Tipper, Ristic, & Ngan, 2004) and includes the superior temporal sulcus (STS) where both the eye gaze and biological motion (walking direction) are encoded (Bruce, Desimone, & Gross, 1981; Campbell, Heywood, Cowey, Regard, & Landis, 1990; Grossman et al., 2000; Grossman, Battelli, & Pascual-Leone, 2005; Grossman & Blake, 2002; Jackson & Blake, 2010; Jiang & He, 2008; Perrett et al., 1985; Puce, Allison, Bentin, Gore, & McCarthy, 1998; Wicker, Michel, Henaff, & Decety, 1998). It is likely that the STS is one of the key neural sites involved in these social cueing effects (Frischen et al., 2007).
In summary, the current study demonstrates that meaningful biological motion walking direction information can trigger reflexive orienting of spatial attention. Our results, together with recent findings on eye gaze and head direction, make a strong case for a specialized attentional mechanism tuned to various aspects of biological signals that are meaningful and important for species’ interactions.
Supplementary Material
Acknowledgments
This research was supported by the Knowledge Innovation Program of Chinese Academy of Sciences (KSCX2-YW-R-248 and 09CX202020), Chinese Ministry of Science and Technology (2007CB512300), and the US National Science Foundation (BCS-0818588) and National Institutes of Health (EY015261). We thank Nikolaus Troje for kindly providing us the point-light biological motion stimuli, and Robert Shannon and Patricia Costello for their help with English proof.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bertenthal B, Pinto J. Global processing of biological motions. Psychol Sci. 1994;5(4):221–225. [Google Scholar]
- Bonda E, Petrides M, Ostry D, Evans A. Specific involvement of human parietal systems and the amygdala in the perception of biological motion. J Neurosci. 1996;16(11):3737–3744. doi: 10.1523/JNEUROSCI.16-11-03737.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Bruce C, Desimone R, Gross C. Visual properties of neurons in a polysensory area in superior temporal sulcus of the macaque. J Neurophysiol. 1981;46(2):369–384. doi: 10.1152/jn.1981.46.2.369. [DOI] [PubMed] [Google Scholar]
- Campbell R, Heywood C, Cowey A, Regard M, Landis T. Sensitivity to eye gaze in prosopagnosic patients and monkeys with superior temporal sulcus ablation. Neuropsychologia. 1990;28(11):1123–1142. doi: 10.1016/0028-3932(90)90050-x. [DOI] [PubMed] [Google Scholar]
- Chang DH, Troje NF. Perception of animacy and direction from local biological motion signals. J Vis. 2008;8(5):3, 1–10. doi: 10.1167/8.5.3. [DOI] [PubMed] [Google Scholar]
- Chang DH, Troje NF. Acceleration carries the local inversion effect in biological motion perception. J Vis. 2009;9(1):19, 11–17. doi: 10.1167/9.1.19. [DOI] [PubMed] [Google Scholar]
- Cutting JE, Kozlowski LT. Recognition of friends by their walk: gait perception without familiarity cues. Bull Psychon Soc. 1977;9:353–356. [Google Scholar]
- Dittrich WH. Action categories and the perception of biological motion. Perception. 1993;22(1):15–22. doi: 10.1068/p220015. [DOI] [PubMed] [Google Scholar]
- Driver J, Davis G, Riccardelli P, Kidd P, Maxwell E, Baron-Cohen S. Shared attention and the social brain: gaze perception triggers automatic visuospatial orienting in adults. Visual Cognition. 1999;6(5):509–540. [Google Scholar]
- Fani L, Prasad S, Harber K, Shiffrar M. Recognizing people from their movements. J Exp Psychol Hum Percept Perform. 2005;31:210–220. doi: 10.1037/0096-1523.31.1.210. [DOI] [PubMed] [Google Scholar]
- Friesen CK, Kingstone A. The eyes have it! Reflexive orienting is triggered by nonpredictive gaze. Psychonomic Bulletin & Review. 1998;5(3):490–495. [Google Scholar]
- Frischen A, Bayliss AP, Tipper SP. Gaze cueing of attention: visual attention, social cognition, and individual differences. Psychol Bull. 2007;133(4):694–724. doi: 10.1037/0033-2909.133.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischen A, Tipper SP. Orienting attention via observed gaze shift evokes longer term inhibitory effects: implications for social interactions, attention, and memory. J Exp Psychol Gen. 2004;133(4):516–533. doi: 10.1037/0096-3445.133.4.516. [DOI] [PubMed] [Google Scholar]
- Gervais WM, Reed CL, Beall PM, Roberts RJ., Jr Implied body action directs spatial attention. Atten Percept Psychophys. 2010;72(6):1437–1443. doi: 10.3758/APP.72.6.1437. [DOI] [PubMed] [Google Scholar]
- Grossman E, Donnelly M, Price R, Pickens D, Morgan V, Neighbor G, Blake R. Brain areas involved in perception of biological motion. J Cogn Neurosci. 2000;12(5):711–720. doi: 10.1162/089892900562417. [DOI] [PubMed] [Google Scholar]
- Grossman ED, Battelli L, Pascual-Leone A. Repetitive TMS over posterior STS disrupts perception of biological motion. Vision Res. 2005;45(22):2847–2853. doi: 10.1016/j.visres.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Grossman ED, Blake R. Brain Areas Active during Visual Perception of Biological Motion. Neuron. 2002;35(6):1167–1175. doi: 10.1016/s0896-6273(02)00897-8. [DOI] [PubMed] [Google Scholar]
- Jackson S, Blake R. Neural integration of information specifying human structure from form, motion, and depth. J Neurosci. 2010;30(3):838–848. doi: 10.1523/JNEUROSCI.3116-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, He S. Neural encoding of walking direction in biological motion: Evidence from direction-specific adaptation and functional neuroimaging. J Vis. 2008;8(6):902a. [Google Scholar]
- Johansson G. Visual perception of biological motion and a model for its analysis. Percept Psychophys. 1973;14:195–204. [Google Scholar]
- Johnson MH. Biological motion: a perceptual life detector? Curr Biol. 2006;16(10):R376–377. doi: 10.1016/j.cub.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Jonides J. Voluntary versus automatic control over the mind’s eye’s movement. 1981. [Google Scholar]
- Kingstone A, Friesen C, Gazzaniga M. Reflexive joint attention depends on lateralized cortical connections. Psychological Science. 2000;11(2):159–166. doi: 10.1111/1467-9280.00232. [DOI] [PubMed] [Google Scholar]
- Kingstone A, Tipper C, Ristic J, Ngan E. The eyes have it!: an fMRI investigation. Brain Cogn. 2004;55(2):269–271. doi: 10.1016/j.bandc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- Kourtzi Z, Kanwisher N. Activation in human MT/MST by static images with implied motion. J Cogn Neurosci. 2000;12(1):48–55. doi: 10.1162/08989290051137594. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, Cutting JE. Recognizing the sex of a walker from a dynamic point light display. Percept Psychophys. 1977;21:575–580. [Google Scholar]
- Kuhlmeier VA, Troje N, Lee V. Young infants detect the direction of biological motion in point-light displays. Infancy. 2010;15(1):83–93. doi: 10.1111/j.1532-7078.2009.00003.x. [DOI] [PubMed] [Google Scholar]
- Langton S, Watt R, Bruce V. Do the eyes have it? Cues to the direction of social attention. Trends in Cognitive Sciences. 2000;4(2):50–59. doi: 10.1016/s1364-6613(99)01436-9. [DOI] [PubMed] [Google Scholar]
- Langton SRH, Bruce V. Reflexive visual orienting in response to the social attention of others. Visual Cognition. 1999;6(5):541–567. [Google Scholar]
- Mather G, Murdoch L. Gender discrimination in biological motion displays based on dynamic cues. Proc R Soc Lond B Biol Sci. 1994;258:273–279. [Google Scholar]
- Mather G, West S. Recognition of animal locomotion from dynamic point-light displays. Perception. 1993;22(7):759–766. doi: 10.1068/p220759. [DOI] [PubMed] [Google Scholar]
- Mori Y, Inagaki M, Wu L, Doi T, Hirasaki E, Kumakura H, Fujita I. Reflexive social attention elicited by biological motion. J Vis. 2006;6(6):966a. [Google Scholar]
- Neri P, Morrone MC, Burr DC. Seeing biological motion. Nature. 1998;395(6705):894–896. doi: 10.1038/27661. [DOI] [PubMed] [Google Scholar]
- Norman JF, Payton SM, Long JR, Hawkes LM. Aging and the perception of biological motion. Psychol Aging. 2004;19(1):219–225. doi: 10.1037/0882-7974.19.1.219. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10(4):437–442. [PubMed] [Google Scholar]
- Perrett D, Smith P, Potter D, Mistlin A, Head A, Milner A, Jeeves M. Visual cells in the temporal cortex sensitive to face view and gaze direction. Proceedings of the Royal Society of London. Series B, Biological Sciences. 1985:293–317. doi: 10.1098/rspb.1985.0003. [DOI] [PubMed] [Google Scholar]
- Posner M. Orienting of attention. The Quarterly Journal of Experimental Psychology. 1980;32(1):3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Bentin S, Gore J, McCarthy G. Temporal cortex activation in humans viewing eye and mouth movements. Journal of Neuroscience. 1998;18(6):2188–2199. doi: 10.1523/JNEUROSCI.18-06-02188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristic J, Friesen CK, Kingstone A. Are eyes special? It depends on how you look at it. Psychon Bull Rev. 2002;9(3):507–513. doi: 10.3758/bf03196306. [DOI] [PubMed] [Google Scholar]
- Ristic J, Kingstone A. Taking control of reflexive social attention. Cognition. 2005;94(3):B55–65. doi: 10.1016/j.cognition.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Saunders DR, Suchan J, Troje NF. Off on the wrong foot: local features in biological motion. Perception. 2009;38(4):522–532. doi: 10.1068/p6140. [DOI] [PubMed] [Google Scholar]
- Simion F, Regolin L, Bulf H. A predisposition for biological motion in the newborn baby. Proc Natl Acad Sci U S A. 2008;105(2):809–813. doi: 10.1073/pnas.0707021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B, Hansen BC, Hess RF, Troje NF. Peripheral vision: good for biological motion, bad for signal noise segregation? J Vis. 2007;7(10):12, 11–17. doi: 10.1167/7.10.12. [DOI] [PubMed] [Google Scholar]
- Thornton I, Vuong Q. Incidental processing of biological motion. Current Biology. 2004;14(12):1084–1089. doi: 10.1016/j.cub.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Thurman SM, Grossman ED. Temporal “Bubbles” reveal key features for point-light biological motion perception. J Vis. 2008;8(3):28, 21–11. doi: 10.1167/8.3.28. [DOI] [PubMed] [Google Scholar]
- Tipples J. Eye gaze is not unique: automatic orienting in response to uninformative arrows. Psychon Bull Rev. 2002;9(2):314–318. doi: 10.3758/bf03196287. [DOI] [PubMed] [Google Scholar]
- Troje NF. Decomposing biological motion: a framework for analysis and synthesis of human gait patterns. J Vis. 2002;2(5):371–387. doi: 10.1167/2.5.2. [DOI] [PubMed] [Google Scholar]
- Troje NF, Westhoff C. The inversion effect in biological motion perception: evidence for a “life detector”? Curr Biol. 2006;16(8):821–824. doi: 10.1016/j.cub.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Troje NF, Westhoff C, Lavrov M. Person identification from biological motion: effects of structural and kinematic cues. Percept Psychophys. 2005;67(4):667–675. doi: 10.3758/bf03193523. [DOI] [PubMed] [Google Scholar]
- Vallortigara G, Regolin L. Gravity bias in the interpretation of biological motion by inexperienced chicks. Curr Biol. 2006;16(8):R279–280. doi: 10.1016/j.cub.2006.03.052. [DOI] [PubMed] [Google Scholar]
- Vanrie J, Verfaillie K. Perception of biological motion: a stimulus set of human point-light actions. Behav Res Methods Instrum Comput. 2004;36(4):625–629. doi: 10.3758/bf03206542. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang K, He S, Jiang Y. Searching for life motion signals: visual search asymmetry in local but not global biological-motion processing. Psychol Sci. 2010;21(8):1083–1089. doi: 10.1177/0956797610376072. [DOI] [PubMed] [Google Scholar]
- Wicker B, Michel F, Henaff M, Decety J. Brain regions involved in the perception of gaze: a PET study. Neuroimage. 1998;8(2):221–227. doi: 10.1006/nimg.1998.0357. [DOI] [PubMed] [Google Scholar]
- Yoon JM, Johnson SC. Biological motion displays elicit social behavior in 12-month-olds. Child Dev. 2009;80(4):1069–1075. doi: 10.1111/j.1467-8624.2009.01317.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.