Abstract
Previous research on vertebrate limb regeneration indicates there are several mediating factors involved during the re-growth process. These factors are both biochemical and biophysical. While the phenomenon of adult limb regeneration does not occur naturally in mammalian species, prior research has focused mainly on biochemical modes of stimulating tissue growth and regeneration. The BioDome was aimed at developing a new experimental tool to permit the more systematic study of the impact of biophysical and biochemical factors on mammalian tissue regeneration. The BioDome is a multi-component sleeve assembly that encompasses the wound site of an amputated murine digit and provides an environment conducive to tissue regeneration. The studies showed that the BioDome was effective in supporting early stages of murine digit tip regeneration when combined with a porcine urinary bladder matrix (UBM) pepsin digest and electrical stimulation. The hydrated inner environment of the BioDome influenced regeneration, with additional effects seen with the application of electrical stimulation and pharmacological treatments.
Keywords: tissue regeneration, BioDome, biophysical, electrical stimulation
INTRODUCTION
Tissue regeneration involves a cascade of biological events that combine to fully rebuild an excision or appendage that was lost during trauma or amputation. There is a distinct difference between a typical wound healing response and a regenerative response. These two-processes, while similar in many aspects, result in completely different end products. During the course of normal wound healing, many complex biological structures, such as sweat glands, ducts, and hair follicles cannot be rebuilt since the biological machinery to do so is not available. In a typical adult mammalian skin wound, these structures are not regenerated since development of these tissues and organs require highly specific processes to occur. Normal wound closure and scar formation does not provide an adequate environment for these structures to regenerate [Brockes 1997]. Epimorphic regeneration, on the other hand, is the process during which all original structures are replaced with replications of the originals [Birnbaum 2008; Tsonis 1996].
While mammals and most higher vertebrates typically express very limited and time-specific regenerative capacities, there are several model systems that exhibit epimorphic regeneration [Vorotnikova 2008]. These organisms and their ability to perform epimorphic regeneration are heavily studied; however, the exact pathways of regeneration remain obscure. Fortunately, several common motifs among regeneration schemes across a variety of species have been pieced together to generate a solid understanding of the underlying principles involved in limb regeneration. Generally, there are three requirements for any system to show epimorphic regeneration [Stocum 2006]. The system must first contain mitotically active cells and, secondly, release signals to promote the proliferation of those cells. Thirdly, the system must be free of factors that can inhibit a regenerative response. These may include a dry external environment, bacterial infection, or an overwhelmingly efficient wound healing process which repairs the wound by scar tissue replacement before a regeneration cascade can begin.
Within the first few minutes after any mammalian epidermal wound, a fibrin clot fills the area of defect. This clot is rich in cross-linked fibrin fibers, platelets, cytokines, growth factors such as epidermal growth factor (EGF), heparin binding epidermal growth factor (HB-EGF), transforming growth factor-α (TGF-α), and other signal molecules that maintain the healing process. The fibrin clot also serves as a matrix through which migratory cells, such as immune or epithelial cells can travel [Martin 1997]. In the event of granulation tissue formation, from a dry skin wound for example, re-epithelialization occurs by migration of the adjacent epidermal cells underneath the fibrin clot. As the re-epithelialized layer develops in response to growth factors and other signal molecules expressed in the fibrin clot, the migrating cells use enzymes to digest the basement layer of the clot, which eventually sloughs off and separates from the new epithelium. In moist wounds, however, re-epithelialization is accelerated and can occur directly over the surface of the wound without granulation tissue formation [Carlson 2007]. Wound healing nears completion during the reformation of the basal lamina and attachment of the new epithelium to the basal lamina. The consequent scar tissue present at completion of the wound healing process is primarily indicated by a dense, partially organized collagenous fiber network that lacks the complete function and characteristics of the original tissue. This network of granulation tissue can be detected using staining and histological imaging.
Previous studies have shown that the formation of the wound epithelium is a necessary step in allowing regeneration to occur. As an example, forced wound closure by sewing a skin flap over an amputated human child’s fingertip halts regeneration. Allowing the injured digits to re-epithelialize on their own in a moist wound dressing promotes full regeneration of human children’s fingertips [Illingworth 1974]. Similar results were shown in urodele and other amphibian models [Borgens 1982].
While wound healing and regeneration share remarkably similar processes early on, such as the re-epithelialization of the wound surface, regeneration can arguably be viewed as a derailment of the wound healing process and vice-versa. As the different stages of both epimorphic regeneration and wound healing progress, there is not only a biochemical response to the trauma, but also a biophysical response that works in tandem with the biochemistry to synergistically repair the defect [Levin 2009]. The biophysical component of a regenerative response involves both physical mechanics and the various bioelectric events and phenomena such as mass depolarization of cellular membranes and the establishment of minute, long-range electric fields and biological wound currents. The presence of the longitudinal electric field both drives an internal wound stump current and provides guidance cues for innervation and the migration of various other cell types near the wound site [Levin 2009]. While these biophysical phenomena do exist in a normal wound healing response, their presence is significantly more profound in the regeneration process.
The goal for this study was to develop a device that could be used to establish a closed and definable macro-environment around a wound or amputation site. This device was termed a ‘BioDome’. The hypothesis was that establishing a hydrated and controlled environment would afford new options to study and stimulation regeneration of tissues, reducing scarring and fostering more rapid and direct tissue regeneration. The animal model used in the study was a surgically amputated murine digit. The BioDome provided an in utero-type environment conducive to promoting a regenerative response. This environment introduced a controlled state of hydration, electrical stimulation at the wound site, and the administration of pharmaceutical cocktails to facilitate the recruitment and/or dedifferentiation of cells and blastema formation.
At the cellular level, it becomes apparent that regenerative systems require some source for new and differentiating cells to form the new tissues. One observation of amputation site behavior in vertebrate model systems is a mass dedifferentiation of local cells near the wound site [Pritchard 1950]. Once dedifferentiated, this mass of cells forms a wound blastema in regenerative species such urodeles and juvenile frogs. This mass resides in the mesenchyme just underneath the surface of the wound epithelium. The blastema is the active site for the regenerating limb, and is a requirement for successful regeneration in all amphibian models. Cells differentiate from the blastema to form all of the constitutive tissues of the new limb—from new bone to new skin and muscle, etc.
The electrical stimulation incorporated in this project is intended to mimic the biophysical processes of limb regeneration that has been observed in spontaneously regenerative vertebrate species. The BioDome system allows for electrical stimulation to occur at the wound site. The incorporation of an external power source and a pair of electrodes—a cathode and an anode, provides a longitudinal electric field and draws current out of the amputation site at the wound core and replicates the stump currents observed in amphibian models [Borgens 1979b; Balakatounis 2008]. Previous studies have shown that artificial electrical stimulation applied during a limb amputation in adult frog and rat models can significantly enhance evidence of regeneration [Sisken 1984]. Unlike the approach taken here, previous studies did not couple electrical stimulation with a controlled macro-environment or pharmaceutical cocktail treatments.
For the treatment scenarios reported here, a 14-day study was the focus to conduct a preliminary examination of the combined effects of porcine urinary bladder matrix (UBM) pepsin digest cocktail and electrical stimulation delivered via the BioDome system. Treatment cocktails are administered with the goal of inducing the accumulation of wound cells either by recruitment, proliferation, or dedifferentation. The use of UBM pepsin digest alone has been shown to increase evidence of regeneration in mammalian systems [Badylak 2004]. The alteration of the cellular transmembrane potential is one possible means of triggering local cells to dedifferentiate or enter a highly mitotic state [Cone Jr. 1971]. Some ongoing studies involving the BioDome system are aimed at investigating this approach to stimulating regenerative responses in mice. Similar biophysical behavior has been observed in model systems where limb regeneration occurs spontaneously. In these systems, cells enter highly mitotic states [Pritchard 1950] and consequently exhibit a depolarization of their transmembrane potentials (Vm). The cells of the blastema, for example, are highly proliferative and maintain a relatively depolarized Vm compared to quiescent cell types [Binggeli 1986]. A depolarization event can quickly change the membrane potential from an average of −70mV in most quiescent cell types to 0mv or higher depending on the nature of the depolarization mechanism.
A series of small-scale pilot tests and studies was conducted to hone the efficacy and streamline the design of the BioDome system. The present study was conducted in an effort to further develop the design of the device and to study the full potential of the BioDome system by combining the controlled macro-environment of the BioDome with artificial electrical stimulation and delivery of specific pharmacological cocktails.
MATERIALS AND METHODS
BioDome System Design and Implementation
The BioDome sleeve has four components: a polyimide inner sleeve or cuff, a silicone septum and retaining band, a nylon reservoir body, and a stainless steel cathode. A temporary implantable stainless steel anode in conjunction with an external constant current source is used to deliver current through the wound for studies. The polyimide cuffs (0.10” long) make physical contact with the digit selected for amputation and align the wound site within the BioDome. Anatomical differences between mice, particularly digit size, require the fabrication of BioDomes with polyimide cuffs with inner diameters of 0.508”, 0.0571” and 0.064”. The specific inner diameter of the polyimide cuff is selected in accordance with the size of the amputated digit at the time of application of the BioDome.
The nylon reservoir is a segment of clear nylon tubing (length = 0.25”, ID = 0.085”). The silicone septum disk of thickness 0.038” is nested within the distal rim of the reservoir to form a seal and allow hypodermic access (Figure 1). All components of the BioDome are manually aligned and secured in place by an epoxy approved for medical device fabrication (Epotek 301). Curing occurs over the course of 2 hours in a 60°C oven or overnight at room temperature.
Figure 1.
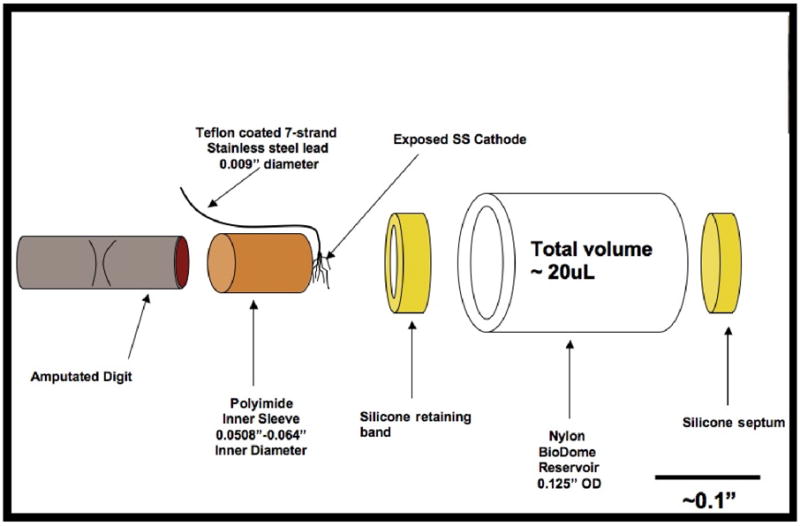
Axially-exploded diagram of BioDome assembly
The silicone retaining band is used to center the smaller diameter polyimide cuff within the larger diameter nylon reservoir tube as well as to provide a watertight seal. The cathode lead is a Teflon coated seven-strand braided stainless steel wire with an overall coated diameter of 0.009”. Cathode leads are cut to 1.0” length. The Teflon coating is removed from each end of the wire segment. 0.25” of Teflon is stripped from the access end of the wire, and 0.1” is removed from the end used as the cathode within the BioDome. Stainless steel is chosen as the preferred material for the cathode since the wire is manufactured with multiple strands that can be easily manipulated to form a fan-shaped electrode. Furthermore, oxidation of the metal at the cathode is not of major concern during the brief electrical stimulation sessions.
The fan-shaped configuration of the cathode maximizes the contact area with the amputation site. The cathode and cathode lead are held in place between the polyimide cuff and the silicone retaining ring (Figure 1). All BioDome components exposed to ethylene oxide gas for 24 hours for sterilization. Materials are then vented for an additional 48–72 hours prior to the surgeries to remove any remaining ethylene oxide.
During surgery, VetBond™ is applied in between the polyimide and the skin of the amputated digit that secures the entire BioDome assembly in place and provides a watertight seal to prevent dehydration of the wound site (Figure 2).
Figure 2.
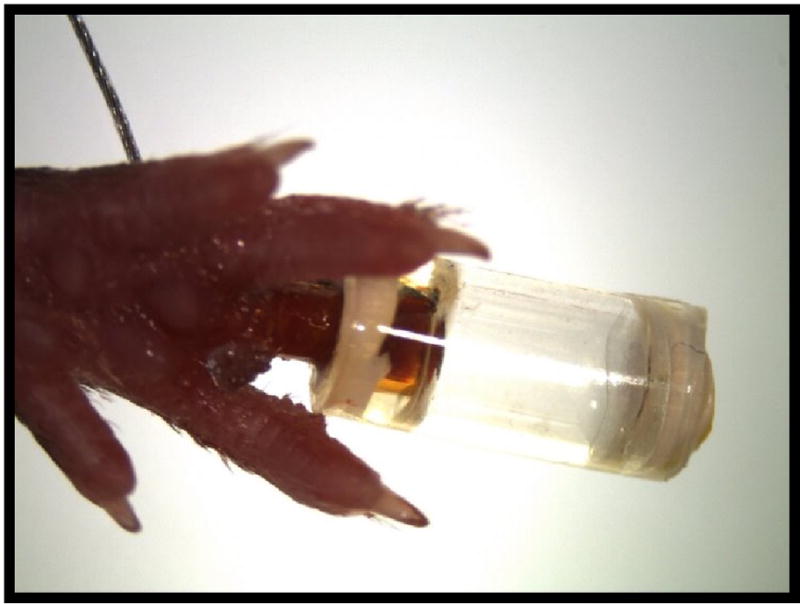
BioDome affixed to subject
The BioDome is designed to stay properly affixed to the digit tip and deliver treatments for up to 6 days to facilitate the start of a regeneration cascade. The BioDome’s adhesion time was engineered for two primary reasons. First, it was anticipated that BioDomes that stay affixed to the digit for longer than 6 days had the potential to cause negative effects such as severe digit irritation, inflammation, or necrosis. Second, after re-epithelialization occurs, few molecules can access the wound site, and the presence of the external fluid treatment would cause no further progress.
Animal Studies
All animal study protocols were in compliance with the University of Massachusetts guidelines and the National Research Council’s criteria for the humane care of animals. Procedures were conducted using approved IACUC procedures. C57BL/6 mice were used in this study under approval of the IACUC at the University of Massachusetts, Lowell (07-03-001-Bra M Mammalian Limb Restoration). 6–8 week old subjects (~20–25g) were used for all studies. Even though female mice are known to demonstrate slightly more developed wound healing and regenerative capacities [Ashcroft 2004], males were selected since the majority of the intended long-term beneficiaries of this study are injured male soldiers than have suffered limb loss in conflict.
The mice were anesthetized via intraperitoneal injection with Ketamine (90–120 mg/kg) and Xylazine (10 mg/kg) prior to surgery. Lubricating eye drops are administered into the eyes of sedated mice to prevent dehydration. After anesthetization, the mice were prepared for surgery by repeatedly cleaning the right hind foot with 70% ethanol and then with a 10% povidone iodine solution. Fur near the anode insertion site is removed with an electric trimmer and razor. The surgical sites are also cleaned with ethanol and povidone iodine after fur removal. The digit amputation was performed at the midline of the 2nd phalange of the right, hind middle digit (digit 3, Standard US Nomenclature) with extra-fine bone scissors. Amputations took place under a Leica EZ 4D microscope. Scissors and other surgical instruments were sterilized between mice using alcohol and a hot glass bead sterilizer. Digit tips were properly discarded after amputation. The surgical time for each mouse averaged between 10 and 15 minutes from the onset of sedation—excluding time required for electrical stimulation.
This investigation involved two treatment groups containing six mice each (Table 1). Group 1 received a BioDome containing the UBM digest control treatment. Group 2 received a BioDome containing the UBM digest treatment. Electrical stimulation (Figure 3) was administered to all subjects on days 0, 1 and 3. Control data obtained from testing conducted in the same manner to compare the response of digits in this study to mice receiving no post-amputation treatment (Figure 4) and to those receiving a BioDome both with and without electrical stimulation (Figures 5 and 6, respectively). A control group that included a completely empty BioDome with electrical stimulation was not performed since the presence of fluid in the BioDome reservoir is an essential factor in permitting electrical conductivity within the system. During testing, consistent current flow was not obtained without the presence of a liquid agent (PBS) within the reservoir.
Table 1.
Treatment Matrix and Result Summary
| Treatment | Day 14 Result Summary | Images |
|---|---|---|
|
Untreated Digit (−) BioDome (−) electrical stimulation (−) pharmacological treatment |
Wound epithelium and new glands: relatively thin wound epithelium with minimal new gland formation within the re-growth region Large mononuclear eosinophilic cells: significant presence of LMECs without any indication of advanced organization |
Figure 4 |
|
BioDome Only and Electrical Stimulation (+) BioDome (−) pharmacological treatment (+) electrical stimulation: 6.4 ua for 15 minutes on days 0, 1, and 3 |
Wound epithelium and new glands: relatively thick wound epithelium with some new gland formation within the re-growth region Large mononuclear eosinophilic cells: significant presence of LMECs with indication of advanced organization and development of lacuna-type regions |
Figure 5 |
|
BioDome Only (+) BioDome (−) pharmacological treatment (−) electrical stimulation |
Wound epithelium and new glands: relatively thin wound epithelium with significant new gland formation within the re-growth region Large mononuclear eosinophilic cells: significant presence of LMECs without any indication of advanced organization |
Figure 6 |
|
Group 1 UBM pepsin digest control (+) BioDome (+) neutralized pepsin buffer (+) electrical stimulation: 6.4 ua for 15 minutes on days 0, 1, and 3 |
Wound epithelium and new glands: slightly thicker wound epithelium with increased new gland formation within the re-growth regions Large mononuclear eosinophilic cells: strong presence of LMECs with some samples showing increased organization and formation of lacuna-type regions |
Figure 7 |
|
Group 2 UBM pepsin digest (+) BioDome (+) UBM pepsin digest cocktail (+) electrical stimulation: 6.4 ua for 15 minutes on days 0, 1, and 3 |
Wound epithelium and new glands: thickest wound epithelium with strongest evidence of new gland formation and vascularization within the re-growth region. Large mononuclear eosinophilic cells: Strongest presence of LMEC’s with a high degree of organization and lacuna-type regions adjacent to original bone. |
Figure 8 |
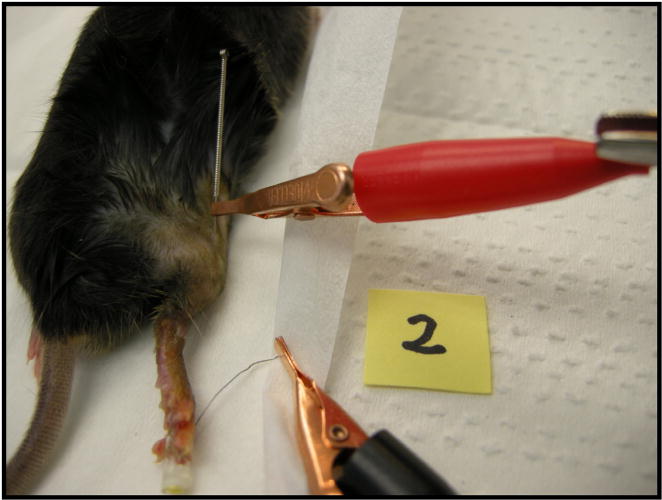
Figure 3. Electrical stimulation.
Mouse 2 receiving electrical stimulation via stainless steel acupuncture anode (red lead)
Figure 4. Day 14 histology of an untreated digit.
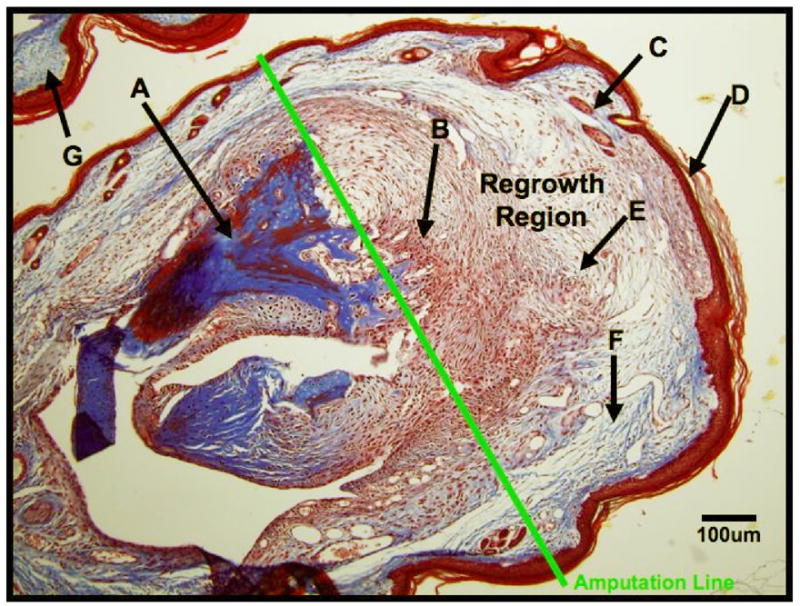
Histological image taken 14 days post amputation from a C57bl/6 mouse that has received no treatment (pharmacological or physical) or wound dressing post amputation. The distal region of the digit is indicated near point (D), and the adjacent digit is indicated at (G). The amputation line shows the approximate line of amputation on Day 0, and the re-growth region is defined as the region of tissue distal to the amputation line. (A) Original, mature bone; (B) Proliferative large mononuclear eosinophilic cells; (C) New hair follicle and sebaceous glands; (D) Wound epithelium; (E) Lymphocytes; (F) New collagen deposition, possible scar tissue formation.
Figure 5. Day 14 histology of BioDome only, with electrical stimulation, without pharmacological treatment.
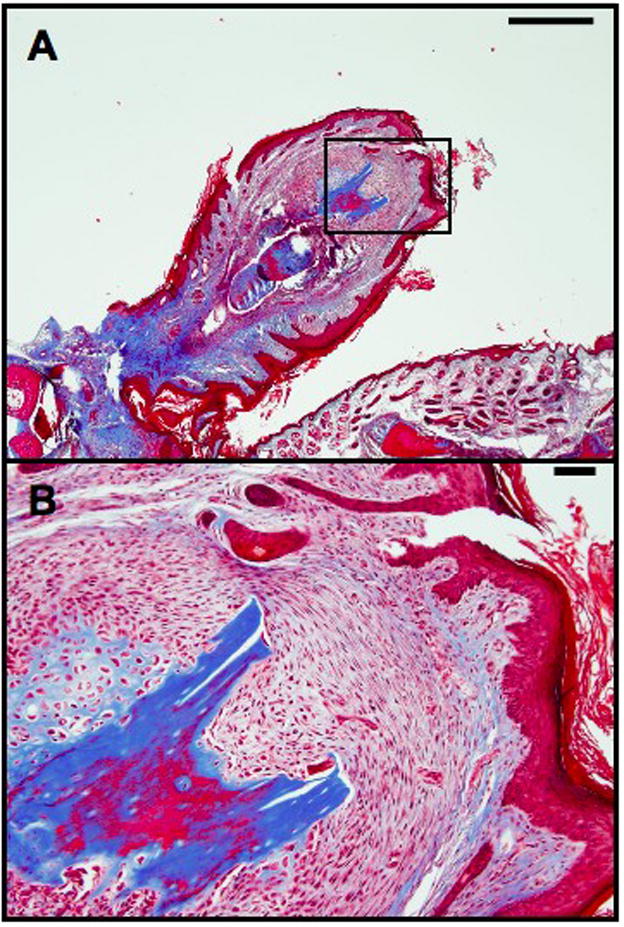
(A, B) Low and high magnification of a digit tip that has received electrical stimulation via a BioDome filled only with PBS and no pharmacological treatment. Scale Bars: 500 um (A), 50 um (B)
Figure 6. Day 14 histology of BioDome only, no electrical stimulation or pharmacological treatment.

(A, B) Low and high magnification of a digit tip that has received a BioDome filled only with PBS and no pharmacological treatment. No electrical stimulation was administered to this subject. Scale Bars: 100 um (A), 50 um (B)
The UBM pepsin digest treatment and control treatment—a neutralized pepsin buffer— were the only pharmacological treatments administered in this study. The UBM treatments have been shown to perform well as scaffolds and promotion of regenerative healing [Badylak 2004, Turner 2009]. The preparation of the UBM treatments was accomplished by harvesting the ECM from a porcine urinary bladder and, then exposing the ECM to a pepsin mediated enzymatic digestion [Badylak 2004; Reing 2009]. While a fully characterized composition the UBM digest remains unknown, various molecules such as collagen, glycosoaminoglycans (GAGs), matrix metalloproteinases (MMPs) and a variety of growth factors are present and serve as a physical scaffold for cell growth.
Treatment Delivery
The ECM digest treatments were administered by puncturing the distal silicone septum of the BioDome with a pair of 30.5 gauge hypodermic syringes. One syringe was used to deliver the liquid cocktail treatment while the second syringe was open-ended and serves as a vent, allowing the air within the BioDome reservoir to escape as treatment is added. Care was taken to avoid the formation of air bubbles within the BioDome reservoir. At this time, the system was checked for leaks. Electrical stimulation was administered while the animals were sedated. Immediately prior to electrical stimulation, the 0.18mm diameter electrical stimulation acupuncture needle was inserted into the haunch ipsilateral with the BioDome. This anode was inserted subcutaneously at an angle to ensure it did not pierce the muscle layer (Figure 3). The stainless steel cathode was built in to the BioDome reservoir as shown in Figure 1. By definition, electrical current flows from the anode towards the cathode.
Electrical stimulation began promptly after the anode was inserted. The electrical connection was made to the anode using a mini alligator clip. Contact between the digit tip and the cathode was visually verified through the transparent nylon reservoir during the administration of electrical stimulation. After the power supply was adjusted to deliver the proper current (6.4 uA), the mouse was electrically connected to the power supply and stimulated for 15 minutes. To check for proper function of the electrical stimulation system as well as contact between the digit tip and the cathode, the initial current flow was verified at the start of the electrical stimulation session using an in-line Ammeter. Since the magnitude of current was on the order of a few microamperes, it was a concern that the ammeter could alter the electrical signal over the course of the electrical stimulation session. The in-line Ammeter was therefore removed from the circuit after proper functionality was verified in order to maintain consistent degree of current flow. After electrical stimulation, the anode was promptly removed and discarded, and the mouse was relocated from the surgical area to the heated stimulation area for recovery. Per protocol, mice received buprenorphine as an analgesic to reduce pain following the surgical procedures. The buprenorphine was administered subcutaneously at 0.05 mg/kg immediately after all surgical procedures have taken place.
Recovery and Euthanasia
Recovery from surgery and electrical stimulation occurred on a heating pad. Mice were intermittently monitored by visual analysis and toe-pinch reflex to determine level of consciousness. After the mice began to move on their own, they were placed in individual housing containers for the duration of the study with free access to food and water in a temperature controlled room. During the initial bedding period, soft Kimwipe® bedding was provided. Euthanasia was performed by CO2 inhalation prior to collections of samples for histology.
Histology
On day 14, the amputated digits along with an adjacent digit for a control were isolated for histology (Figure 4). Due to the presence of the bone in the tissue samples, all samples are decalcified using the Decalcifier I® treatment (Surgipath Inc.) for 24 hours. After decalcification, the samples were rinsed and then placed in 10% formalin for at least 24 additional hours prior to paraffin embedding. The digits were then sectioned along the proximal-distal axis, stained with trichrome, and imaged at low and high magnifications.
RESULTS
The BioDome devices were tolerated well by the test animals, and fulfilled their design specifications by maintaining a controlled wound site environment. The installation procedure for each device averaged approximately 10 minutes per subject once the anesthesia had taken effect. The design of the device allowed each to be installed with minimal effort. While some initial gnawing and scratching of the device was observed in some subjects, acclimatization occurred within the first few hours after regaining consciousness with minimal tampering thereafter. As expected, the devices remained attached to the digits for up to 6 days to provide adequate exposure of the wound site to the provided stimulating factors while avoiding the negative effects of digit constriction and necrosis.
Histological images from previous amputation studies conducted by the BioDome team show the normal healing response at day 14 for mice that do not receive any treatment or wound dressing immediately following digit amputation (Figure 4). A summary of all results can be found in Table 1. Figure 4 shows a typical wound healing response as indicated by a very thin wound epithelium, significant collagen deposition in the form of scar tissue, the presence of large mononuclear eosinophilic cells (LMECs), lymphocytes, and no evidence of advanced bone remodeling.
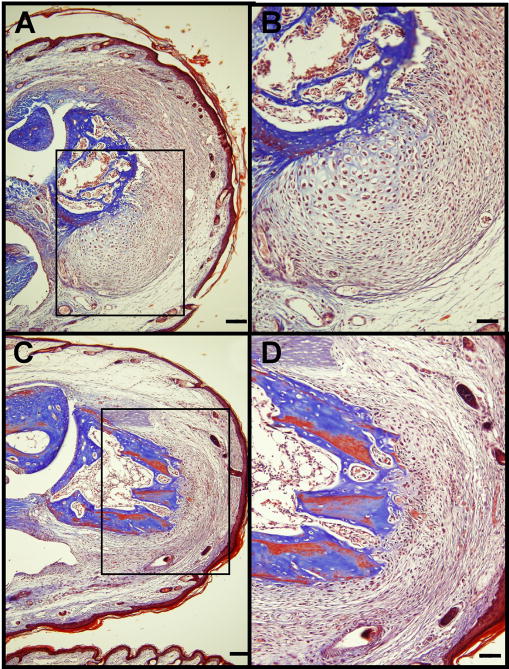
The subject shown in Figure 6 serves as a control and demonstrates the effects of the BioDome without added pharmacological treatment or electrical stimulation. This BioDome only treatment shows little evidence of advanced constructive re-growth as indicated by the thin wound epithelium and the weak organization of LMECs. The subject shown in Figure 5 also serves as a control and demonstrates the effects of the electrical stimulation treatment when administered with a BioDome containing no pharmacological treatment. This subject exhibits evidence of advanced constructive re-growth as indicated by a thick wound epithelium and high degree of organization of LMECs including a region showing the development of a lacuna-type structure adjacent to the original bone.
Mice receiving the UBM pepsin digest control treatment with electrical stimulation demonstrated thicker wound epithelia, large areas of new collagen deposition and bone remodeling with lacunae, and the strong presence of large mononuclear eosinophilic cells, and hair follicles as shown in Figure 7.
Figure 7. Day 14 histology of UBM control treatment group, with electrical stimulation.
(A, C) Low and (B, D) high magnification images of two different digit tips in the UBM pepsin digest control with electrical stimulation group. Scale Bars: 100 um (A, C), 50 um (B, D)
Mice receiving the UBM pepsin digest treatment with electrical stimulation demonstrated a highly pronounced organization of LMECs, bone remodeling, and thick wound epithelia with hair follicle re-development and generally large re-growth regions as shown in Figure 8. Bone remodeling occurred in an asymmetrical pattern in all subjects.
Figure 8. Day 14 histology of UBM treatment group, with electrical stimulation.
(A, C) Low and (B, D) high magnification images of two different digit tips in the UBM pepsin digest with electrical stimulation group. Scale Bars: 100 um (A, C), 50 um (B, D)
DISCUSSION
The BioDome system was designed to address the following issues in a murine model system: wound site hydration, drug delivery, electrical stimulation, subject ambulation and stress management, tamper prevention, and simplicity of installation. Wound site dehydration can affect the host response to injury. To prevent dehydration of the wound site, the BioDome maintains a watertight seal around the severed digit, and operates as a sealed environment. Additionally, this sealed environment aids in the prevention of contamination of the inner chamber and infection. The internal chamber or reservoir of the BioDome is accessible via a small diameter self-sealing septum located at the distal end of the device. A hypodermic syringe is used to both apply pharmacological agents into the sleeve and remove old treatment or bodily fluids such as excess blood or plasma. The types and amounts of drugs and agents to be administered are varied among the different study groups. Since regeneration tissue regenerative response has been shown to be augmented by the presence of small electrical currents passing through the wound core [Borgens 1979a], artificial current can be supplied by a small network of electrodes.
The main goal of this project was to develop a device that could control selected microenvironmental conditions at the wound site in a mouse model of partial digit amputation. Investigations on this matter are becoming increasingly important due to the rising number of limb losses and other traumas that are affecting soldiers as a result of current warfare practices and technologies. The materials and configuration for our device were chosen to minimize potential damage caused by gnawing, scratching, normal movement and exposure to the rodent housing environment. The BioDome system was streamlined to minimize complexity of the device for fabrication and handling reasons and to reduce the number and severity of possible complications that may arise during any part of the surgical procedure.
The system fulfills this goal. It provides a protected and hydrated environment by encompassing the wound site. Access to this inner environment is accomplished through the use of the distal silicone septum and a pair or hypodermic needles. In previous studies, devices have been fabricated and implanted that only deliver electrical stimulation without serving as a vehicle for other pharmacological treatments. These devices [Smith 1981; Borgens 1979a; 1979b] utilize a similar electrode pair to set up a longitudinal electrical field in effort to drive an artificial current through the wound core. In these studies, the presence of the small artificial current significantly enhanced evidence of tissue regeneration without the aid of pharmacological treatments. While the devices in previous studies have been developed to either deliver electrical stimulation or a pharmacological treatment, the BioDome accomplishes both while maintaining a low profile, and facilitating a relatively simple surgical installation procedure that takes approximately 5–10 minutes per subject to complete. Fabrication of the device is completed by hand, and sterilization is performed using ethylene oxide gas to account for the delicate plastic nature of the system’s components. The use of the stainless steel acupuncture needles as the anode was effective and efficient means of electrode configuration (Figure 3).
The device was utilized to study limb amputation responses in a limited set of screens. Administration of the BioDome presented evidence of enhanced regeneration even without electrical stimulation or treatment with a pharmacological cocktail as indicated in Figure 6. As hypothesized, this response was enhanced upon application of electrical stimulation and liquid treatments. There are, however, numerous flaws to the design that will be addressed in future investigations. The minimal observed digit necrosis was attributed to the selection of BioDomes with wider polyimide cuffs. The large cuffs provide a looser fit, and accommodate more inflammation than the previous smaller versions (data not provided). Implementation of an expandable and flexible cuff is a possible solution to enhance regeneration and eliminate or reduce digit necrosis.
In general, BioDomes have a short adhesion time, and many are removed or punctured within the first 72 hours as a result of subject tampering and normal ambulation. This duration is desirable, since the overall goal of this study is to kick-start the regeneration cascade rather than tend to the cascade over a long period of time.
The small reservoir volume (~30 uL) needs modification. Contamination of the reservoir by biological fluids such as blood or plasma can occur even if bleeding has been stopped prior to installation of the BioDome. While implementing an external bladder and pump has been discussed, the bulkiness of any external pack on such a small animal has considerable impact on stress levels since estimations of any sort of external system has a minimum mass of approximately 5.0 g which is 20% of the body weight of a 20.0 g mouse. An external apparatus is possible for larger animals; however, a slight physical size increase of the BioDome reservoir for future designs along with frequent manual replenishment of the treatment fluid in the BioDome are more realistic system modifications for future murine studies.
CONCLUSIONS
From the qualitative analysis of the histological data, it can be concluded that the presence of the BioDome’s well-hydrated environment plays a crucial role in enhancing digit regeneration. Administration of electrical stimulation to the wound site also enhances this response to the extent where highly organized structures indicative of bone remodeling were observed as early as day 14 in most subjects (Figures 5, 7 and 8). Subjects receiving a BioDome with UBM pepsin digest control solution with electrical stimulation showed evidence of enhanced regeneration over both the untreated control digit shown in Figure 4 as well as the subject receiving a BioDome with no electrical stimulation or pharmacological treatment as shown in Figure 6. Subjects receiving a BioDome with UBM pepsin digest and electrical stimulation exhibited even greater evidence of regeneration over the control groups as indicated by a more pronounced network of collagen deposition and large mononuclear eosinophilic cells. Keeping in mind that this is a preliminary study, positive indicators of advanced regeneration were obtained; however, further design modifications are required in order to increase reservoir volume and minimize digit irritation for future studies that utilize this unique approach.
Acknowledgments
We thank Tony Zhang, Dept. Biomedical Engineering, Tufts University; Patricia Arnold, University of Massachusetts Lowell, Dept. of Biology; Dr. Joel Therrien, University of Massachusetts Lowell, Dept. of Electrical Engineering; Dr. Ekaterina Vorotnikova, University of Massachusetts Lowell, Dept. of Biology; Jianping Zhang, University of Massachusetts Lowell, Dept. of Biology for support of various aspects of this study. Funding for the project was originally provided by the National Institutes of Health (NIH) and the U.S. government’s Defense Advanced Research Project Agency (DARPA).
LIST OF ABBREVIATIONS
- ECM
Extracellular matrix
- EGF
Epidermal growth factor
- GAG
Glycosaminoglycan
- HP-EGF
Heparin-binding epidermal growth factor
- LMEC
Large mononuclear eosinophilic cell
- MMP
Matrix metalloproteinase
- TGF-α
Transforming growth factor α
- UBM
(porcine) urinary bladder matrix
Footnotes
CONFLICT OF INTEREST STATEMENT
There are no financial and personal relationships with other people or organizations that could inappropriately influence (bias) this work.
AUTHORS’ CONTRIBUTIONS
DGH designed and assembled the BioDome device and drafted the manuscript. AD performed the animal surgeries and provided input contributing to the design of the device. SB provided use of animal facilities and together with ML and DLK assisted in the design of the BioDome device as well as the development of the pharmacological treatments and interpretation of data. All authors were involved in writing the manuscript and have read and approved the final manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashcroft GS. Sex difference in wound healing. Advances in Molecular and Cell Biology. 2004;34:321–328. [Google Scholar]
- Badylak SF. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transplant Immunology. 2004;12:367–377. doi: 10.1016/j.trim.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Balakatounis KC, et al. Low-intensity Electrical Stimulation in Wound Healing: Review of the Efficacy of Externally Applied Currents Resembling the Current of Injury. ePlasty. 2008;8:283–291. [PMC free article] [PubMed] [Google Scholar]
- Binggeli R, Wienstein R. Membrane potentials and sodium channels: hypothesis for growth regulation and cancer formation based on changes in sodium channels and gap junctions. Journal of Theoretical Biology. 1986;123:377–401. doi: 10.1016/s0022-5193(86)80209-0. [DOI] [PubMed] [Google Scholar]
- Birnbaum KD. Slicing Across Kingdoms: Regeneration in Plants and Animals. Cell. 2008;132:687–710. doi: 10.1016/j.cell.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes JP. Amphibian Limb Regeneration: Rebuilding a Complex Structure. Science. 1997;276:81–87. doi: 10.1126/science.276.5309.81. [DOI] [PubMed] [Google Scholar]
- Borgens RB, et al. Small Artificial Currents Enhance Xenopus Limb Regeneration. Journal of Experimental Zoology. 1979a;207:217–226. [Google Scholar]
- Borgens RB, et al. Role of Subdermal Current Shunts in the Failure of Frogs to Regenerate. Journal of Experimental Zoology. 1979b;209:49–56. doi: 10.1002/jez.1402090106. [DOI] [PubMed] [Google Scholar]
- Borgens RB. What Is the Role of Naturally Produced Electric Current in Vertebrate Regeneration and Healing? International Review of Cytology. 1982;76:245–296. doi: 10.1016/s0074-7696(08)61793-3. [DOI] [PubMed] [Google Scholar]
- Carlson BM. Principles of Regenerative Biology. New York: Elsevier Inc; 2007. [Google Scholar]
- Cone CD., Jr Control of Somatic Cell Mitosis by Simulated Changes in the Transmembrane Potential Level. Oncology. 1971;25:168–182. doi: 10.1159/000224567. [DOI] [PubMed] [Google Scholar]
- Illingworth CM. Trapper fingers and amputated finger tips in children. Journal of Pediatric Surgery. 1974;9:853–858. doi: 10.1016/s0022-3468(74)80220-4. [DOI] [PubMed] [Google Scholar]
- Levin M. Large-scale biophysics: ion flows and regeneration. Trends in Cell Biology. 2007;17:251–310. doi: 10.1016/j.tcb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Levin M. Bioelectric mechanisms in regeneration: Unique aspects and future perspectives. Seminars in Cell & Development Biology. 2009;20:543–546. doi: 10.1016/j.semcdb.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. Wound Healing—Aiming of Perfect Skin Regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Pritchard JJ, Ruzicka AJ. Comparison of fracture repair in the frog, lizard and rat. Journal of Anatomy. 1950;84:236–261. [PMC free article] [PubMed] [Google Scholar]
- Reing JE, Zhang L, Myers-Irvin J, Cordero KE, Freytes DO, Heber-Katz E, Bedelbaeva K, McIntosh D, Abiche D, Braunhut SJ, Badylak SF. Degradation Products of Extracellular Matrix Affect Cell Migration and Proliferation. Tissue Engineering. 2009 Mar;15(3):605–14. doi: 10.1089/ten.tea.2007.0425. [DOI] [PubMed] [Google Scholar]
- Sisken BF. Repsonse of Amputated Rat Limbs to Fetal Nerve Tissue Implants and Direct Current. Journal of Orthopaedic Research. 1984;2:177–189. doi: 10.1002/jor.1100020209. [DOI] [PubMed] [Google Scholar]
- Smith SD. The Role of Electrode Position in the Electrical Induction of Limb Regeneration in Subadult Rats. Bioelectrochemistry and Bioenergetics. 1981;8:661–670. [Google Scholar]
- Stocum DM. Regenerative Biology and Medicine. New York: Elsevier Inc; 2006. [Google Scholar]
- Tsonis PA. Limb Regeneration. New York: Cambridge University Press; 1996. [Google Scholar]
- Turner NJ, Yates AJ, Jr, Weber DJ, Qureshi IR, Stolz DB, Gilbert TW, Badylak SF. Xenogeneic extracellular matrix as an inductive niche for regeneration of functional skeletal muscle. PNAS. doi: 10.1089/ten.TEA.2010.0169. (Submitted, August 2009) [DOI] [PubMed] [Google Scholar]
- Vorotnikova E, McIntosh D, Dewilde A, Zhang J, Reing JE, Zhang L, Cordero K, Bedelbaeva K, Gourevitch D, Heber-Katz E, Badylak SF, Braunhut SJ. MRL cell matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Wound Healing and Tissue Regeneration. doi: 10.1016/j.matbio.2010.08.007. (Submitted, September 2008) [DOI] [PubMed] [Google Scholar]