Abstract
BACKGROUND & AIMS
Heparan sulfate proteoglycans (HSPGs) act as co-receptors or storage sites for growth factors and cytokines such as FGF and Wnts. Glypican 3 (GPC3) is the most highly expressed HSPG in hepatocellular carcinomas (HCCs). Sulfatase 2 (SULF2), an enzyme with 6-O desulfatase activity on HSPGs, is upregulated in 60% of primary HCCs and associated with a worse prognosis. We have previously shown that the oncogenic effect of SULF2 in HCC may be mediated in part through up-regulation of GPC3. Here we demonstrate that GPC3 stimulates the Wnt/β-catenin pathway and mediates SULF2 oncogenic function in HCC.
METHODS
Wnt signaling in vitro and in vivo was assessed in SULF2-negative Hep3B HCC cells transfected with SULF2 and SULF2-expressing Huh7 cells transfected with shRNA targeting SULF2. The interaction between GPC3, SULF2, and Wnt3a was assessed by co-immunoprecipitation and flow cytometry. β-catenin-dependent transcriptional activity was assessed by the TOPFLASH luciferase assay.
RESULTS
In HCC cells, SULF2 increased cell surface GPC3 and Wnt3a expression, stabilized β-catenin, and activated TCF transcription factor activity and expression of the Wnt/β-catenin target gene cyclin D1. Opposite effects were observed in SULF2-knockdown models. In vivo, nude mouse xenografts established from SULF2-transfected Hep3B cells showed enhanced GPC3, Wnt3a and β-catenin levels.
CONCLUSIONS
Together, these findings identified a novel mechanism mediating the oncogenic function of SULF2 in HCC including GPC3-mediated activation of Wnt signaling via the Wnt3a/GSK3β axis.
Keywords: SULF2, heparan sulfate proteoglycan (HSPG), heparan sulfate glycosaminoglycan (HSGAG), glypican 3 (GPC3), Wnt signaling pathway, beta catenin, hepatocellular carcinoma, oncogene
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third most frequent cause of cancer death worldwide (1). Survival of HCC patients is poor and only 10–20% of HCCs are detected at an early enough stage for potentially curative therapy. Loco-regional therapies are usually palliative, and there are limited options for chemotherapy. Therefore, new agents are needed for effective treatment of the majority of HCCs (2).
The Wnt/Frizzled/β-catenin pathway is activated in about 50% of HCCs. The Wnt ligands Wnt3, Wnt3a, Wnt4 and Wnt5a and the Frizzled receptors Frizzled 3, 6 and 7 have been implicated in development of HCC and up to 95% of HCCs show potential Wnt/Frizzled activating events (3–5). The Wnt/β-catenin pathway is regulated by heparan sulfate proteoglycans (HSPGs), which modulate cell surface signaling by acting as co-receptors or storage sites for Wnt proteins. HSPGs consist of a protein core to which is attached heparan sulfate glycosaminoglycan (HSGAG) chains that are variably sulfated at the 2-O-, 3-O-, and 6-O- positions of their component disaccharides. Glypicans are cell surface-anchored HSPGs that regulate the activity of Wnts (6, 7). In particular, glypican 3 (GPC3) is highly overexpressed in HCCs and is under development as a target for therapy of HCC (8, 9). Wnt3a has been shown to mediate the GPC3-induced growth of HCCs via the canonical Wnt/β-catenin pathway (5, 10).
Sulfated HSGAG chains of GPC3 and other HSPGs are potential substrates for desulfation at the 6-O position by human sulfatase 2 (SULF2). The sulfation state of HSGAGs is critical for growth factor binding; hence SULF2 may regulate tumor growth by releasing growth factors from HSGAG storage sites at the cell surface and in the extracellular matrix, thus increasing the local concentration of growth factors available to bind to cell surface receptors and enhancing cell signaling. We have shown that SULF2 up-regulates FGF signaling in a heparan sulfate- and GPC3-dependent manner in HCC cells, both in vitro and in vivo (11).
Since GPC3 activates Wnt signaling and is a potential substrate for desulfation by SULF2, we hypothesized that desulfation by SULF2 releases stored Wnts from HSGAG sites on GPC3. Released Wnt then binds to Frizzled receptors and activates the Wnt/β-catenin pathway. We investigated the roles of SULF2 and GPC3 in Wnt3a signaling by addressing the following questions: 1) Does SULF2 enhance Wnt/β-catenin activation in HCC cells? 2) Are Wnt3a binding to HCC cells and Wnt/β-catenin activation dependent on heparan sulfate and GPC3? 3) Does Wnt3a associate with SULF2 and GPC3? 4) Does SULF2 drive Wnt/β-catenin signaling in the absence of exogenous Wnt? 5) Does knockdown of SULF2 decrease GPC3 and Wnt3a expression and inhibit Wnt/β-catenin signaling? 6) Is the association between SULF2, GPC3 and Wnt3a demonstrable in vivo? We show that SULF2 activates Wnt/β-catenin signaling in HCC cells and that this process is GPC3-dependent and can be independent of exogenous Wnts.
MATERIALS AND METHODS
Chemicals and antibodies
Heparan sulfate, anti-actin antibody, horseradish peroxidase-conjugated mouse IgG and rabbit IgG were purchased from Sigma Chemical Co. (St Louis, MO); anti-GPC3 antibody from BioMosaics (Burlington, VT); recombinant human Wnt3a and anti-Wnt3a antibody from R&D Systems (Minneapolis, MN). Plasmid vectors pSS-H1p and pG-SUPER were gifts from Dr. Daniel D. Billadeau and Dr. Shin-ichiro Kojima, respectively. TOPFLASH and FOPFLASH plasmids were from Dr. Wanguo Liu. The rabbit polyclonal anti-SULF2 antibody was previously reported (11).
HCC cell lines
The Hep3B, Huh-7, PLC/PRF/5 and HepG2 cell lines were from the American Type Culture Collection (Manassas, VA) and cultured as recommended.
SULF2 stable transfectant clones
SULF2-negative Hep3B cells were stably-transfected with SULF2 expressing plasmids. SULF2-expressing Huh7 cells were stably-transfected with plasmids expressing short hairpin RNA (shRNA) sequences targeting SULF2 (11). Two SULF2-transfected Hep3B clones were selected for the experiments, a low SULF2-expressing clone (Hep3B-SULF2-L) and high SULF2-expressing clone (Hep3B-SULF2-H). Similarly, two SULF2 knockdown Huh7 clones, Huh7 SULF2 shRNA-3 and Huh7 SULF2 shRNA-4, were selected. Target sequences used for SULF2 shRNA constructs (shRNA-a and shRNA-b) were as reported (11).
Wnt3a binding by flow cytometry
Wnt3a was biotinylated using EZ-Link Sulfo-NHS-LC-Biotin (Pierce). 100,000 Hep3B cells were transiently transfected with control pcDNA3.1 (Invitrogen) or full-length hSULF2 in pcDNA3.1 for 48 hours using Fugene 6, then pelleted and resuspended at 4×106 cells/ml in PBS. Cells were treated with PBS (control), 10 µg or 50 µg heparan sulfate for 5 minutes on ice. 5 ng biotinylated Wnt3a was added (a background control with no added Wnt3a was used for each condition), cells were incubated on ice for 30 minutes, and 5 µl of 1:10 dilution of Streptavidin PE (in PBS + 0.1%BSA) (Jackson Immunoresearch) was added before re-incubation on ice for another 30 minutes in the dark. Cells were washed with PBS + 2% BSA, pelleted and resuspended in 0.4 ml 4% paraformaldehyde. Flow cytometry was performed using a FAC-Scan analyzer (BD Biosciences, San Jose, CA). Similar experiments used Hep3B SULF2-H transfected with shRNA against GPC3 or control scrambled shRNA and 20 ng/ml heparan sulfate.
Immunocytochemistry and confocal microscopy
SULF2-positive or negative Huh7 and Hep3B cells were seeded on glass cover slips in six-well plates and incubated for 24 h. Immunocytochemistry and confocal microscopy were performed using antibodies against SULF2, GPC3, Wnt3a and β-catenin (12).
Western immunoblotting
SULF2-positive or negative Huh7 and Hep3B cells were cultured for 24 hours and whole-cell lysates prepared (12). 20 µg/lane of protein was separated by electrophoresis and transferred onto a PVDF membrane. Western immunoblotting was performed using antibodies against SULF2, GPC3, Wnt3a, β-catenin, phospho-β-catenin, GSK3β, phospho-GSK3β and cyclin D1, with β-actin as the loading control.
Immunoprecipitation using anti-SULF2 and anti-GPC3 antibodies
Hep3B vector and Hep3B SULF2-H cells in 10-cm dishes were washed twice with ice-cold PBS and lysed on ice for 30 min in 1 ml modified RIPA lysis buffer supplemented with Complete-Mini Protease Inhibitor Mixture. After determining the protein concentration and diluting the lysate to approximately 2 µg/µl total cell protein with PBS, the lysate was pre-cleared by adding 20 µl of protein G sepharose bead slurry per ml of lysate and incubating at 4°C for 1 hour on a rocker. SULF2 and GPC3 proteins were immunoprecipitated by incubating pre-cleared lysate with rabbit anti-SULF2 antibody or mouse anti-glypican 3 antibody and protein G-Sepharose (40 µl) overnight at 4°C. Immune complexes were pelleted by centrifugation for 1 min at 14,000 × g, washed three times with lysis buffer, and released from the beads by boiling for 5 min in 40 µl 2× sample buffer. The beads were collected by centrifugation, and the supernatants were resolved by SDS-PAGE. Western immunoblot analysis was performed as described above.
Luciferase assay for TOPFLASH/FOPFLASH reporter activity
TOPFLASH
Hep3B and PLC/PRF/5 cells plated in 24-well plates at a density of 6×104 cells/well were allowed to adhere overnight. The following day, cells were transfected with 0.025 µg/well TOPFLASH reporter construct and 0.1 µg/well either SULF2-expressing construct or empty vector using lipofectamine. After 5 hr, serum-containing media was added and the cells were cultured overnight. Cells were then serum-starved in media containing 0.5% BSA overnight followed by treatment with Wnt3a ligand (R&D System, Minneapolis, MN) for the indicated times. Cell lysates were assayed using the Luciferase Assay System (Promega). Luciferase activity was normalized to total protein content.
TOPFLASH/FOPFLASH
SULF2-positive or negative Hep3B and Huh7 cells were plated into 12 well plates and cultured to 60–70% confluency. The cells were transfected with either 0.5 µg TOPFLASH plasmid (Tcf Reporter) or 0.5 µg FOPFLASH plasmid (with a mutant Tcf binding site) using Lipofectamine Plus (13, 14). To adjust for transfection efficiency, 10 ng pRL-CMV vector (Promega) was co-transfected. Twenty-four hours later, cell lysates were prepared, and firefly and Renilla luciferase activities were quantitated using the Dual-Luciferase Reporter Assay System (Promega).
Mouse xenografts and immunohistochemistry
BALB/c nu/nu nude mouse xenografts were derived from SULF2-negative Hep3B Vector and SULF2-positive Hep3B SULF2–5 cells (11). Immunohistochemistry was performed using antibody against SULF2, GPC3, Wnt3a or β-catenin (11). Primary antibody was replaced with 1% BSA/trishydroxymethylaminomethane-buffered saline for negative controls. The Institutional Animal Care and Use Committee approved the protocols.
Ki-67 and Caspase-3 Assays
Tissue sections were stained with antibody against Ki-67 (Dako, 1/100) and Caspase 3 (Cell Signaling, 1:800) using the Dako Autostainer Plus and counterstained with hematoxylin.
Terminal Deoxynucleotidyl Transferase–Mediated dUTP Nick-End Labeling (TUNEL) Assay
Liver sections were TUNEL stained using the In Situ Cell death Detection Kit, POD (Roche Diagnosis GmbH, Mannheim, Germany). The number of TUNEL-positive cells per 10 high power fields was quantified.
Statistical analysis
All data represent at least three independent experiments and are expressed as the mean ± SEM. Differences between groups were compared using an unpaired two-tailed t test.
RESULTS
Wnt3a induced activation of the Wnt pathway is both SULF2- and GPC3-dependent
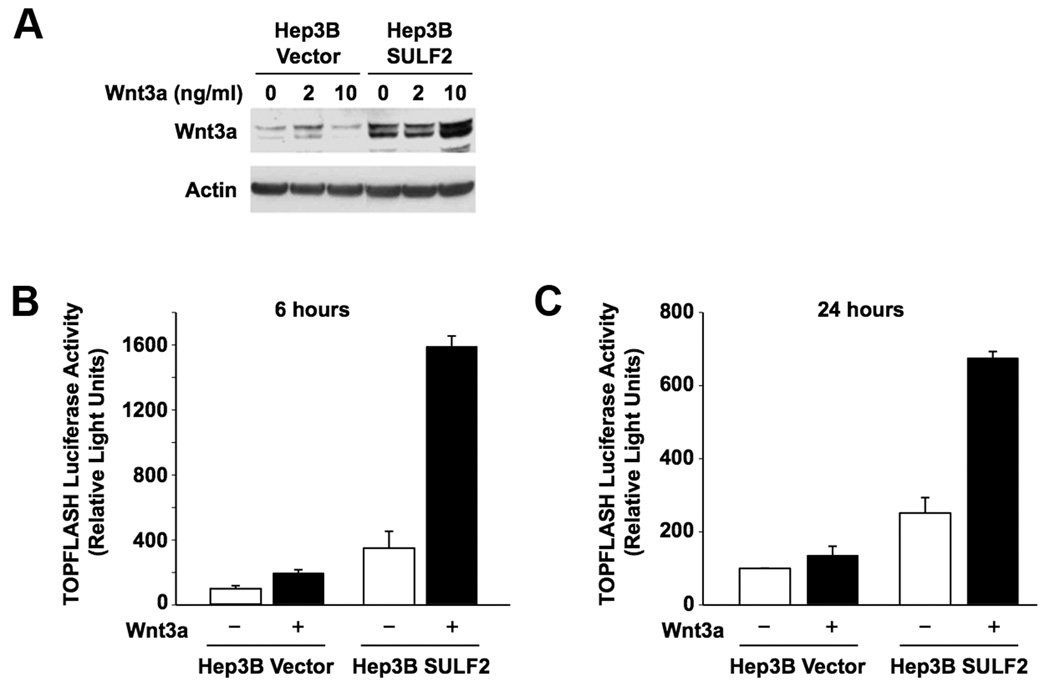
Wnt3a is an important regulator of HCC growth (5). Desulfation of cell surface HSPGs by quail sulfatase 1 was proposed to release sequestered Wnt ligands bound to HSPGs at the cell surface and thus enhance binding of released Wnts to their Frizzled receptors (15). We investigated i) the effects of SULF2 on Wnt signaling in HCC cells upon exposure to exogenous Wnt3a, and ii) whether SULF2 activation of Wnt signaling is dependent on heparan sulfate. Hep3B-Vector and Hep3B-SULF2-H cells were treated with 0, 2, and 10 ng/ml of Wnt3a ligand for 24 hours and washed extensively. Wnt3a levels in cell lysates were then compared by Western immunoblotting. In Hep3B-Vector cells there was a small increase in Wnt3a when cells were treated with 2 ng/ml Wnt3a, but no further increase at 10 ng/ml. In Hep3B-SULF2-H cells, the basal level of Wnt3a was higher. Treatment with 2 ng/ml Wnt3a did not increase Wnt3a, however, 10 ng/ml Wnt3a led to a substantial increase in Wnt3a, suggesting that SULF2 increases endogenous Wnt3a levels (Figure 1A). Moreover, the TOPFLASH luciferase reporter assay showed that Wnt3a stimulation of transiently transfected Hep3B-SULF2 cells induced significant Wnt/β-catenin pathway activity (p<0.0002) as early as 6 hours after transfection and was sustained over 24 hours (Figure 1B and 1C). Similar SULF2 enhancement of Wnt3a-induced TOPFLASH expression occurred in PLC/PRF/5 cells, which also have low SULF2 expression (p<0.03) (Supplementary Data Figure 1).
Figure 1. SULF2 increases Wnt3a expression and enhances Wnt/β-catenin signaling in HCC cells.
(A) Hep3B Vector and Hep3B SULF2-H cells were treated with 0, 2, and 10 ng/ml of Wnt3a ligand for 24 hours, washed and lysed, and Western immunoblotting performed using antibodies against Wnt3a and actin (loading control). SULF2 increased basal and Wnt3a-induced Wnt3a expression. (B and C) TOPFLASH luciferase assay showing the effect of SULF2 on Wnt3a-induced Tcf/Lef transcriptional activity in HCC cells. Hep3B cells were transfected with a TOPFLASH reporter construct and either SULF2-expressing construct or an empty vector. After serum-starvation, cells were treated with 5 ng/ml Wnt3a and TOPFLASH luciferase activity was measured after 6 hours (B) or 24 hours (C). SULF2 enhanced Wnt3a-induced luciferase activity as early as 6 hours and the effect was sustained over 24 hours (p<0.0002).
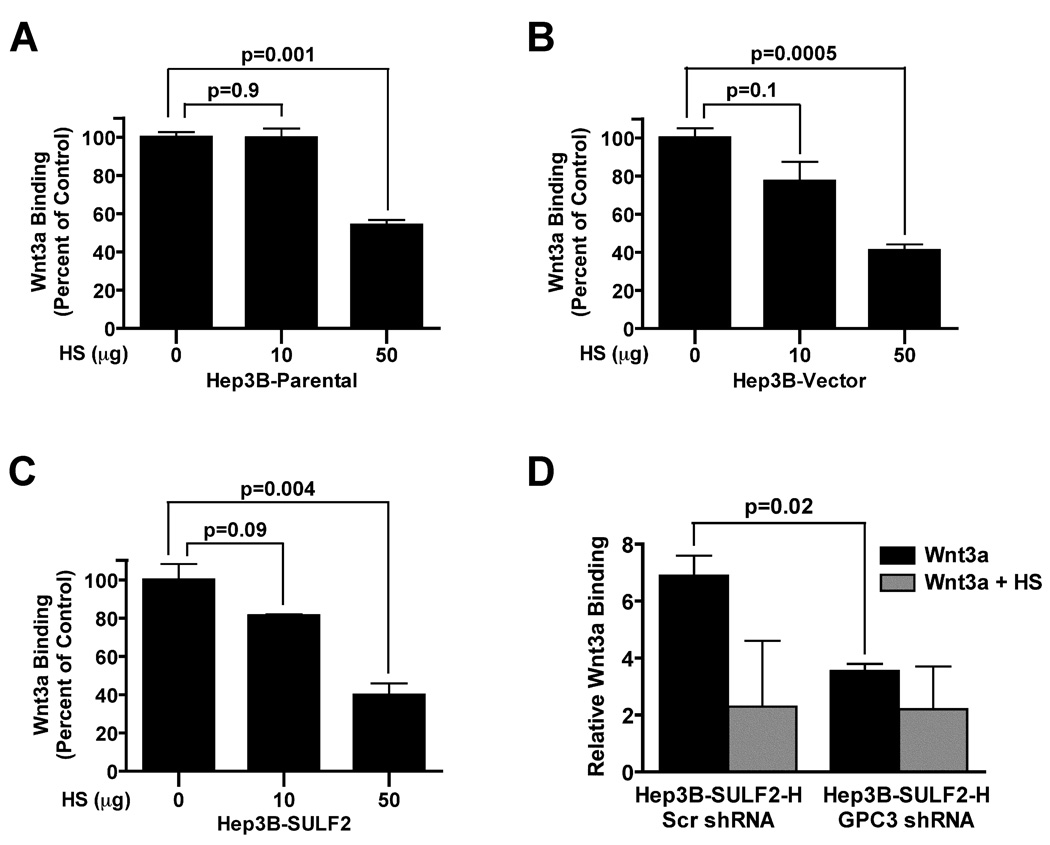
Next, we determined if Wnt3a binding to HCC cells is heparan sulfate-dependent. Wnt3a binding was inhibited by heparan sulfate in a dose-dependent manner (Figure 2A – 2C). Since GPC3 is the most highly upregulated HSPG in HCC and has been shown to bind Wnt3a and activate the Wnt/β-catenin pathway (5, 10), we hypothesized that knockdown of GPC3 would abrogate binding of Wnt3a at the cell surface. To test this hypothesis, we transiently transfected GFP plasmid constructs co-expressing shRNA targeting the GPC3 mRNA or control scrambled shRNA into Hep3B SULF2-H cells. GPC3-knockdown significantly decreased Wnt3a binding to Hep3B cells. Wnt3a binding was also further decreased by heparan sulfate (Figure 2D).
Figure 2. Wnt3a binding to HCC cells is heparan sulfate-dependent and mediated by GPC3.
(A) Wnt3a binding to Hep3B Parental, Hep3B Vector and Hep3B SULF2 cells was assessed by flow cytometry. Cells were incubated with 5 ng of biotinylated Wnt3a without or with 10 or 50 µg heparan sulfate. After staining with streptavidin, 20,000 cells were counted by flow cytometry. There was dose-dependent inhibition of Wnt3a binding by heparan sulfate in all cells. (D) Wnt3a binding to Hep3B SULF2-H cells is GPC3-dependent. Hep3B SULF2-H cells were transiently transfected with a GFP-coexpressing plasmid encoding either a scrambled control shRNA or an shRNA targeting the GPC3 mRNA. GPC3 suppression decreased Wnt3a binding to Hep3B SULF2-H cells. Addition of heparan sulfate (HS) decreased binding further.
SULF2, GPC3 and Wnt3a associate in a possible ternary complex
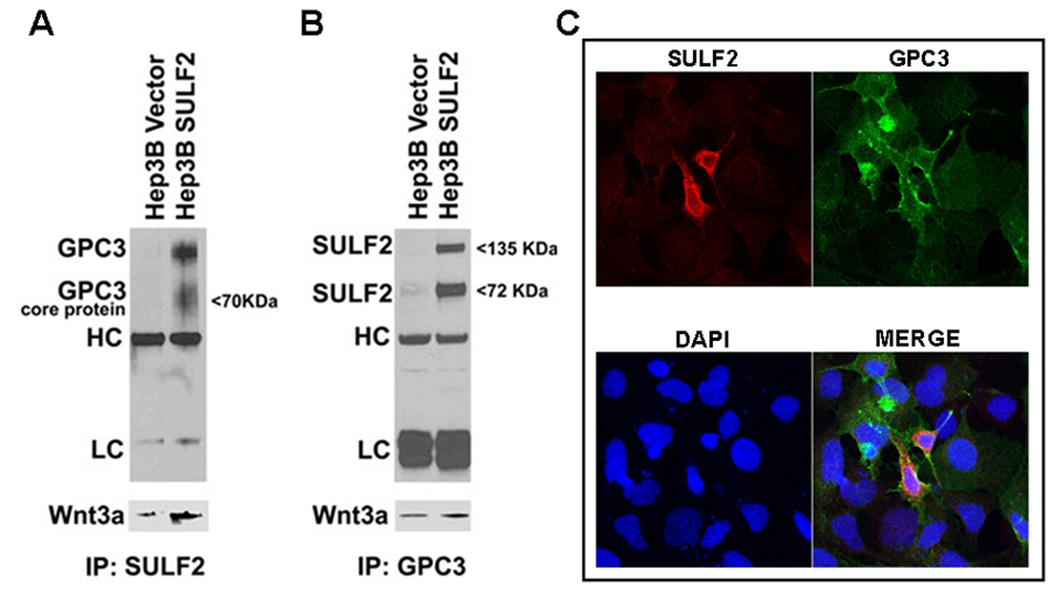
To determine whether SULF2, GPC3 and Wnt3a associate in HCC cells, we treated Hep3B Vector and Hep3B SULF2-H cells with 10 ng/ml Wnt3a ligand and performed immunoprecipitations using antibodies against SULF2 and GPC3. The SULF2 antibody pulled down GPC3 and Wnt3a (Figure 3A) and the GPC3 antibody pulled down SULF2 and Wnt3a (Figure 3B), suggesting that all three molecules associate in a molecular complex. Since GPC3 and SULF2 are primarily located at the cell surface, we confirmed the cell surface co-localization of SULF2 and GPC3 by immunocytochemistry (Figure 3C).
Figure 3. SULF2, GPC3 and Wnt3a associate at the cell surface in a possible ternary complex.
(A and B) Lysates from Hep3B Vector and Hep3B SULF2 cells were used for immunoprecipitation using anti-SULF2 and anti-GPC3 antibodies. Western immunoblotting was performed using antibody against GPC3 and SULF2. The HC and LC bands refer to immunoglobulin heavy chains and light chains, respectively. The blots were stripped and reprobed with antibody against Wnt3a (lower panels of Figure 2A and 2B). (C) Immunocytochemistry showing co-localization of SULF2 and GPC3 in Hep3B cells transiently transfected with SULF2. Nuclei were counterstained with DAPI.
SULF2 up-regulates Wnt3a and GPC3 and activates the Wnt/β-catenin pathway
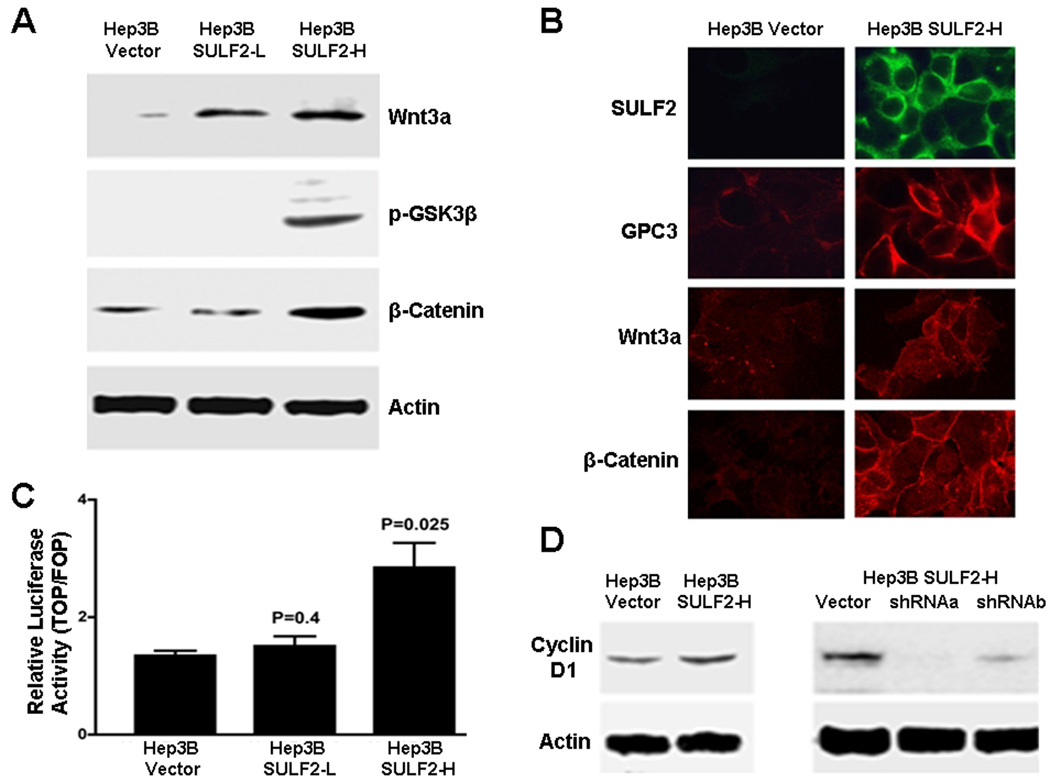
GPC3-dependent Wnt/β-catenin pathway activation and consequent HCC cell proliferation has been demonstrated using exogenous Wnt3a (5, 10). Since SULF2-expressing Hep3B cells have higher Wnt3a expression and may activate the Wnt/β-catenin pathway in an autocrine fashion (Figure 1A – 1C), we investigated the relationship between SULF2, GPC3 and Wnt signaling in the absence of exogenous Wnt3a. We have previously shown by Western immunoblotting that SULF2-induces up-regulation of GPC3 protein (11). SULF2-induced changes in expression of Wnt3a and the Wnt/β-catenin molecules phospho-GSK3β and β-catenin were assessed by Western immunoblotting. Forced expression of SULF2 increased Wnt3a, increased phospho-GSK3β, and increased total β-catenin, consistent with canonical Wnt/β-catenin activation (Figure 4A). Total GSK3β was unchanged and inactive phospho-β-catenin was decreased (Supplementary Data Figure 2). Immunocytochemistry showed increased cell surface localization of SULF2, GPC3, and Wnt3a and membrane, cytoplasmic and nuclear accumulation of β-catenin in Hep3B SULF2-H cells (Figure 4B) (Supplementary Data Figure 3).
Figure 4. Expression of SULF2 up-regulates cell surface Wnt3a and activates the Wnt/β-catenin signaling pathway.
(A) Western immunoblotting was performed on whole cell lysates from Hep3B Vector (control), low-expressing Hep3B SULF2-L and high-expressing Hep3B SULF2-H cells using antibodies against Wnt3a, phospho-GSK3β, β-catenin and actin. SULF2 expression resulted in increases in Wnt3a, phospho-GSK3β, and β-catenin (B) Immunocytochemistry showed that SULF2 increased expression of GPC3, Wnt3a and β-catenin. (C) Transient transfection with TOPFLASH and FOPFLASH plasmids. SULF2 enhanced Tcf-mediated transcriptional activity in Hep3B cells (p=0.025); (D) SULF2 increased cyclin D1 expression; conversely, downregulation of SULF2 decreased cyclin D1 levels.
To determine the functional effects of SULF2 downstream of β-catenin, we measured β-catenin-dependent Tcf/Lef transcriptional activity using the TOPFLASH reporter plasmid. Forced expression of SULF2 significantly increased Tcf/Lef transcription in Hep3B SULF2-H cells (p<0.05) (Figure 4C) and also increased expression of the target gene, Cyclin D1 (Figure 4D). Furthermore, the increase in Cyclin D1 was reversed by knockdown of SULF2 in Hep3B SULF2-H cells (Figure 4D).
Knockdown of SULF2 down-regulates Wnt3a and inhibits Wnt/β-catenin signaling
Since most HCC cell lines over-express SULF2, we examined the effects of down-regulation of SULF2 on Wnt/β-catenin signaling in SULF2-positive Huh7 cells. We have previously shown that knockdown of SULF2 down-regulates GPC3 in Huh7 cells (11). Knockdown of SULF2 decreased the levels of Wnt3a, phospho-GSK3β and β-catenin by Western immunoblotting (Figure 5A), and the level of β-catenin by immunocytochemistry (Figure 5B). Knockdown of SULF2 also significantly decreased Tcf/Lef transcriptional activity in Huh7 cells (p<0.05) (Figure 5C), with an associated decrease in the expression of Cyclin D1 (5D).
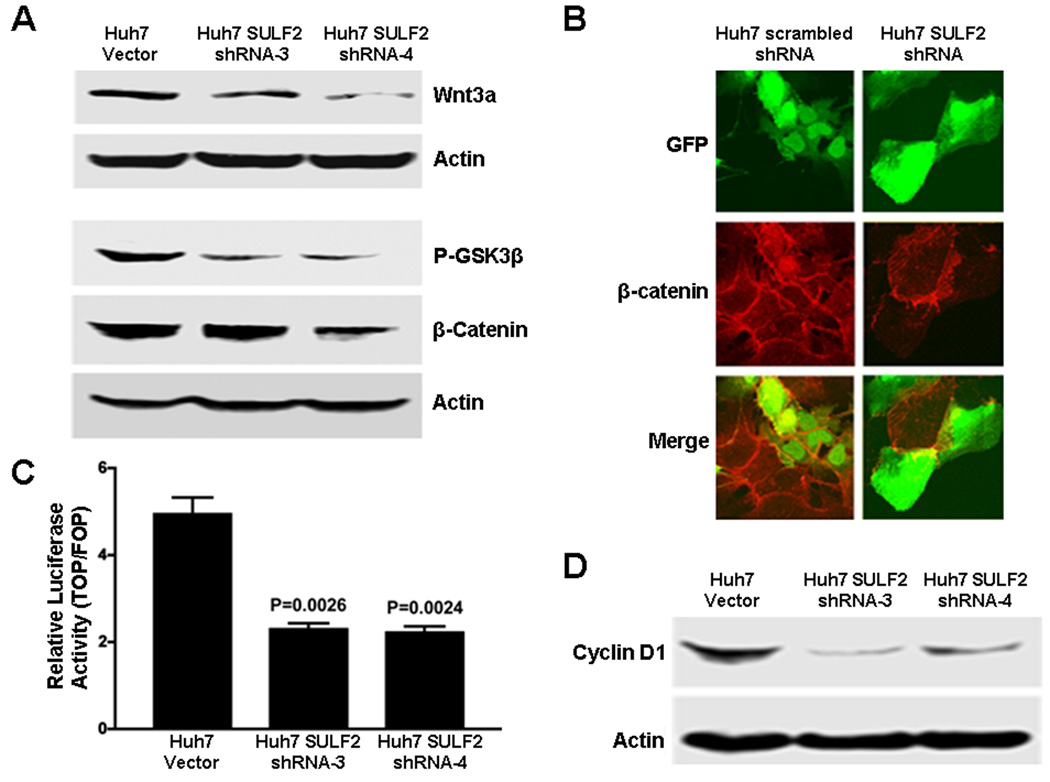
Figure 5. Knockdown of SULF2 down-regulates GPC3 expression and inhibits Wnt signaling in Huh7 cells.
(A) Whole cell lysates of Huh7 Vector cells and two stable clones of Huh7 cells transfected with shRNA targeting SULF2, Huh7 SULF2 shRNA-3 and Huh7 SULF2 shRNA-4, were prepared. Western immunoblotting was performed using antibodies against Wnt3a, phospho-GSK3β, β-catenin and actin (loading control). Downregulation of SULF2 decreased Wnt3a, phospho-GSK3β, and β-catenin (B) Huh7 cells were transiently transfected with GFP-expressing plasmids carrying either a control scrambled shRNA sequence or shRNA targeting SULF2 mRNA (shRNA-a). After 24 hours, cells were immunostained for β-catenin. Cells expressing the scrambled shRNA (left column, green fluorescent cells) showed no difference in β-catenin levels; in contrast, cells expressing the SULF2 shRNA (right column, green fluorescence) showed substantially decreased β-catenin as compared to the untransfected cells without green fluorescence. (C) Huh7 Vector and Huh7 SULF2 shRNA stable clones were transiently transfected with TOPFLASH and FOPFLASH plasmids. Downregulation of SULF2 inhibited Tcf-mediated transcriptional activity in Huh7 cells (p=0.002). (D) Suppression of SULF2 expression decreased cellular levels of cyclin D1 as detected by Western immunoblotting.
SULF2 up-regulates GPC3 and Wnt3a and increases cell proliferation in nude mouse xenografts in vivo
We have previously shown that SULF2 increases proliferation and viability of HCC cell lines in vitro. To confirm this observation in vivo, we inoculated stably-transfected HCC cells subcutaneously in nude mice. SULF2 significantly increased tumor growth, decreasing the median time to reach a tumor size of 1000 mm3 by 28 days (11). To confirm the SULF2-induced changes in GPC3 and Wnt signaling in vivo, we performed immunohistochemistry using antibodies against SULF2, GPC3, Wnt3a and β-catenin in consecutive sections of xenografts derived from Hep3B Vector and Hep3B SULF2-H cells. SULF2-induced up regulation of GPC3, Wnt3a and β-catenin in vivo (Figure 6A). Further, the dominant effect of SULF2 on HCC cell growth was through increased proliferation (Ki67 assay, Figure 6B and 6C) rather than from decreased apoptosis (Cleaved caspase-3 and TUNEL assays, Figure 7).
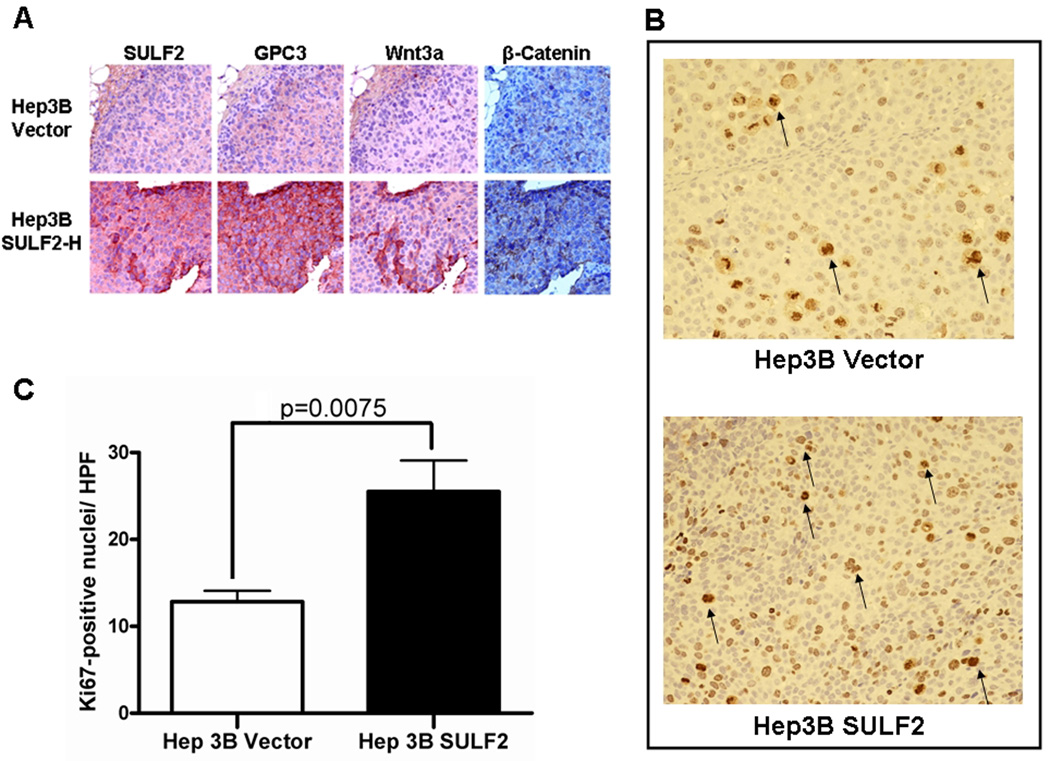
Figure 6. SULF2 up-regulates GPC3 and Wnt3a and promotes tumor growth in HCC nude mouse xenografts mainly through increased cell proliferation.
Hep3B Vector and Hep3B SULF2-H clones were subcutaneously inoculated into the flanks of ten nude mice. SULF2 significantly enhanced tumor growth in vivo (11). (A) Successive sections from paraffin-embedded xenografts from Hep3B Vector (upper panel) and Hep3B SULF2-H cells (lower panel) were immunostained with antibodies against SULF2, GPC3 and Wnt3a respectively; nuclei were counterstained with hematoxylin. SULF2 expression was associated with increases in tumor cell GPC3, Wnt3a, and β-catenin. (B) Ki-67 staining from Hep3B SULF2-derived xenografts compared with Hep3B Vector. Xenografts from Hep3 SULF2 cells showed smaller sized but increased number of brown-staining proliferative cells (arrows) (magnification ×200). (C) Graph quantitating increased cell proliferation in Hep3B SULF2-derived xenografts as compared to Hep3B Vector xenografts (average of 6 high power fields).
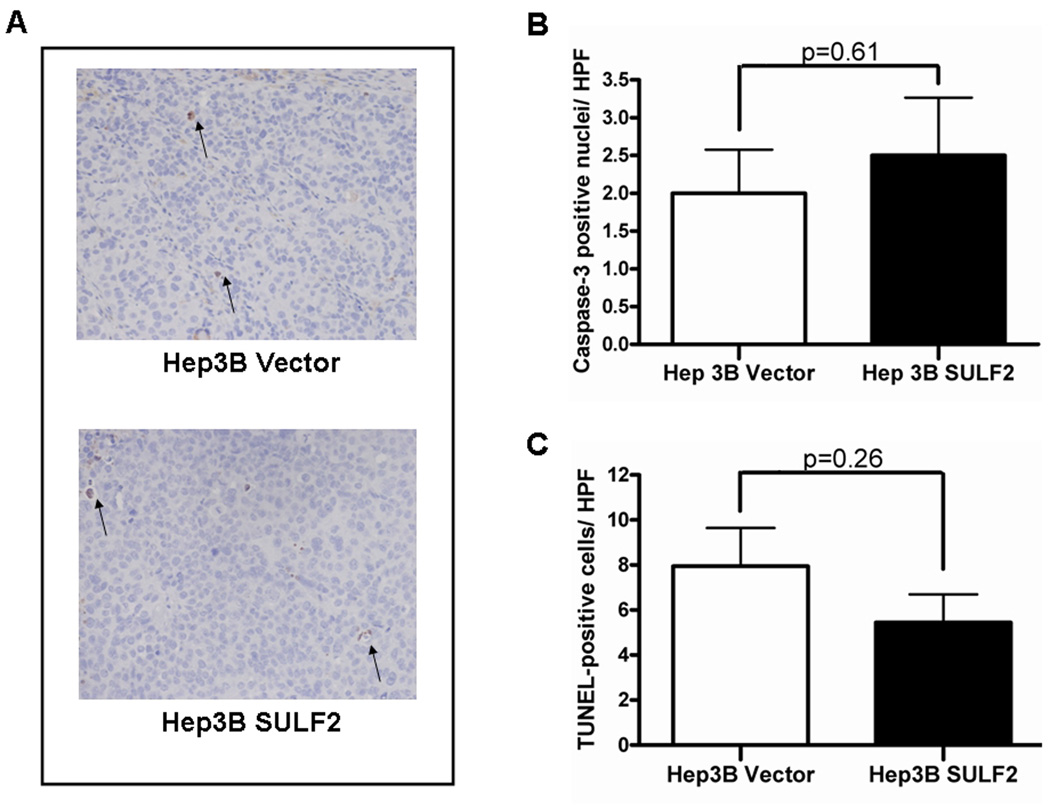
Figure 7. SULF2 does not decrease apoptosis in HCC mouse xenografts.
Sections from paraffin embedded xenografts from Hep3B Vector and Hep3B SULF2-H cells were stained for apoptotic cells (arrows). (A) Caspase-3 assay showed few apoptotic cells in both Hep3B Vector- and Hep3B SULF2-derived xenograft sections (magnification ×200). (B) There was no significant difference in active caspase-3 between the 2 groups. (C) TUNEL staining also showed no difference between the groups. Values represent the mean of TUNEL-positive cells in 6 high power fields.
DISCUSSION
The mechanisms regulating Wnt/β-catenin pathway activation in HCC are not completely elucidated (2). Many cell growth signaling pathways have ligands for which cell surface and extracellular matrix proteoglycans serve as co-receptors or storage sites. GPC3 is a cell surface HSPG that is highly over-expressed in HCC and can sequester growth factor ligands and cytokines via its sulfated HSGAG side chains. GPC3 has been shown to mediate activation of the canonical Wnt/β-catenin pathway, and anchorage of GPC3 to the cell membrane has been shown to be critical for Wnt/β-catenin activation and growth of HCC cells (5, 16, 17). The heparan sulfate-degrading endosulfatase SULF2 may release sequestered factors from HSGAGs and allow binding to their receptors, thus enhancing growth signaling (18). We therefore hypothesized that GPC3-mediated activation of the Wnt/β-catenin pathway in human HCCs would be enhanced by SULF2. In this paper we have explored the contribution of SULF2 expression to GPC3-mediated Wnt pathway activation in HCC.
The principal findings of this study are that: 1) SULF2 increases endogenous Wnt3a expression and stimulates basal and Wnt3a-induced Tcf/Lef transcriptional activation in SULF2-negative Hep3B HCC cells; 2) binding of exogenous Wnt3a to the cell surface is dependent on the heparan sulfate proteoglycan GPC3; 3) SULF2, GPC3 and Wnt3a associate in a possible ternary complex; and 4) the canonical Wnt pathway is activated by SULF2, which increases GPC3 and Wnt3a expression, GSK3β phosphorylation, membrane, cytosolic and nuclear β-catenin, and downstream Cyclin D1 expression. Down regulation of SULF2 in the SULF2-positive Huh7 cell line leads to opposite effects on Wnt/β-catenin signaling. The SULF2-induced increase in GPC3, Wnt3a and β-catenin occurs in HCC xenografts in vivo and is primarily due to activation of cell proliferation.
Previous work on GPC3 mediated Wnt/β-catenin signaling has used exogenous Wnt3a. However, since we found that SULF2 up-regulates endogenous Wnt3a, we assessed changes in Wnt/β-catenin pathway activity both in HCC cell lines treated with exogenous Wnt3a (as would occur in paracrine signaling) and in HCC cells transfected with SULF2 (mimicking autocrine signaling). Using SULF2-transfected and GPC3-knockout cell models, we demonstrated that the effect of heparan sulfate on Wnt3a binding at the cell surface is dose-dependent and that GPC3 is a mediator of Wnt3a binding. In addition, by immunoprecipitation and immunocytochemistry using antibodies against SULF2 and GPC3 we provide evidence for the cellular interaction of SULF2, GPC3 and Wnt3a.
To determine the functional consequences of the cell surface association of GPC3, SULF2 and Wnt3a, we examined the effect of forced expression of SULF2 in the SULF2-negative Hep3B HCC cell line and also studied the impact of SULF2 knockdown in Huh7 HCC cells, which endogenously express SULF2 (11). In Hep3B cells, SULF2 expression increased GPC3 and Wnt3a expression and activated the Wnt/β-catenin pathway as evidenced by increased phosphorylation of GSK3β and consequent accumulation of β-catenin. Conversely, knockdown of SULF2 in Huh7 cells decreased GPC3, Wnt3a, phosphorylated GSK3β and β-catenin. The functional significance of these changes in β-catenin expression was confirmed by measuring the β-catenin-dependent Tcf/Lef transcriptional activity using the TOPFLASH/FOPFLASH luciferase reporter assay and the corresponding expression of the target gene Cyclin D1. These findings demonstrate that Wnt/β-catenin pathway activation is mediated by both SULF2 and the HSPG GPC3 in a complex involving Wnt3a.
Finally, we have provided in vivo evidence of SULF2-induced up-regulation of GPC3, Wnt3a and β-catenin expression in HCC xenografts from nude mice. Together, our results support a working model that SULF2-mediated desulfation of GPC3 HSGAGs at the cell surface releases Wnt from “storage” type HSGAGs to enable Wnt activation of its Frizzled receptors and downstream Wnt//β-catenin signaling (Figure 8). Since the primary action of the sulfatases is on the HSGAG chains attached to core proteins, this model suggests that the HSGAG chains of GPC3 play a role in GPC3-mediated activation of the Wnt pathway in HCC. This supports earlier work that described HSGAG chains as essential in GPC3-mediated Wnt signaling in both canonical and non-canonical Wnt pathway activation (19). Although it has been suggested that the HSGAG chains of GPC3 are not absolutely required for canonical Wnt signaling in HCC (5), our findings strongly suggest that SULF2 induced changes in the sulfation state of GPC3 HSGAGs modulate GPC3–mediated Wnt/β-catenin signaling in HCC cells, both in vitro and in vivo.
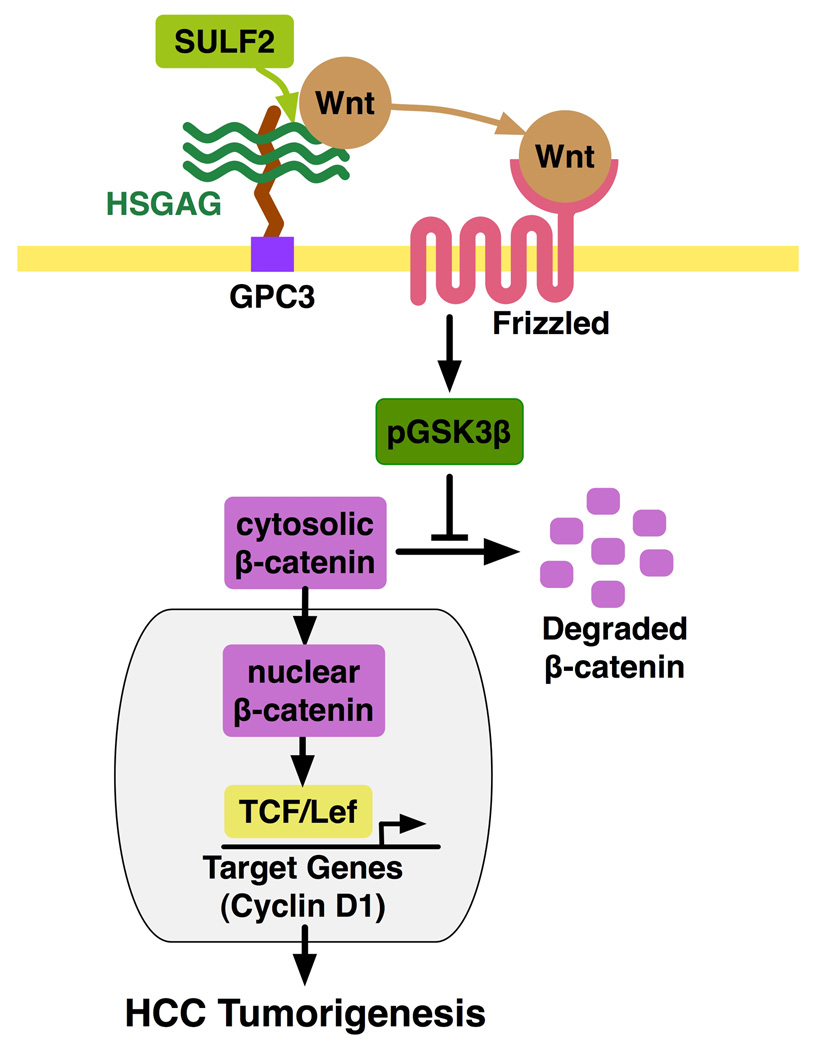
Figure 8. Working model for the effect of SULF2 on Wnt/β-catenin signaling in HCC cells.
Expression of SULF2 in HCC cells up-regulates cell surface GPC3 and Wnt3a. SULF2 desulfates GPC3 HSGAGs, leading to release of Wnt, binding and activation of the Wnt receptor Frizzled and phosphorylation of GSK3β. Phosphorylation of GSK3β results in the dissolution of the complex responsible for degradation of β-catenin, thus allowing β-catenin to accumulate in the cytosol and translocate to the nucleus. This results in increased Tcf/Lef transcription and up-regulation of target genes, including cyclin D1, with consequent promotion of cell proliferation in vitro and tumor growth in vivo.
In summary, this work supports the hypothesis that SULF2 acts as an oncogenic protein in HCCs at least in part by increasing Wnt3a and GPC3 expression and activating the Wnt/β-catenin pathway, thus promoting growth of HCC cell lines and xenografts. We have previously shown that SULF2 also enhances signaling by receptor tyrosine kinases such as FGF2 (11). This ability of SULF2 to modulate FGF2 and Wnt binding at the cell surface, and hence activate the MAPK, AKT and Wnt pathways likely accounts for the high tumor recurrence rate and poor survival of HCC patients whose tumors express high levels of SULF2 (11). Our findings also confirm the concept that, in contrast to its tumor suppressor-like homolog, SULF1, SULF2 has an oncogenic effect in human HCCs. Agents that inhibit SULF2 may therefore be effective for prevention and/or treatment of HCCs (12, 20, 21). A recent study has also shown an analogous oncogenic effect of SULF2 in lung cancer (22).
We are currently pursuing studies to determine the exact mechanism by which desulfation regulates GPC3 function and to determine how this modulates Wnt3a-Frizzled 7 binding and Wnt pathway activation at the cell surface. In particular we propose that 6-O desulfation of the heparan sulfate chains of GPC3 by SULF2 will release more Wnt proteins from their storage sites, making them available to bind to and stimulate their cognate Frizzled receptors.
Supplementary Material
Acknowledgements
Supported by NIH grants CA100882 and CA128633 (to L.R.R.), CA136526 and CA102701 (Mayo Clinic Pancreas SPORE) (to M.E.F.-Z.), and P30DK084567 (Mayo Clinic Center for Cell Signaling in Gastroenterology). The authors thank Dr. Shin-Ichiro Kojima for pG-SUPER vector, Dr. Daniel D. Billadeau for pSSH1p and TOPFLASH vectors, Dr. Wanguo Liu for TOPFLASH and FOPFLASH vectors, Patrick L. Splinter and Linda M. Murphy for technical assistance, Victoria Campion for secretarial assistance, and Drs. Gregory J. Gores and Rosebud O. Roberts for critical review of the manuscript.
Abbreviations
- DAPI
4’,6-diamidino-2-phenylindole dihydrochloride
- GFP
green fluorescent protein
- GPC3
glypican 3
- HCC
hepatocellular carcinoma
- HRP
horseradish peroxidase
- HSGAG
heparan sulfate glycosaminoglycan
- HSPG
heparan sulfate proteoglycan
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- NP-40
nonidet P-40
- TUNEL
Terminal Deoxynucleotidyl Transferase–Mediated dUTP Nick-End Labeling Assay
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Roberts LR, Gores GJ. Hepatocellular carcinoma: molecular pathways and new therapeutic targets. Semin Liver Dis. 2005;25:212–225. doi: 10.1055/s-2005-871200. [DOI] [PubMed] [Google Scholar]
- 3.Bengochea A, de Souza MM, Lefrancois L, Le Roux E, Galy O, Chemin I, Kim M, et al. Common dysregulation of Wnt/Frizzled receptor elements in human hepatocellular carcinoma. Br J Cancer. 2008;99:143–150. doi: 10.1038/sj.bjc.6604422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee HC, Kim M, Wands JR. Wnt/Frizzled signaling in hepatocellular carcinoma. Front Biosci. 2006;11:1901–1915. doi: 10.2741/1933. [DOI] [PubMed] [Google Scholar]
- 5.Capurro MI, Xiang YY, Lobe C, Filmus J. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res. 2005;65:6245–6254. doi: 10.1158/0008-5472.CAN-04-4244. [DOI] [PubMed] [Google Scholar]
- 6.Filmus J, Capurro M, Rast J. Glypicans. Genome Biol. 2008;9:224. doi: 10.1186/gb-2008-9-5-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song HH, Filmus J. The role of glypicans in mammalian development. Biochim Biophys Acta. 2002;1573:241–246. doi: 10.1016/s0304-4165(02)00390-2. [DOI] [PubMed] [Google Scholar]
- 8.Zhu ZW, Friess H, Wang L, Abou-Shady M, Zimmermann A, Lander AD, Korc M, et al. Enhanced glypican-3 expression differentiates the majority of hepatocellular carcinomas from benign hepatic disorders. Gut. 2001;48:558–564. doi: 10.1136/gut.48.4.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sung YK, Hwang SY, Park MK, Farooq M, Han IS, Bae HI, Kim JC, et al. Glypican-3 is overexpressed in human hepatocellular carcinoma. Cancer Sci. 2003;94:259–262. doi: 10.1111/j.1349-7006.2003.tb01430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capurro MI, Shi W, Sandal S, Filmus J. Processing by convertases is not required for glypican-3-induced stimulation of hepatocellular carcinoma growth. J Biol Chem. 2005;280:41201–41206. doi: 10.1074/jbc.M507004200. [DOI] [PubMed] [Google Scholar]
- 11.Lai JP, Sandhu DS, Yu C, Han T, Moser CD, Jackson KK, Guerrero RB, et al. Sulfatase 2 up-regulates glypican 3, promotes fibroblast growth factor signaling, and decreases survival in hepatocellular carcinoma. Hepatology. 2008;47:1211–1222. doi: 10.1002/hep.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai JP, Chien JR, Moser DR, Staub JK, Aderca I, Montoya DP, Matthews TA, et al. hSulf1 Sulfatase promotes apoptosis of hepatocellular cancer cells by decreasing heparin-binding growth factor signaling. Gastroenterology. 2004;126:231–248. doi: 10.1053/j.gastro.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 13.Kim K, Pang KM, Evans M, Hay ED. Overexpression of beta-catenin induces apoptosis independent of its transactivation function with LEF-1 or the involvement of major G1 cell cycle regulators. Mol Biol Cell. 2000;11:3509–3523. doi: 10.1091/mbc.11.10.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 15.Dhoot GK, Gustafsson MK, Ai X, Sun W, Standiford DM, Emerson CP., Jr Regulation of Wnt signaling and embryo patterning by an extracellular sulfatase. Science. 2001;293:1663–1666. doi: 10.1126/science.293.5535.1663. [DOI] [PubMed] [Google Scholar]
- 16.Zittermann SI, Capurro MI, Shi W, Filmus J. Soluble glypican 3 inhibits the growth of hepatocellular carcinoma in vitro and in vivo. Int J Cancer. 126:1291–1301. doi: 10.1002/ijc.24941. [DOI] [PubMed] [Google Scholar]
- 17.Hsu HC, Cheng W, Lai PL. Cloning and expression of a developmentally regulated transcript MXR7 in hepatocellular carcinoma: biological significance and temporospatial distribution. Cancer Res. 1997;57:5179–5184. [PubMed] [Google Scholar]
- 18.Lai JP, Thompson JR, Sandhu DS, Roberts LR. Heparin-degrading sulfatases in hepatocellular carcinoma: roles in pathogenesis and therapy targets. Future Oncol. 2008;4:803–814. doi: 10.2217/14796694.4.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baeg GH, Lin X, Khare N, Baumgartner S, Perrimon N. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development. 2001;128:87–94. doi: 10.1242/dev.128.1.87. [DOI] [PubMed] [Google Scholar]
- 20.Lai JP, Yu C, Moser CD, Aderca I, Han T, Garvey TD, Murphy LM, et al. SULF1 inhibits tumor growth and potentiates the effects of histone deacetylase inhibitors in hepatocellular carcinoma. Gastroenterology. 2006;130:2130–2144. doi: 10.1053/j.gastro.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 21.Lai JP, Sandhu DS, Shire AM, Roberts LR. The tumor suppressor function of human sulfatase 1 (SULF1) in carcinogenesis. J Gastrointest Cancer. 2008;39:149–158. doi: 10.1007/s12029-009-9058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemjabbar-Alaoui H, van Zante A, Singer MS, Xue Q, Wang YQ, Tsay D, He B, et al. Sulf-2, a heparan sulfate endosulfatase, promotes human lung carcinogenesis. Oncogene. 29:635–646. doi: 10.1038/onc.2009.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.