Abstract
We tested the hypothesis that insulin-like growth factor-I (IGF-I) stimulates ovarian androgen production by increasing theca-interstitial cell luteinizing hormone (LH) binding affinity and/or binding capacity. We then investigated the role of transcriptional and translational events in mediating these actions of IGF-I. LH bound to saturable, high affinity binding sites on rat ovarian theca-interstitial cells. Preincubation with LH produced a decrease in LH binding capacity with no effect on LH binding affinity. Treatment with IGF-I, both in the absence and presence of LH, increased LH binding capacity 1.5- to 2-fold with no change in LH binding affinity. Androgen production was increased progressively by LH, suggesting that LH-stimulated steroidogenesis is not tightly coupled to LH receptor downregulation. IGF-I increased androgen synthesis in proportion to its upregulation of LH binding capacity. Transcriptional inhibition with dichlorobenzimidazole riboside inhibited the IGF-I-mediated increase in LH binding capacity but had no effect on androgen production. Translational inhibition with cycloheximide inhibited both the IGF-I-mediated increase in LH binding and stimulation of androgen synthesis. We conclude that IGF-I increases theca-interstitial cell LH binding capacity and reverses the LH-induced downregulation of LH binding sites. The enhancement of LH binding by IGF-I is compatible with transcriptional mediation whereas the effect of IGF-I on androgen synthesis appears to be mediated by a direct effect of the peptide on the translational process(es) involved in steroidogenesis.
Full text
PDF


Images in this article
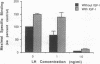
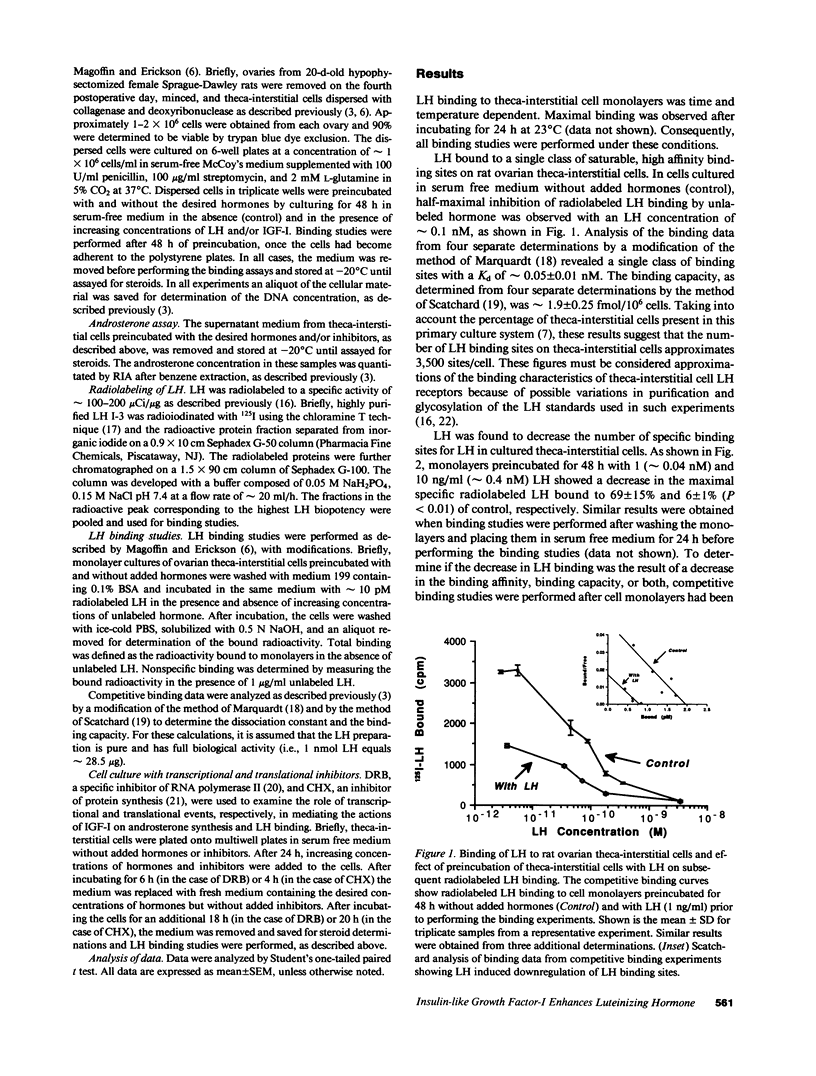
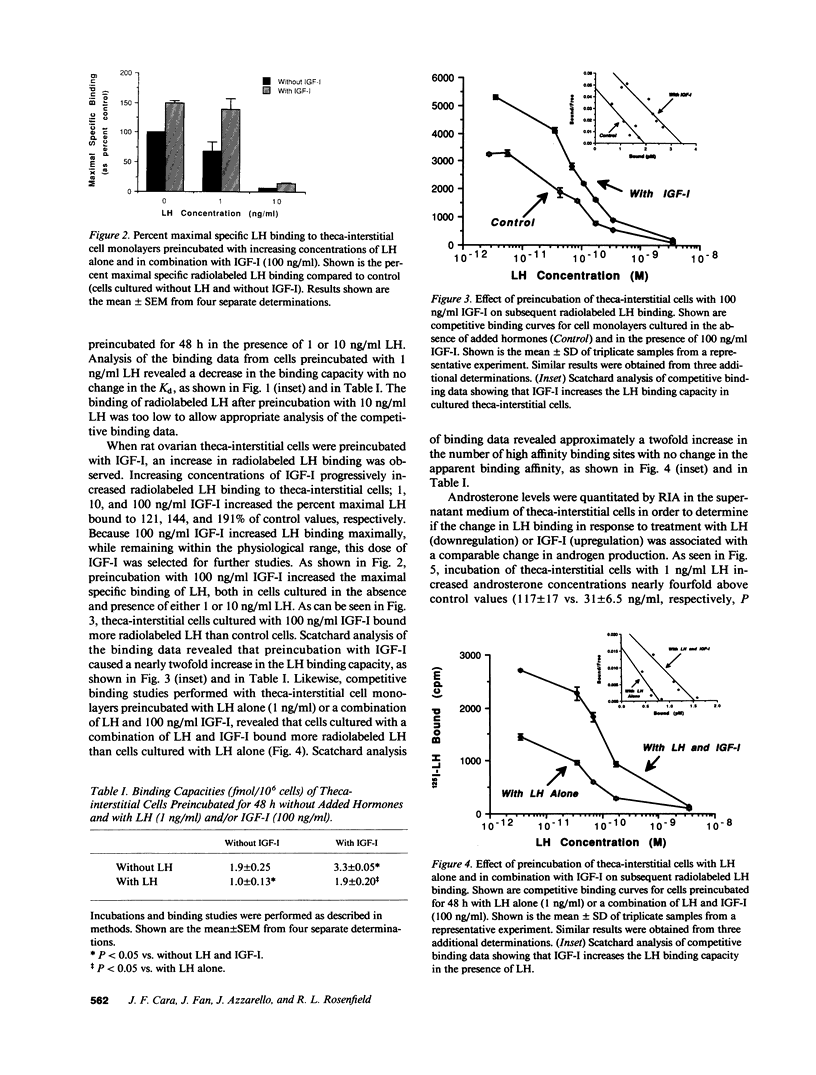
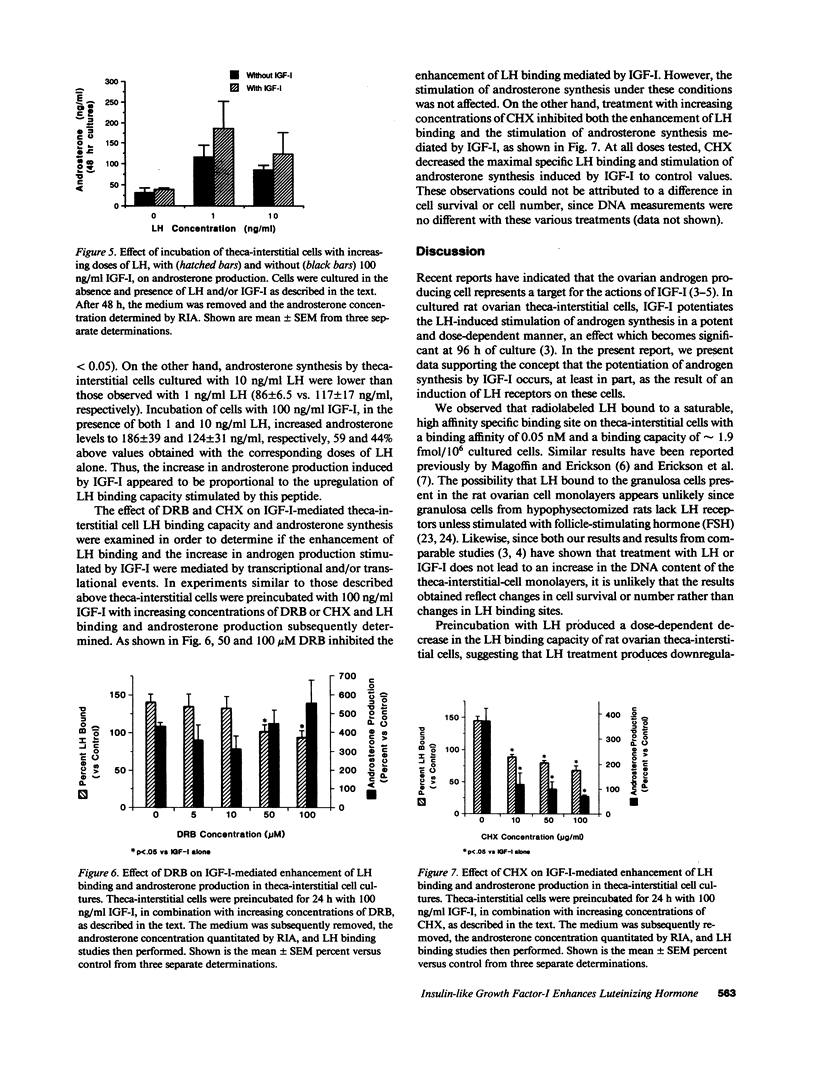
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adashi E. Y., Resnick C. E., Svoboda M. E., Van Wyk J. J. Somatomedin-C enhances induction of luteinizing hormone receptors by follicle-stimulating hormone in cultured rat granulosa cells. Endocrinology. 1985 Jun;116(6):2369–2375. doi: 10.1210/endo-116-6-2369. [DOI] [PubMed] [Google Scholar]
- Barbieri R. L., Makris A., Ryan K. J. Insulin stimulates androgen accumulation in incubations of human ovarian stroma and theca. Obstet Gynecol. 1984 Sep;64(3 Suppl):73S–80S. doi: 10.1097/00006250-198409001-00019. [DOI] [PubMed] [Google Scholar]
- Barnes R., Rosenfield R. L. The polycystic ovary syndrome: pathogenesis and treatment. Ann Intern Med. 1989 Mar 1;110(5):386–399. doi: 10.7326/0003-4819-110-5-386. [DOI] [PubMed] [Google Scholar]
- Bernier M., Chatelain P., Mather J. P., Saez J. M. Regulation of gonadotropin receptors, gonadotropin responsiveness, and cell multiplication by somatomedin-C and insulin in cultured pig Leydig cells. J Cell Physiol. 1986 Nov;129(2):257–263. doi: 10.1002/jcp.1041290218. [DOI] [PubMed] [Google Scholar]
- Bogovich K., Richards J. S., Reichert L. E., Jr Obligatory role of luteinizing hormone (LH) in the initiation of preovulatory follicular growth in the pregnant rat: specific effects of human chorionic gonadotropin and follicle-stimulating of hormone on LH receptors and steroidogenesis in theca, granulosa, and luteal cells. Endocrinology. 1981 Sep;109(3):860–867. doi: 10.1210/endo-109-3-860. [DOI] [PubMed] [Google Scholar]
- Burstein S., Schaff-Blass E., Blass J., Rosenfield R. L. The changing ratio of bioactive to immunoreactive luteinizing hormone (LH) through puberty principally reflects changing LH radioimmunoassay dose-response characteristics. J Clin Endocrinol Metab. 1985 Sep;61(3):508–513. doi: 10.1210/jcem-61-3-508. [DOI] [PubMed] [Google Scholar]
- Cara J. F., Rosenfield R. L. Insulin-like growth factor I and insulin potentiate luteinizing hormone-induced androgen synthesis by rat ovarian thecal-interstitial cells. Endocrinology. 1988 Aug;123(2):733–739. doi: 10.1210/endo-123-2-733. [DOI] [PubMed] [Google Scholar]
- Chodosh L. A., Fire A., Samuels M., Sharp P. A. 5,6-Dichloro-1-beta-D-ribofuranosylbenzimidazole inhibits transcription elongation by RNA polymerase II in vitro. J Biol Chem. 1989 Feb 5;264(4):2250–2257. [PubMed] [Google Scholar]
- Daughaday W. H., Hall K., Salmon W. D., Jr, Van den Brande J. L., Van Wyk J. J. On the nomenclature of the somatomedins and insulin-like growth factors. J Clin Endocrinol Metab. 1987 Nov;65(5):1075–1076. doi: 10.1210/jcem-65-5-1075. [DOI] [PubMed] [Google Scholar]
- Dufau M. L., Winters C. A., Hattori M., Aquilano D., Barañao J. L., Nozu K., Baukal A., Catt K. J. Hormonal regulation of androgen production by the Leydig cell. J Steroid Biochem. 1984 Jan;20(1):161–173. doi: 10.1016/0022-4731(84)90203-6. [DOI] [PubMed] [Google Scholar]
- Erickson G. F., Magoffin D. A., Dyer C. A., Hofeditz C. The ovarian androgen producing cells: a review of structure/function relationships. Endocr Rev. 1985 Summer;6(3):371–399. doi: 10.1210/edrv-6-3-371. [DOI] [PubMed] [Google Scholar]
- Erickson G. F., Wang C., Hsueh A. J. FSH induction of functional LH receptors in granulosa cells cultured in a chemically defined medium. Nature. 1979 May 24;279(5711):336–338. doi: 10.1038/279336a0. [DOI] [PubMed] [Google Scholar]
- Froesch E. R., Schmid C., Schwander J., Zapf J. Actions of insulin-like growth factors. Annu Rev Physiol. 1985;47:443–467. doi: 10.1146/annurev.ph.47.030185.002303. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez E. R., Resnick C. E., Holtzclaw W. D., Payne D. W., Adashi E. Y. Insulin as a regulator of androgen biosynthesis by cultured rat ovarian cells: cellular mechanism(s) underlying physiological and pharmacological hormonal actions. Endocrinology. 1988 May;122(5):2034–2043. doi: 10.1210/endo-122-5-2034. [DOI] [PubMed] [Google Scholar]
- Hernandez E. R., Resnick C. E., Svoboda M. E., Van Wyk J. J., Payne D. W., Adashi E. Y. Somatomedin-C/insulin-like growth factor I as an enhancer of androgen biosynthesis by cultured rat ovarian cells. Endocrinology. 1988 Apr;122(4):1603–1612. doi: 10.1210/endo-122-4-1603. [DOI] [PubMed] [Google Scholar]
- Hill D. J., Milner R. D. Insulin as a growth factor. Pediatr Res. 1985 Sep;19(9):879–886. doi: 10.1203/00006450-198509000-00001. [DOI] [PubMed] [Google Scholar]
- Magoffin D. A., Erickson G. F. An improved method for primary culture of ovarian androgen-producing cells in serum-free medium: effect of lipoproteins, insulin, and insulinlike growth factor-I. In Vitro Cell Dev Biol. 1988 Sep;24(9):862–870. doi: 10.1007/BF02623895. [DOI] [PubMed] [Google Scholar]
- Magoffin D. A., Erickson G. F. Primary culture of differentiating ovarian androgen-producing cells in defined medium. J Biol Chem. 1982 Apr 25;257(8):4507–4513. [PubMed] [Google Scholar]
- Mondschein J. S., Schomberg D. W. Growth factors modulate gonadotropin receptor induction in granulosa cell cultures. Science. 1981 Mar 13;211(4487):1179–1180. doi: 10.1126/science.6258228. [DOI] [PubMed] [Google Scholar]
- Pestka S. Inhibitors of ribosome functions. Annu Rev Microbiol. 1971;25:487–562. doi: 10.1146/annurev.mi.25.100171.002415. [DOI] [PubMed] [Google Scholar]
- Poretsky L., Kalin M. F. The gonadotropic function of insulin. Endocr Rev. 1987 May;8(2):132–141. doi: 10.1210/edrv-8-2-132. [DOI] [PubMed] [Google Scholar]
- Richards J. S., Hedin L. Molecular aspects of hormone action in ovarian follicular development, ovulation, and luteinization. Annu Rev Physiol. 1988;50:441–463. doi: 10.1146/annurev.ph.50.030188.002301. [DOI] [PubMed] [Google Scholar]
- Robertson D. M., Diczfalusy E. Biological and immunological characterization of human luteinizing hormone: II. A comparison of the immunological and biological activities of pituitary extracts after electrofocusing using different standard preparations. Mol Cell Endocrinol. 1977 Nov;9(1):57–67. doi: 10.1016/0303-7207(77)90046-6. [DOI] [PubMed] [Google Scholar]
- Stuart C. A., Prince M. J., Peters E. J., Meyer W. J., 3rd Hyperinsulinemia and hyperandrogenemia: in vivo androgen response to insulin infusion. Obstet Gynecol. 1987 Jun;69(6):921–925. [PubMed] [Google Scholar]
- Taylor S. I. Insulin action and inaction. Clin Res. 1987 Sep;35(5):459–472. [PubMed] [Google Scholar]
- Urdl W. Polycystic ovarian disease: endocrinological parameters with specific reference to growth hormone and somatomedin-C. Arch Gynecol Obstet. 1988;243(1):13–36. doi: 10.1007/BF00931548. [DOI] [PubMed] [Google Scholar]
- Yamaoka K., Tanigawara Y., Nakagawa T., Uno T. A pharmacokinetic analysis program (multi) for microcomputer. J Pharmacobiodyn. 1981 Nov;4(11):879–885. doi: 10.1248/bpb1978.4.879. [DOI] [PubMed] [Google Scholar]
- Zipf W. B., Payne A. H., Kelch R. P. Dissociation of lutropin-induced loss of testicular lutropin receptors and lutropin-induced desensitization of testosterone synthesis. Biochim Biophys Acta. 1978 May 3;540(2):330–336. doi: 10.1016/0304-4165(78)90146-0. [DOI] [PubMed] [Google Scholar]