Abstract
The non-structural proteins encoded by the orf- I, II, III, and IV genes of the human T-cell leukemia/lymphoma virus type 1 (HTLV-1) genome, are critical for the modulation of cellular genes expression and T-cell proliferation, the escape from cytotoxic T-cells and natural killer cells, and virus expression. In here, we review the main functions of the HTLV-1 Orf -I products. The 12 kDa product from orf-I (p12) is proteolytically cleaved within the endoplasmic reticulum (ER) to generate the 8 kDa protein (p8). At the steady state, both proteins are expressed at similar levels in transfected T-cells. The p12 protein remains in the ER and cis-Golgi, whereas the p8 protein traffics to the cell surface and is recruited to the immunological synapse. The p12 and the p8 proteins have seemingly opposite effects on T-cells; the ER resident p12, modulates T-cell activation and proliferation, whereas p8 induces T-cell anergy. The p8 protein also increases the formation of cellular conduits, is transferred to neighboring T-cells, and increases virus transmission. The requirement for HTLV-1 infectivity of orf-I is demonstrated by the loss of virus infectivity in macaques exposed to an engineered virus, whereby expression of orf-I was ablated. Altogether the current knowledge demonstrates that the concerted activity of p8 and p12 is essential for the persistence of virus infected cells in the host.
Keywords: HTLV-1, p12, p8, T-cells, orf-I, virus transmission, immunological synapse
1. Introduction
Human T-cell leukemia/lymphoma virus type 1 (HTLV-1) is an oncogenic retrovirus discovered in 1980 from a patient with cutaneous T-cell lymphoma (Poiesz, Ruscetti et al. 1980a;Poiesz, Ruscetti et al. 1980b). It is estimated that 20 million people may be infected by HTLV-1 worldwide, and although the majority of infected individuals remain asymptomatic with 1-2% developing adult T-cell leukemia/lymphoma (ATLL) (Takatsuki,2005;Poiesz, Ruscetti, Gazdar, Bunn, Minna, and Gallo,1980a), or HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP) in their lifetime (Gessain,1996).
In addition to the typical retroviral structural genes gag, pol, and env, the HTLV-1 genome encodes the regulatory Tax and Rex proteins as well as several other proteins from four overlapping open reading frames (orf) at the 3′end of the viral genome (Franchini and Lairmore,2007). The Tax and Rex proteins are essential transcriptional and posttranscriptional positive regulators of viral expression. Four additional proteins, p12/p8 and p30/p13, are produced by alternatively spliced mRNAs from orfs I and II, respectively (Berneman, Gartenhaus et al. 1992;Ciminale, Pavlakis et al. 1992;Koralnik, Gessain et al. 1992). Moreover, an anti-sense RNA, transcribed from the minus DNA strand, encodes the HTLV-I bZIP protein (HBZ) protein (Gaudray, Gachon et al. 2002).
The p12, p13, and p30 proteins are dispensable for in vitro human T-cell immortalization (Derse, Mikovits et al. 1997;Robek, Wong et al. 1998). However, when their expression is ablated individually, HTLV-1 remains infectious in rabbits but not in macaques (Valeri, Hryniewicz et al. 2010). Up to date, no direct evidence exists of protein expression from orf-I and orf-II. However, their importance is suggested by the requirement for these genes in HTLV-1 infectivity, in animal models, the presence of cross-reactive antibodies, and cytotoxic T-cells responses in HTLV-1-infected patients (Dekaban, Peters et al. 2000;Pique, Ureta-Vidal et al. 2000), and the detection of mRNA from ex vivo samples. The level of mRNA expression for these viral genes is 100-to-1000 fold less than the tax and rex genes in HTLV-1-infected cell lines, in ATLL, HAM/TSP, and asymptomatic carriers (Berneman, Gartenhaus, Reitz, Jr., Blattner, Manns, Hanchard, Ikehara, Gallo, and Klotman ,1992;Ciminale, Pavlakis, Derse, Cunningham, and Felber ,1992;Koralnik, Gessain, Klotman, Lo Monico, Berneman, and Franchini ,1992;Ciminale, D’Agostino et al. 1995;Cereseto, Berneman et al. 1997;Dekaban, Peters, Mulloy, Johnson, Trovato, Rivadeneira, and Franchini ,2000). A recent study, based on a real-time RT-PCR approach, has demonstrated that the mRNA level for p12 and p30 is also much lower than that of the structural genes, gag, and env (Li, Kesic et al. 2009) in the blood of HTLV-1-infected animals.
The p30 protein is a nucleolar protein encoded by a doubly spliced mRNA that places the env and tax genes AUG initiating codon in frame with orf II (Ciminale, Pavlakis, Derse, Cunningham, and Felber ,1992;Koralnik, Gessain, Klotman, Lo Monico, Berneman, and Franchini ,1992) and acts as both a transcriptional and posttranscriptional regulator of gene expression(Michael, Nair et al. 2004;Nicot, Dundr et al. 2004). The p13 protein contains the last 87 amino acids of p30 but is encoded by a private singly spliced mRNA (Ciminale, Pavlakis, Derse, Cunningham, and Felber ,1992;Koralnik, Gessain, Klotman, Lo Monico, Berneman, and Franchini ,1992). The p13 increases production of reactive oxygen species (ROS) by the mitochondria, and affects cell turnover (Silic-Benussi, Marin et al. 2010). In the presence of Tax, however, a portion of p13 undergoes unconventional ubiquitination and is re-routed to nuclear speckles (Andresen, Pise-Masison et al. 2010). Another negative regulator of Tax-mediated viral expression is the HBZ protein. HBZ mRNA also induces T-cell proliferation and is expressed in ATLL cells (Gaudray, Gachon, Basbous, Biard-Piechaczyk, Devaux, and Mesnard,2002;Matsuoka and Green ,2009;Satou, Yasunaga et al. 2006).
2. The proteins encoded by orf-I
A singly spliced mRNA from orf-I (Ciminale, Pavlakis, Derse, Cunningham, and Felber,1992;Koralnik, Gessain, Klotman, Lo Monico, Berneman, and Franchini ,1992) encodes p12/p8. The Orf-I amino acid sequence is conserved in HTLV-1-infected individuals and is highly hydrophobic (Franchini ,1995;Martins, Soares et al. 2002;Iniguez, Otsuki et al. 2005;Fukumoto, Andresen et al. 2009). Because of the p12 hydrophobicity, it has been difficult to generate antibodies able to recognize the natural Orf-I protein products. Hydropathy and immunogenicity plots predict few soluble regions and two putative transmembrane domains extending from amino acid 12 to 30 and amino acid 48 to 67(Franchini ,1995) (Figure 1). The p12 protein contains four putative proline-rich (PXXP) Src homology 3 (SH3)-binding domains, two putative leucine zipper (LZ) motifs, a putative adaptin motif, and a noncanonical ER retention/retrieval motif at the N-terminus (Fukumoto, Andresen, Bialuk, Cecchinato, Walser, Valeri, Nauroth, Gessain, Nicot, and Franchini ,2009;Franchini,1995) (Figure 1). These structural features may contribute to the p12 membrane localization, homodimerization (Trovato, Mulloy et al. 1999), and protein-protein interactions. Immunoprecipitation and immunoblot data have demonstrated that the p12 protein, tagged at the C-terminus with either AU1 or HA1 epitopes, forms dimers in HeLa-Tat cells (Trovato, Mulloy, Johnson, Takemoto, de Oliveira, and Franchini ,1999). Two allelic forms of p12, which differ substantially in their stability, have been described. The p12 allele that carries a lysine at position 88, p12K88, is ubiquitinated and has a half life of 30 minutes, while the allelic variant, p12R88, is not ubiquitinated and has an 8 hour half life (Trovato, Mulloy, Johnson, Takemoto, de Oliveira, and Franchini ,1999).
Figure 1. Amino acid sequence and domains within the p12 and p8 proteins.
HTLV-1 p12 is a highly hydrophobic protein that contains an amino terminus noncanonical ER retention/retrieval motif (MLFRL), four putative proline-rich (PXXP) Src homology 3 (SH3)-binding domains, two putative leucine zipper (LZ) motifs, an adaptin motif, and a calcinuerin binding motif. The arrows indicate the two cleavage sites between amino acid positions 9 and 10; 29 and 30, respectively.
3. Post-translational cleavage of the Orf-I protein product generates the p12 and p8 proteins that have distinct cellular localization and functions
Early observations have shown that expression of the p12 cDNA consistently yields two proteins (Koralnik, Gessain, Klotman, Lo Monico, Berneman, and Franchini ,1992). The Orf-I protein products were originally described to localize in the ER and the cis-Golgi apparatus (Johnson, Nicot et al. 2001;Koralnik, Fullen et al. 1993;Ding, Albrecht et al. 2001). Recently, it has been found that p12 contains two proteolytic cleavage sites: the first, between amino acids 9 and 10 and the second, between amino acids 29 and 30 which produces an 8 kDa protein (Fukumoto, Andresen, Bialuk, Cecchinato, Walser, Valeri, Nauroth, Gessain, Nicot, and Franchini ,2009) (Figure 1). Both cleavage sites appear to be important for the removal of a non canonical ER retention/retrieval signal. These studies, guided by computer-based prediction of amino acid motifs (software PSPORT and ELM30), were conducted through site-directed mutagenesis and the construction of fused proteins. Cleavage of the 12 kDa form results in an 8 kDa protein, p8, that localizes to the lipid rafts and is recruited to the immunological synapse upon T-cell receptor ligation (Fukumoto, Andresen, Bialuk, Cecchinato, Walser, Valeri, Nauroth, Gessain, Nicot, and Franchini ,2009). A mutation from glycine to serine at position 29 (G29->S), found in natural HTLV-1 strains, prevents cleavage at the second site and results in ER retention of the full-length p12 protein. In contrast, a mutation at position 26 from glutamate to asparagine (D26N) increases cleavage and results in the predominance of the cleaved the p8 protein (our unpublished data). The enzyme that processes p12 has not been identified to date.
The demonstration that ER retention of p12 is depends on its N-terminus was obtained using transfected constructs, whereby p12 was fused to neuromodulin plasma membrane targeting signal, and green fluorescent protein (GFP-Mem) (Fukumoto, Andresen, Bialuk, Cecchinato, Walser, Valeri, Nauroth, Gessain, Nicot, and Franchini ,2009). Expressed alone, GFP-Mem is targeted to the plasma membrane, however fusion of the first 15 amino acids of p12 with the GFP-Mem protein resulted in localization of the protein to the plasma membrane. Interestingly, when the serine in position 10 was mutated to alanine, the fused protein was retained in the ER. Similarly, the presence of the first 32 amino acids of the wild-type p12 resulted in plasma membrane localization of p8, whereas the 32 amino acids of the p12G29S mutant resulted in retention/retrieval of the uncleaved p12G29S-GFP-Mem protein in the ER. Indeed, the first 5 amino acids of p12 alone (MLFRL), when fused with the GFP-Mem protein, caused retention of the fused protein in the ER, demonstrating that this amino acid stretch functions as a non-canonical ER retention/retrieval motif (Fukumoto, Andresen, Bialuk, Cecchinato, Walser, Valeri, Nauroth, Gessain, Nicot, and Franchini ,2009).
4. ER function of p12
4.1. p12 binds to the IL-2 Receptor γ and β chains and increases T-cell proliferation
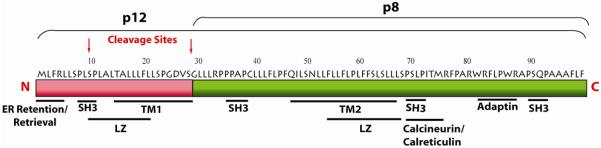
HTLV-1 infects and immortalizes T-cells and full T-cell transformation is associated with T-cell growth in an interleukin-2 (IL-2) independent manner. The switch to IL-2-independence correlates with the constitutive activation of the Jak/signal transducers and activators of transcription (STAT) pathway {Migone, 1995 MIGONE1995 /id}. Tax protein is believed to facilitate T-cell activation and proliferation by the induction of IL-2, the IL-2 receptor (IL-2R) α chain, c-fos, and other genes (Johnson, Harrod et al. 2001;Xu, Kang, Heidenrich, Okerholm, O’Shea, and Nerenberg ,1995). The IL-2R is composed of three chains: the α chain, which increases ligand binding affinity, and the signaling molecules β and γc chains, which recruit Jak3 and STAT5α and STAT5β (Smith, Jacobson et al. 1999). STAT-5 activation results in IL-2 expression and cell division. The ER resident p12 protein binds to the immature form of the IL-2R β and γc chains. It is speculated that p12, by aggregating the two IL-2R chains, mimics the ability of IL-2 to induce STAT5 phosphorylation, thereby lowering the threshold of IL-2 requirement for T-cell growth(Mulloy, Crowley et al. 1996;Nicot, Mulloy et al. 2001) (Figure 2). Thus, p12 expression may favor the entry of T-cells into the S phase even in conditions of suboptimal antigen stimulation and IL-2 production (Nicot, Mulloy, Ferrari, Johnson, Fu, Fukumoto, Trovato, Fullen, Leonard, and Franchini,2001). Therefore, the combined ability of p12 and Tax to promote STAT-5 activation and increase TCR signaling increases T-cell responsiveness to IL-2 and that in turn, increases virus production because IL-2 increases CREB/ATF and Tax transcription (Lin, Hickey et al. 2005).
Figure 2. p12 induces the dimerization of the ILR β and γ chains and increases STAT5 activation.
In the ER, p12 binds to both the IL-2 the β and γc chains and decreases the trafficking of the two IL-2R chains to the cell surface. p12 increases STAT5 phosphorylation and IL-2 gene transcription. This leads to the decreased requirement for IL-2 in HTLV-1-infected cells.
4.2 p12 modulates calcium release and T-cell activation
Intracellular calcium (Ca2+) release is known to be required for the transcriptional activity of nuclear factor of activated T-cells (NFAT) (Negulescu, Shastri et al. 1994). An increase of intracellular Ca2+ concentration activates the phosphatase calcineurin that dephosphorylates NFAT (Zhu and McKeon ,2000). Dephosphorylated NFAT translocates to the nucleus and induces expression of IL-2. p12 protein increases the basal level of cytosolic Ca2+ (Ding, Albrecht et al. 2002) and this event results in the dephosphorylation of NFAT, enhancement of IL-2 production, and T-cell proliferation (Ding, Kim et al. 2003) (Figure 3). p12 physically binds to resident chaperones calreticulin and calnexin proteins that facilitate appropriate protein folding, as well as modulate Ca2+ storage. Indeed it has been suggested that the binding of calreticulin and calnexin to p12 increased the intracellular Ca2+ concentration (Ding, Albrecht, Luo, Zhang, Stanley, Newbound, and Lairmore ,2001). The p12-mediated increase of NFAT activation is observed with agents that stimulate T-cells bypassing the T-cell receptor (TCR), such as phorbol myristate acetate (PMA). PMA stimulation directly activates a protein kinase C (PKC) isoform downstream of the TCR (Albrecht, D’Souza et al. 2002;Ding, Albrecht, Kelley, Muthusamy, Kim, Altschuld, and Lairmore,2002). Therefore, as expected, treatment with a Ca2+ chelator (BAPTA-AM or cyclosporine A) or with a dominant negative mutant of NFAT, blocks the p12 mediated Ca2+ release and NFAT activation. The effect of p12 on Ca2+ release can be recapitulated by the treatment of T-cells with thapsigargin, a drug that depletes intracellular Ca2+ stores (Albrecht, D’Souza, Ding, Tridandapani, Coggeshall, and Lairmore ,2002;Ding, Albrecht, Kelley, Muthusamy, Kim, Altschuld, and Lairmore,2002). The p12-mediated increase in NFAT activation is also dependent on inositol triphosphate (IP3) and partially dependent on Ca2+ release-activated Ca2+ channel (CRAC), a channel that facilitates Ca2+ influx across the plasma membrane (Ding, Albrecht, Kelley, Muthusamy, Kim, Altschuld, and Lairmore ,2002). Altogether, these data suggest that in the ER, p12 increases calcium influx, NFAT activation, and IL-2 production.
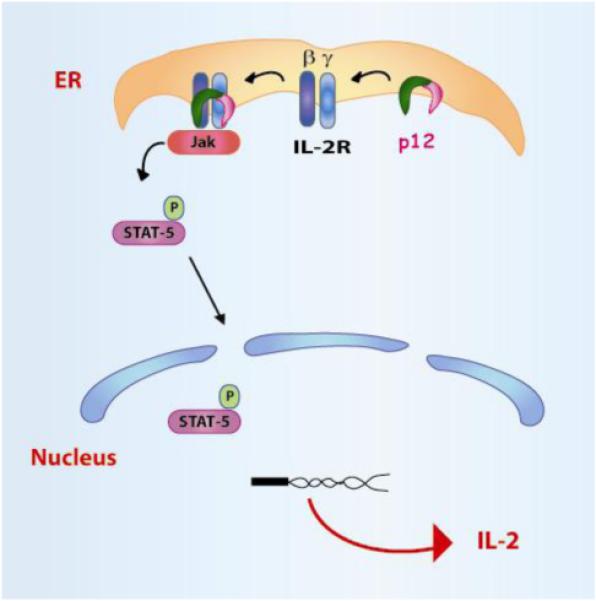
Figure 3. Opposing effects of the Orf-I products on NFAT activation.
Upon phorbol myristate acetate (PMA) stimulation, which releases ER calcium-stores in a TCR-independent mechanism, p12 increases NFAT dephosphorylation. The dephosphorylated NFAT is translocated into the nucleus, where it activates IL-2 production and favors T-cell growth (left part of the Figure). The Orf-I product(s), however, also bind(s) to calcineurin, competes with calcineurin for binding to NFAT and decrease T-cell activation (right part of the Figure).
Surprisingly, the Orf-I protein products also inhibit NFAT through competitive-binding to calcineurin (Kim, Ding et al. 2003). The p12 and p8 proteins carry a highly conserved calcineurin-binding motif calcineurin-binding motif PXIXIT that could compete for binding of calcineurin to NFAT (Figure 1)(Kim, Ding, Albrecht, Green, and Lairmore ,2003). Point mutations of the four SH3 like-binding motifs in orf-I demonstrated two positive (SH3-2,4) and two negative regions (SH3-1,3) in NFAT regulation (Figure 1) (Ding, Kim, Nair, Michael, Boris-Lawrie, Tripp, Feuer, and Lairmore ,2003). Thus, the Orf-I proteins regulates positively NFAT transcriptional activity by increasing ER Ca2+ release and negatively, by competing with NFAT for calcineurin binding (Figure 4). It is logical to speculate that the modulation of Ca2+ release is mediated by the ER resident p12 protein. At present, whether p12 or p8 or both bind to calcineurin remains unclear.
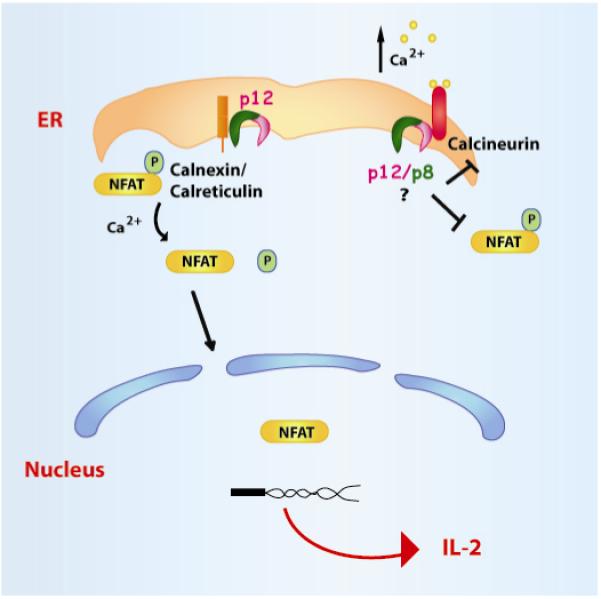
Figure 4. p12 binds to and reroutes the MHC I Heavy chain for degradation to the proteasome.
The p12 protein interacts with the MHC I heavy chain (Hc) in the ER and prevents its association with the beta-2-microglobulin (β2m). The lack of association of MHC I HC with the β2m causes its retrotranslocation into the cytosol and degradation by the proteasome. This ultimately leads to a decreased MHC I cell surface expression.
4.3 p12 re-routes the major histocompatibility complex class I to the proteasome for degradation
The immune system has evolved highly sophisticated mechanisms to recognize “self” and “non-self” antigens in order to eliminate pathogens without causing tissue damage. Toll-like receptors on dendritic cells (Fitzgerald and Golenbock ,2007) are able to sense specific pathogens. Dendritic cells engulf microbial antigens and prime T-cells for recognition of pathogen-specific peptides presented by the major histocompatibility complex class I (MHC-I) on the surface of infected cells. Once primed, the cytotoxic T-cells (CTL) continuously scan somatic cell surfaces in search for pathogens. Once a “non-self” antigen from a pathogen is identified, the CD8+ T-cells are activated and rapidly kill the infected cell. The MHC I complex includes a 44 kDa transmembrane glycoprotein heavy chain, the β2-microglobulin (β2m), and a short peptide of 8–12 amino acids. In the ER, p12 physically interacts with the MHC I Heavy chain (Hc) and prevents its association with the β2m (Johnson, Nicot, Fullen, Ciminale, Casareto, Mulloy, Jacobson, and Franchini ,2001;Johnson, Mulloy et al. 2000). This interaction induces the MHC I Hc retro-translocation into the cytosol for degradation by the proteasome (Figure 4). Thus, p12 decreases MHC I on the cell surface. In addition, p12 has been shown to target HLA-A2 and HLA-B7 (Johnson, Nicot, Fullen, Ciminale, Casareto, Mulloy, Jacobson, and Franchini,2001) but other MHC-I types have not been tested. Of importance, peripheral mononuclear lymphocytes isolated from HTLV-1-infected patients, have altered expression of MHC I alleles (Mann, Popovic et al. 1983;Sonoda, Yashiki et al. 1987;Uno, Matsuoka et al. 1995). Likely, the ability of p12 to interfere, with the assembly and trafficking of the MHC I complex to the cell surface, contributes to viral persistence by helping infected cells to evade immune surveillance.
4.4 p12 and natural killer cells
Decreased expression of MHC I on the surface of cells pose the risk of recognition and killing by natural killer cells (NK) cells. Thus, pathogens that reduce MHC-I expression on the cell surface, have evolved strategies that also counteract the function of NK cells and HTLV-1 is no exception. NK cells require ICAM receptors to adhere to T-cells through the leukocyte function antigen-1 (LFA-1) adhesion molecule. The Orf-I proteins have been shown to impair NK killing of infected cells by decreasing the expression of intercellular adhesion molecule ICAM-1 and ICAM-2 on the cell surface (Banerjee, Feuer et al. 2007). This down-modulation is selective since the Orf-I proteins do not alter the expression of ICAM-3 on CD4+ T-cells (Banerjee, Feuer, and Barker,2007). The mechanism of downregulation of ICAM-1 and ICAM-2 by either p12 and/or p8 is unknown. Nevertheless, the down-regulation of MHC class I and ICAM-1 and ICAM-2, induced by the Orf-I proteins, likely helps the HTLV-1-infected cells to escape immune recognition by both CTL and NK cell.
5. Cell Membrane functions of p8
5.1 p8 downregulates proximal T-Cell Receptor signaling
The immunological synapse (IS) is a highly structured region of contact between the T-cells and the antigen presenting cells (APC), such as dendritic cells. IS is formed following the engagement of the T-cell receptor (TCR), expressed on T-cells, to the MHC-I complex expressed on the APC (Wulfing and Davis ,1998). Such an event leads to the activation of the protein tyrosine kinases Lck and Fyn, which phosphorylates tyrosines located on the cytosolic domains of TCR (Figure 5). The downstream phosporylation of ZAP70 results in activation of LAT, that in turn, recruits Grb2, phospholipase C-1 (PLC-1), and the p85 subunit of phosphatidylinositol 3-kinase and indirectly, Vav, Cb1, and SLP76 (Manz and Groves ,2010). The recruitment of these molecules to the plasma membrane is essential for calcium release from the ER, nuclear translocation of NFAT, enhancement of transcription, IL-2 production, and T-cell proliferation. Interestingly, HTLV-1 infection is associated with a significant degree of immune deficiency (Bunn, Jr., Schechter et al. 1983;Yarchoan, Guo et al. 1986). Indeed, the adaptive immune response to HTLV-1 varies among infected people (Yarchoan, Guo, Reitz, Jr., Maluish, Mitsuya, and Broder ,1986;Jacobson, Shida et al. 1990;Parker, Daenke et al. 1992;Kannagi, Matsushita et al. 1993;Kannagi, Matsushita et al. 1994). The exact nature of the wide variation in levels and quality of the immune responses to HTLV-1 among infected individuals is unclear. Insights regarding these early findings were provided by the realization that the HTLV-1 p8 protein is recruited to the lipid rafts within the IS upon engagement of TCR and causes T-cell anergy (Fukumoto, Andresen, Bialuk, Cecchinato, Walser, Valeri, Nauroth, Gessain, Nicot, and Franchini ,2009). Lipid rafts are a heterogeneous mixture of sphingolipid and cholesterol that are tightly packed and form microdomains in cell membranes and serve as organizational centers for the clustering of signaling molecules (Taner, Onfelt et al. 2004). p12 binds to LAT and decreases LAT phoshorylation, that in turn, results in decreased phosphorylation of the downstream T-cell signaling molecules PLC-γ1 and Vav and dowregulation of NFAT activity (Figure 5) (Fukumoto, Dundr et al. 2007). This contrasts with p12-mediated NFAT activation following PMA stimulation, which, by bypassing TCR, is independent of LAT (Figure 3). The opposing effects of p12 and p8 on NFAT may occur at different stages of the cell cycle since TCR stimulation drives T-cells from a resting state to early G1 phase, while responsiveness to IL-2 is linked with the S phase. The effect of p8 on the immunological synapse has been recently confirmed by imaging techniques that demonstrate a reduction in the strength of the immunological synapse in the presence of p8 {Van Prooyen N, 2010 362 /id}. An important finding is that p8, by inhibiting NFAT, also inhibits viral replication (Fukumoto, Andresen, Bialuk, Cecchinato, Walser, Valeri, Nauroth, Gessain, Nicot, and Franchini ,2009), since NFAT activation increase Tax activity (Lin, Hickey, Hsu, Medina, and Rabson ,2005). Collectively, these data suggest that HTLV-1, through the timely expression of the Orf-I proteins, may finely modulate T-cell activation as well as its own expression.
Figure 5. p8 localizes at the immunological synapse and decreases proximal T-cell signaling.
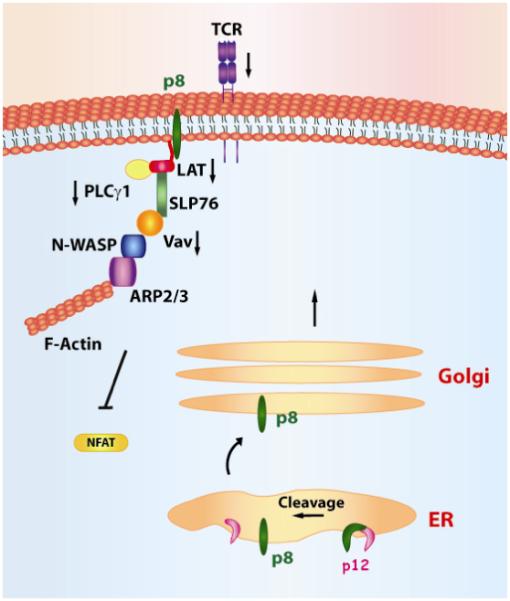
p8 decreases T-cell activation after TCR-stimulation through its interaction with LAT, which causes the decrease in phosphorylation of LAT, PLC-γ1, Vav, and Lck and decreased activity of NFAT.
5.2 p8 promotes T-cell contact and HTLV-1 transmission
HTLV-1 infects dendritic cell and T-cells and virus transmission is more efficient through direct cell-to-cell contact (Jones, Petrow-Sadowski et al. 2008). Upon cell contact HTLV-1 mediates rapid reorientation of the microtubule organizing center (MTOC) towards the cell-cell junction (Igakura, Stinchcombe et al. 2003). The formation of the virological synapse requires the polarization of cytoskeleton and adhesion receptors, such as LFA-1 and ICAM receptors (Majorovits, Nejmeddine et al. 2008). Prior data demonstrated that the Orf-I protein products increased LFA-1 clustering on T-cells (Kim, Nair et al. 2006) and more recent evidence, demonstrates that the increased clustering of LFA-1 in lipid rafts is mediated by p8, and not by p12 protein (Van Prooyen N, Gold H, Andresen V, Schwartz O, Jones K, Rucetti F, Lockett S, Prabhakar G, Venzon D, and Franchini G ,2010) (Figure 6).
Figure 6. p8 enhances viral spread by increasing cell-cell contact.
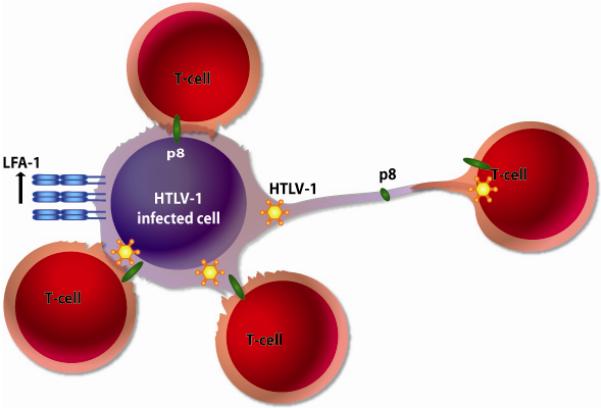
The p8 protein increases LFA-1 clustering at the cell surface leading to increased cell-cell contact. In addition, p8 stimulates the formation of cellular conduits. These modes of cell contact lead to increased p8 transfer to neighboring cells and ultimately viral transmission.
p8 increases also the number and length of cellular conduits (Watkins and Salter ,2005;Sowinski, Jolly et al. 2008) that favor communication among several types of cells, and p8 is transferred, within minutes, to neighboring T-cells {Van Prooyen N, 2010 355 /id}. Importantly, p8 augments the envelope-dependent transfer of HTLV-I to neighboring T-cells. These data are consistent with recent reports demonstrating that the Orf-I protein products increases viral transmission in an IL-2-dependent manner (Taylor, Brown et al. 2009).
6. p12/p8 and the H+ vacuolar ATPase
H+ vacuolar ATPase (V-ATPase) regulates pH in cellular organelles and affects enzymes activity, membrane fusions, and the dissociation of internalized ligand-receptor complexes in endosomes (Toei, Saum et al. 2010). Acidification is achieved through regulation of V-ATPase activity by its isoform composition, as well as the specific targeting and trafficking of V-ATPase to organelles. Activation of V-ATPase causes the gradual acidification of compartments along the secretory pathway, ranging from the ER at pH 7.2 to secretory granules at pH 5.2. In addition, V-ATPases acidify the vesicles of the endocytic pathway from early endosomes at pH 6.3 to lysosomes at pH 5.5 (Marshansky and Futai ,2008).
Co-immunoprecipitation experiments demonstrated that both the p12 and p8 proteins bind to the 16 kDa component of the V-ATPase, the pore forming subunits of the enzyme complex (Franchini, Mulloy et al. 1993;Koralnik, Mulloy et al. 1995). Further work demonstrated that the two putative transmembrane domains of p12 do not participate in this interaction. The proline-rich region amino acid located between amino acid 36 and 48, present in both p12 and p8 (Figure 1), is important of V-ATPase binding (Koralnik, Mulloy, Andresson, Fullen, and Franchini ,1995).
The interaction between p12 and the 16 kDa protein of the V-ATPase likely occurs in the ER and may prevent the assembly of the mature form of the V-ATPase and acidification of the secretory pathway. This effect may have important functional implications. For example, late endosomal compartments require a low compartmental pH in order for MHC II to bind a peptide. MHC II is expressed on professional antigen presenting cells, such as dendritic cells that are infected by HTLV-1 (Jones, Petrow-Sadowski, Huang, Bertolette, and Ruscetti ,2008). MHC II presents exogenous foreign peptides to CD4+ helper T-cells. Therefore, by inhibiting the V-ATPase, p12 could block MHC II presentation to T-cells and reduce immune surveillance (Chow and Mellman ,2005). A similar effect could be exerted on MHC-I antigen presentation.
Recent work shows that the p8 protein traffics to the cell surface viable secretory pathway (Fukumoto, Andresen, Bialuk, Cecchinato, Walser, Valeri, Nauroth, Gessain, Nicot, and Franchini ,2009) and also localizes at early endosomes and recycling endosomes (our unpublished results). Thus, p8 association with the V-ATPase could alter, not only the secretory and the endocytic pathway, but also affect receptor recycling on the cell membrane. Functional data on the effect of p8 or p12 on these pathways are not available at present. It is likely that the biological consequences of p12 and p8 interaction with the V-ATPase vary in different cellular compartments as well as in T-cells versus dendritic cells.
7. Role of Orf-I in viral infectivity in vivo
The orf-I mRNA is detected in vivo in HTLV-1 in infected patients as well as in asymptomatic carriers (Furukawa, Furukawa et al. 1991;Berneman, Gartenhaus, Reitz, Jr., Blattner, Manns, Hanchard, Ikehara, Gallo, and Klotman ,1992;Ciminale, Pavlakis, Derse, Cunningham, and Felber ,1992;Koralnik, Gessain, Klotman, Lo Monico, Berneman, and Franchini ,1992;Ciminale, D’Agostino, Zotti, Franchini, Felber, and Chieco-Bianchi ,1995;Cereseto, Berneman, Koralnik, Vaughn, Franchini, and Klotman ,1997). Thus, orf-I expression appears to be important throughout the course of natural HTLV-I infection. Initial studies that ablated the orf-I splice acceptor site from an HTLV-I molecular clone, resulted in a significant loss of HTLV-1 infectivity in the White New Zealand rabbit model (Collins, Newbound et al. 1998). However, that mutation also affected HBZ, whose ablation alone decreases virus levels (Arnold, Yamamoto et al. 2006). Therefore, the results of the early work using this orf-I mutant could have been in part due to the alteration of the HBZ protein. Indeed, a more recent study, whereby the expression of the orf-I gene was ablated by a single point mutation in the initiation codon without truncating HBZ, demonstrated that this mutant virus, defective in orf-I expression, was able to replicate in rabbits as the wild type HTLV-1 (Valeri, Hryniewicz, Andresen, Jones, Fenizia, Bialuk, Chung, Fukumoto, Parks, Ferrari, Nicot, Cecchinato, Ruscetti, and Franchini ,2010). Of interest, the same orf-I mutant virus was unable to establish infection in primary human dendritic cell in vitro and in macaques in vivo (Valeri, Hryniewicz, Andresen, Jones, Fenizia, Bialuk, Chung, Fukumoto, Parks, Ferrari, Nicot, Cecchinato, Ruscetti, and Franchini ,2010). This data demonstrates the expression of orf-I gene is essential in nonhuman primates and suggests that HTLV-1 infection of macaques may be a more relevant model for humans. This animal species is infected with HTLV-1 in the wild and develop leukemia, whereas rabbits do not (Beilke, Traina-Dorge et al. 1996;Traina-Dorge, Martin et al. 2007;Lairmore, Silverman et al. 2005;Allan, Leland et al. 2001). Collectively, these data support an important role for Orf-I protein products in the establishment and the maintenance of HTLV-1 infection in vivo.
8. Naturally Occurring genetic Orf-I polymorphism and Disease Associations
Lysine is a known target for covalent binding of ubiquitin, allowing lysine-containing proteins to be targeted for degradation by the proteasome (Varshavsky ,1996). HTLV-1 Orf-I contains a rare genetic polymorphism that results in mutation of arginine at position 88 to a lysine (p12K88). This allelic variant is ubiquitinated and rapidly degradated whereas the more frequent p12R88 is more stable (Trovato, Mulloy, Johnson, Takemoto, de Oliveira, and Franchini ,1999). The p12K88 variant was initially found in HAM/TSP patients (5 out of 8 patients), raising the hypothesis that the presence of the p12K88 mutation could be associated with HAM/TSP. (Trovato, Mulloy, Johnson, Takemoto, de Oliveira, and Franchini,1999). However, further sequence analysis of proviral DNA from Brazilian and Japanese patients (Martins, Soares, Ribas, Thorun, Johnson, Kroon, Carneiro-Prioetti, and Bonjardim,2002) (Furukawa, Usuku et al. 2004) did not support this hypothesis. Thus, because the p12K88 variant is rare (2.7% of 37 patients and 1.4% of 144 patients, respectively) in HAM/TSP patients, the significance of this allelic polymorphism in TSP/HAM, if any, remains unclear.
A large study performed on 231 HTLV-1 infected individuals demonstrated the preservation of the orf I genetic sequence in 46 asymptomatic patients. Interestingly however, premature termination that truncated the carboxy terminus of 17 or of 12 amino acids was found in 0.7% and 4.97%, respectively, of TSP/HAM patients from Japan. In the same study, a female TSP/HAM was also found to have a mutation in the initiating methionine to isolencine (M->I) (Furukawa et al., 2004) and the provirus of the infected sister carried the same mutation.
These data “perse” do not suggest that orf I is not necessary for infection. Rather they indicate that the last 17 amino acid may not be necessary to maintain HTLV-I infection. Similarly, the finding of a substitution of M->I in the three HTLV-I infected siblings does not necessarily prove that the orf I products are dispensable for viral infectivity, since doubly spliced mRNA pX-Rex-orf I encodes the orf I product that undergoes cleavage as demonstrated by Koralink et al. 1992.
Sicca syndrome is described as an autoimmune disorder that affects the ability of the lachrymal and the salivary glands to produce tears and saliva (Wu and Fox ,1994). The sicca syndrome has been frequently associated with HAM/TSP and it has been suggested that it may be an early event in progression to HAM/TSP (Vernant, Buisson et al. 1988;Hajjar, Sainte-Foie et al. 1995;Nakamura, Eguchi et al. 1997). Sequence analysis of Caribbean or Guianese HTLV-1 infected patients with sicca syndrome demonstrated that p12 was identical in several isolates from patients regardless of their different clinical statuses. Several amino acid changes were identified within the putative transmembrane domains of p12, located between amino acid 12-32 and amino acid 48-68 (Beby-Defaux, Frugier et al. 1999). However, the functional significance of these mutations and their relevance to disease, if any, remains to be established.
More recent studies, suggest that some natural orf-I alleles may differ functionally. Reverse genetic studies on natural variants of the HTLV-1 orf-I have demonstrated mutations that alter the proteolytic cleavage of the Orf-I protein product. Proviruses with orf-I that expresses mainly p12 are much more common than those that predominantly express p8 (Fukumoto, Andresen, Bialuk, Cecchinato, Walser, Valeri, Nauroth, Gessain, Nicot, and Franchini ,2009)(our unpublished data). Few patients have been shown to carry an allelic polymorphism that favors cleavage to the 8kDa form. Further studies will be necessary to determine whether there is a correlation between the presence of specific orf-I alleles and virus levels or disease progression in HTLV-1-infected individuals.
9. Future work and conclusions
Although significant progress has been made in our understanding of the functional importance of p12/p8 in vitro and in vivo, several questions remain unanswered. Future studies need to identify cellular partners that mediate the effect of p12 and p8 on MHC-I, STAT5, ICAM-1 at ICAM 2, and NFAT proteins. The identification of these cellular proteins will help to address, mechanistically the functions of p12 and p8 and also to uncover other pathways affected by the Orf-I products. The timing of p8 and p12 expression within T-cells and dendritic cells is also of great interest because these proteins have opposing effects on T-cell growth and may differently affect virus replication in vitro and in vivo. However, this represents a difficult goal. The mRNA for orf-I is expressed at very low levels and because of the hydrophobicity of p12 and p8, it has been difficult to generate specific antibodies to the natural protein products of orf-I.
Very little is known regarding the functional effect of p12 and p8 interactions with the V-ATPase enzymatic complex. The V-ATPases are enzymes composed of multiple subunits that acidifies intracellular organelles, secretory pathway and regulate receptor recycling and degradation(Toei, Saum, and Forgac ,2010). The p12 and p8 proteins might have different effects on the V-ATPase located in endosomal compartments or in the cellular membrane. Modulation of the V- ATPase, activity might be particularly important for virus entry and egress in T-cell and dendritic cells.
The use of reverse genetic, in combination with functional studies of natural orf-I mutants of HTLV- 1 infected individuals, will hopefully elucidate the impact of the p12 and p8 functional domains that affect the level of provirus DNA in the host. This information is of great importance because HTLV-1 provirus levels predict disease progression in humans. Thus, the understanding of the underlying mechanisms of p12 and p8 function may facilitate the development of therapeutic approaches interfere with virus persistence in the infected host.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. We thank Teresa Habina for editorial assistance. We are very grateful to Dustin Edwards and Shari Gordon for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Albrecht B, D’Souza CD, Ding W, Tridandapani S, Coggeshall KM, Lairmore MD. Activation of nuclear factor of activated T cells by human T-lymphotropic virus type 1 accessory protein p12(I) J.Virol. 2002;76:3493–3501. doi: 10.1128/JVI.76.7.3493-3501.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan JS, Leland M, Broussard S, Mone J, Hubbard G. Simian T-cell lymphotropic Viruses (STLVs) and lymphomas in African nonhuman primates. Cancer Invest. 2001;19:383–395. doi: 10.1081/cnv-100103133. [DOI] [PubMed] [Google Scholar]
- Andresen V, Pise-Masison C, Sinha-Datta U, Parks RW, Cecchinato V, Fukumoto R, Nicot C, Franchini G. Suppression of HLV-I Replication by Tax –mediated Re-routing of the p13 Viral Protein to Nuclear Speckles. 2010 doi: 10.1182/blood-2010-06-293340. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold J, Yamamoto B, Li M, Phipps AJ, Younis I, Lairmore MD, Green PL. Enhancement of infectivity and persistence in vivo by HBZ, a natural antisense coded protein of HTLV-1. Blood. 2006;107:3976–3982. doi: 10.1182/blood-2005-11-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee P, Feuer G, Barker E. Human T-cell leukemia virus type 1 (HTLV-1) p12I down-modulates ICAM-1 and -2 and reduces adherence of natural killer cells, thereby protecting HTLV-1-infected primary CD4+ T cells from autologous natural killer cell-mediated cytotoxicity despite the reduction of major histocompatibility complex class I molecules on infected cells. J.Virol. 2007;81:9707–9717. doi: 10.1128/JVI.00887-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baydoun HH, Bellon M, Nicot C. HTLV-1 Yin and Yang: Rex and p30 master regulators of viral mRNA trafficking. AIDS Rev. 2008;10:195–204. [PMC free article] [PubMed] [Google Scholar]
- Beby-Defaux A, Frugier F, Bourgoin A, Moynet D, Hajjar C, Sainte-Foie S, Guillemain B, Agius G. Nucleotide sequence analysis of human T-cell lymphotropic virus type I pX and LTR regions from patients with sicca syndrome. J.Med.Virol. 1999;59:245–255. doi: 10.1002/(sici)1096-9071(199910)59:2<245::aid-jmv20>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Beilke MA, Traina-Dorge V, England JD, Blanchard JL. Polymyositis, arthritis, and uveitis in a macaque experimentally infected with human T lymphotropic virus type I. Arthritis Rheum. 1996;39:610–615. doi: 10.1002/art.1780390410. [DOI] [PubMed] [Google Scholar]
- Berneman ZN, Gartenhaus RB, Reitz MS, Jr., Blattner WA, Manns A, Hanchard B, Ikehara O, Gallo RC, Klotman MK. Expression of alternatively spliced human T-lymphotropic virus type I (HTLV-I) pX mRNA in infected cell lines and in primary uncultured cells from patients with adult T-cell leukemia/lymphoma and healthy carriers. Proc.Natl.Acad.Sci.USA. 1992;89:3005–3009. doi: 10.1073/pnas.89.7.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn PA, Jr., Schechter GP, Jaffe E, Blayney D, Young RC, Matthews MJ, Blattner W, Broder S, Robert-Guroff M, Gallo RC. Clinical course of retrovirus-associated adult T-cell lymphoma in the United States. N.Engl.J.Med. 1983;309:257–264. doi: 10.1056/NEJM198308043090501. [DOI] [PubMed] [Google Scholar]
- Cereseto A, Berneman Z, Koralnik I, Vaughn J, Franchini G, Klotman ME. Differential expression of alternatively spliced pX mRNAs in HTLV-I-infected cell lines. Leukemia. 1997;11:866–870. doi: 10.1038/sj.leu.2400665. [DOI] [PubMed] [Google Scholar]
- Chow AY, Mellman I. Old lysosomes, new tricks: MHC II dynamics in DCs. Trends Immunol. 2005;26:72–78. doi: 10.1016/j.it.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Ciminale V, D’Agostino DM, Zotti L, Franchini G, Felber BK, Chieco-Bianchi L. Expression and characterization of proteins produced by mRNAs spliced into the X region of the human T-cell leukemia/lymphotropic virus type II. Virology. 1995;209:445–456. doi: 10.1006/viro.1995.1277. [DOI] [PubMed] [Google Scholar]
- Ciminale V, Pavlakis GN, Derse D, Cunningham CP, Felber BK. Complex splicing in the human T-cell leukemia virus (HTLV) family of retroviruses: novel mRNAs and proteins produced by HTLV-I. J.Virol. 1992;66:1737–1745. doi: 10.1128/jvi.66.3.1737-1745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciminale V, Zotti L, D’Agostino DM, Ferro T, Casareto L, Franchini G, Bernardi P, Chieco-Bianchi L. Mitochondrial targeting of the p13II protein coded by the x-II ORF of human T-cell leukemia/lymphotropic virus type I (HTLV-I) Oncogene. 1999;18:4505–4514. doi: 10.1038/sj.onc.1203047. [DOI] [PubMed] [Google Scholar]
- Collins ND, Newbound GC, Albrecht B, Beard JL, Ratner L, Lairmore MD. Selective ablation of human T-cell lymphotropic virus type 1 p12I reduces viral infectivity in vivo. Blood. 1998;91:4701–4707. [PubMed] [Google Scholar]
- Dekaban GA, Peters AA, Mulloy JC, Johnson JM, Trovato R, Rivadeneira E, Franchini G. The HTLV-I orf I protein is recognized by serum antibodies from naturally infected humans and experimentally infected rabbits. Virology. 2000;274:86–93. doi: 10.1006/viro.2000.0406. [DOI] [PubMed] [Google Scholar]
- Derse D, Mikovits J, Ruscetti F. X-I and X-II open reading frames of HTLV-I are not required for virus replication or for immortalization of primary T-cells in vitro. Virology. 1997;237:123–128. doi: 10.1006/viro.1997.8781. [DOI] [PubMed] [Google Scholar]
- Ding W, Albrecht B, Kelley RE, Muthusamy N, Kim SJ, Altschuld RA, Lairmore MD. Human T-cell lymphotropic virus type 1 p12(I) expression increases cytoplasmic calcium to enhance the activation of nuclear factor of activated T cells. J.Virol. 2002;76:10374–10382. doi: 10.1128/JVI.76.20.10374-10382.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Albrecht B, Luo R, Zhang W, Stanley JR, Newbound GC, Lairmore MD. Endoplasmic reticulum and cis-Golgi localization of human T-lymphotropic virus type 1 p12(I): association with calreticulin and calnexin. J Virol. 2001;75:7672–7682. doi: 10.1128/JVI.75.16.7672-7682.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Kim SJ, Nair AM, Michael B, Boris-Lawrie K, Tripp A, Feuer G, Lairmore MD. Human T-cell lymphotropic virus type 1 p12I enhances interleukin-2 production during T-cell activation. J.Virol. 2003;77:11027–11039. doi: 10.1128/JVI.77.20.11027-11039.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KA, Golenbock DT. Immunology. The shape of things to come. Science. 2007;316:1574–1576. doi: 10.1126/science.1144483. [DOI] [PubMed] [Google Scholar]
- Franchini G. Molecular mechanisms of human T-cell leukemia/lymphotropic virus type I infection. Blood. 1995;86:3619–3639. [PubMed] [Google Scholar]
- Franchini G, Lairmore MD. Fields Virology. L.W.&.W. (Ed.); Philadelphia: 2007. Human T-cell leukemia/lymphoma virus types 1 and 2; pp. 2071–2106. [Google Scholar]
- Franchini G, Mulloy JC, Koralnik IJ, Lo Monico A, Sparkowski JJ, Andresson T, Goldstein DJ, Schlegel R. The human T-cell leukemia/lymphotropic virus type I p12I protein cooperates with the E5 oncoprotein of bovine papillomavirus in cell transformation and binds the 16-kilodalton subunit of the vacuolar H+ ATPase. J.Virol. 1993;67:7701–7704. doi: 10.1128/jvi.67.12.7701-7704.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto R, Andresen V, Bialuk I, Cecchinato V, Walser JC, Valeri VW, Nauroth JM, Gessain A, Nicot C, Franchini G. In vivo genetic mutations define predominant functions of the human T-cell leukemia/lymphoma virus p12I protein. Blood. 2009;113:3726–3734. doi: 10.1182/blood-2008-04-146928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto R, Dundr M, Nicot C, Adams A, Valeri VW, Samelson LE, Franchini G. Inhibition of T-Cell Receptor Signal Transduction and Viral Expression by the Linker for Activation of T Cells-Interacting p12I Protein of Human T-Cell Leukemia/Lymphoma Virus Type 1. J.Virol. 2007;81:9088–9099. doi: 10.1128/JVI.02703-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K, Furukawa K, Shiku H. Alternatively spliced mRNA of the pX region of human T-lymphotropic virus type 1 proviral genome. FEBS Lett. 1991;295:141–145. doi: 10.1016/0014-5793(91)81404-v. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Usuku K, Izumo S, Osame M. Human T cell lymphotropic virus type I (HTLV-I) p12I is dispensable for HTLV-I transmission and maintenance of infection in vivo. AIDS Res.Hum.Retroviruses. 2004;20:1092–1099. doi: 10.1089/aid.2004.20.1092. [DOI] [PubMed] [Google Scholar]
- Gaudray G, Gachon F, Basbous J, Biard-Piechaczyk M, Devaux C, Mesnard JM. The complementary strand of the human T-cell leukemia virus type 1 RNA genome encodes a bZIP transcription factor that down-regulates viral transcription. J.Virol. 2002;76:12813–12822. doi: 10.1128/JVI.76.24.12813-12822.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessain A. Virological aspects of tropical spastic paraparesis/HTLV-I associated myelopathy and HTLV-I infection. J Neurovirol. 1996;2:299–306. doi: 10.3109/13550289609146894. [DOI] [PubMed] [Google Scholar]
- Hajjar C, Sainte-Foie S, Savin J, Lacave J, Berlet F, Teron-Aboud B, Batelier L, Guillemin B. [HTLV1 infection and sicca syndrome] J.Fr.Ophtalmol. 1995;18:597–602. [PubMed] [Google Scholar]
- Igakura T, Stinchcombe JC, Goon PK, Taylor GP, Weber JN, Griffiths GM, Tanaka Y, Osame M, Bangham CR. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science. 2003;299:1713–1716. doi: 10.1126/science.1080115. [DOI] [PubMed] [Google Scholar]
- Iniguez AM, Otsuki K, Magalhaes GP, Silva EA, Vicente AC. Genetic markers on the HTLV-1 p12I protein sequences from Brazilian HAM/TSP patients and asymptomatic HTLV-1 carrier isolates. AIDS Res.Hum.Retroviruses. 2005;21:580–582. doi: 10.1089/aid.2005.21.580. [DOI] [PubMed] [Google Scholar]
- Jacobson S, Shida H, McFarlin DE, Fauci AS, Koenig S. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature. 1990;348:245–248. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- Johnson J, Nicot C, Fullen J, Ciminale V, Casareto L, Mulloy JC, Jacobson S, Franchini G. Free Major Histocompatibility Complex Class I Heavy Chain Is Preferentially Targeted for Degradation by Human T-Cell Leukemia/Lymphotropic Virus Type 1 p12I Protein. J.Virol. 2001:6086–6094. doi: 10.1128/JVI.75.13.6086-6094.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JM, Harrod R, Franchini G. Molecular biology and pathogenesis of the human T-cell leukaemia/lymphotropic virus Type-1 (HTLV-1) Int J Exp Pathol. 2001;82:135–147. doi: 10.1046/j.1365-2613.2001.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JM, Mulloy JC, Ciminale V, Fullen J, Nicot C, Franchini G. The MHC class I heavy chain is a common target of the small proteins encoded by the 3′ end of HTLV type 1 and HTLV type 2. AIDS Res.Hum.Retroviruses. 2000;16:1777–1781. doi: 10.1089/08892220050193308. [DOI] [PubMed] [Google Scholar]
- Jones KS, Petrow-Sadowski C, Huang YK, Bertolette DC, Ruscetti FW. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4(+) T cells. Nat.Med. 2008;14:429–436. doi: 10.1038/nm1745. [DOI] [PubMed] [Google Scholar]
- Kannagi M, Matsushita S, Harada S. Expression of the target antigen for cytotoxic T lymphocytes on adult T-cell-leukemia cells. Int.J.Cancer. 1993;54:582–588. doi: 10.1002/ijc.2910540411. [DOI] [PubMed] [Google Scholar]
- Kannagi M, Matsushita S, Shida H, Harada S. Cytotoxic T cell response and expression of the target antigen in HTLV-I infection. Leukemia. 1994;8(Suppl 1):S54–S59. [PubMed] [Google Scholar]
- Kim SJ, Ding W, Albrecht B, Green PL, Lairmore MD. A conserved calcineurin-binding motif in human T lymphotropic virus type 1 p12I functions to modulate nuclear factor of activated T cell activation. J.Biol.Chem. 2003;278:15550–15557. doi: 10.1074/jbc.M210210200. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Nair AM, Fernandez S, Mathes L, Lairmore MD. Enhancement of LFA-1-mediated T cell adhesion by human T lymphotropic virus type 1 p12I1. J.Immunol. 2006;176:5463–5470. doi: 10.4049/jimmunol.176.9.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koralnik I, Gessain A, Klotman ME, Lo Monico A, Berneman ZN, Franchini G. Protein isoforms encoded by the pX region of the human T-cell leukemia/lymphotropic virus type I. Proc.Natl.Acad.Sci.USA. 1992;89:8813–8817. doi: 10.1073/pnas.89.18.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koralnik I, Mulloy JC, Andresson T, Fullen J, Franchini G. Mapping of the intermolecular association of the human T-cell leukemia/lymphotropic virus type 1 p12I and the vacuolar H+ATPase 16 kDa subunit protein. J.Gen.Virol. 1995;76:1909–1916. doi: 10.1099/0022-1317-76-8-1909. [DOI] [PubMed] [Google Scholar]
- Koralnik IJ, Fullen J, Franchini G. The p12I, p13II, and p30II proteins encoded by human T-cell leukemia/lymphotropic virus type I open reading frames I and II are localized in three different cellular compartments. J.Virol. 1993;67:2360–2366. doi: 10.1128/jvi.67.4.2360-2366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lairmore MD, Silverman L, Ratner L. Animal models for human T-lymphotropic virus type 1 (HTLV-1) infection and transformation. Oncogene. 2005;24:6005–6015. doi: 10.1038/sj.onc.1208974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Kesic M, Yin H, Yu L, Green PL. Kinetic analysis of human T-cell leukemia virus type 1 gene expression in cell culture and infected animals. J.Virol. 2009;83:3788–3797. doi: 10.1128/JVI.02315-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HC, Hickey M, Hsu L, Medina D, Rabson AB. Activation of human T cell leukemia virus type 1 LTR promoter and cellular promoter elements by T cell receptor signaling and HTLV-1 Tax expression. Virology. 2005;339:1–11. doi: 10.1016/j.virol.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Majorovits E, Nejmeddine M, Tanaka Y, Taylor GP, Fuller SD, Bangham CR. Human T-lymphotropic virus-1 visualized at the virological synapse by electron tomography. PLoS.One. 2008;3:e2251. doi: 10.1371/journal.pone.0002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DL, Popovic M, Sarin P, Murray C, Reitz MS, Jr., Strong DM, Haynes BF, Gallo RC, Blattner WA. Cell lines producing human T-cell lymphoma virus show altered HLA expression. Nature. 1983;305:58–60. doi: 10.1038/305058a0. [DOI] [PubMed] [Google Scholar]
- Manz BN, Groves JT. Spatial organization and signal transduction at intercellular junctions. Nat.Rev.Mol.Cell Biol. 2010;11:342–352. doi: 10.1038/nrm2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshansky V, Futai M. The V-type H+-ATPase in vesicular trafficking: targeting, regulation and function. Curr.Opin.Cell Biol. 2008;20:415–426. doi: 10.1016/j.ceb.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins ML, Soares BC, Ribas JG, Thorun GW, Johnson J, Kroon EG, Carneiro-Prioetti AB, Bonjardim CA. Frequency of p12K and p12R alleles of HTLV Type 1 in HAM/TSP patients and in asymptomatic HTLV type 1 carriers. AIDS Res.Hum.Retroviruses. 2002;18:899–902. doi: 10.1089/088922202760265560. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Green PL. The HBZ gene, a key player in HTLV-1 pathogenesis. Retrovirology. 2009;6:71. doi: 10.1186/1742-4690-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael B, Nair AM, Hiraragi H, Shen L, Feuer G, Boris-Lawrie K, Lairmore MD. Human T lymphotropic virus type-1 p30II alters cellular gene expression to selectively enhance signaling pathways that activate T lymphocytes. Retrovirology. 2004;1:39. doi: 10.1186/1742-4690-1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migone TS, Lin JX, Cereseto A, Mulloy JC, O’Shea JJ, Franchini G, Leonard WJ. Constitutively activated JAK-STAT pathway in T-cells transformed with HTLV-I. Science. 1995;269:79–81. doi: 10.1126/science.7604283. [DOI] [PubMed] [Google Scholar]
- Mulloy JC, Crowley RW, Fullen J, Leonard WJ, Franchini G. The human T-cell leukemia/lymphotropic virus type I p12I protein binds the interleukin-2 receptor β and γc chains and affects their expression on the cell surface. J.Virol. 1996;70:3599–3605. doi: 10.1128/jvi.70.6.3599-3605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Eguchi K, Nakamura T, Mizokami A, Shirabe S, Kawakami A, Matsuoka N, Migita K, Kawabe Y, Nagataki S. High prevalence of Sjogren’s syndrome in patients with HTLV-I associated myelopathy. Ann.Rheum.Dis. 1997;56:167–172. doi: 10.1136/ard.56.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negulescu PA, Shastri N, Cahalan MD. Intracellular calcium dependence of gene expression in single T lymphocytes. Proc.Natl.Acad.Sci.U.S.A. 1994;91:2873–2877. doi: 10.1073/pnas.91.7.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicot C, Dundr M, Johnson JM, Fullen JR, Alonzo N, Fukumoto R, Princler GL, Derse D, Misteli T, Franchini G. HTLV-1-encoded p30(II) is a post-transcriptional negative regulator of viral replication. Nat.Med. 2004;10:197–201. doi: 10.1038/nm984. [DOI] [PubMed] [Google Scholar]
- Nicot C, Mulloy JC, Ferrari MG, Johnson JM, Fu K, Fukumoto R, Trovato R, Fullen J, Leonard WJ, Franchini G. HTLV-1 p12(I) protein enhances STAT5 activation and decreases the interleukin-2 requirement for proliferation of primary human peripheral blood mononuclear cells. Blood. 2001;98:823–829. doi: 10.1182/blood.v98.3.823. [DOI] [PubMed] [Google Scholar]
- Parker CE, Daenke S, Nightingale S, Bangham CR. Activated, HTLV-1-specific cytotoxic T-lymphocytes are found in healthy seropositives as well as in patients with tropical spastic paraparesis. Virology. 1992;188:628–636. doi: 10.1016/0042-6822(92)90517-s. [DOI] [PubMed] [Google Scholar]
- Pique C, Ureta-Vidal A, Gessain A, Chancerel B, Gout O, Tamouza R, Agis F, Dokhelar MC. Evidence for the chronic in vivo production of human T cell leukemia virus type I Rof and Tof proteins from cytotoxic T lymphocytes directed against viral peptides. J.Exp.Med. 2000;191:567–572. doi: 10.1084/jem.191.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc.Natl.Acad.Sci.USA. 1980a;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz BJ, Ruscetti FW, Mier JW, Woods AM, Gallo RC. T-cell lines established from human T-lymphocytic neoplasias by direct response to T-cell growth factor. Proc.Natl.Acad.Sci.U.S.A. 1980b;77:6815–6819. doi: 10.1073/pnas.77.11.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robek MD, Wong F-H, Ratner L. Human T-cell leukemia virus type 1 pX-I and pX-II open reading frames are dispensable for the immortalization of primary lymphocytes. J.Virol. 1998;72:4458–4462. doi: 10.1128/jvi.72.5.4458-4462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satou Y, Yasunaga J, Yoshida M, Matsuoka M. HTLV-I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc.Natl.Acad.Sci.U.S.A. 2006;103:720–725. doi: 10.1073/pnas.0507631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silic-Benussi M, Cannizzaro E, Venerando A, Cavallari I, Petronilli V, La RN, Marin O, Chieco-Bianchi L, Di LF, D’Agostino DM, Bernardi P, Ciminale V. Modulation of mitochondrial K(+) permeability and reactive oxygen species production by the p13 protein of human T-cell leukemia virus type 1. Biochim.Biophys.Acta. 2009;1787:947–954. doi: 10.1016/j.bbabio.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Silic-Benussi M, Marin O, Biasiotto R, D’Agostino DM, Ciminale V. Effects of human T-cell leukemia virus type 1 (HTLV-1) p13 on mitochondrial K+ permeability: A new member of the viroporin family? FEBS Lett. 2010;584:2070–2075. doi: 10.1016/j.febslet.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KA, Jacobson EL, Emert R, Giordano M, Kovacs E, Mumneh N, Pilaro F, Sohn T, Warren D. Restoration of immunity with interleukin-2 therapy. AIDS Read. 1999;9:563–572. [PubMed] [Google Scholar]
- Sonoda S, Yashiki S, Takahashi K, Arima N, Daitoku Y, Matsumoto M, Matsumoto T, Tara M, Shinmyozu K, Sato K. Altered HLA antigens expressed on T and B lymphocytes of adult T-cell leukemia/lymphoma patients and their relatives. Int.J.Cancer. 1987;40:629–634. doi: 10.1002/ijc.2910400510. [DOI] [PubMed] [Google Scholar]
- Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Kohler K, Oddos S, Eissmann P, Brodsky FM, Hopkins C, Onfelt B, Sattentau Q, Davis DM. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat.Cell Biol. 2008;10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- Takatsuki K. Discovery of adult T-cell leukemia. Retrovirology. 2005;2:16. doi: 10.1186/1742-4690-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taner SB, Onfelt B, Pirinen NJ, McCann FE, Magee AI, Davis DM. Control of immune responses by trafficking cell surface proteins, vesicles and lipid rafts to and from the immunological synapse. Traffic. 2004;5:651–661. doi: 10.1111/j.1600-0854.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- Taylor JM, Brown M, Nejmeddine M, Kim KJ, Ratner L, Lairmore M, Nicot C. Novel role for interleukin-2 receptor-Jak signaling in retrovirus transmission. J Virol. 2009;83:11467–11476. doi: 10.1128/JVI.00952-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toei M, Saum R, Forgac M. Regulation and isoform function of the V-ATPases. Biochemistry. 2010;49:4715–4723. doi: 10.1021/bi100397s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traina-Dorge VL, Martin LN, Lorino R, Winsor EL, Beilke MA. Human T cell leukemia virus type 1 up-regulation after simian immunodeficiency virus-1 coinfection in the nonhuman primate. J Infect.Dis. 2007;195:562–571. doi: 10.1086/510914. [DOI] [PubMed] [Google Scholar]
- Trovato R, Mulloy JC, Johnson JM, Takemoto S, de Oliveira MP, Franchini G. A Lysine-to-Arginine change found in natural alleles of the HTLV-I p12I protein greatly influences its stability. J.Virol. 1999;73:6460–6467. doi: 10.1128/jvi.73.8.6460-6467.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno H, Matsuoka H, Suzuki M, Tsuda K, Tsubouchi H. Altered expression of class I HLA antigen on peripheral mononuclear cells in patients with adult T-cell leukemia: inverse relationship with natural killer susceptibility. Cancer Epidemiol.Biomarkers Prev. 1995;4:367–372. [PubMed] [Google Scholar]
- Valeri V, Hryniewicz A, Andresen V, Jones KS, Fenizia C, Bialuk I, Chung HK, Fukumoto R, Parks RW, Ferrari MG, Nicot C, Cecchinato V, Ruscetti F, Franchini G. Requirement of the Human T-cell Leukemia Virus p12 and p30 Genes for Infectivity of Human Dendritic Cells and Macaques but not Rabbits. Blood. 2010 doi: 10.1182/blood-2010-05-284141. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Prooyen N, Gold H, Andresen V, Schwartz O, Jones K, Rucetti F, Lockett S, Prabhakar G, Venzon D, Franchini G. The HTLV-1 p8 protein increases cellular conduits and virus transmission. 2010 doi: 10.1073/pnas.1009635107. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. The N-end rule: functions, mysteries, uses. Proc.Natl.Acad.Sci.USA. 1996;93:12142–12149. doi: 10.1073/pnas.93.22.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernant JC, Buisson G, Magdeleine J, De TJ, Jouannelle A, Neisson-Vernant C, Monplaisir N. T-lymphocyte alveolitis, tropical spastic paresis, and Sjogren syndrome. Lancet. 1988;1:177. doi: 10.1016/s0140-6736(88)92744-4. [DOI] [PubMed] [Google Scholar]
- Watkins SC, Salter RD. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 2005;23:309–318. doi: 10.1016/j.immuni.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Wu AJ, Fox PC. Sjogren’s syndrome. Semin.Dermatol. 1994;13:138–143. [PubMed] [Google Scholar]
- Wulfing C, Davis MM. A receptor/cytoskeletal movement triggered by costimulation during T cell activation. Science. 1998;282:2266–2269. doi: 10.1126/science.282.5397.2266. [DOI] [PubMed] [Google Scholar]
- Xu X, Kang SH, Heidenrich O, Okerholm M, O’Shea JJ, Nerenberg MI. Constitutive activation of different Jak tyrosine kinases in human T-cell leukemia virus type 1 (HTLV-1) tax protein or virus-transformed cells. J.Clin.Invest. 1995;96:1548–1555. doi: 10.1172/JCI118193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarchoan R, Guo HG, Reitz M, Jr., Maluish A, Mitsuya H, Broder S. Alterations in cytotoxic and helper T cell function after infection of T cell clones with human T cell leukemia virus, type I. J.Clin.Invest. 1986;77:1466–1473. doi: 10.1172/JCI112459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, McKeon F. Nucleocytoplasmic shuttling and the control of NF-AT signaling. Cell Mol.Life Sci. 2000;57:411–420. doi: 10.1007/PL00000703. [DOI] [PMC free article] [PubMed] [Google Scholar]