Abstract
Background
To assess the risk and identify risk factors of Hodgkin lymphoma (HL) in solid organ transplant recipients. Prior research has been limited by the rarity of HL and the requirement for extended follow-up after transplantation.
Methods
Using data from the Scientific Registry of Transplant Recipients (SRTR), we conducted a retrospective cohort study of U.S. solid organ transplant recipients (1997–2007). We estimated hazard ratios (HRs) for HL risk factors using proportional hazards regression. Standardized incidence ratios (SIRs) compared HL risk in the transplant cohort with the general population.
Results
The cohort included 283,190 transplant recipients (average follow-up 3.7 years after transplantation). Based on 73 cases, HL risk factors included male gender (HR 2.1, 95%CI 1.2–3.7), young age (4.0, 2.3–6.8), and EBV seronegativity at the time of transplantation (3.1, 1.2–8.1). Among tumors with EBV status information, 79% were EBV positive, including all tumors in recipients who were initially seronegative. Overall, HL risk was higher than in the general population (SIR 2.2) and increased monotonically over time following transplantation (SIR 4.1 at 8–10 years post-transplant). Excess HL risk was especially high following heart and/or lung transplantation (SIR 3.2).
Conclusion
HL is a late complication of solid organ transplantation. The high HL risk in recipients who were young or EBV seronegative at the time of transplant, and the fact that most HL tumors were EBV positive, highlight the role of primary EBV infection and poor immune control of this virus. The occurrence of HL may rise with improved long-term survival in transplant recipients.
Keywords: Hodgkin lymphoma, transplantation, Epstein-Barr virus, United States
Introduction
The long-term health consequences of solid organ transplantation have taken on greater importance due to improvements in patient and graft survival (1). Due in large part to long-term immunosuppression, solid organ transplant recipients are at greatly elevated risk of a number of malignancies, including non-melanoma skin cancer and non-Hodgkin lymphoma (NHL) (1–5). NHL and Hodgkin lymphoma (HL) both comprise part of a spectrum of post-transplant lymphoproliferative disease (PTLD) arising in transplant recipients (6).
The impact of solid organ transplantation on the incidence of HL has not been extensively evaluated, but earlier studies have demonstrated that solid organ transplantation is associated with increased HL risk compared to the general population (1–5). Previous studies, which have typically been limited to small case series, describe post-transplant HL as an aggressive, late complication of solid organ transplantation, with tumors typically manifesting mixed cellularity pathology and almost uniform Epstein-Barr virus (EBV) positivity (7–10). The HLs described in transplant recipients are thus similar to those arising in the setting of human immunodeficiency virus (HIV) infection, providing evidence that disturbances in immune function play an important etiologic role in this malignancy (11).
A better understanding of HL risk following solid organ transplantation will provide additional clues to the role of immunosuppression and EBV infection in the etiology of this malignancy. The U.S. Scientific Registry of Transplant Recipients (SRTR) is a unique resource for evaluating the epidemiology of post-transplant HL, because detailed follow-up data are available on a large number of transplant recipients. We used these data to conduct a retrospective cohort study examining the risk factors and timing of HL following solid organ transplantation.
Methods
Study design and subjects
We conducted a retrospective cohort study of U.S. transplant recipients using data provided to the SRTR by transplantation centers and organ procurement organizations that together comprise the Organ Procurement and Transplantation Network (OPTN). Baseline and follow-up data are available on all solid organ transplants performed in the U.S. since 1986. Follow-up data are available at 6 and 12 months after transplantation, and annually thereafter. In our cohort we included all recipients of first organ transplants conducted between October 1, 1987, and August 31, 2007, who had no evidence of HIV infection and had at least 30 days of post-transplant follow-up.
Exposure assessment
For each transplant recipient we obtained data from the SRTR baseline file regarding demographic and transplant characteristics. The transplant characteristics included the type of organ transplanted (kidney and/or pancreas, liver, heart and/or lung, other) and the total number of HLA mismatches with the donor at the A, B, and DR loci (range: 0–6). We also obtained baseline viral serology data for EBV (EBV IgG) and cytomegalovirus (CMV IgG). Updated EBV serostatus was obtained from SRTR follow-up files.
The SRTR immunosuppression file was used for data on the initial immunosuppressive regimen prescribed for each transplant recipient prior to hospital discharge. The data included medications prescribed to induce or maintain immunosuppression, or to treat initial rejection episodes. We created variables for categories of medication, including antibody induction therapy, steroid-based maintenance therapy, and anti-rejection therapy.
Outcome ascertainment
We used the SRTR follow-up files to identify recipients with HL. At each follow-up visit, transplant center providers were required to report any malignancies diagnosed since transplantation. If a recipient was diagnosed with HL, the date of diagnosis was recorded, along with the EBV status of the tumor. The SRTR follow-up files were also used to identify occurrence of death, graft failure, re-transplantation, and loss to follow-up.
Reporting of HL to the SRTR changed over time. HL diagnosis information was not collected on lung transplant recipients until April 1, 1994, and on all other recipients until March 1, 1997.
Statistical analysis
Follow-up for all recipients started at 30 days post-transplantation or July 1, 1997, whichever occurred later, and continued until they developed HL or were censored due to PTLD other than HL, graft failure, re-transplantation, death, loss to follow-up, or 10 years post-transplantation. We included only follow-up time and HL events starting on July 1, 1997 to allow for uniform ascertainment for transplants of all organs. Recipients transplanted prior to July 1, 1997, only contributed follow-up time and HL events starting on July 1, 1997.
To estimate hazard ratios and 95% confidence intervals (CI) for risk factors for HL, we developed univariate proportional hazards regression models with time since transplantation as the time metric. HL risk factors examined in the analyses included demographic characteristics, organ transplanted, overall number of HLA mismatches, baseline viral serologies, and initial immunosuppressive regimen. By evaluating an interaction between each risk factor and follow-up time, we demonstrated that all of the models that we present satisfied the proportional hazards assumption. The statistical significance of HL risk factors was based on the whether the estimated HR 95% CI overlapped the null value of 1.0.
We utilized population-based incidence data from the Surveillance, Epidemiology, and End Results (SEER) network of U.S. cancer registries (http://seer.cancer.gov/) to calculate the expected numbers of HL cases in our cohort using rates specific to gender, race/ethnicity (non-Hispanic white, non-Hispanic black, and Hispanic), age, and calendar year. We then calculated standardized incidence ratios (SIRs) as the ratio of the observed number of HL cases between 1997 and 2007, to the expected number calculated by summing the expected cases across the categories. For this analysis, we included only transplant recipients of white, black, or Hispanic race/ethnicity, since incidence data were available from SEER to calculate expected case numbers.
Results
The cohort included 283,190 recipients followed for a mean of 3.7 years (standard deviation +/− 2.7 years) after transplantation (Table 1). The majority of transplant recipients were male (61.5%), non-Hispanic white (66.6%), and age 20 years or older (91.9%). The most common organ transplanted was kidney and/or pancreas (63.7%), followed by liver (20.6%).
Table 1.
Hodgkin lymphoma risk factors among U.S. transplant recipients during 1997–2007
| Recipients, n (%) | Hodgkin lymphoma cases, n | Hazard ratio (95% CI) | |
|---|---|---|---|
| Total | 283,190 (100%) | 73 | - - |
| Gender | |||
| Male | 174,194 (61.5%) | 56 | 2.1 (1.2–3.7) |
| Female | 108,996 (38.5%) | 17 | 1.0 (ref) |
| Age at transplant, years | |||
| 0–19 | 22,870 (8.1%) | 21 | 4.0 (2.3–6.8) |
| 20–50 | 139,430 (49.2%) | 32 | 1.0 (ref) |
| >50 | 120,890 (42.7%) | 20 | 0.9 (0.5–1.5) |
| Median age | 48.0 | ||
| Race/ethnicity | |||
| White, non-Hispanic | 188,648 (66.6%) | 57 | 1.5 (0.9–2.6) |
| Other | 94,542 (33.4%) | 16 | 1.0 (ref) |
| EBV serostatus at transplant | |||
| Positive | 87,291 (30.8%) | 10 | 1.0 (ref) |
| Negative | 17,612 (6.2%) | 7 | 3.1 (1.2–8.1) |
| Missing/unknown | 178,287 (63.0%) | 56 | 1.0 (0.5–2.1) |
| CMV serostatus at transplant | |||
| Positive | 93,726 (33.1%) | 9 | 1.0 (ref) |
| Negative | 57,213 (20.2%) | 9 | 1.6 (0.6–4.0) |
| Missing/unknown | 132,251 (46.7%) | 55 | 1.6 (0.8–3.5) |
| Type of organ transplanted | |||
| Kidney and/or pancreas | 180,449 (63.7%) | 40 | 1.0 (ref) |
| Liver | 58,235 (20.6%) | 15 | 1.1 (0.6–2.0) |
| Heart and/or lung | 42,447 (15.0%) | 18 | 1.6 (0.9–2.9) |
| Other | 2,059 (0.7%) | 0 | 0 |
| HLA mismatch, number of alleles* | |||
| 0–2 | 54,948 (22.4%) | 17 | 1.0 (ref) |
| 3–4 | 103,708 (42.2%) | 28 | 0.9 (0.5–1.7) |
| 5–6 | 87,106 (35.4%) | 19 | 0.8 (0.4–1.6) |
| Antibody induction† | |||
| Yes | 119,505 (43.1%) | 25 | 1.0 (0.6–1.7) |
| No | 157,549 (56.9%) | 46 | 1.0 (ref) |
| Steroid maintenance† | |||
| Yes | 246,799 (89.1%) | 67 | 1.0 (0.4–2.8) |
| No | 30,255 (10.9%) | 4 | 1.0 (ref) |
| Anti-rejection therapy† | |||
| Yes | 34,628 (12.5%) | 13 | 1.2 (0.7–2.2) |
| No | 242,426 (87.5%) | 58 | 1.0 (ref) |
Notes
Abbreviations: CI confidence interval, HLA human leukocyte antigen, EBV Epstein-Barr virus, CMV cytomegalovirus.
HLA information was missing for 37,428 (13.2%) transplant recipients.
Medication information was missing for 6,136 (2.2%) transplant recipients.
Recipients with missing data for a particular covariate were not included in the Cox proportional hazards regression model for that covariate.
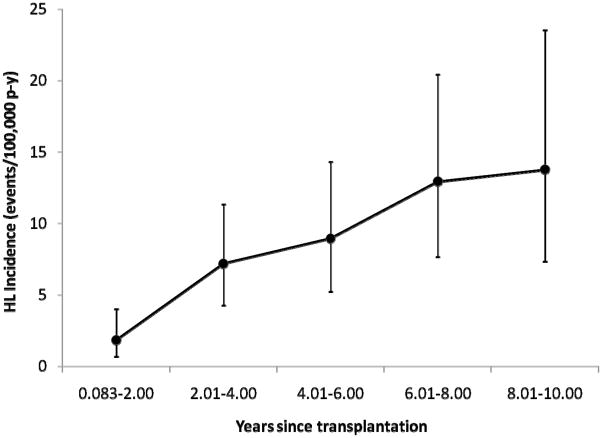
During follow-up, 73 HL cases were diagnosed. As shown in Figure 1, HL incidence was low in the first two years after transplant (1.8 cases per 100,000 person-years) and increased steadily thereafter, reaching 13.8 cases per 100,000 person-years 8–10 years after transplant. Risk factors for HL are presented in Table 1. Risk factors included male gender (hazard ratio [HR] 2.1, 95% CI 1.2–3.7) and young age at transplantation (HR 4.0, 95% CI 2.3–6.8, for age 0–19 vs. 20–50 years at transplant). Based on limited data, EBV seronegativity at transplant was associated with elevated subsequent HL risk (HR 3.1, 95% CI 1.2–8.1). Recipient race/ethnicity, CMV serostatus, type of organ transplanted, HLA mismatch, and immunosuppressive regimen were not significantly associated with HL risk.
Figure 1.
Incidence of Hodgkin lymphoma among U.S. transplant recipients during 1997–2007. Incidence rates and their 95% confidence intervals are shown as a function of time since transplantation and are displayed as Hodgkin lymphoma cases per 100,000 person-years. Follow-up for all recipients began 30 days (0.083 years) after transplantation.
Of the 73 HL cases, 7 were diagnosed in transplant recipients who were EBV seronegative at the time of transplantation. Information on EBV status of the tumors was available in 6 of these 7 cases, of which all were EBV positive. Also, of the 7 HL cases who were EBV seronegative at the time of transplantation, 3 had follow-up EBV serology data, and all showed evidence of EBV seroconversion. Finally, EBV status was available for 32 of the remaining 66 HL tumors, of which 24 (75%) were EBV positive. Overall, 79% of tumors with available information were EBV positive.
Overall, HL risk was doubled in solid organ transplant recipients compared to the general population (SIR 2.2, 95%CI 1.7–2.7). The excess risk compared to the general population was greatest in children and adolescents (SIRs 93.7 and 11.1 for ages 0–9 and 10–19 years at transplant, respectively) and recipients who were EBV seronegative at transplant (SIR 4.7, 95% CI 1.9–9.6). Heart and/or lung transplant recipients experienced especially high HL risk compared to the general population (SIR 3.2, 95% CI 1.9–5.0), whereas the excess risk associated with kidney and/or pancreas or liver transplantation was more modest (SIRs 1.8 and 2.3, respectively). While the risk was not elevated in the first two years post-transplant (SIR 0.6, 95% CI 0.2–1.2), by 8–10 years post-transplant HL risk was increased 4-fold compared with the general population (SIR 4.1 95% CI 2.2–7.0).
Discussion
In this large cohort study of transplant recipients, the incidence of HL was twice the incidence in the general population. The excess HL risk was especially high for recipients who were young or EBV seronegative at the time of transplant, and most HL tumors were EBV positive. HL incidence increased steadily over time following transplant, highlighting the importance of HL as a late complication of solid organ transplantation.
Previous research on this malignancy has been limited by the rarity of HL and the requirement for a long period of post-transplant follow-up. Our risk estimate appears lower than reported previously (SIR 2.2 in our study, vs. 3.9 in a recent meta-analysis) (2). Losses to follow-up may have partly contributed to underascertainment of HL in our study, or some cases of HL could have been reported incorrectly as other types of PTLD (e.g., NHL). Unfortunately, we could not retrieve tumor tissue from reported cases of HL or other PTLD to perform additional pathological review.
The monotonic increase of HL incidence with time since transplantation contrasts with the bimodal incidence pattern of PTLD overall (mostly NHL) (12,13). The highest incidence of PTLD is typically observed in the period immediately following transplantation (12,14), and is believed to be the result of primary infection or reactivation of EBV in this period of intense immunosuppression (15,16).
Several observations in our study support the importance of EBV in development of post-transplant HL. First, although EBV serostatus was missing for most recipients, we found that seronegative recipients had an elevated HL risk. This observation is consistent with a model in which primary EBV infection following transplant, when recipients are unable to mount an effective initial immune response, greatly increases HL risk. Second, young transplant recipients were at especially high risk of HL, likely because a large proportion of young recipients are EBV seronegative at the time of transplantation. In western countries, many people acquire EBV during adolescence, and an elevated risk of HL following primary EBV infection in adolescence is seen in the general population(17). Third, as reported in previous studies (7–10), we found that a large proportion of HL tumors were EBV positive. Most HLs in the setting of HIV infection are also EBV positive (11). Within HL tumors, EBV can be detected in the malignant Hodgkin Reed Sternberg cells (18), where it has been shown to inhibit apoptosis (19). Additionally, a high frequency of tumors following transplantation and in the setting of HIV infection manifest mixed cellularity pathology (7,8,11). These characteristics of transplant-related HL differ from HL cases in the U.S. general population, within which the most common subtype is nodular sclerosis and many cases are EBV-negative (18,20). Overall, the late timing of HL in transplant recipients and the associations with EBV infection suggest that the impact of EBV primary infection on the development of HL may be delayed compared to its effect on transplant-associated NHL.
Additional findings deserve some brief comments. HL risk was more than twice as high in male compared to female transplant recipients. This gender difference was greater than observed in the general population, where HL incidence is only slightly higher in males than females (21). The suggestion of higher HL risk following heart and/or lung transplantation likely reflects the greater intensity of immunosuppression following heart and lung transplantation, and it mirrors the increased risk of NHL following heart transplantation compared to kidney transplantation (14), however the difference in risk across organ types did not achieve statistical significance. We did not find any other significant risk factors for post-transplant HL. One previous large-scale study that used data from the United States Renal Data System found few risk factors for post-transplant HL, but this study was limited to 3 years of post-transplant follow-up and focused only on adult transplant recipients (22).
Although the pattern of HL incidence post-transplant is very different from the bimodal pattern observed for other PTLD (mostly polymorphic lymphoproliferations and high-grade NHL), there are several similarities that deserve mention. Both post-transplant HL and other PTLD are associated with young age and EBV seronegativity at the time of transplantation, and both types are predominately EBV positive (16). These similarities highlight the importance of EBV infection in the etiology of various post-transplant lymphoproliferations including HL.
Our study had a number of strengths. It is the first large-scale study to examine HL incidence following all types of solid organ transplantation. All recipients of first organ transplants performed in the U.S. during the study interval were included, allowing us to evaluate a large number of cases and facilitating the generalizability of our results. The large size and extended follow-up were especially important since HL is a rare outcome and has been observed to occur late after transplantation.
Limitations also need to be considered. As noted above, the occurrence of HL may have been underascertained. Previous research indicates that losses to follow-up are minimal in the first 1–2 years post-transplant but can exceed 10% three or more years from transplantation (23). This pattern could have contributed to underascertainment of HL late after transplantation. Incomplete reporting by transplant providers may also have contributed. However, underascertainment of HL incidence is unlikely to have biased the observed associations with risk factors that we examined. In addition, we had limited data on viral serostatus of transplant recipients and the EBV status of HL tumors, making it difficult to assess the impact of EBV on post-transplant HL etiology in detail.
To conclude, our results add to limited previous research indicating that HL is a rare but notable late complication associated with solid organ transplantation, and risk is especially high for transplant recipients who are young, male, or EBV seronegative Because HL incidence increases monotonically with time since transplantation, it is likely that the occurrence of HL will continue to rise as post-transplant survival improves. Our results highlight the importance of EBV infection and immunosuppression in the development of this cancer, but additional research is needed to further clarify its etiology.
Acknowledgments
The data reported here have been supplied by the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the authors and do not reflect official policy of or interpretation by the SRTR or the U.S. Government.
This work was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health. The Scientific Registry of Transplant Recipients (SRTR) is supported by contract 231–00-0116 from the U.S. Department of Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services.
Abbreviations
- CI
Confidence interval
- CMV
Cytomegalovirus
- EBV
Epstein-Barr virus
- HIV
Human immunodeficiency virus
- HL
Hodgkin lymphoma
- HLA
Human leukocyte antigen
- HR
Hazard ratio
- NHL
non-Hodgkin lymphoma
- OPTN
Organ Procurement and Transplantation Network
- PTLD
post-transplant lymphoproliferative disorder
- SEER
Surveillance, Epidemiology and End Results
- SIR
Standardized incidence ratio
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
Author Contributions
O.L. and L.M.M. participated in research design; S.C.Q. and E.E. participated in research design, data analysis, and writing of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Reference List
- 1.Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation. 2005;80:S254–S264. doi: 10.1097/01.tp.0000186382.81130.ba. [DOI] [PubMed] [Google Scholar]
- 2.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 3.Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4:905–913. doi: 10.1111/j.1600-6143.2004.00450.x. [DOI] [PubMed] [Google Scholar]
- 4.Vajdic CM, McDonald SP, McCredie MR, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823–2831. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 5.Vajdic CM, van Leeuwen MT. Cancer incidence and risk factors after solid organ transplantation. Int J Cancer. 2009;125:1747–1754. doi: 10.1002/ijc.24439. [DOI] [PubMed] [Google Scholar]
- 6.Harris NL, Swerdlow SH, Frizzera G, Knowles DM. Post-transplant lymphoproliferative disorders. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. Pathology and Genetics - Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001. pp. 264–269. [Google Scholar]
- 7.Bierman PJ, Vose JM, Langnas AN, et al. Hodgkin's disease following solid organ transplantation. Ann Oncol. 1996;7:265–270. doi: 10.1093/oxfordjournals.annonc.a010570. [DOI] [PubMed] [Google Scholar]
- 8.Garnier JL, Lebranchu Y, Dantal J, et al. Hodgkin's disease after transplantation. Transplantation. 1996;61:71–76. doi: 10.1097/00007890-199601150-00015. [DOI] [PubMed] [Google Scholar]
- 9.Knight JS, Tsodikov A, Cibrik DM, Ross CW, Kaminski MS, Blayney DW. Lymphoma after solid organ transplantation: risk, response to therapy, and survival at a transplantation center. J Clin Oncol. 2009;27:3354–3362. doi: 10.1200/JCO.2008.20.0857. [DOI] [PubMed] [Google Scholar]
- 10.Semakula B, Rittenbach JV, Wang J. Hodgkin lymphoma-like posttransplantation lymphoproliferative disorder. Arch Pathol Lab Med. 2006;130:558–560. doi: 10.5858/2006-130-558-HLPLD. [DOI] [PubMed] [Google Scholar]
- 11.Biggar RJ, Jaffe ES, Goedert JJ, Chaturvedi A, Pfeiffer R, Engels EA. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood. 2006;108:3786–3791. doi: 10.1182/blood-2006-05-024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faull RJ, Hollett P, McDonald SP. Lymphoproliferative disease after renal transplantation in Australia and New Zealand. Transplantation. 2005;80:193–197. doi: 10.1097/01.tp.0000165098.49658.f3. [DOI] [PubMed] [Google Scholar]
- 13.Morton LM, Landgren O, Chatterjee N, et al. Hepatitis C virus infection and risk of posttransplantation lymphoproliferative disorder among solid organ transplant recipients. Blood. 2007;110:4599–4605. doi: 10.1182/blood-2007-07-101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Opelz G, Dohler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4:222–230. doi: 10.1046/j.1600-6143.2003.00325.x. [DOI] [PubMed] [Google Scholar]
- 15.Epstein-Barr virus and lymphoproliferative disorders after transplantation. Am J Transplant. 2004;4 (Suppl 10):59–65. doi: 10.1111/j.1600-6135.2004.00728.x. [DOI] [PubMed] [Google Scholar]
- 16.Cockfield SM. Identifying the patient at risk for post-transplant lymphoproliferative disorder. Transpl Infect Dis. 2001;3:70–78. doi: 10.1034/j.1399-3062.2001.003002070.x. [DOI] [PubMed] [Google Scholar]
- 17.Hjalgrim H, Askling J, Rostgaard K, et al. Characteristics of Hodgkin's lymphoma after infectious mononucleosis. N Engl J Med. 2003;349:1324–1332. doi: 10.1056/NEJMoa023141. [DOI] [PubMed] [Google Scholar]
- 18.Hjalgrim H, Engels EA. Infectious aetiology of Hodgkin and non-Hodgkin lymphomas: a review of the epidemiological evidence. J Intern Med. 2008;264:537–548. doi: 10.1111/j.1365-2796.2008.02031.x. [DOI] [PubMed] [Google Scholar]
- 19.Ambinder RF. Epstein-Barr virus and Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program. 2007:204–209. doi: 10.1182/asheducation-2007.1.204. [DOI] [PubMed] [Google Scholar]
- 20.Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landgren O, Caporaso NE. New aspects in descriptive, etiologic, and molecular epidemiology of Hodgkin's lymphoma. Hematol Oncol Clin North Am. 2007;21:825–840. doi: 10.1016/j.hoc.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Caillard S, Agodoa LY, Bohen EM, Abbott KC. Myeloma, Hodgkin disease, and lymphoid leukemia after renal transplantation: characteristics, risk factors and prognosis. Transplantation. 2006;81:888–895. doi: 10.1097/01.tp.0000203554.54242.56. [DOI] [PubMed] [Google Scholar]
- 23.Levine GN, McCullough KP, Rodgers AM, Dickinson DM, Ashby VB, Schaubel DE. Analytical methods and database design: implications for transplant researchers, 2005. Am J Transplant. 2006;6:1228–1242. doi: 10.1111/j.1600-6143.2006.01277.x. [DOI] [PubMed] [Google Scholar]