Abstract
We investigated whether acute cold-induced vagal activation through brainstem thyrotropin-releasing hormone (TRH) signaling influences abdominal surgery-induced delayed gastric emptying (GE) in fasted rats. Laparotomy and cecal palpation or sham (short anesthesia alone) was performed 10 min before or 30 min after cold exposure (4–6°C) lasting 90 min. Non-nutrient GE was assessed during 70–90 min of cold exposure. Control groups remained at room temperature (RT). The stable TRH analog, RX-77368 (50 ng/rat) was injected intracisternally directly before surgery and GE monitored 30–50 min post surgery in rats maintained at RT. Plasma acyl (AG) and total ghrelin levels were assessed using the new RAPID blood processing method and radioimmunoassays. Desacyl ghrelin (DAG) was derived from total minus AG. In rats maintained at RT, abdominal surgery decreased GE by 60% compared to sham. Cold before or after surgery or RX-77368 normalized the delayed GE. In non-fasted rats, cold exposure increased plasma AG and DAG levels at 2h (2.4- and 2.7-times respectively) and 4h (2.2- and 2.0-times respectively) compared to values in rats maintained at RT. In fasted rats, abdominal surgery decreased AG and DAG levels by 2.4- and 2.1-times respectively at 90 min. Cold for 90 min after surgery normalized AG and DAG levels to those observed in sham-treated animals kept at RT. These data indicate that endogenous (cold exposure) and exogenous (TRH analog) activation of medullary TRH vagal signaling prevents abdominal surgery-induced delayed GE. The restoration of circulating AG levels inhibited by abdominal surgery may contribute to alleviate postoperative gastric ileus.
Keywords: abdominal surgery, acyl ghrelin, cold, desacyl ghrelin, gastric emptying, RAPID method, rat, TRH analog
1. Introduction
A decrease in ambient temperature induces a multitude of endocrine and thermogenic responses. The activation of the three amino acid hormone, thyrotropin-releasing hormone (TRH) signaling pathways in the brain, plays a pivotal role in the response to cold exposure [2, 25, 46]. Synthesis and release of TRH, produced in the hypothalamic paraventricular nucleus (PVN) and brainstem raphe pallidus (Rpa), raphe obscurus and parapyramidal regions, are augmented under conditions of acute exposure to cold ambient temperature [2, 55]. Moreover, cold exposure increases vagal efferent activity [10], activates 93% of submucosal and 97% of gastric myenteric cholinergic neurons as shown by Fos expression and double labeling [57] and induces a vagal-dependent stimulation of gastric functions including acceleration of gastric emptying in rats [25]. The demonstration that acute cold exposure-induced stimulation of gastric emptying was abolished by an intracisternal (ic) injection of a TRH receptor1 antisense oligonucleotide in rats [25] is indicative of a physiological role of medullary TRH-TRH1 receptor signaling pathways in the cold-induced vagal activation of gastric function [46]. This is further supported by brainstem tracing studies in rats showing that TRH immunoreactive fibers innervating the dorsal vagal complex originate exclusively from TRH-synthesizing cell bodies in the Rpa, raphe obscurus and parapyramidal nucleus [19, 34] and electrophysiological reports showing that TRH activates dorsal motor nucleus of the vagus nerve (DMV) neurons [27, 48] resulting in increased gastric vagal efferent activity [33, 45].
Abdominal surgery in animals is a well established model to study postoperative ileus, a condition that occurs after gastrointestinal tract surgery in the clinical setting and is characterized by the suppression of effective transit of bowel contents including delayed gastric emptying [58]. Abdominal surgery consisting of laparotomy with manipulation of the bowel [5, 51] activates endocrine markers of the stress response including corticotropin-releasing factor (CRF), adrenocorticotropic hormone (ACTH), and cortisol in experimental animals and humans [4, 8, 18, 31]. CRF microinjected into the dorsal vagal complex was shown to suppress the vagally-mediated activation of gastric contractions [14] induced by the stable TRH analogue, RX-77368 [28], giving rise to an antagonistic interaction between the CRF and TRH signaling systems.
In the present study, we investigated in rats whether medullary activation of TRH signaling pathways either endogenously by short exposure to cold ambient temperature or exogenously by ic injection of the stable TRH analogue, RX-77368 [28] would influence the abdominal surgery-induced delayed gastric emptying. As there is evidence that brainstem TRH regulates total ghrelin serum levels through vagally-dependent mechanisms in anesthetized rats [1] and that abdominal surgery is improved by peripheral administration of a ghrelin agonist [50], we investigated changes in circulating ghrelin levels in response to abdominal surgery in conscious rats exposed to normal ambient or cold temperature. Both major forms of ghrelin, acyl and desacyl ghrelin (derived from total minus acyl ghrelin) were assessed using the recently described RAPID method of blood processing that results in a higher ratio of acyl/desacyl ghrelin compared to standard methods [43].
2. Materials and Methods
2.1. Animals
Adult male Sprague-Dawley rats (Harlan, San Diego, CA, USA, body weight: 280–320 g) were housed 4 animals/cage under conditions of controlled illumination (12:12 h light/dark cycle, lights on/off: 6.00 h/18.00 h) and temperature (22 ± 2 °C) except otherwise stated. Animals were fed with a standard rodent diet (Prolab RMH 2500; LabDiet, PMI Nutrition, Brentwood, MO, USA) and tap water ad libitum. Animal care and experimental procedures followed institutional ethic guidelines and conformed to the requirements of the federal authority for animal research conduct. All procedures were approved by the Animal Research Committee at Veterans Affairs Greater Los Angeles Healthcare System (animal protocol # 05058-02).
2.2. Abdominal surgery
Rats housed in single cages were fasted for 17 h with free access to tap water prior to the experiment carried out between 9.00 h and 11.00 h. Abdominal surgery was performed in rats anesthetized with isoflurane (4.5% vapor concentration in oxygen; VSS, Rockmart, GA, USA) as in our previous studies [5, 6, 41]. After a median laparotomy (2–3 cm), the cecum was exteriorized, placed in saline-soaked gauze and gently manipulated between two fingers for 1 min. Thereafter, the cecum was replaced into the abdominal cavity and the peritoneum, muscle and skin were sutured. Anesthesia and surgery lasted for approximately 10 min and animals woke up within 2–3 min after removal of isoflurane. The sham group consisted of rats undergoing exposure to anesthesia alone for 10 min. Afterwards, animals were housed singly without access to food or water.
2.3. Intravenous catheterization
Intravenous catheterization was performed as described before [40]. Briefly, rats were anesthetized with a mixture of ketamine (75 mg/kg ip; Fort Dodge Laboratories, Fort Dodge, IA) and xylazine (5 mg/kg ip; Mobay, Shawnee, KS) and a sterile PE-50 catheter was inserted into the right external jugular vein. The saline rinsed catheter was exteriorized between the scapulae via subcutaneous tunneling, then secured to the skin and filled with heparin solution (200 units/ml) to maintain lumen patency and closed using a wire. Rats were single housed after surgery and allowed to recover for three days during which they were accustomed to the experimental procedures including light hand restraint for blood withdrawal and measurement of rectal temperature. Body weight was monitored before intravenous catheterization and during the recovery period.
2.4. Measurements
2.4.1. Gastric emptying
Gastric emptying of a non-nutrient viscous solution was determined by the phenol red/methyl cellulose method as previously described [11]. Rats were food- but not water-deprived overnight for 17 h and received an orogastric gavage of a viscous solution (1.5 ml). Animals were euthanized 20 min later by CO2 inhalation followed by cardiac incision. The abdominal cavity was opened, gastric pylorus and cardia were clamped, and the stomach removed and gastric emptying assessed as detailed previously [11].
2.4.2. Blood measurements
Blood samples were processed for ghrelin measurements according to the recently developed RAPID method as detailed before [43]. Briefly, immediately after withdrawal, blood was diluted 1:10 in ice-cold buffer (pH 3.6) containing 0.1 M ammonium acetate, 0.5 M NaCl, and enzyme inhibitors (diprotin A, E-64-d, antipain, leupeptin, chymostatin, 1 µg/ml; Peptides International, Louisville, KY), and centrifuged at 3000 rpm for 10 min at 4 °C. Sep-Pak C18 cartridges (360 mg, 55–105µm, product-no. WAT051910, Waters Corporation, Milford, MA) were charged with 5 ml 100% acetonitrile and equilibrated with 10 ml 0.1% trifluoroacetate (TFA). The equilibrated cartridges were loaded with sample, rinsed with 3 ml 0.1% TFA and eluted with 2 ml 70% acetonitrile containing 0.1% TFA. The eluted samples were dried by vacuum centrifugation and powder stored at −80 °C until further processing. Immediately before radioimmunoassay, samples were re-suspended in double distilled H2O according to the original volume of plasma. Total and acyl ghrelin levels were measured using specific radioimmunoassay kits (# GHRT-89HK and GHRA-88HK, Millipore, Billerica, MA). Acyl and total ghrelin levels will also comprise other forms of ghrelin such as des-Gln14-ghrelin which have been detected in low amounts in the stomach [15] since the antibodies used were raised against the N- and C-terminus respectively (according to manufacturer’s information). Desacyl ghrelin was calculated as the difference of total minus acyl ghrelin for each individual sample. Blood glucose levels were assessed by commercial test strips (One-Touch Ultra; LifeScan, Milpitas, CA).
2.4.3. Rectal temperature
Rectal temperature was assessed using a thermometer (Lumiscope Co., Inc., Piscataway, NJ) lubricated with chlorhexidine gluconate (Surgilube, E. Fougera & Co., Atlanta Inc., NY), inserted 3 cm from the anus into the distal colon and left for 10 sec in lightly hand restrained animals to obtain stable reading.
2.5. Experimental protocols
2.5.1. Effect of acute cold exposure on abdominal surgery-induced delayed gastric emptying
Overnight fasted rats were subjected to either abdominal surgery or sham procedure and then placed in semi-restraint Bollman cages and maintained either under normal (21–23 °C) or cold (4–6 °C) ambient temperature for 90 min. Conscious rats received an orogastric gavage of 1.5 ml methyl cellulose-phenol red viscous solution at 70 min after the start of exposure to different ambient temperatures and gastric emptying was monitored 20 min later. In another experiment, rats were placed in semi-restraint Bollman cages for 30 min before sham procedure or abdominal surgery and thereafter were maintained at either normal (21–23 °C) or cold (4–6 °C) ambient temperature for another 60 min. At 20 min before the end of this period, rats received an orogastric gavage of 1.5 ml viscous solution and gastric emptying was monitored 20 min later.
2.5.2. Effect of intracisternal injection of TRH analog on abdominal surgery-induced delayed gastric emptying
Overnight fasted rats were injected ic under isoflurane anesthesia with either the stable TRH analogue, RX-77368 (Ferring Pharmaceuticals, Feltham, Middlesex, U.K.) at the dose of 50 ng/10 µl saline or vehicle (10 µl sterile saline), underwent abdominal surgery or sham procedure and then were maintained singly at room temperature. Animals received the orogastric gavage of a viscous solution 30 min after the end of the surgery or sham procedure and gastric emptying was monitored 20 min later. The accuracy of the delivery into the cisterna magna was ascertained before the injection by withdrawal of cerebrospinal fluid into the Hamilton syringe as in our previous studies [21]. The RX-77368 dose was based on our previous dose-response studies showing sub-maximal stimulation of gastric emptying [20].
2.5.3. Effect of cold exposure on plasma ghrelin and blood glucose levels
Rats with a chronic intravenous catheter and fed ad libitum were placed in semi-restraint Bollman cages and maintained either at normal (21–23 °C) or cold (4–6 °C) ambient temperature for a period of 4 h without access to food and water. Blood (0.5 ml) was withdrawn before placing the rats into the Bollman cages and at 2 and 4 h after the start of the experiment and immediately processed for total and acyl ghrelin and glucose determinations. At the same time points, rectal temperature was assessed.
2.5.4. Effect of abdominal surgery on plasma ghrelin levels and influence of cold exposure
Overnight fasted rats with a chronic intravenous catheter were subjected to abdominal surgery and then placed in semi-restraint Bollman cages either maintained at normal (21–23 °C) or cold (4–6 °C) ambient temperature. A fasted sham control group was also placed in Bollman cages and maintained at room temperature. Blood (0.5 ml) was withdrawn at 30 and 90 min after the end of the surgery and processed for total and acyl ghrelin measurements.
2.6. Statistical analysis
Data are expressed as mean ± SEM and analyzed by one way analysis of variance (ANOVA) followed by Tukey post hoc test or two-way ANOVA followed by Holm-Sidak method. The correlations between individual values of plasma levels of acyl and desacyl ghrelin and those of blood glucose or rectal temperature were determined by univariate linear regression. Differences between groups were considered significant when p < 0.05.
3. Results
3.1. Cold ambient temperature before or after abdominal surgery reverses or prevents delayed gastric emptying
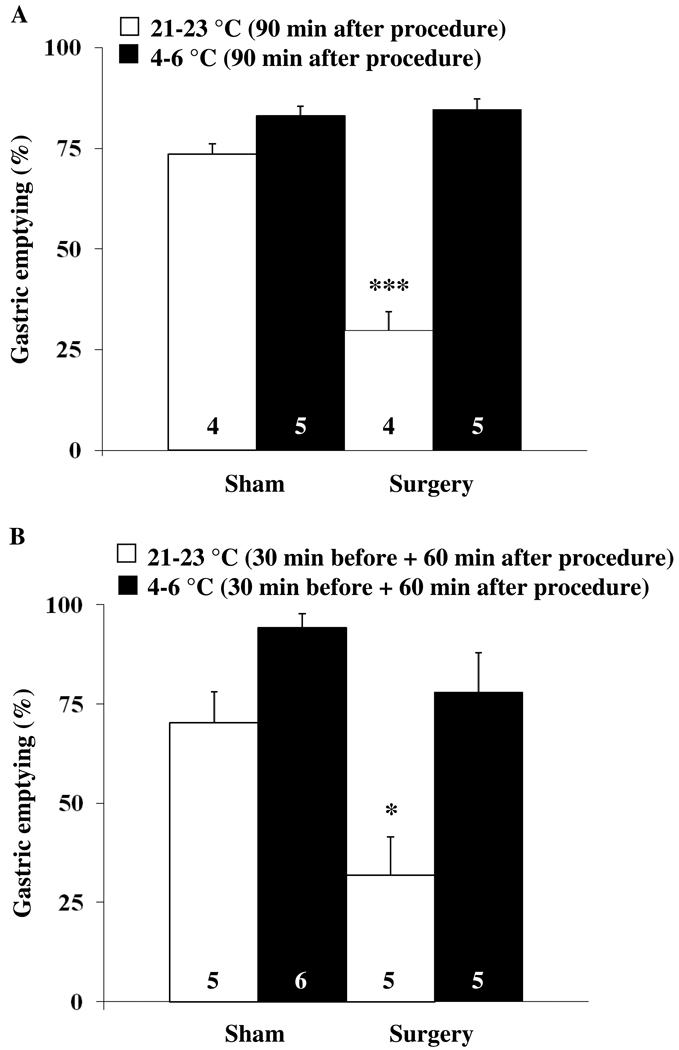
Abdominal surgery performed under short inhalation anesthesia significantly reduced the gastric emptying of a non-nutrient viscous solution to 29.8 ± 4.6 % compared to 73.5 ± 2.5 % in rats exposed to anesthesia alone (sham, p < 0.001) as monitored during the 70–90 min period post anesthesia/surgery in fasted rats maintained at room temperature (Fig. 1A). Exposure to cold ambient temperature for 90 min after the end of the surgery completely reversed the abdominal surgery-induced delayed gastric emptying and values (84.6 ± 2.7 %) were similar to those of the sham group also kept at 4–6 °C (83.0 ± 2.5 %, p > 0.05; Fig. 1A). Gastric emptying of the non-nutrient viscous solution was 10 % higher in the sham group maintained at 4–6 °C compared to the sham treated rats maintained at normal ambient temperature (83.0 ± 2.5 vs. 73.5 ± 2.5 %); however, the difference did not reach statistical significance (p > 0.05; Fig. 1A). Two-way ANOVA indicated a significant influence of procedure (F(1,14)=45.7, p < 0.001), temperature (F(1,14)=106.5, p < 0.001) and procedure × temperature (F(1,14)=52.9, p < 0.001).
Fig. 1.
Cold ambient temperature reverses or prevents the abdominal surgery-induced delay of gastric emptying. (A) Overnight fasted rats were subjected to abdominal surgery (laparotomy and cecal palpation) or sham treatment (anesthesia alone) and thereafter kept in semi-restraint Bollman cages under normal (21–23 °C) or cold (4–6 °C) ambient temperature for 90 min. Animals received an orogastric gavage of 1.5 ml viscous solution 70 min after the start of the different ambient temperatures and gastric emptying was monitored 20 min later. (B) Rats were placed in semi-restraint Bollman cages and kept at normal (21–23 °C) or cold (4–6 °C) ambient temperature starting at 30 min before sham procedure or abdominal surgery. After undergoing sham procedure or abdominal surgery, animals were housed for another 60 min at normal or cold ambient temperature. At 20 min before the end of this period, rats received an orogastric gavage of a liquid non-nutrient solution and gastric emptying was assessed 20 min later. Each bar represents the mean ± sem of the number of rats indicated at the bottom of the column. * p < 0.05 and *** p < 0.001 vs. all other groups.
Likewise, when rats were exposed to cold ambient temperature for 30 min before and 60 min after the abdominal surgery, gastric emptying (78.0 ± 9.9 %) was similar to that of the sham group maintained at room temperature (70.3 ± 7.7 %) as monitored during the 40–60 min period post surgery (Fig. 1B). By contrast, in rats maintained at normal ambient temperature, abdominal surgery induced a significant decrease in gastric emptying (31.9 ± 9.7 %) compared to the sham group (p < 0.05; Fig. 1B). Exposure to cold for 30 min before and 60 min after sham treatment resulted in a non-significant trend towards a higher gastric emptying compared to sham-treated rats maintained at room temperature (94.2 ± 3.7 vs. 70.3 ± 7.7 %, p > 0.05; Fig. 1B). Two-way ANOVA indicated a significant influence of procedure (F(1,17)=12.1, p < 0.01) and temperature (F(1,17)=19.9, p < 0.001).
3.2. The TRH analogue RX-77368 injected intracisternally blocks abdominal surgery-induced delayed gastric emptying
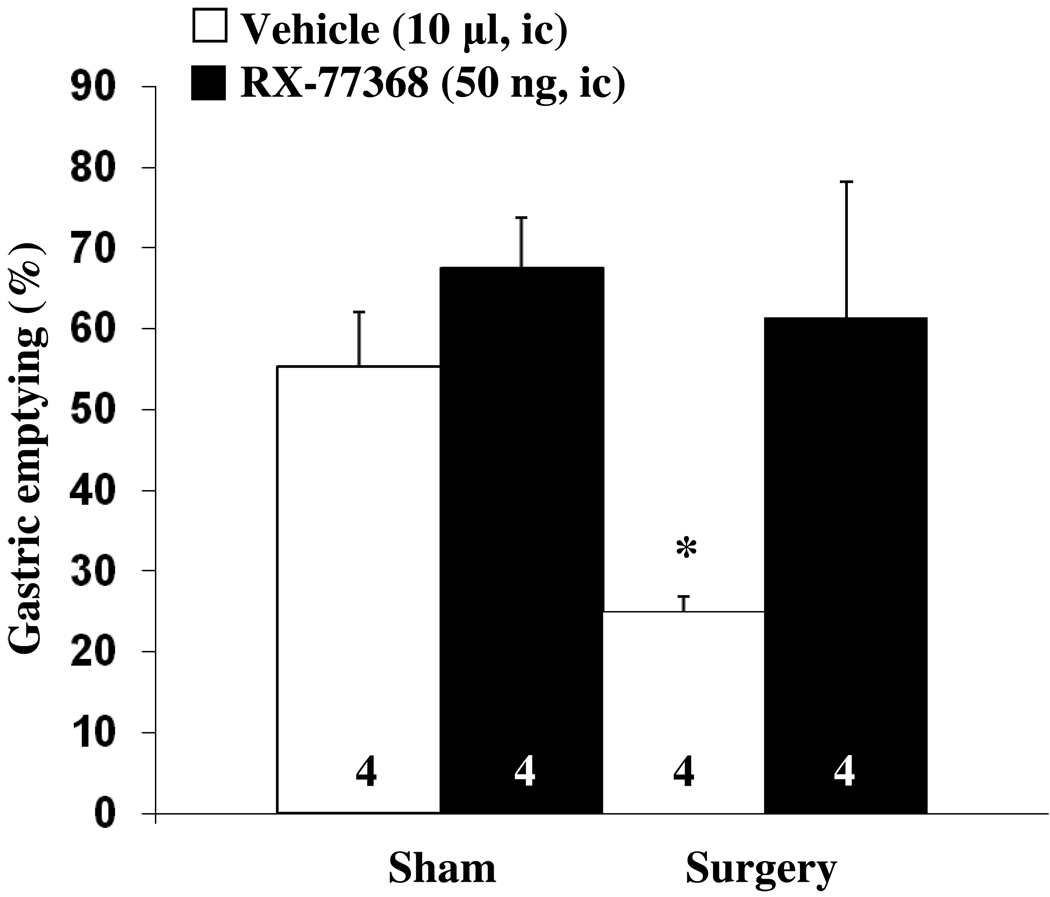
In ic saline injected fasted rats, abdominal surgery significantly delayed gastric emptying of a viscous non-nutrient solution compared to the sham group (25.0 ± 1.9 vs. 55.3 ± 6.8 %, p < 0.01, Fig. 2) as monitored during the 30–50 min period after ic injection. The stable TRH analogue, RX-77368 (50 ng, ic) completely blocked the abdominal surgery-induced delayed gastric emptying (61.3 ± 16.8 %). In the sham group injected ic with RX-77368, gastric emptying values were 12 % higher compared to vehicle, although the difference did not reach significance (67.5 ± 6.2 %, p > 0.05, Fig. 2). Two-way ANOVA indicated a significant influence of procedure (F(1,12)=4.9, p < 0.05) and treatment (F(1,12)=8.7, p < 0.05).
Fig. 2.
Intracisternal injection of the TRH analogue RX-77368 prevents the abdominal surgery-induced delayed gastric emptying. Rats fasted overnight were injected intracisternally with either the TRH analogue, RX-77368 (50 ng/10 µl saline) or vehicle (10 µl saline) and afterwards subjected to abdominal surgery or sham treatment. Animals received an orogastric gavage of 1.5 ml viscous solution 30 min after the end of the surgery or sham and gastric emptying was monitored 20 min later. Each bar represents the mean ± sem of the number of rats indicated at the bottom of the column. * p < 0.05 vs. all other groups.
3.3. Cold ambient temperature increases plasma acyl and desacyl ghrelin and blood glucose levels in non-fasted rats
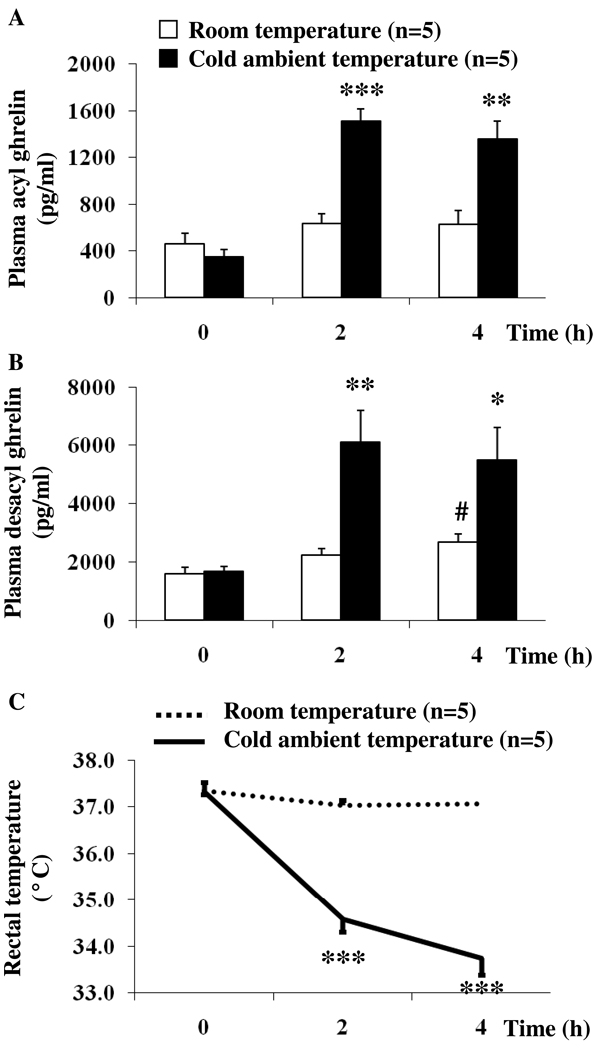
Ad libitum fed rats to be assigned to normal (21–23 °C) or cold (4–6 °C) ambient temperature had similar basal plasma levels of acyl ghrelin (460.8 ± 88.1 and 345.6 ± 62.9 pg/ml respectively, p > 0.05; Fig. 3A). Exposure to cold ambient temperature for 4 h significantly increased acyl ghrelin levels at 2 h (1513.5 ± 103.0 vs. 633.0 ± 85.6 pg/ml, p < 0.001) and 4 h (1354.3 ± 156.3 vs. 626.9 ± 118.9 pg/ml, p < 0.01) compared to rats kept at room temperature (Fig. 3A). During the same time period, there were no significant changes in plasma acyl ghrelin in rats maintained at room temperature (Fig. 3A). Two-way ANOVA indicated a significant influence of treatment (F(1,24)=32.7, p < 0.001), time (F(2,24)=23.5, p < 0.001) and treatment × time (F(2,24)=12.6, p < 0.001).
Fig. 3.
Cold ambient temperature increases acyl and desacyl ghrelin levels and decreases rectal temperature. Chronically intravenously cannulated, ad libitum fed rats were divided into two groups and kept in semi-restraint Bollman cages at either normal (21–23 °C) or cold (4–6 °C) ambient temperature for 4 h. Blood was withdrawn before and at 2 and 4h after start of the experiment. Acyl ghrelin (A) and total ghrelin levels were assessed by radioimmunoassay. Desacyl ghrelin (B) levels were obtained by calculating the difference of total ghrelin minus acyl ghrelin. Rectal temperature was assessed at the same time before and at 2 and 4h (C). Each bar or line represents the mean ± sem of 5 rats/group. * p < 0.05, ** p < 0.01 and *** p < 0.001 vs. control at the respective time point. # p < 0.05 vs. same group at time point 0 h.
As observed for acyl ghrelin, desacyl ghrelin levels did not differ between groups before subjecting them to normal or cold ambient temperature (1607.6 ± 207.0 and 1670.0 ± 188.9 pg/ml respectively, p > 0.05; Fig. 3B). Likewise, desacyl ghrelin levels significantly rose under conditions of cold ambient temperature at 2 h (6119.7 ± 1076.0 vs. 2250.2 ± 208.2 pg/ml, p < 0.01) and 4 h (5508.9 ± 1092.0 vs. 2692.9 ± 275.8 pg/ml, p < 0.05) compared to levels measured in rats maintained at normal room temperature (Fig. 3B). In the group kept at room temperature, plasma desacyl ghrelin levels were significantly increased at 4 h compared to levels at time point 0 h (2692.9 ± 275.8 vs. 1607.6 ± 207.0 pg/ml, p < 0.05), whereas at 2 h no changes were observed (2250.2 ± 208.2 pg/ml, p > 0.05; Fig. 3B). Two-way ANOVA showed a significant influence of treatment (F(1,24)=17.9, p < 0.001), time (F(2,24)=9.9, p < 0.001) and treatment × time (F(2,24)=4.6, p < 0.05).
Rectal temperature did not differ between the two groups before subjecting them to either normal or cold ambient temperature (37.3 ± 0.2 and 37.3 ± 0.1 °C respectively, p > 0.05, Fig. 3C). Cold ambient temperature for 4 h resulted in a significant decrease of rectal temperature at 2 h (34.6 ± 0.3 vs. 37.0 ± 0.1 °C, p < 0.001) and 4 h (33.7 ± 0.4 vs. 37.1 ± 0.1 °C, p < 0.001) compared to rats kept at room temperature (Fig. 3C). Two-way ANOVA indicated a significant influence of treatment (F(1,36)=130.0, p < 0.001), time (F(2,36)=48.2, p < 0.001) and treatment × time (F(2,36)=34.2, p < 0.001). No correlation was observed between rectal temperature and acyl ghrelin or desacyl ghrelin at any time point (p > 0.05).
Similarly, blood glucose did not differ between the two groups before maintaining them on either normal or cold ambient temperature (106.4 ± 1.7 and 108.9 ± 2.2 mg/dl respectively, p > 0.05). The normal temperature group showed an increase of blood glucose at 2 h (117.3 ± 2.8 mg/dl, p < 0.01) and 4 h (120.4 ± 2.9 mg/dl, p < 0.01) after the start of the experiment compared to time point 0 h. Cold ambient temperature further increased blood glucose at 2 h (170.4 ± 10.4 mg/dl, p < 0.001) and 4 h (176.0 ± 17.3 mg/dl, p < 0.01) compared to rats maintained at normal temperature. Two-way ANOVA indicated a significant influence of treatment (F(1,36)=28.5, p < 0.001), time (F(2,36)=13.7, p < 0.001) and treatment × time (F(2,36)=6.2, p < 0.01). No correlation was observed between blood glucose and acyl ghrelin or desacyl ghrelin at any time point (p > 0.05).
3.4. Cold ambient temperature blocks abdominal surgery-induced decrease in plasma acyl and desacyl ghrelin levels
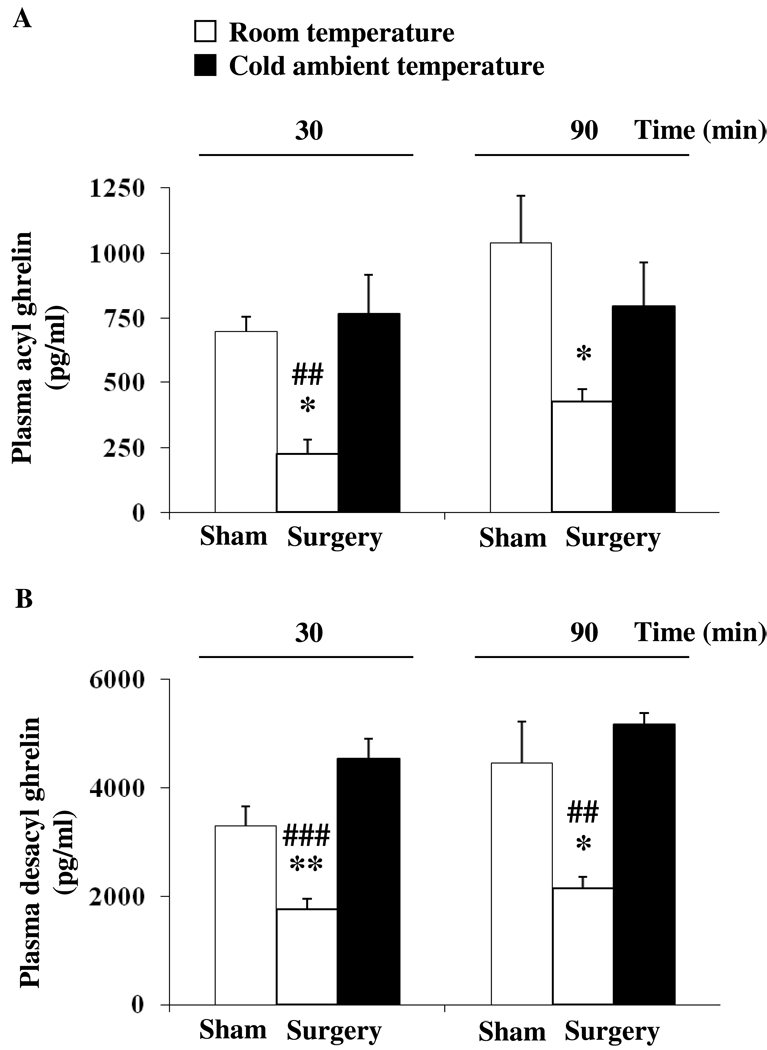
Rats fasted overnight and subjected to abdominal surgery showed a significant decrease in plasma acyl ghrelin compared to sham-treated rats (anesthesia alone) at 30 min (225.8 ± 53.9 vs. 697.3 ± 59.5 pg/ml, p < 0.05) and 90 min (427.8 ± 46.5 vs. 1038.1 ± 183.6 pg/ml, p < 0.05; Fig. 4A) and desacyl ghrelin levels at 30 min (1756.0 ± 199.5 vs. 3287.7 ± 366.1 pg/ml, p < 0.01) and 90 min (2142.8 ± 220.1 vs. 4462.5 ± 754.2 pg/ml, p < 0.05; Fig. 4B). Cold ambient temperature immediately after abdominal surgery normalized plasma acyl ghrelin levels at 30 min (767.6 ± 147.9 pg/ml) and 90 min (796.0 ± 169.5 pg/ml) reaching levels similar to those observed in sham-treated animals kept at normal ambient temperature (Fig. 4A). Likewise, in rats exposed to cold following abdominal surgery, plasma desacyl ghrelin levels were not significantly different from those of the sham group maintained at normal room temperature as assessed at 30 min (4535.7 ± 369.2 vs. 3287.7 ± 366.1 pg/ml, p > 0.05) and 90 min (5166.0 ± 281.8 vs. 4462.5 ± 754.2 pg/ml, p > 0.05) post abdominal surgery (Fig. 4B).
Fig. 4.
Cold ambient temperature reverses the abdominal surgery-induced decrease of acyl and desacyl ghrelin levels. Overnight fasted rats equipped with a chronic intravenous catheter underwent abdominal surgery and were kept in semi-restraint Bollman cages at normal (21–23 °C) or cold (4–6 °C) ambient temperature for 90 min. A control group was sham treated (anesthesia alone) and kept at room temperature afterwards. Blood was withdrawn at 30 and 90 min post treatment. Acyl ghrelin (A) and total ghrelin levels were assessed by radioimmunoassay. Desacyl ghrelin (B) was obtained by calculating the difference of total ghrelin minus acyl ghrelin. Each bar represents the mean ± sem of 4–5 rats per group. * p < 0.05 and ** p < 0.01 vs. sham at the respective time point. ## p < 0.01 and ### p < 0.001 vs. abdominal surgery/cold at the respective time point.
4. Discussion
In the present study, we demonstrated in rats that central vagal stimulation induced by cold ambient temperature or intracisternal injection of a TRH analogue blocked the abdominal surgery-induced delayed gastric emptying of a non-nutrient viscous meal and this is associated with the normalization of the acyl and desacyl ghrelin plasma levels that were decreased by abdominal surgery.
It is well established that abdominal surgery delays gastric emptying in experimental animals [3, 4, 22, 23, 44] and humans [29]. In line with these previous reports, gastric emptying of a viscous non-nutrient solution was reduced by 60% compared to sham (anesthesia alone) when monitored either during the 30–50 min or 70–90 min periods post surgery. In addition, we showed that the postoperative gastric ileus was completely reversed or prevented when semi-restrained rats were maintained at cold ambient temperature of 4–6 °C for 90 min post surgery or already housed at cold ambient temperature starting at 30 min before surgery, while sham animals exposed to cold showed a 10–20% increase which did not reach statistical significance compared to rats kept at room temperature. Converging previous and present data support that the cold exposure-induced complete prevention of abdominal surgery-induced delayed gastric emptying is likely to be mediated by activation of medullary TRH signaling pathways and related vagal stimulation of gastric functions [45, 46]. We previously established that rats exposed to cold for 1–3 h display increased TRH mRNA expression in the medulla oblongata as monitored by quantitative Northern blot analysis with a 110% plateau elevation at 90 and 120 min along with elevated TRH gene expression signals in TRH-synthesizing neurons namely the Rpa, raphe obscurus and parapyramidal region as assessed by in situ hybridization [55, 56]. Cold exposure for 90 min also induces Fos expression, a marker for neuronal activation [37], in TRH-expressing neurons of the Rpa, raphe obscurus and parapyramidal region and at their projection sites such as the nucleus of the solitary tract and DMV, whereas rats placed in semi-restraint cages at room temperature showed none or very few Fos positive cells in these nuclei indicating a specific effect of the cold ambient temperature [7, 24, 53]. Since the DMV is the major nucleus sending vagal projections to the stomach [13], its activation by cold exposure and related vagal-dependent activation of gastric cholinergic myenteric neurons, as shown by Fos expression and double labeling in cold exposed rats [57], is very likely to be part of the underlying mechanisms restoring gastric emptying and counteracting the postoperative gastric ileus in cold exposed rats. This is further supported by our previous report that cold exposure induces a vagal cholinergic-dependent stimulation of gastric emptying via a brainstem TRH signaling pathway as indicated by the complete blockade of accelerated gastric emptying by pretreatment with a TRH receptor1 antisense oligonucleotide injected into the cisterna magna as well as by vagotomy or atropine [25]. Moreover, in the present study exogenous stimulation of brainstem TRH signaling by intracisternal injection of the TRH analogue, RX-77368 mimicked the effect of cold exposure and completely restored the abdominal surgery-induced 55% delay of gastric emptying. These data also indicate the retained activation of DMV-vagal pathways under conditions of postoperative gastric ileus by exogenous TRH.
The stomach-derived peptide ghrelin is the only known peripherally produced and centrally acting hormone that stimulates food intake [42] and also gastric motility [26] resulting in the acceleration of gastric emptying [12]. In addition, there is converging evidence that ghrelin and the ghrelin receptor agonists, RC-1139 and TZP-101 injected intravenously after the end of the surgery reverse the abdominal surgery-induced delay of gastric emptying in rats [35, 49, 50]. Similarly, ghrelin infused intravenously increased gastric emptying in patients with diabetic gastroparesis [30] and the ghrelin agonist, TZP-101 significantly shortened the time to first bowel movement, reduced nausea and vomiting, accelerated recovery and allowed earlier hospital discharge in patients following major abdominal surgery [36]. These data are promising for a broad use of ghrelin and agonists as prokinetics in patients with gastroparesis and postoperative ileus [9]. Previous reports indicate that the medullary TRH signaling system stimulates ghrelin release as shown by the increased serum levels of total ghrelin after ic injection of the TRH analog, RX-77368 in pentobarbital anesthetized rats [1]. In the present study, we found that acute cold exposure induces a robust and long-lasting rise in plasma acyl ghrelin and desacyl ghrelin reaching a 2.4- and 2.7-times increase respectively at 2 h which stayed similarly elevated at 4 h in ad libitum fed conscious rats. The rise in acyl ghrelin induced by cold in ad libitum fed rats is of similar magnitude as that induced by 17–24-h fasting [43] (present study) indicative that cold exposure restores a fasting state of circulating ghrelin levels in fed rats. Such adaptive response may have a bearing with integrating energy demand and digestive processes induced by acute cold exposure.
Simultaneously with the rise in acyl and desacyl ghrelin plasma levels, cold ambient temperature decreased rectal temperature with a nadir decrease of 3.3 °C observed at 4 h as established before [38]. However changes in rectal temperature did not correlate with acyl or desacyl ghrelin levels at any time point as observed in our previous study after injection of lipopolysaccharide [40]. This suggests that changes in rectal temperature do not directly influence the increase in circulating levels of acyl and desacyl ghrelin. Supportive of this assumption, mechanisms related to acute cold-induced thermoregulatory changes and ghrelin regulation are different. Activation of hypothalamic nuclei such as the suprachiasmatic nucleus associated with an activation of the sympathetic nervous system increases heat production in the brown adipose tissue to counteract cold-induced hypothermia [47], whereas cold-induced vagal stimulation of gastric secretory functions involves medullary TRH [45, 46]. Cold exposure robustly increased blood glucose levels with a peak increase of 1.5-times at 4 h. As observed for rectal temperature, blood glucose levels did not correlate with those of acyl or desacyl ghrelin at any time point investigated. Although a negative correlation between ghrelin and insulin has been reported before [16, 17], glucose levels are not always correlated with ghrelin [40, 52]. In addition, the ic TRH agonist-induced hyperglycemia is mediated via sympathetic activation of the adrenal glands in contrast to the stimulatory effect on circulating ghrelin, which is vagally mediated in rats [1]. Taken together, differential underlying mechanisms and the lack of correlation suggest that the rise in blood glucose levels does not directly account for the increased acyl and desacyl ghrelin plasma levels induced by acute cold exposure.
The present study also provides the first evidence that abdominal surgery markedly inhibits fasting plasma levels of ghrelin with a 3.1- and 1.9-times reduction in acyl ghrelin and desacyl ghrelin respectively observed after 30 min which was maintained at 90 min post abdominal surgery. The acyl and desacyl ghrelin levels induced by abdominal surgery in fasted rats were similar to, or even below, those observed previously in intact rats fed ad libitum [43] (present study) indicating that abdominal surgery induces a fed state level of acyl ghrelin in fasted animals. Such a drop in circulating ghrelin was completely reversed in rats exposed to cold ambient temperature suggesting that medullary TRH signaling pathways activated by acute cold exposure may underlie the stimulation of ghrelin release under these conditions. Since atropine and vagotomy were previously shown to completely block the ic TRH analog injection-induced increased total serum ghrelin levels [1], the effect of cold is likely to be exerted through vagal efferent cholinergic stimulation of ghrelin-producing X/A-like cells. In support of this pathway, in vitro studies showed that ghrelin release from the vascularly perfused isolated rat stomach is increased by acetylcholine [39] and that a muscarinic receptor blocker substantially inhibits ghrelin release in fasted rats [54].
In summary, we show that the activation of medullary TRH - vagal signaling pathways, induced either endogenously by acute cold exposure or exogenously by intracisternal injection of a TRH analogue, prevents abdominal surgery-induced delayed gastric emptying as assessed during the acute postsurgical phase. These data strengthen the assumption of an important role of restoring central vagal activity during abdominal surgery which may have a bearing with recent clinical trials indicating that chewing gum and related vagal stimulation shortened the time to the first flatus and bowel movements and reduced the period of hospitalization [32]. The peripheral mechanisms underlying cold ambient temperature and intracisternal TRH-induced prevention of postoperative gastric ileus may involve the rapid vagal-dependent increase in plasma levels of acyl ghrelin known to have prokinetic effects under conditions of postoperative ileus. These data provide strong evidence for a medullary TRH vagal regulation of gastric endocrine X/A-like cells stimulating acyl ghrelin release and open new venues to counteract the inhibition of acyl ghrelin induced by surgery.
Acknowledgements
This work was supported by German Research Foundation Grants STE 1765/1-1 (A.S.), GO 1718/1-1 (M.G.), Veterans Administration Research Career Scientist Award, VA Merit Award, Center Grant NIH DK-41301 (Animal Core) and R01 NIH DK 33061 (Y.T). We are grateful to Mrs. Honghui Liang for the excellent technical support and Ms. Eugenia Hu for reviewing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors have nothing to disclose. No conflicts of interest exist.
References
- 1.Ao Y, Go VL, Toy N, Li T, Wang Y, Song MK, et al. Brainstem thyrotropin-releasing hormone regulates food intake through vagal-dependent cholinergic stimulation of ghrelin secretion. Endocrinology. 2006;147:6004–6010. doi: 10.1210/en.2006-0820. [DOI] [PubMed] [Google Scholar]
- 2.Arancibia S, Rage F, Astier H, Tapia-Arancibia L. Neuroendocrine and autonomous mechanisms underlying thermoregulation in cold environment. Neuroendocrinology. 1996;64:257–267. doi: 10.1159/000127126. [DOI] [PubMed] [Google Scholar]
- 3.Barquist E, Bonaz B, Martinez V, Rivier J, Zinner MJ, Taché Y. Neuronal pathways involved in abdominal surgery-induced gastric ileus in rats. Am J Physiol. 1996;270:R888–R894. doi: 10.1152/ajpregu.1996.270.4.R888. [DOI] [PubMed] [Google Scholar]
- 4.Barquist E, Zinner M, Rivier J, Taché Y. Abdominal surgery-induced delayed gastric emptying in rats: role of CRF and sensory neurons. Am J Physiol. 1992;262:G616–G620. doi: 10.1152/ajpgi.1992.262.4.G616. [DOI] [PubMed] [Google Scholar]
- 5.Bonaz B, Plourde V, Taché Y. Abdominal surgery induces Fos immunoreactivity in the rat brain. J Comp Neurol. 1994;349:212–222. doi: 10.1002/cne.903490205. [DOI] [PubMed] [Google Scholar]
- 6.Bonaz B, Taché Y. Corticotropin-releasing factor and systemic capsaicin-sensitive afferents are involved in abdominal surgery-induced Fos expression in the paraventricular nucleus of the hypothalamus. Brain Res. 1997;748:12–20. doi: 10.1016/s0006-8993(96)01281-4. [DOI] [PubMed] [Google Scholar]
- 7.Bonaz B, Taché Y. Induction of Fos immunoreactivity in the rat brain after cold-restraint induced gastric lesions and fecal excretion. Brain Res. 1994;652:56–64. doi: 10.1016/0006-8993(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 8.Calogero AE, Norton JA, Sheppard BC, Listwak SJ, Cromack DT, Wall R, et al. Pulsatile activation of the hypothalamic-pituitary-adrenal axis during major surgery. Metabolism. 1992;41:839–845. doi: 10.1016/0026-0495(92)90164-6. [DOI] [PubMed] [Google Scholar]
- 9.Camilleri M, Papathanasopoulos A, Odunsi ST. Actions and therapeutic pathways of ghrelin for gastrointestinal disorders. Nat Rev Gastroenterol Hepatol. 2009;6:343–352. doi: 10.1038/nrgastro.2009.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho CH, Qui BS, Bruce IC. Vagal hyperactivity in stress induced gastric ulceration in rats. J Gastroenterol Hepatol. 1996;11:125–128. doi: 10.1111/j.1440-1746.1996.tb00048.x. [DOI] [PubMed] [Google Scholar]
- 11.Czimmer J, Million M, Taché Y. Urocortin 2 acts centrally to delay gastric emptying through sympathetic pathways while CRF and urocortin 1 inhibitory actions are vagal dependent in rats. Am J Physiol Gastrointest Liver Physiol. 2006;290:G511–G518. doi: 10.1152/ajpgi.00289.2005. [DOI] [PubMed] [Google Scholar]
- 12.Dornonville de la Cour C, Lindstrom E, Norlen P, Hakanson R. Ghrelin stimulates gastric emptying but is without effect on acid secretion and gastric endocrine cells. Regul Pept. 2004;120:23–32. doi: 10.1016/j.regpep.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Fox EA, Powley TL. Longitudinal columnar organization within the dorsal motor nucleus represents separate branches of the abdominal vagus. Brain Res. 1985;341:269–282. doi: 10.1016/0006-8993(85)91066-2. [DOI] [PubMed] [Google Scholar]
- 14.Heymann-Mönnikes I, Taché Y, Trauner M, Weiner H, Garrick T. CRF microinjected into the dorsal vagal complex inhibits TRH analog- and kainic acid-stimulated gastric contractility in rats. Brain Res. 1991;554:139–144. doi: 10.1016/0006-8993(91)90181-t. [DOI] [PubMed] [Google Scholar]
- 15.Hosoda H, Kojima M, Matsuo H, Kangawa K. Purification and characterization of rat des-Gln14-Ghrelin, a second endogenous ligand for the growth hormone secretagogue receptor. J Biol Chem. 2000;275:21995–22000. doi: 10.1074/jbc.M002784200. [DOI] [PubMed] [Google Scholar]
- 16.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Oikawa S. Effects of insulin, leptin, and glucagon on ghrelin secretion from isolated perfused rat stomach. Regul Pept. 2004;119:77–81. doi: 10.1016/j.regpep.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Lippl F, Kircher F, Erdmann J, Allescher HD, Schusdziarra V. Effect of GIP, GLP-1, insulin and gastrin on ghrelin release in the isolated rat stomach. Regul Pept. 2004;119:93–98. doi: 10.1016/j.regpep.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Luckey A, Wang L, Jamieson PM, Basa NR, Million M, Czimmer J, et al. Corticotropin-releasing factor receptor 1-deficient mice do not develop postoperative gastric ileus. Gastroenterology. 2003;125:654–659. doi: 10.1016/s0016-5085(03)01069-2. [DOI] [PubMed] [Google Scholar]
- 19.Lynn RB, Kreider MS, Miselis RR. Thyrotropin-releasing hormone-immunoreactive projections to the dorsal motor nucleus and the nucleus of the solitary tract of the rat. J Comp Neurol. 1991;311:271–288. doi: 10.1002/cne.903110208. [DOI] [PubMed] [Google Scholar]
- 20.Maeda-Hagiwara M, Taché Y. Central nervous system action of TRH to stimulate gastric emptying in rats. Regul Pept. 1987;17:199–207. doi: 10.1016/0167-0115(87)90063-2. [DOI] [PubMed] [Google Scholar]
- 21.Martinez V, Barquist E, Rivier J, Taché Y. Central CRF inhibits gastric emptying of a nutrient solid meal in rats: the role of CRF2 receptors. Am J Physiol. 1998;274:G965–G970. doi: 10.1152/ajpgi.1998.274.5.G965. [DOI] [PubMed] [Google Scholar]
- 22.Martinez V, Rivier J, Taché Y. Peripheral injection of a new corticotropin-releasing factor (CRF) antagonist, astressin, blocks peripheral CRF- and abdominal surgery-induced delayed gastric emptying in rats. J Pharmacol Exp Ther. 1999;290:629–634. [PubMed] [Google Scholar]
- 23.Martinez V, Rivier J, Wang L, Taché Y. Central injection of a new corticotropin-releasing factor (CRF) antagonist, astressin, blocks CRF- and stress-related alterations of gastric and colonic motor function. J Pharmacol Exp Ther. 1997;280:754–760. [PubMed] [Google Scholar]
- 24.Martinez V, Wang L, Taché Y. Central TRH receptor 1 antisense blocks cold-induced gastric emptying but not brain c-Fos induction. Peptides. 2001;22:81–90. doi: 10.1016/s0196-9781(00)00359-4. [DOI] [PubMed] [Google Scholar]
- 25.Martinez V, Wu SV, Taché Y. Intracisternal antisense oligodeoxynucleotides to the thyrotropin-releasing hormone receptor blocked vagal-dependent stimulation of gastric emptying induced by acute cold in rats. Endocrinology. 1998;139:3730–3735. doi: 10.1210/endo.139.9.6195. [DOI] [PubMed] [Google Scholar]
- 26.Masuda Y, Tanaka T, Inomata N, Ohnuma N, Tanaka S, Itoh Z, et al. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun. 2000;276:905–908. doi: 10.1006/bbrc.2000.3568. [DOI] [PubMed] [Google Scholar]
- 27.McCann MJ, Hermann GE, Rogers RC. Thyrotropin-releasing hormone: effects on identified neurons of the dorsal vagal complex. J Auton Nerv Syst. 1989;26:107–112. doi: 10.1016/0165-1838(89)90158-6. [DOI] [PubMed] [Google Scholar]
- 28.Metcalf G, Dettmar PW, Fortune D, Lynn AG, Tulloch IF. Neuropharmacological evaluation of RX 77368--a stabilised analogue of thyrotropin-releasing hormone (TRH) Regul Pept. 1982;3:193–206. doi: 10.1016/0167-0115(82)90125-2. [DOI] [PubMed] [Google Scholar]
- 29.Miedema BW, Johnson JO. Methods for decreasing postoperative gut dysmotility. Lancet Oncol. 2003;4:365–372. doi: 10.1016/s1470-2045(03)01118-5. [DOI] [PubMed] [Google Scholar]
- 30.Murray CD, Martin NM, Patterson M, Taylor SA, Ghatei MA, Kamm MA, et al. Ghrelin enhances gastric emptying in diabetic gastroparesis: a double blind, placebo controlled, crossover study. Gut. 2005;54:1693–1698. doi: 10.1136/gut.2005.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naito Y, Fukata J, Tamai S, Seo N, Nakai Y, Mori K, et al. Biphasic changes in hypothalamo-pituitary-adrenal function during the early recovery period after major abdominal surgery. J Clin Endocrinol Metab. 1991;73:111–117. doi: 10.1210/jcem-73-1-111. [DOI] [PubMed] [Google Scholar]
- 32.Noble EJ, Harris R, Hosie KB, Thomas S, Lewis SJ. Gum chewing reduces postoperative ileus? A systematic review and meta-analysis. Int J Surg. 2009;7:100–105. doi: 10.1016/j.ijsu.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 33.O-Lee TJ, Wei JY, Taché Y. Intracisternal TRH and RX 77368 potently activate gastric vagal efferent discharge in rats. Peptides. 1997;18:213–219. doi: 10.1016/s0196-9781(96)00281-1. [DOI] [PubMed] [Google Scholar]
- 34.Palkovits M, Mezey E, Eskay RL, Brownstein MJ. Innervation of the nucleus of the solitary tract and the dorsal vagal nucleus by thyrotropin-releasing hormone-containing raphe neurons. Brain Res. 1986;373:246–251. doi: 10.1016/0006-8993(86)90338-0. [DOI] [PubMed] [Google Scholar]
- 35.Poitras P, Polvino WJ, Rocheleau B. Gastrokinetic effect of ghrelin analog RC-1139 in the rat. Effect on post-operative and on morphine induced ileus. Peptides. 2005;26:1598–1601. doi: 10.1016/j.peptides.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 36.Popescu I, Fleshner PR, Pezzullo JC, Charlton PA, Kosutic G, Senagore AJ. The Ghrelin agonist TZP-101 for management of postoperative ileus after partial colectomy: a randomized, dose-ranging, placebo-controlled clinical trial. Dis Colon Rectum. 53:126–134. doi: 10.1007/DCR.0b013e3181b54166. [DOI] [PubMed] [Google Scholar]
- 37.Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- 38.Shimada SG, Stitt JT. Inhibition of shivering during restraint hypothermia. Can J Physiol Pharmacol. 1983;61:977–982. doi: 10.1139/y83-146. [DOI] [PubMed] [Google Scholar]
- 39.Shrestha YB, Wickwire K, Giraudo SQ. Direct effects of nutrients, acetylcholine, CCK, and insulin on ghrelin release from the isolated stomachs of rats. Peptides. 2009;30:1187–1191. doi: 10.1016/j.peptides.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stengel A, Goebel M, Wang L, Reeve JR, Jr, Taché Y, Lambrecht NW. Lipopolysaccharide differentially decreases plasma acyl and desacyl ghrelin levels in rats: Potential role of the circulating ghrelin-acylating enzyme GOAT. Peptides. 2010;31:1689–1696. doi: 10.1016/j.peptides.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stengel A, Goebel M, Wang L, Taché Y. Abdominal surgery activates nesfatin-1 immunoreactive brain nuclei in rats. Peptides. 2010;31:263–270. doi: 10.1016/j.peptides.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stengel A, Goebel M, Wang L, Taché Y. Ghrelin, des-acyl ghrelin and nesfatin-1 in gastric X/A-like cells: role as regulators of food intake and body weight. Peptides. 2010;31:357–369. doi: 10.1016/j.peptides.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stengel A, Keire D, Goebel M, Evilevitch L, Wiggins B, Taché Y, et al. The RAPID method for blood processing yields new insight in plasma concentrations and molecular forms of circulating gut peptides. Endocrinology. 2009;150:5113–5118. doi: 10.1210/en.2009-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taché Y, Barquist E, Stephens RL, Rivier J. Abdominal surgery- and trephination-induced delay in gastric emptying is prevented by intracisternal injection of CRF antagonist in the rat. J Gastrointest Motil. 1991;3:19–25. [Google Scholar]
- 45.Taché Y, Yang H, Kaneko H. Caudal raphe-dorsal vagal complex peptidergic projections: role in gastric vagal control. Peptides. 1995;16:431–435. doi: 10.1016/0196-9781(94)00212-o. [DOI] [PubMed] [Google Scholar]
- 46.Taché Y, Yang H, Miampamba M, Martinez V, Yuan PQ. Role of brainstem TRH/TRH-R1 receptors in the vagal gastric cholinergic response to various stimuli including shamfeeding. Auton Neurosci. 2006;125:42–52. doi: 10.1016/j.autneu.2006.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taniguchi H, Tanida M, Okumura N, Hamada J, Sano S, Nagai K. Regulation of sympathetic and parasympathetic nerve activities by BIT/SHPS-1. Neurosci Lett. 2006;398:102–106. doi: 10.1016/j.neulet.2005.12.071. [DOI] [PubMed] [Google Scholar]
- 48.Travagli RA, Gillis RA, Vicini S. Effects of thyrotropin-releasing hormone on neurons in rat dorsal motor nucleus of the vagus, in vitro. Am J Physiol. 1992;263:G508–G517. doi: 10.1152/ajpgi.1992.263.4.G508. [DOI] [PubMed] [Google Scholar]
- 49.Trudel L, Tomasetto C, Rio MC, Bouin M, Plourde V, Eberling P, et al. Ghrelin/motilin-related peptide is a potent prokinetic to reverse gastric postoperative ileus in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G948–G952. doi: 10.1152/ajpgi.00339.2001. [DOI] [PubMed] [Google Scholar]
- 50.Venkova K, Fraser G, Hoveyda HR, Greenwood-Van Meerveld B. Prokinetic effects of a new ghrelin receptor agonist TZP-101 in a rat model of postoperative ileus. Dig Dis Sci. 2007;52:2241–2248. doi: 10.1007/s10620-007-9783-7. [DOI] [PubMed] [Google Scholar]
- 51.Venkova K, Mann W, Nelson R, Greenwood-Van Meerveld B. Efficacy of ipamorelin, a novel ghrelin mimetic, in a rodent model of postoperative ileus. J Pharmacol Exp Ther. 2009;329:1110–1116. doi: 10.1124/jpet.108.149211. [DOI] [PubMed] [Google Scholar]
- 52.Wang L, Basa NR, Shaikh A, Luckey A, Heber D, St-Pierre DH, et al. LPS inhibits fasted plasma ghrelin levels in rats: role of IL-1 and PGs and functional implications. Am J Physiol Gastrointest Liver Physiol. 2006;291:G611–G620. doi: 10.1152/ajpgi.00533.2005. [DOI] [PubMed] [Google Scholar]
- 53.Wang L, Cardin S, Martinez V, Taché Y. Intracerebroventricular CRF inhibits cold restraint-induced c-fos expression in the dorsal motor nucleus of the vagus and gastric erosions in rats. Brain Res. 1996;736:44–53. doi: 10.1016/0006-8993(96)00726-3. [DOI] [PubMed] [Google Scholar]
- 54.Williams DL, Grill HJ, Cummings DE, Kaplan JM. Vagotomy dissociates short- and long-term controls of circulating ghrelin. Endocrinology. 2003;144:5184–5187. doi: 10.1210/en.2003-1059. [DOI] [PubMed] [Google Scholar]
- 55.Yang H, Wu SV, Ishikawa T, Taché Y. Cold exposure elevates thyrotropin-releasing hormone gene expression in medullary raphe nuclei: relationship with vagally mediated gastric erosions. Neuroscience. 1994;61:655–663. doi: 10.1016/0306-4522(94)90442-1. [DOI] [PubMed] [Google Scholar]
- 56.Yang H, Yuan PQ, Wang L, Taché Y. Activation of the parapyramidal region in the ventral medulla stimulates gastric acid secretion through vagal pathways in rats. Neuroscience. 2000;95:773–779. doi: 10.1016/s0306-4522(99)00490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan PQ, Kimura H, Million M, Bellier JP, Wang L, Ohning GV, et al. Central vagal stimulation activates enteric cholinergic neurons in the stomach and VIP neurons in the duodenum in conscious rats. Peptides. 2005;26:653–664. doi: 10.1016/j.peptides.2004.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeinali F, Stulberg JJ, Delaney CP. Pharmacological management of postoperative ileus. Can J Surg. 2009;52:153–157. [PMC free article] [PubMed] [Google Scholar]