Abstract
Lissencephaly is a severe human neuronal migration defect characterized by a smooth cerebral surface, mental retardation and seizures. The two most common genes mutated in patients with lissencephaly are LIS1 and DCX. LIS1 was the first gene cloned that was important for neuronal migration in any organism, and heterozygous mutations or deletions of LIS1 are found in the majority of patients with lissencephaly, while DCX mutations were found in males with X-linked lissencephaly. In this review, we will discuss how an understanding of the molecular and cellular pathways disrupted in model organisms with Lis1 and Dcx mutations or knock-down not only provide insights into the normal processes of neuronal migration, including neurogenesis, but they also may lead to potential novel therapeutic strategies for these severe cortical malformations.
Keywords: Neurogenetic disease, Neuronal migration, Neurogenesis, Lissencephaly, Therapy
The human brain is the most elaborate structure known and comprised of an integrated network of more than 100 billion neuronal cells. The formation of this intricate neuronal network requires the precise choreography of neurogenesis, neuronal migration, pruning and synaptogenesis during development. A fundamental problem in developmental neurobiology is to understand the genetic and biochemical pathways that regulate the carefully choreographed production and migration of immature neurons to their final location in the adult brain. In this review, we will briefly discuss normal neurogenesis and neuronal migration. Then we will discuss the cloning the two most common genes mutated in the severe neuronal migration lissencephaly, LIS1 and DCX. Then, functional studies of these genes in model organisms will be described, which have led to a basic understanding of many of the pathways and processes that these genes involved in. Remarkably, these studies also led to two therapeutic approaches in model organisms that provided substantial phenotypic rescue, and provide additional support for the argument that a complete understanding of mechanisms of actions of disease genes can lead to novel therapeutic strategies, even when those diseases are severe neurogenetic disorders.
Neurogenesis and neuronal migration during cortical development
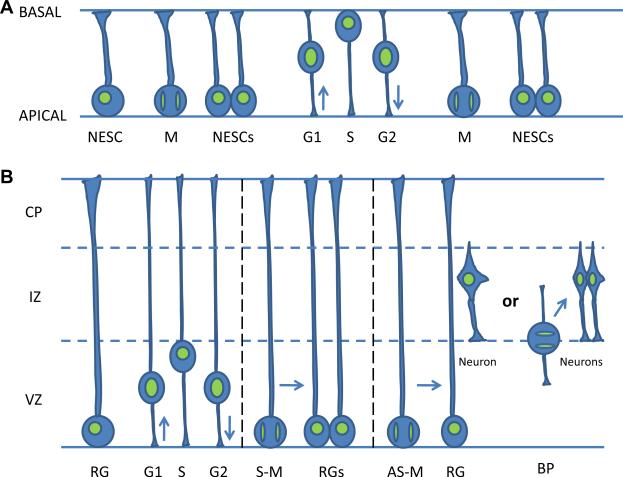
Neurogenesis and neuronal migration have been studied extensively for over thirty years in diverse mammalian species from the mouse to human (reviewed in Gupta et al. 2002; Tsai and Gleeson 2005; Ayala et al. 2007). The basic sequence of events that occurs during cortical development is shared by these species, although the timing of events is species dependent: in humans they occur between 5th and 22nd gestational weeks, while in the mouse they occur between embryonic day 11 (E11) and 19 (E19). Prior to E11 in the mouse, the neural tube consists of a single layer of neuroepithelial stem cells (NESCs) that proliferate rapidly. Each NESC stretches from the apical to the basal surface of the neuroepithelium, and as the cell progresses through the cell cycle, the nuclei display interkinetic nuclear migration (Fig. 1). Nuclei divide at the apical surface, and synthesize DNA at the basal surface of the neural epithelium. Consequently, nuclei from cells in G1 migrate from the apical to basal surface, while nuclei from cells in G2 move from the basal to apical surface. At about E11, shortly after closure of the rostral neural tube, the neuroepithelial stem cells further differentiate into a more restricted progenitor, radial glial progenitor cells (RGPCs), that also display interkinetic nuclear migration, similar to the neuroepithelial stem cells, but only in the VZ, a proliferative zone at the apical surface (Fig. 1). A subpopulation of these cells become postmitotic and begin to move radially out from the VZ at about E12 along the extensions of the radial glial progenitors, which are bipolar-shaped in the VZ but then convert to a multipolar morphology within the subventricular zone (SVZ) and migrate into the intermediate zone (IZ). A switch from the multipolar state back to a bipolar morphology accompanies the radial glia guided locomotion of projection neurons towards the cortical plate (CP), with the trailing process concomitantly developing into the axon. Once the neuron arrives in the CP, the leading process attaches to the pial surface and the neuron undergoes a terminal somal translocation step to reach its final location (Nadarajah et al., 2001; Noctor et al., 2004; Tsai et al., 2005). Classical studies have established that the cerebral cortex is formed by an “inside-out” migration of cells from the ventricular zone to form layers 6 through 2. The early arising neuroblasts that migrate first to the cortical plate occupy the deepest cortical layers in the adult. The later born migrating young neurons migrate past the earlier established cortical plate cells to occupy more superficial layers of the adult cortex. In addition to radial migration of cortical projection neurons, the inhibitory interneurons migrate tangentially from the medial (MGE) and lateral (LGE) ganglionic eminences.
Fig 1.
Neurogenesis in (A) neuroepithelial stem cells (NESC) and (B) radial glial progenitor cells (RGPC). In NESC, nuclei undergo interkinetic nuclear migration from apical to basal surfaces, and divide symmetrically and vertically to produce NESCs. In RGPCs, interkinetic nuclear migration occurs only in the ventricular zone (VZ). At the ventricular zone, the RGPCs divide mostly vertically, but this division can be symmetric (S-M) to produce two RGPCs, or asymmetric (AS-M) to produce one RGPC and either one neuron or one basal progenitor (BP). This BP moves to the subventricular zone at the border of the VZ and intermediate zone (IZ), where it divides once symmetrically and horizontally to produce two neurons. Neurons produced by either of these two ways migrate into the cortical plate (CP), where they set up the cortical layers in an inside-out fashion.
Human lissencephaly: identification of LIS1 and DCX as causative genes
One of the most striking abnormalities of human neuronal migration is lissencephaly (or “smooth brain”), a term originally used to describe the smooth cerebral surface of lower mammals (reviewed in Kato and Dobyns 2003). Lissencephaly of the human brain is a severe malformation in which the brain has a smooth cerebral surface rather than the characteristic gyri and sulci that make the human and primate brain instantly recognizable. The primary defect underlying the smoothening of the brain of lissencephalic patients is defective neuronal migration, although defective neurogenesis may also contribute. The inability of postmitotic neurons to reach their final destination and correctly populate the cortical plate of the cerebral cortex consequently leads to abnormal cortical thickness and reduced or absent gyri and sulci of its surface. There are two major types of classical lissencephaly: isolated lissencephaly sequence (ILS) and Miller-Dieker syndrome (MDS). Isolated lissencephaly sequence (ILS) is a heterogeneous disorder consisting of variably severe lissencephaly with no other major malformations such as craniofacial dysmorphism. Miller-Dieker syndrome (MDS) consists of more severe lissencephaly than ILS patients, characteristic facial anomalies (high forehead, a small nose with anteverted nares, thin vermilion border, and micrognathia), and occasionally other malformations (Dobyns et al. 1984). Children with ILS and MDS are severely retarded and suffer from epilepsy (Dobyns et al. 1992). These disorders are fatal in early childhood. MDS (100%) and some cases of ILS (40%) are the result of haploinsufficiency at human chromosome 17p13.3, with visible or submicroscopic deletions detectable by FISH (Dobyns et al. 1994). The LIS1 gene was cloned from this region (Reiner et al. 1993). LIS1 was disrupted in an ILS patient with a translocation, and several other key MDS patients (Chong et l. 1997; LoNigro et al. 1997).
There are X-linked forms of lissencephaly. The major cause of X-linked lissencephaly is mutation of the Doublecortin (DCX) gene (des Portes et al. 1998; Gleeson et al. 1998). Mutations in the DCX gene cause gross neocortical disorganization and lissencephaly in hemizygous males, while heterozygous females show a mosaic phenotype with a normal cortex as well as a second band of misplaced (heterotopic) neurons beneath the cortex (“double cortex syndrome”). Although several other lissencephaly genes have been identified, the majority of patients with lissencephaly display mutations in either LIS1 or DCX (Pilz et al. 1998).
Mouse models for Lis1 and Dcx: in vivo functional studies
Lis1
Mouse models for lissencephalies have aided in the understanding of the function of LIS1 and the pathways associated with it during brain development (Vallee and Tsai 2006; Wynshaw-Boris 2007). We produced two knock-out and one conditional knock-out Lis1 mutant alleles by gene targeting in the mouse (Hirotsune et al. 1998). The two knock-out alleles (one from germline Cre-mediated deletion of the conditional knock-out allele) are nulls, while the conditional allele is hypomorphic, due to the disruption of transcription or splicing by the insertion of PGKneo in intron 3. By mating mice with various Lis1 alleles, mice were produced with graded reduction in LIS1 dosage. These mice exhibited a LIS1 dose-dependent disorganized cortical layers, hippocampus, cerebellum and olfactory bulb due to cell autonomous neuronal migration defects, and are a good model for the human disorder. Complete loss of LIS1 results in peri-implantation lethality, a result confirmed in another Lis1 knock-out (Cahana et al. 2001), demonstrating that Lis1 is an essential gene. Further studies demonstrated impairments of motor coordination and cognition in Lis1-null heterozygotes and a severe disruption of hippocampal cellular and synaptic physiology in Lis1 mutant mice (Paylor et al. 1999; Fleck et al. 2000). Several lines of evidence support the conclusion that there are in vivo migrational defects in mice with reduction of LIS1 dosage, including histological analysis during development, BrdU birthdating experiments, and in vitro migration of granule cells from cerebellar cell reaggregates (Hirotsune et al. 1998; Gambello et al. 2003). It appears that LIS1 is required for nuclear movement during neuronal migration by coupling the nucleus to the centrosome (Tanaka et al. 2004a). LIS1 is localized to the centrosome with some extension toward the nucleus. Consistent with an important role in dynein function (see below), inhibition of dynein by overexpression of dynamitin resulted in a similar phenotype. More recently, it has been possible to directly examine neuronal migration in slice cultures from embryonic cortex. Lipophillic dyes or in utero transfection with GFP are used to label the migrating cells and migration is recorded by confocal time-lapse videomicroscopy. These studies definitively demonstrated that LIS1 is required for neuronal migration and nuclear movements (Shu et al. 2004; Tsai et al. 2005; Youn et al. 2009).
LIS1 also is required for the normal generation and survival of cortical ventricular zone neuroblasts (Gambello et al. 2003), suggesting that LIS1 is important for cell proliferation during neurogenesis as well as for neuronal survival. LIS1 knock-down was associated with neural stem cell division (Tsai et al. 2005). LIS1 is colocalized with cytoplasmic dynein at the mitotic kinetochores, indicating a role of LIS1 in chromosomal behavior and microinjection of anti-Lis1 antibody resulted in a delay of mitotic progression accompanied with chromosomal defects at the metaphase plate. LIS1 is also expressed at the cortical (marginal) area of proliferating epithelial cells, and its over-expression results in disruption of the mitotic spindle organization as a result of reductions in cortical distribution of dynein (Faulkner et al. 2000). More recently, we have uncovered an essential role for Lis1 in neurogenesis and spindle orientation (Yingling et al. 2008, see below).
Dcx
We produced a Dcx mutant mouse (Corbo et al. 2002) carrying a targeted mutation in the Dcx gene. Hemizygous male Dcx mice display postnatal lethality, and the few that survive to adulthood are variably fertile. Dcx mutant mice show neocortical lamination that is largely indistinguishable from wild-type and show normal patterns of neocortical neurogenesis and neuronal migration. In contrast, the hippocampus of both heterozygous females and hemizygous males shows disrupted lamination that is most severe in the CA3 region. Behavioral tests show defects in context and cued conditioned fear tests, suggesting deficits in hippocampal learning that accompany the abnormal cytoarchitecture. By contrast, siRNA inhibition of Dcx in rat embryonic cortical slices results in severe defects in neuronal migration (Bai et al. 2003). DCX is part of a gene family that includes DCKL. Dcx and Dckl display functional redundancy, since double Dcx/Dckl mutants display more severe developmental defects in the cortex and hippocampus (Koizumi et al. 2006; Tanaka et al. 2006)
LIS1: a conserved nuclear distribution (Nud) pathway and the regulation of cytoplasmic dynein
Neuronal migration is driven by complex mechanisms that are orchestrated by many proteins that interact mainly, but not exclusively, to promote the recruitment, organization, stability and movement/function of microtubules and actin cytoskeleton. LIS1 is an atypical microtubule associated protein (MAP), and is part of a highly conserved pathway that regulates nuclear migration in the bread mold Aspergillus nidulans (Morris 2000). In this organism, nuclei migrate towards the growing tip of hyphae to establish regular spacing, a process termed nucleokinesis. The LIS1 homologue in A. nidulans, NudF is part of a signaling pathway that regulates nucleokinesis along microtubules via the regulation of dynein motor function. Several nuclear distribution mutants (nud mutants) have been generated in this organism, and besides NudF (LIS1), other nud mutants directly implicated cytoplasmic dynein and dynactin in this process.
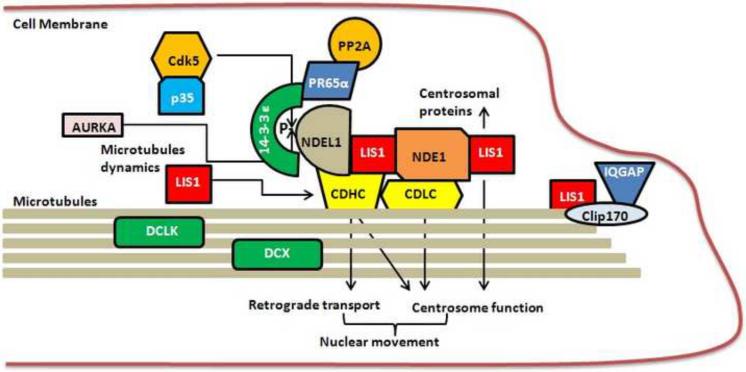
The nud pathway is remarkably conserved in eukaryotes, and the regulation of dynein motor function by LIS1 and microtubule organization is conserved in mammalian cells (Fig. 2). Several groups, including our own, reported the cloning of mammalian NudE homologues from yeast two hybrid screens using LIS1 as bait (Feng et al. 2000; Niethammer et al. 2000; Sasaki et al 2000; Kitagawa et al. 2000), providing a direct link between LIS1 and dynein motors. There are two mammalian NudE homologues: mNudE (now termed NDE1) and NUDEL (now termed NDEL1). Both NDEL1 and NDE1 proteins co-localize with LIS1 at the centrosome or microtubule organizing center (MTOC) in mitotic embryonic neuroblasts or fibroblasts. NDE1, NDEL1 and LIS1 directly interact with cytoplasmic dynein. NDEL1 is a phosphoprotein. It is a substrate for CDK5/p35 in vitro and in vivo at three sites: S198, T219 and S231 (Niethammer et al. 2000; Sasaki et al. 2000). CDK5 phosphorylation of NDEL1 controls its cellular localization and probably influences dynein motor function. In addition to CDK5 (or CDK1 in fibroblasts), NDEL1 is phosphorylated by the mitotic kinase Aurora A at S251 (Mori et al. 2006, see below). Coordinate phosphorylation of NDEL1 by two mitotic kinases suggests that NDEL1 is a central component of the LIS1 complex to regulate proliferation, and NDEL1 activity appears to be tightly regulated by a typical signal transduction pathway (Fig. 2). The role of these phosphorylation events during neurogenesis and neuronal migration require further investigation. These same serine residues are conserved in mNudE (NDE1), suggesting that NDE1 may be a target of CDK5. However, no direct in vitro and in vivo experiments have addressed whether NDE1 is a substrate for the CDK5 complex or Aurora A kinase. Finally, LIS1 also interacts with IQGAP and CLIP170 to promote neuronal motility (Kholmanskikh et al. 2006). This interaction also bridges the MT and actin cytoskeletal networks.
Fig. 2.
The LIS1/NDE/dynein complex, conserved from Aspergillus through mammals. LIS1 interacts directly with NDE1 and NDEL1, which interact with the cytoplasmic dynein light (CDLC) and heavy (CDHC) chains to regulate centrosomal protein localization and function as well as microtubule dynamics. These interactions are critical for nuclear movements and neuronal migration. NDEL1 is phosphorylated by two different mitotic kinases: CDK5 with its required coactivator p35 phosphorylates NDEL1 on three sites (S198, T219, S231); and Aurora A kinase (AURKA) phosphorylates NDEL1 on S251. CDK5-phosphorylated NDEL1 binds to 14-3-3ε, which protects NDEL1 from dephosphorylation, perhaps by phosphatase 4 (PP4) or phosphatase 2A (PP2A), since NDEL1 binds to the PP2A subunit PR65α. LIS1 also interacts with IQGAP and CLIP170.
We made a knock-out and conditional knock-out of Ndel1 (Sasaki et al. 2005). Complete loss of Ndel1 results in early embryonic lethality, demonstrating that Ndel1 is an essential gene, and consistent with NDEL1 having a critical and central role in mediating the effects of LIS1. RNAi knock-down studies of Ndel1, Lis1 or dynein were performed by in utero electroporation into the developing neocortex (Shu et al. 2004), resulting in impaired neuronal positioning and caused the uncoupling of the centrosome and nucleus, similar to our studies with Lis1 mutants (Tanaka et al. 2004a). Overexpression of LIS1 partially rescued the positioning defect caused by Ndel1 RNAi but not dynein RNAi, whereas overexpression of NDEL1 did not rescue the phenotype induced by Lis1 RNAi. These findings with NDEL1 are in contrast to genetic ablation of the LIS1-interacting protein NDE1 in mouse (Feng and Walsh 2004). Complete loss of Nde1 results in viable mice with microcephaly, especially in the cerebral cortex. Cortical lamination was mostly preserved, but the mutant cortex had fewer neurons and very thin superficial cortical layers (layers 2 to 4), with dramatic defects in mitotic progression, mitotic orientation, and mitotic chromosome localization in cortical progenitors. The small cerebral cortex seems to reflect both reduced progenitor cell division and altered neuronal cell fates. These results provide strong evidence that NDEL1 and NDE1 interaction with LIS1 are particularly important for microtubule organization, nuclear translocation, neuronal positioning and development.
While investigating differences between ILS and MDS, we examined the function of genes deleted in MDS, but not ILS. YWHAE, the gene that codes for 14-3-3εis always deleted in MDS patients (Cardoso et al 2003). The 14-3-3 family of highly conserved regulatory proteins bind as dimers to sequence-specific phosphoserine and phosphothreonine motifs, resulting in modulation of function of those proteins (Darling et al. 2005). We produced mice deficient for 14-3-3ε (Toyo-oka et al. 2003). These mice display developmental brain and neuronal migration defects similar to Lis1 heterozygous mutant mice, and 14-3-3ε/Lis1 double heterozygotes display more severe migration defects than single heterozygotes, providing genetic evidence that 14-3-3ε may be responsible for most severe form of lissencephaly, complete agyria, seen in MDS. 14-3-3ε binds directly to CDK5/p35-phosphorylated NDEL1 and this binding maintains NDEL1 phosphorylation (Fig. 2) to protect P-NDEL1 from phosphatase attack. These results establish a crucial role for 14-3-3ε in neuronal development by sustaining the effects of CDK5 phosphorylation, provide a molecular explanation for the differences in severity of human neuronal migration defects with 17p13.3 deletions, and place 14-3-3ε in the LIS1 pathway.
DCX: a phosphoprotein regulating microtubule bundling with potential functional overlap with LIS1
Little is known of the nature of involvement of DCX in neuronal migration. This is partly because DCX appears to be present only in mammals, and so it has not been possible to study its function in fungi or Drosophila. However, both LIS1 and DCX are known to associate with microtubules in mammalian cells. LIS1 appears to increase microtubule polymerization and to decrease microtubule depolymerization rates in vitro (Sapir et al. 1997), and DCX appears to bind to and polymerize microtubules both in vitro and in heterologous cells (Gleeson et al. 1999; Francis et al. 1999; Horesh et al. 1999). It seems then that LIS1 and DCX are both working in concert to maintain microtubule polymerization, indicating that these two proteins may act in similar pathways. Consistent with this hypothesis, an interaction between DCX and LIS1 has been reported in vitro and in mouse embryonic brain extracts (Caspi et al. 2000), although it is unknown if DCX has any effect on dynein motor function. DCX and LIS1 might function together to increase the pool of polymerized microtubules to promote neuronal migration, although how microtubule polymerization might facilitate successful neuronal migration remains to be elucidated. In addition, DCX is a phosphoprotein, a target of CDK5 (Tanaka et al. 2004b) and PKA and/or MARK/Par-1 (Schaar et al. 2004). Phosphorylation of DCX by CDK5 at S297 or by MARK/Par-1 at S47 lowers its affinity to microtubules in vitro, reduces its effect on polymerization, and displaces it from microtubules in cultured neurons. These findings suggest that the function of DCX during neuronal migration is regulated by phosphorylation. Finally, we have recently provided evidence that Lis1 and Dcx are in common pathways regulating not only neuronal migration, but also neurogenesis (Pramparo et al. 2010).
Distinct effects of NDEL1 Phosphorylation by CDK5 and Aurora A
NDEL1 is a substrate for two different kinases: CDK5/p35 in neurons (or CDK1 in mitotic cells) at three sites: S198, T219 and S231 (Niethammer et al. 2000; Sasaki et al. 2000); and the mitotic kinase aurora-A at S251 (Mori et al. 2007). At the G2/M transition and during early stages of mitosis, Plks have been implicated in the maturation of centrosomes, the activation of Cdk1/cyclin B, disassembly of the Golgi complex and removal of cohesin subunits from chromatin (Nigg 2001). We demonstrated that Aurora-A kinase efficiently phosphorylates NDEL1 at S251 at the beginning of mitotic entry coincidently with Aurora-A activation at the G2-prophase transition, while S251 phosphorylation suddenly disappears after prophase (Mori et al. 2007). CDK1 mediated T219 NDEL1 phosphorylation of NDEL1 inhibited further phosphorylation at S251 by Aurora-A, suggesting that Aurora-A mediated phosphorylation of NDEL1 is transient and may trigger the entrance into prophase, followed by degradation and CDK1 mediated phosphorylation. Persistent and simultaneous phosphorylation of NDEL1 by both Aurora-A and CDK1 was inhibitory to the generation of normal spindle and chromosome segregation. Phosphorylation of NDEL1 by CDK1 facilitates Katanin p60 recruitment to the centrosome to trigger microtubule remodeling and shortening (Toyo-oka at al. 2005). NDEL1 phosphorylation by Aurora-A is essential for mitotic entry and centrosome separation by recruitment of TACC3 and XMAP215 at the centrosome (Mori et al. 2007; Mori et al. 2009). These are critical events for centrosome-mediated MT assembly in mitosis (Kinoshita et al. 2005). In Dictyostelium, XMAP215 acts with LIS1 and dynein to capture MTs at the cortex (Rehberg et al. 2005). The coordinate regulation of NDEL1 phosphorylation by two mitotic kinases suggests that NDEL1 is a central component of the LIS1 complex to regulate proliferation.
We identified an atypical protein kinase C (aPKC)-Aurora-A-NDEL1 pathway critical for the regulation of microtubule organization during neurite extension (Mori et al. 2009). aPKC phosphorylates Aurora-A at T287, which augments interaction with TPX2 and facilitates activation of Aurora-A at the neurite hillock, followed by S251 phosphorylation of NDEL1 and recruitment. Suppression of aPKC, Aurora-A, TPX2, or disruption of Ndel1 results in severe impairment of neurite extension. Analysis of microtubule dynamics using a microtubule plus-end marker revealed that suppression of aPKC-Aurora-A-NDEL1 pathway resulted in significant reduction of the frequency of microtubule emanation from the microtubule organizing center (MTOC), suggesting that Aurora-A is downstream of aPKC for regulation of microtubule dynamics. These findings demonstrate a surprising role of aPKC-Aurora-A-NDEL1 pathway on microtubule remodeling during neurite extension, and implies that mitotic kinases play important roles in post-mitotic neurons.
Lis1 is essential for neuroepithelial stem cell and radial glial neurogenesis
Complete loss of Lis1 results in early peri-implantation lethality. We used a conditional Cre-loxP strategy to study the complete loss of Lis1 in defined spatial-temporal patterns in the brain (Yingling et al. 2008). Mice homozygous for a hypomorphic conditional allele of Lis1 (Lis1hc) were crossed with a variety of Cre lines, and the analysis of brains from these embryonic and neonatal mice revealed that loss of Lis1 in NESCs resulted in complete loss of the those cells, while in the RGPCs of the cortex, loss of Lis1 resulted in depletion of progenitors without catastrophic loss. These studies demonstrate an immediate and essential role for Lis1 in dividing neural progenitor cells of the neuroepithelium, while the loss of Lis1 in radial glial progenitors undergoing neurogenesis and neuronal migration have less immediate consequences for survival. Further studies demonstrated that these NESC and RGPC neurogenesis defects result from loss of the tight control of vertical spindle orientation, which in turn result from defective cortical dynein localization and microtubule capture. In mouse embryo fibroblasts (MEFs) with reduced levels of LIS1, we observed shortened and sparse astral MTs while in interphase cells MTs do not fully extend to the cell cortex. LIS1 is required for dynein and dynactin localization to the cell cortex, suggesting that LIS1 stabilizes MTs by plus-end capture at the cell cortex via localization of dynein components, providing an explanation for the spindle orientation defects seen in NESCs and RGPCs.
We proposed a model to explain these findings (Yingling et al. 2008). Apical spindle cleavage planes in the NESCs are tightly controlled to be more symmetric and vertical than in RGPCs, where both symmetric and asymmetric divisions occur to produce daughter cells that are progenitors (symmetric) or one progenitor and one neuron (or glial cell), respectively. In NESCs from mice completely deficient for Lis1, the tight control of spindle orientation is disrupted, resulting in massive loss of those cells. In RGPCs of mouse mutants for Nde1, Ags3 or Dclk, by contrast, dysregulation of plane of division results in depletion of progenitor pools and an eventual decrease in the production of neurons (Feng and Walsh 2004, Sanada and Tsai 2005; Shu et al. 2006), but resulting in small brain size without catastrophic consequences. This appears to be the case when Lis1 is lost in RGPCs. We also proposed that loss of LIS1 result in profound spindle orientation defects since LIS1 is essential for proliferation and the specification of mitotic spindle orientation via its critical role in cortical MT capture and stability, which results from a critical role for LIS1 in dynein and dynactin localization to the cell cortex. This model suggests that LIS1 stabilizes MTs by plus-end capture at the cell cortex via localization of dynein components, providing an explanation for the spindle orientation defects seen in NESCs and RGPCs.
Mechanism of defective cortical dynein localization
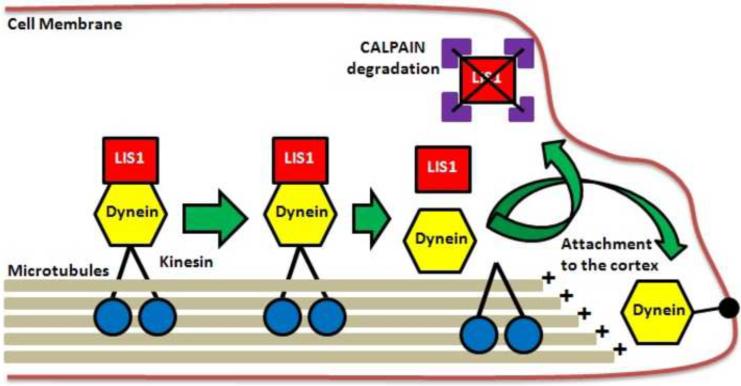
The next question we addressed was how does loss of LIS1 result in defects in the cortical localization of dynein (Yamada et al. 2008)? We used purified tubulin, LIS1 and NDEL1 to demonstrate that LIS1 suppresses the motility of cytoplasmic dynein on microtubules (MTs), whereas NDEL1 releases the blocking effect of LIS1 on cytoplasmic dynein. LIS1, cytoplasmic dynein and MT fragments co-migrate in an anterograde direction in dorsal root ganglion (DRG) cells in vitro by Fluorescence Recovery after Photobleaching (FRAP), a surprising finding since dynein is a retrograde motor. Suppression of LIS1 function by a blocking antibody resulted in cytoplasmic dynein. Cytoplasmic dynein forms a complex with LIS1, tubulins and kinesin heavy chain, and immunoabsorption of LIS1 resulted in disappearance of co-precipitated tubulins and kinesin. We proposed a novel model (Fig. 3) of the regulation of cytoplasmic dynein by LIS1, in which LIS1 mediates anterograde transport of cytoplasmic dynein to the plus-end of cytoskeletal MTs as a dynein-LIS1 complex on transportable microtubules (tMTs). LIS1 holds dynein on the tMT to make a complex, which is transported to plus-end of MTs. This model is consistent with our observations demonstrating that once Lis1 is disrupted, plus-end directed dynein transport is severely impaired, resulting in excessive accumulation around the centrosome associated with peripheral depletion. The unbalanced distribution of cytoplasmic dynein would likely be the causative mechanism of the defect of N-C coupling and nucleokinesis defects displayed by migrating neurons (Tanaka et al., 2004). The role of this complex in anterograde transport of cytoplasmic dynein provides an explanation for the normal broad cytoplasmic distribution of cytoplasmic dynein are localized in a perinuclear pattern when LIS1 is reduced (Sasaki et al 2000; Niethammer et al. 2000; Toyo-oka et al. 2003; Yingling et al 2008). It also provides an explanation for the observation that loss of LIS1 results in reduction of astral microtubules, reduction of cortical dynein and impairment of cortical microtubule capture in mitotic cells (Faulkner et al. 2000; Yingling et al 2008). More generally, our model provides a general mechanism for the transport of cytoplasmic dynein to the plus-end of cytoskeletal MTs via a novel form of soluble tubulin clusters, tMTs which may have a different origin and distinct mode of regulation from cytoskeletal MTs. Our imaging analysis suggests that these tMTs exist as stable entities rather than intermediates from the breakdown or remodeling of cytoskeletal MTs. We propose the possibility that these unique tubulin clusters may be distinctly regulated and may also have unique functions such as “freighter” for the transport of other molecules such as dyneins. The use of such freighter MTs provides a simple and flexible mechanism to transport dynein from inside the cell to the periphery, and may be used more generally for other transportable cargoes. Although the use of tMT is a possibility supported by the data, the precise structure of tMTs used for the dynein transport is not clear.
Fig. 3.
LIS1 binding partipates in the anterograde transport of dynein via kinesin motors to the surface of the cell (cortex). At the cell cortex, the dynein is unloaded at the plus-end of microtubules allowing dynein to attach to the cortex while LIS1 is degraded via calpain-dependent proteolysis.
We also found that mNUDC, another component in the conserved nuclear distribution pathway first defined is A. nidulans, is an essential molecule for anterograde transport of cytoplasmic dynein and dynactins (Yamada et al 2010). Our data suggest that mNUDC mediates the binding of cargo molecules with kinesin-1, and is required for anterograde transport of separate cytoplasmic dynein and dynactin complexes. This provides a molecular mechanism for the inactivation of cytoplasmic dynein during anterograde transport, but cytoplasmic dynein is then activated after reaching the plus-end of MTs by reuniting with dynactin.
This may not be the only function of LIS1 and its complex regarding dynein function. Others have shown that in cell free systems (McKenney et al. 2010) that LIS1 and NDEL1 cooperatively regulate a persistent force state of dynein that improves the ensemble function of multiple dyneins for transport under high load conditions. This type of role for the LIS1 complex may support some of the phenotypes observed in RNAi knock-down experiments regarding nuclear movements during neuronal migration (Tsai et al. 2007). It is possible that LIS1 and its complex perform multiple roles in regulating dynein function and localization, since these two distinct functions are not mutually exclusive.
Therapeutic strategies for treating defects found in rodent models of human lissencephaly
One of the major reasons for investigating the mechanism of action of genes involved in human genetic disease is that a detailed understanding may lead to potential therapeutic targets. It may be difficult to see how this might apply to severe neurodevelopmental disorders, but there have been two recent studies suggesting that it may be possible. First, the phenotype of a Dcx-knock-down rat model was partially rescued by reexpression of DCX after birth (Manent et al. 2009). In addition, we have recently found a novel therapeutic intervention that resulted in phenotypic improvement in our Lis1 mutant mice based on the mechanistic studies described above (Yamada et al. 2009). We will briefly discuss these two exciting studies.
Postnatal expression of DCX partially rescues brain defects after embryonic knock-down of Dcx in the rat
In a rat model of subcortical band heterotopia (SBH) generated by in utero RNA interference of the Dcx gene, aberrantly positioned neurons can be stimulated to migrate by reexpressing Dcx after birth. Restarting migration in this way both reduces neocortical malformations and restores neuronal patterning (Manent et al. 2009). The capacity to reduce SBH continues into early postnatal development. These studies suggest that disorders of neuronal migration may be eventually treatable by reengaging developmental programs both to reduce the size of cortical malformations and to rescue clinical manifestations.
Rescue of cortical dynein localization and phenotypic defects in Lis1 mutants by inhibiting LIS1 degradation
In the course of studying the role of LIS1/NDEL1/mNUDC in the transport of dynein to the cortex of cell, as discussed above, it appeared that LIS1 was degraded at the cortex. For example, FRAP analysis indicated that the retrograde flux of LIS1 is significantly smaller than anterograde flux. This suggests that as much as half of LIS1 disappears and is likely to be degraded after reaching to the plus-end of MTs. Interestingly, we found that LIS1 protein is in fact degraded at the cell cortex after transport to the plus-end of MTs via calpain-dependent proteolysis. We demonstrated that the calpain inhibitors ALLN e64d protected LIS1 from proteolysis resulting in the augmentation of LIS1 levels in Lis1+/− mouse embryonic fibroblast (MEF) cells and dorsal root ganglia (DRG) neurons (Yamada et al. 2009). This led to the rescue of the aberrant distribution of cytoplasmic dynein and intracellular components including mitochondria and Golgi vesicles. Furthermore, the presence of ALLN improved neuronal migration of Lis1+/− cerebellar granular neurons. The most striking finding, though, was that intra-peritoneal injection of ALLN to pregnant Lis1+/− dams rescued apoptotic neuronal cell death, reduction of brain weight and neuronal migration defects in Lis1+/− offspring. Lis1+/− mice exhibited laminar splitting and discontinuities of pyramidal cells in the CA3 and CA2 region of the hippocampus. After administration of ALLN in utero, Lis1+/− mice also displayed splitting and diffuse pyramidal cells of the hippocampus, but these defects were markedly improved. These experiments suggest a potential therapeutic intervention for lissencephaly. Cortical dynein localization can be rescued by inhibiting LIS1 degradation with the calpain inhibitor ALLN, providing a proof-of-principle for the notion that detailed understanding of mechanism of action of human disease genes can lead to novel therapeutic targets for even severe neurodevelopmental diseases.
Conclusions
The identification of genes responsible for a varitety of human neurogenetic diseases has accelerated rapidly over the past 20 years. Mouse models have been productively been use in order to understand the detailed mechanisms of action of these genes and the pathophysiological abnormalities that result from mutations in these genes. A potential long-term goal of such studies has been that such studies may lead to new avenues of therapies for severe neurogenetic disorders that would not be possible without the detailed understanding that such an approach would provide. As discussed in this review, the detailed understanding of the mechanims of action of these disease genes in relevant animal models has led to potential therapies for the severe human neurogenetic disorder lissencephaly in these animal models – a “bench-to-cageside” model. It will likely be difficult to move these animal studies into human patients, for a variety of reasons, and these difficulties cannot be overstated. However, they do provide an avenue by which one can start to think about translating these animal studies to human patients with severe neurogenetic disorders such as lissencephaly. When this may occur is difficult to predict, but it is likely that such “bench-to-cageside-to-bedside” studies will happen with greater frequency in the near future.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ayala R, Shu T, Tsai LH. Trekking across the brain: the journey of neuronal migration. Cell. 2007;128:29–43. doi: 10.1016/j.cell.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Bai J, Ramos RL, Ackman JB, Thomas AM, Lee RV, LoTurco JJ. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nat Neurosci. 2003;12:1277–83. doi: 10.1038/nn1153. [DOI] [PubMed] [Google Scholar]
- Cahana A, Escamez T, Nowakowski RS, Hayes NL, Giacobini M, von Holst A, Shmueli O, Sapir T, McConnell SK, Wurst W, Martinez S, Reiner O. Targeted mutagenesis of Lis1 disrupts cortical development and LIS1 homodimerization. Proc Natl Acad Sci U S A. 2001;98:6429–6434. doi: 10.1073/pnas.101122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso C, Leventer RJ, Ward HL, Toyo-oka K, Chung J, Gross A, Martin CL, Allanson J, Pilz DT, Olney AH, Mutchinick OM, Hirotsune S, Wynshaw-Boris A, Dobyns WB, Ledbetter DH. Refinement of a 400-kb critical region allows genotypic differentiation between isolated lissencephaly, Miller-Dieker syndrome and other phenotypes secondary to deletions of 17p13.3. Amer J Hum Genet. 2003;72:918–930. doi: 10.1086/374320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi M, Atlas R, Kantor A, Sapir T, Reiner O. Interaction between LIS1 and doublecortin, two lissencephaly gene products. Hum. Mol. Genet. 2002;9:2205–2213. doi: 10.1093/oxfordjournals.hmg.a018911. [DOI] [PubMed] [Google Scholar]
- Chong SS, Pack SD, Roschke AV, Tanigami A, Carrozzo R, Smith AC, Dobyns WB, Ledbetter DH. A revision of the lissencephaly and Miller-Dieker syndrome critical regions in chromosome 17p13.3. Hum Mol Genet. 1997;6:147–155. doi: 10.1093/hmg/6.2.147. 1997. [DOI] [PubMed] [Google Scholar]
- Corbo JC, Deuel TA, Long JM, LaPorte P, Tsai E, Wynshaw-Boris A, Walsh CA. Doublecortin is required in mice for lamination of the hippocampus but not the neocortex. J Neuro. 2002;22:7548–7557. doi: 10.1523/JNEUROSCI.22-17-07548.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling DL, Yingling J, Wynshaw-Boris A. Role of 14-3-3 proteins in eukaryotic signaling and development. Curr Topics Develop Biol. 2005;68:281–315. doi: 10.1016/S0070-2153(05)68010-6. [DOI] [PubMed] [Google Scholar]
- des Portes V, Pinard JM, Billuart P, Vinet MC, Koulakoff A, Carrie A, Gelot A, Dupuis E, Motte J, Berwald-Netter Y, Catala M, Kahn A, Beldjord C, Chelly J. A novel CNS gene required for neuronal migration and involved in X- linked subcortical laminar heterotopia and lissencephaly syndrome. Cell. 1998b;92:51–61. doi: 10.1016/s0092-8674(00)80898-3. [DOI] [PubMed] [Google Scholar]
- Dobyns WB, Carrozzo R, Ledbetter DH. Frequent deletions of the LIS1 gene in classic lissencephaly. Ann. Neurol. 1994;36:489–490. [Google Scholar]
- Dobyns WB, Elias ER, Newlin AC, Pagon RA, Ledbetter DH. Causal heterogeneity in isolated lissencephaly. Neurology. 1992;42:1375–1388. doi: 10.1212/wnl.42.7.1375. 1992. [DOI] [PubMed] [Google Scholar]
- Dobyns WB, Stratton RF, Greenberg F. Syndromes with lissencephaly. I: Miller-Dieker and Norman-Roberts syndromes and isolated lissencephaly. Am J Med Genet. 1984;18:509–526. doi: 10.1002/ajmg.1320180320. [DOI] [PubMed] [Google Scholar]
- Faulkner NE, Dujardin DL, Tai C, Vaughan KT, O'Connell CB, Wang Y, Vallee RB. A role for the lissencephaly gene LIS1 in mitosis and cytoplasmic dynein function. Nat Cell Biol. 2000;2:784–791. doi: 10.1038/35041020. [DOI] [PubMed] [Google Scholar]
- Feng Y, Olson EC, Stukenberg PT, Flanagan LA, Kirschner MW, Walsh CA. Interactions between LIS1 and mNudE, a central component of the centrosome, are required for CNS lamination. Neuron. 2000;28:665–679. doi: 10.1016/s0896-6273(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Feng Y, Walsh CA. Mitotic spindle regulation by Nde1 controls cerebral cortical size. Neuron. 2004;44:279–293. doi: 10.1016/j.neuron.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Fleck MW, Hirotsune S, Gambello MJ, Phillips-Tansy E, Suarez G, Mervis RF, Gleeson JG, Wynshaw-Boris A, McBain CJ. Hippocampal abnormalities in Lis1 mutant mice provide a neuronal basis for epileptogenesis in neuronal migration defects. J Neurosci. 2000;20:2439–2450. doi: 10.1523/JNEUROSCI.20-07-02439.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet MC, Friocourt G, McDonnell N, Reiner O, Kahn A, McConnell SK, Berwald-Netter Y, Denoulet P, Chelly J. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23:247–256. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- Gambello MJ, Darling DL, Yingling J, Tanaka T, Gleeson JG, Wynshaw-Boris A. Multiple dose-dependent effects of Lis1 on cerebral cortical development. J Neurosci. 2003;23:1719–1729. doi: 10.1523/JNEUROSCI.23-05-01719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson JG, Allen KM, Fox JW, Lamperti ED, Berkovic S, Scheffer I, Cooper EC, Dobyns WB, Minnerath SR, Ross ME, Walsh CA. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92:63–72. doi: 10.1016/s0092-8674(00)80899-5. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- Gupta A, Tsai LH, Wynshaw-Boris A. Life is a journey: a genetic look at neocortical development. Nature Reviews Genetics. 2002;3:342–355. doi: 10.1038/nrg799. [DOI] [PubMed] [Google Scholar]
- Hirotsune S, Fleck MW, Gambello MJ, Bix GJ, Chen A, Clark GD, Ledbetter DH, McBain CJ, Wynshaw-Boris A. Graded reduction of Pafah1b1 (Lis1) activity results in neuronal migration defects and early embryonic lethality. Nature Genet. 1998;19:333–339. doi: 10.1038/1221. [DOI] [PubMed] [Google Scholar]
- Horesh D, Sapir T, Francis F, Wolf SG, Caspi M, Elbaum M, Chelly J, Reiner O. Doublecortin, a stabilizer of microtubules. Hum. Mol. Genet. 1999;8:1599–610. doi: 10.1093/hmg/8.9.1599. [DOI] [PubMed] [Google Scholar]
- Kato M, Dobyns WB. Lissencephaly and the molecular basis of neuronal migration. Hum Mol Genet. 2003;1:R89–96. doi: 10.1093/hmg/ddg086. [DOI] [PubMed] [Google Scholar]
- Kholmanskikh SS, Begum H, Wynshaw-Boris A, Gomez T, Letourneau P, Ross ME. Calcium-dependent interaction of Lis1 with IQGAP and Cdc42 promotes neuronal motility. Nat Neuro. 2006;9:50–57. doi: 10.1038/nn1619. [DOI] [PubMed] [Google Scholar]
- Kinoshita K, Noetzel TL, Pelletier L, Mechtler K, Drechsel DN, Schwager A, Lee M, Raff JW, Hyman AA. Aurora A phosphorylation of TACC3/maskin is required for centrosome-dependent microtubule assembly in mitosis. J Cell Biol. 2005;170:1047–1055. doi: 10.1083/jcb.200503023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa M, Umezu M, Aoki J, Koizumi H, Arai H, Inoue K. Direct association of LIS1, the lissencephaly gene product, with a mammalian homologue of a fungal nuclear distribution protein, rNUDE. FEBS Lett. 2000;479:57–62. doi: 10.1016/s0014-5793(00)01856-1. [DOI] [PubMed] [Google Scholar]
- Koizumi H, Tanaka T, Gleeson JG. Doublecortin-like kinase functions with doublecortin to mediate fiber tract decussation and neuronal migration. Neuron. 2006;49:55–66. doi: 10.1016/j.neuron.2005.10.040. [DOI] [PubMed] [Google Scholar]
- LoNigro C, Chong CS, Smith AC, Dobyns WB, Carrozzo R, Ledbetter DH. Point mutations and an intragenic deletion in LIS1, the lissencephaly causative gene in isolated lissencephaly sequence and Miller-Dieker syndrome. Hum Mol Genet. 1997;6:157–164. doi: 10.1093/hmg/6.2.157. [DOI] [PubMed] [Google Scholar]
- Manent JB, Wang Y, Chang Y, Paramasivam M, LoTurco JJ. Dcx reexpression reduces subcortical band heterotopia and seizure threshold in an animal model of neuronal migration disorder. Nat Med. 2009;15:84–90. doi: 10.1038/nm.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori D, Yamada M, Mimori-Kiyosue Y, Shirai Y, Suzuki A, Ohno S, Saya H, Wynshaw-Boris A, Hirotsune S. An essential role of the aPKC-Aurora A-NDEL1 pathway on neurite elongation via modulation of microtubule dynamics. Nat Cell Biol. 2009;11:1057–1068. doi: 10.1038/ncb1919. [DOI] [PubMed] [Google Scholar]
- Mori D, Yano Y, Toyo-oka K, Yoshida N, Yamada M, Mutamatsu M, Zhang D, Saya H, Toyoshima YY, Kinoshita K, Wynshaw-Boris A, Hirotsune S. NDEL1 phosphorylation by Aurora-A kinase is essential for centrosome maturation, separation and TACC3 recruitment. Mol Cell Biol. 2007;27:352–367. doi: 10.1128/MCB.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris NR. Nuclear migration: from fungi to the mammalian brain. J Cell Biol. 2000;148:1097–1101. doi: 10.1083/jcb.148.6.1097. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadarajah B, Brunstrom JE, Grutzendler J, Wong RO, Pearlman AL. Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci. 2001;2:143–50. doi: 10.1038/83967. [DOI] [PubMed] [Google Scholar]
- Niethammer M, Smith DS, Ayala R, Peng J, Ko J, Lee M, Morabito M, Tsai LH. The Lis1 interacting protein Nudel is a Cdk5 substrate that interacts with cytoplasmic dynein. Neuron. 2000;28:697–711. doi: 10.1016/s0896-6273(00)00147-1. [DOI] [PubMed] [Google Scholar]
- Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol. 2001;2:21–32. doi: 10.1038/35048096. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Pawlisz A, Wynshaw-Boris A, Walsh CA, Feng Y. Lis1-Nde1 dependent neuronal fate control determines cerebral cortical size and lamination. Hum Mol Genet. 2008;17:2441–2455. doi: 10.1093/hmg/ddn144. PMC2486443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paylor R, Hirotsune S, Gambello MJ, Yuva-Paylor L, Crawley J, Wynshaw-Boris A. Impaired learning and motor behavior in heterozygous Pafah1b1 (Lis1) mutant mice. Learn Memory. 1999;6:521–537. doi: 10.1101/lm.6.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz DT, Matsumoto N, Minnerath S, Mills P, Gleeson JG, Allen KM, Walsh CA, Barkovich AJ, Dobyns WB, Ledbetter DH, Ross ME. LIS1 and XLIS (DCX) mutations cause most classical lissencephaly, but different paterns of malformation. Hum Mol Genet. 1998;7:2029–2037. doi: 10.1093/hmg/7.13.2029. [DOI] [PubMed] [Google Scholar]
- Pramparo T, Youn YH, Hirotsune S, Wynshaw-Boris A. Novel embryonic neuronal migration and neurogenesis defects in Dcx mutant mice are exacerbated by Lis1 reduction. J Neuro. 2010;30:3002–3012. doi: 10.1523/JNEUROSCI.4851-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehberg M, Kleylein-Sohn J, Faix J, Ho TH, Schulz I, Graf R. Dictyostelium LIS1 is a centrosomal protein required for microtubule/cell cortex interactions, nucleus/centrosome linkage, and actin dynamics. Mol. Biol Cell. 2005;16:2759–2771. doi: 10.1091/mbc.E05-01-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner O, Carrozzo R, Shen Y, Wehnert M, Faustinella F, Dobyns WB, Caskey CT, Ledbetter DH. Isolation of a Miller-Dieker lissencephaly gene containing G protein beta-subunit-like repeats. Nature. 1993;364:717–721. doi: 10.1038/364717a0. [DOI] [PubMed] [Google Scholar]
- Sanada K, Tsai LH. G protein betagamma subunits and AGS3 control spindle orientation and asymmetric cell fate of cerebral cortical progenitors. Cell. 2005;122:119–131. doi: 10.1016/j.cell.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Sapir T, Elbaum M, Reiner O. Reduction of microtubule catastrophe events by LIS1, platelet-activating factor acetylhydrolase subunit. EMBO J. 1997;16:6977–84. doi: 10.1093/emboj/16.23.6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S, Shionoya A, Ishida M, Gambello MJ, Yingling J, Wynshaw-Boris A, Hirotsune S. A LIS1/NUDEL/cytoplasmic dynein heavy chain complex in the developing and adult nervous system. Neuron. 2000;28:681–696. doi: 10.1016/s0896-6273(00)00146-x. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Mori D, Toyo-oka K, Chen A, Garrett-Beal L, Muramatsu M, Miyagawa S, Hiraiwa N, Yoshiki A, Wynshaw-Boris A, Hirotsune S. Disruption of Ndel1 causes neuronal migration defects and early embryonic lethality. Mol Cell Biol. 2005;25:7812–7827. doi: 10.1128/MCB.25.17.7812-7827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaar BT, Kinoshita K, McConnell SK. Doublecortin microtubule affinity is regulated by a balance of kinase and phosphatase activity at the leading edge of migrating neurons. Neuron. 2004 Jan 22;41(2):203–13. doi: 10.1016/s0896-6273(03)00843-2. [DOI] [PubMed] [Google Scholar]
- Shu T, Ayala R, Nguyen MD, Xie Z, Gleeson JG, Tsai LH. Ndel1 operates in a common pathway with LIS1 and cytoplasmic dynein to regulate cortical neuronal positioning. Neuron. 2004;44:263–277. doi: 10.1016/j.neuron.2004.09.030. [DOI] [PubMed] [Google Scholar]
- Shu T, Tseng HC, Sapir T, Stern P, Zhou Y, Sanada K, Fischer A, Coquelle FM, Reiner O, Tsai LH. Doublecortin-like kinase controls neurogenesis by regulating mitotic spindles and M phase progression. Neuron. 2006;1:25–39. doi: 10.1016/j.neuron.2005.10.039. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Serneo FF, Higgins C, Gambello MJ, Wynshaw-Boris A, Gleeson JG. Lis1 and doublecortin function with dynein to mediate coupling of the nucleus to the centrosome in neuronal migration. J Cell Biol. 2004a;165:709–721. doi: 10.1083/jcb.200309025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Serneo FF, Tseng HC, Kulkarni AB, Tsai LH, Gleeson JG. Cdk5 phosphorylation of doublecortin ser297 regulates its effect on neuronal migration. Neuron. 2004b;41(2):215–27. doi: 10.1016/s0896-6273(03)00852-3. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Koizumi H, Gleeson JG. The doublecortin and doublecortin-like kinase 1 genes cooperate in murine hippocampal development. Cereb Cortex. 2006;16(1):i69–73. doi: 10.1093/cercor/bhk005. [DOI] [PubMed] [Google Scholar]
- Tsai JW, Chen Y, Kriegstein AR, Vallee RB. LIS1 RNA interference blocks neural stem cell division, morphogenesis, and motility at multiple stages. J Cell Biol. 2005;170:935–945. doi: 10.1083/jcb.200505166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai LH, Gleeson JG. Nucleokinesis in neuronal migration. Neuron. 2005;46:383–388. doi: 10.1016/j.neuron.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Tsai JW, Bremner KH, Vallee RB. Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live tissue. Nat Neurosci. 2007;10:970–979. doi: 10.1038/nn1934. [DOI] [PubMed] [Google Scholar]
- Toyo-oka K, Mori D, Yano Y, Shiota M, Iwao H, Goto H, Inagaki M, Hiraiwa N, Mimori-Kiyosue Y, Muramatsu M, Wynshaw-Boris A, Yoshiki A, Hirotsune S. Protein phosphatase 4 catalytic subunit (PP4c) regulates CDK1 activity and organization of microtubules through dephosphorylation of NDEL1. J Cell Biol. 2008;180:1133–1147. doi: 10.1083/jcb.200705148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyo-oka K, Sasaki S, Yano Y, Mori D, Kobayashi T, Toyoshima YY, Tokuoka SM, Ishii S, Shimizu T, Muramatsu M, Hiraiwa N, Yoshiki A, Wynshaw-Boris A, Hirotsune S. Recruitment of katanin p60 by phosphorylated NDEL1, an LIS1 interacting protein, is essential for mitotic cell division and neuronal migration. Hum Mol Genet. 2005;14:3113–3128. doi: 10.1093/hmg/ddi339. [DOI] [PubMed] [Google Scholar]
- Toyo-oka K, Shionoya A, Gambello MJ, Cardoso C, Leventer RJ, Ward H, Ayala R, Tsai LH, Dobyns WB, Ledbetter DH, Hirotsune S, Wynshaw-Boris A. 14-3-3ε is important for neuronal migration via binding to NUDEL: a molecular explanation for Miller-Dieker syndrome. Nature Genet. 2003;34:274–285. doi: 10.1038/ng1169. [DOI] [PubMed] [Google Scholar]
- Vallee RB, Tsai JW. The cellular roles of the lissencephaly gene LIS1, and what they tell us about brain development. Genes Dev. 2006;20:1384–1393. doi: 10.1101/gad.1417206. [DOI] [PubMed] [Google Scholar]
- Wynshaw-Boris A. Lissencephaly and LIS1: insights into the molecular mechanisms of neuronal migration and development. Clin Genet. 2007;72:296–204. doi: 10.1111/j.1399-0004.2007.00888.x. [DOI] [PubMed] [Google Scholar]
- Xiang X, Morris NR. Hyphal tip growth and nuclear migration. Curr Opin Microbiol. 1999;2:636–640. doi: 10.1016/s1369-5274(99)00034-x. [DOI] [PubMed] [Google Scholar]
- Yamada M, Toba S, Yoshida Y, Haratani K, Mori D, Yano Y, Mimori-Kiyosue Y, Nakamura T, Ito K, Fushiki S, Setou M, Wynshaw-Boris A, Torisawa T, Toyoshima YY, Hirotsune S. LIS1 and NDEL1 coordinate the plus end directed transport of cytoplasmic dynein. EMBO J. 2008;27:2471–2483. doi: 10.1038/emboj.2008.182. PMC2567412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Yoshida Y, Mori D, Takitoh T, Kengaku M, Takao K, Miyakawa T, Sato M, Sorimachi H, Wynshaw-Boris A, Hirotsune S. Inhibition of calpain increases LIS1 and partially rescues in vivo phenotypes in Lis1 mutant mice: a potential therapy for lissencephaly. 2009;15:1202–1208. doi: 10.1038/nm.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Toba S, Takitoh T, Yoshida Y, Mori D, Nakamura T, Iwane AH, Yanagida T, Imai H, Yu-Lee LY, Schroer T, Wynshaw-Boris A, Hirotsune S. mNUDC is required for plus-end directed transport of cytoplasmic dynein and dynactins by kinesin-1. EMBO J. 2010;29:517–31. doi: 10.1038/emboj.2009.378. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yingling Y, Youn YH, Darling D, Toyo-oka K, Pramparo T, Hirotsune S, Wynshaw-Boris A. Neuroepithlial stem cell proliferation requires LIS1 for precise spindle orientation and symmetric division. Cell. 2008;132:474–486. doi: 10.1016/j.cell.2008.01.026. PMC2265303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn YH, Pramparo T, Hirotsune S, Wynshaw-Boris A. Distinct dose-dependent neuronal migration defects in Lis1 and Ndel1 mutant mice. 2009 doi: 10.1523/JNEUROSCI.4630-09.2009. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]