Abstract
Obesity is rapidly becoming a pandemic and is associated with increased carcinogenesis. Obese populations have higher circulating levels of leptin in contrast to low concentrations of adiponectin. Hence, it is important to evaluate the dynamic role between adiponectin and leptin in obesity-related carcinogenesis. Recently, we reported the oncogenic role of leptin including its potential to increase tumor invasiveness and migration of hepatocellular carcinoma (HCC) cells. In the present study, we investigated whether adiponectin could antagonize the oncogenic actions of leptin in HCC. We employed HCC cell-lines HepG2 and Huh7, nude mice-xenograft model of HCC and immunohistochemistry-data from tissue-microarray to demonstrate the antagonistic role of adiponectin on the oncogenic actions of leptin. Adiponectin treatment inhibited leptin-induced cell proliferation of HCC cells. Using scratch-migration and electric cell-substrate impedance-sensing based migration assays, we found that adiponectin inhibited leptin-induced migration of HCC cells. Adiponectin treatment effectively blocked leptin-induced invasion of HCC cells in matrigel invasion assays. While leptin inhibited apoptosis in HCC cells, we found that adiponectin treatment induced apoptosis even in the presence of leptin. Analysis of the underlying molecular mechanisms revealed that adiponectin treatment reduced leptin-induced Stat3 and Akt phosphorylation. Adiponectin also increased suppressor of cytokine signaling (SOCS3), a physiologic negative regulator of leptin signal transduction. Importantly, adiponectin significantly reduced leptin-induced tumor burden in nude mice. In HCC samples, leptin expression significantly correlated with HCC proliferation as evaluated by Ki-67 while adiponectin expression correlated significantly with increased disease-free-survival and inversely with tumor size and local recurrence.
Conclusion
Collectively, these data demonstrate that adiponectin has the molecular potential to inhibit the oncogenic actions of leptin by blocking downstream effector molecules.
Keywords: Adipocytokines, Obesity, HCC, Invasion, Migration
Introduction
Epidemiological studies suggest that obesity is rapidly becoming a global pandemic. This pandemic has significant potential to influence risk, prognosis and progression of various cancers including colon, prostate, breast, endometrial and hepatocellular (1, 2). Obesity is associated with an increase in white adipose tissue (WAT) that greatly alters the local and systemic secretion of biologically active polypeptides, adipocytokines including leptin and adiponectin (3).
Leptin was originally discovered as an afferent satiety signal (4). Studies over the last few years have provided important clues about its apheliotropic actions, its role in the pathogenesis of atherosclerotic vascular disease (5), as well as carcinogenesis (6). Leptin circulates as 16-kD protein and functions through specific cell surface receptors (7, 8). Leptin increases proliferation of breast, endometrial, hepatocellular and many other cancer cells via multiple signaling pathways including Stat3/ERK/Akt signaling (8–12). Our recent research also shows a direct stimulatory effect of leptin on cancer cell migration and invasion (9). The therapeutic potential of inhibition of leptin has been evaluated to some extent in diseases associated with metabolic syndrome (13), but inhibition of leptin signaling in carcinogenesis needs to be appraised.
Adiponectin is an important adipocytokine (14–17) that has a protective role against obesity-related disorders namely, metabolic syndrome, type-2-diabetes and cardiovascular disease (18–20). Adiponectin can directly bind certain growth factors to control their bioavailability (21). Recent research has expanded a role for adiponectin in cancer (22). Adiponectin Receptor 1, Adiponectin Receptor 2 (23) and T-cadherin (24) have been identified as adiponectin receptors that mediate the cellular functions of adiponectin in a tissue-dependent manner (25). Importantly, epidemiological studies have linked low levels of plasma adiponectin with obesity and many cancers(1, 25). Most importantly, some studies have suggested that tumors arising in patients with low-serum adiponectin levels may have a more aggressive phenotype (large tumor-size, high histological grade, and increased metastasis). Several recent studies have shown that adiponectin also mediates anti-proliferative response in cancer cells (26).
In the present study, we specifically investigated the protective effect of adiponectin against oncogenic actions of leptin on HCC. Intriguingly, we show that adiponectin inhibits leptin-induced malignant properties of HCC cells, including migration and invasion. Adiponectin also inhibits important downstream molecules of leptin signaling. Adiponectin inhibits leptin-induced HCC tumorigenesis in vivo. In agreement with our in vitro and in vivo data, we show that leptin expression significantly correlates with HCC proliferation in a large number of HCC TMA, as evaluated by Ki-67 expression. Importantly, we show that adiponectin expression significantly and inversely correlates with tumor size, and local recurrence while positively correlating with disease free survival.
Materials and Methods
Antibodies
Antibodies for Phospho-AKT, AKT were purchased from Cell-Signaling Technology (Danvers, MA). Phospho-Stat3, Stat3, SOCS3, leptin and adiponectin antibodies were procured from SantaCruz Biotechnology (SantaCruz, CA). Anti-Ki-67 was purchased from DAKO (Carpinteria, CA). Anti-PPH3 was purchased from Epitomics Inc. (Burlingame, CA).
Cell culture, reagents and treatments
Human HCC cell lines, HepG2 (ATCC, Manassas, VA) and Huh7, were maintained in MEM (ATCC) and DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% FBS (Gemini Bioproducts, Woodland, CA), respectively. Cells were seeded at a density of 1 × 106/100-mm tissue-culture dish, serum starved for 16 h followed by treatment with 100 ng/ml (9) human recombinant leptin (Sigma-Aldrich, St. Louis, MO) and/or 10 μg/ml human recombinant full-length adiponectin (Biovendor, Candler, NC) as indicated. For determination of optimum inhibitory dose of adiponectin against leptin, cells were treated with various doses of adiponectin (1.25–30 μg/ml) with 100 ng/ml leptin as indicated. For electric cell-substrate impedance sensing (ECIS) migration-assay, ECIS cell culture-ware was procured from Applied Biophysics, Troy, NY.
Western Blot
Western blot analysis and immunodetection was performed following our established protocols (9, 10) using specific antibodies as described.
Quantification of DNA/cell proliferation assay by bromodeoxyuridine (BrdU) incorporation
BrdU incorporation analysiswas performed using an ELISA (Roche Diagnostics, Indianapolis, IN) following our previous protocol (27).
Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labeling Assay
TUNEL analysis was performed following our established protocol (32).
Tumor cell invasion assay
For an in vitro model system for metastasis, we performed a Matrigel invasion assay using a Matrigel invasion chamber from BD Biocoat Cellware (San Jose, CA) following our previously standardized protocol (9).
Migration Assay
Migration assays were performed following our standardized scratch-migration protocol (10).
Electric cell-substrate impedance sensing (ECIS) (Wound-healing assays for cell migration)
Quantitative wound-healing assays were performed with ECIS (Applied BioPhysics) technology following our previously published method (9).
HCC tumorigenesis assay
HepG2 (5×106) cells in 0.1ml of HBSS were injected subcutaneously into the right gluteal region of 4–6-week-old female athymic NCr-nu/nu mice, (National Cancer Institute, Frederick, MD). Two weeks after initial implantation, animals placed into five experimental groups. Mice were treated with intratumoral injections of 1) recombinant adenovirus [108 plaque-forming units (pfu)] expressing adiponectin (Ad-Adn), 2) luciferase (Ad-Luc) (as vector control) [kind gift from Dr. Yu Wang (28) Assistant Professor of Pharmacology & Pharmacy, University of Hong Kong], 3) saline, 4) intraperitoneal injections of recombinant leptin (dosage of 5 mg/kg), 5) leptin and Ad-Adn together, every 36 hours for the duration of the experiment. Plasma adiponectin levels were monitored regularly using ELISA. Ad-Adn treatment significantly increased plasma adiponectin levels as compared to respective Ad-luc treated cells (data not shown). Tumors were measured using vernier calipers, with tumor volume calculated using the formula (V= a/2 × b2), where V is the tumor volume in mm3, a and b are the largest and smallest diameters in mm, respectively. All animals were sacrificed after 5 weeks of treatment. Tumors were collected; weighed, fixed in 10% neutral-buffered formalin; and subjected to further analysis by immunoblot and immunohistochemistry. All animal studies were conducted in accordance with the guidelines of Emory University IACUC.
Immunohistochemistry of human HCC TMA
Tissue microarrays (TMA’s) were constructed with two 1-mm cores from each of 135 cases of HCC and 5 nonneoplastic adjacent livers from archived specimens obtained from Tumor Tissue Bank, Department of Pathology, Emory University. These specimens were archived between 1985–2002. Detailed clinicopathological information including but not limited to tumor size, histological grade, solitary/multiple tumors, lymph node involvement, angioinvasion, local recurrence, metastasis, mitosis, Ki-67, PPH3, disease free survival, NASH and non-NASH was available to the Pathologist. None of the patients’ samples were from transplant explants. Immunohistochemical staining with antibodies against leptin (1:50), adiponectin (1:20), Ki-67 (1:160) and PHH3 (1:6400) were performed on 5μm sections of 3 TMA’s. Leptin and adiponectin stained TMA’s were visually interpreted by trained pathologists for intensity (0–4+) and percent positivity of HCC cells. Ki-67 and PPH3 stained TMA’s were analyzed visually by trained pathologists as the mean of the two tissue cores (positive cells/0.79 μm2). Leptin and adiponectin immunostains were correlated with important clinicopathologic prognostic factors, proliferative markers (Ki-67, PPH3, and mitotic activity index), and follow-up data in order to assess their role in prognosis, proliferation, and outcome.
These studies were approved by the Institutional Review Board at Emory University.
Statistical Analysis
All experiments were performed thrice in triplicates. Statistical analysis was performed using Microsoft Excel software. Significant differences were analyzed using student’s t test and two-tailed distribution. Data were considered to be statistically significant if p<0.05. Data were expressed as mean ± SE between triplicate experiments performed thrice. For TMA the data were analyzed using a combination of Chi-Square, Fisher’s exact test, t-tests, two-tailed distribution and analysis of variance (ANOVA). Statistical analysis for TMA was performed with SPSS 18.0 using two-tailed univariate calculations. For categorical variables, a Chi-Square (sufficient sample size) or Fisher’s exact test (small sample size) was performed. For continuous variables, t-test comparing means were used. Kaplan-Meier survival curves were created using follow-up data to assess for differences in time to recurrence and death. Comparisons in mean time to event were computed using log rank analysis. P-values less than 0.05 were considered statistically significant.
Results
Adiponectin treatment increases apoptosis and decreases proliferation opposing the pro-proliferative and anti-apoptotic actions of leptin
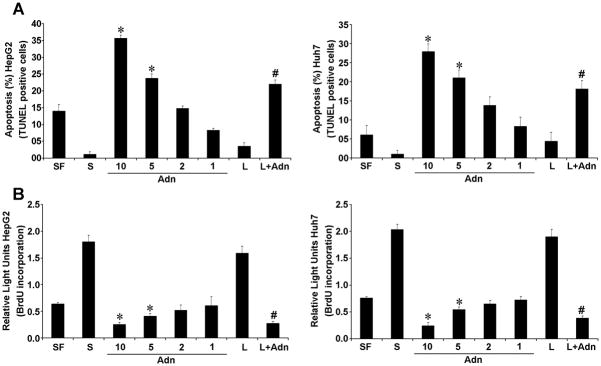
Recently, we and others have shown that leptin increases proliferation and growth of various cancer cells (8–11). Low adiponectin levels are significantly associated with an increased tumor growth and metastasis (22, 29–31) indicating an anti-oncogenic role for adiponectin. We examined the effect of adiponectin on pro-proliferative and anti-apoptotic actions of leptin using BrdU and TUNEL assay. Adiponectin increased apoptosis in a dose-dependent manner (Figure 1A). Kinetics of increasing/decreasing doses of adiponectin in combination with leptin showed that 10 ug/ml adiponectin efficiently inhibited the effect of leptin (Supplementary Figure 1). Adiponectin eliminated the anti-apoptotic effect of leptin (Figure 1A). Adiponectin treatment significantly increased caspase-3 activity even in the presence of leptin (Supplementary Figure 3). Importantly, adiponectin inhibited proliferation of HCC cells in a dose dependent manner in contrast to leptin treatment which increased proliferation. Combined treatment with adiponectin and leptin also resulted in significant inhibition of leptin-induced proliferation (Figure 1B).
Figure 1. Adiponectin reduces the effect of leptin on apoptosis and proliferation in hepatocellular carcinoma cells.
Cells were treated with adiponectin (Adn) (1–10 μg/ml) or leptin (L) (100 ng/ml) alone or in combination (L+ 10 μg/ml Adn) for 24h. Cells grown in 10% FBS (S) and in serum-free condition (SF) were used as controls. *p<0.01, compared to untreated (SF) control; #p<0.01, compared to leptin (L) treated cells. (A) TUNEL assay was performed to determine apoptosis. The percentage of apoptosis was calculated as percent ratio between TUNEL-positive number of cells and total number of cells in each field. (B) BrdU incorporation assay was performed to determine proliferation.
Adiponectin inhibits leptin-induced migration and invasion
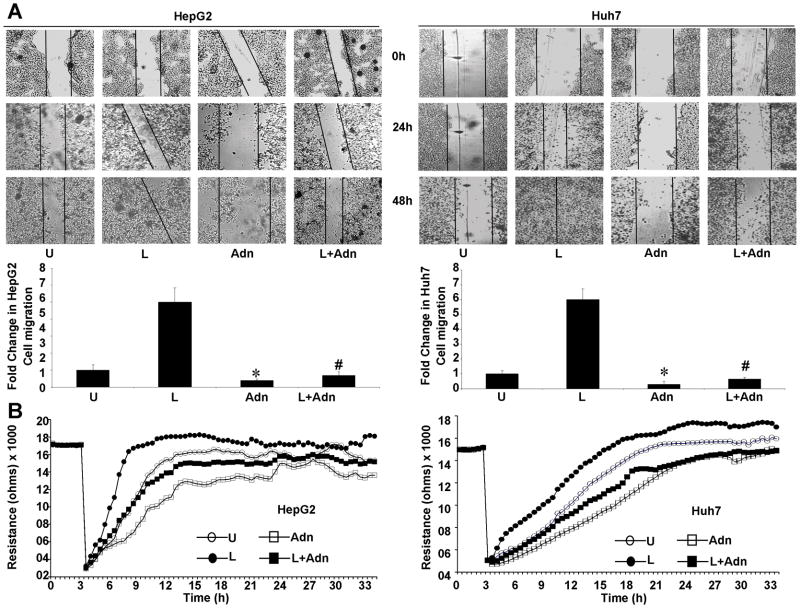
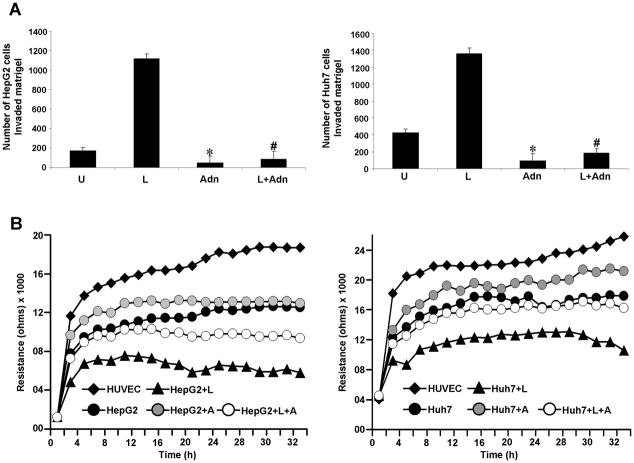
Cancer progression is a multistep process that involves invasion of the basement membrane by tumor cells and migration to points far from a given primary tumor mass leading to metastasis (32). We examined the effect of adiponectin on leptin-induced invasion and migration of HCC cells. Leptin increased migration of HCC cells while adiponectin inhibited migration in a conventional scratch-migration assay. Adiponectin treatment also inhibited migration of cancer cells in the presence of leptin overcoming its pro-migratory potential (Figure 2A). In a quantitative real-time assay using an ECIS-based technique to follow migration of HCC cells, we found that cells treated with leptin showed increased resistance whereas adiponectin treatment inhibited cell migration (showing low resistance). Cells co-treated with both adiponectin and leptin displayed a decreased resistance showing that adiponectin could inhibit leptin-induced migration (Figure 2B). Next, we performed matrigel invasion assays to examine the effect of adiponectin on leptin-induced invasion potential of HCC cells. Leptin treatment increased invasion of cancer cells through matrigel in comparison to untreated cells whereas adiponectin treatment inhibited invasion of HCC cells. Importantly, adiponectin treatment significantly inhibited leptin-induced invasion of cancer cells (Figure 3A). In an ECIS-based invasion assay, established HUVEC cell layers were challenged with HCC cells. The drop in the resistance showed direct interactions of the tumor cells with HUVEC cells and extravasation of HCC cells on the substratum. Leptin treatment induced a steep drop in resistance than no treatment control demonstrating that leptin increased invasive potential. Adiponectin inhibited invasive potential of HCC even in the presence of leptin (Figure 3B). These results showed that adiponectin could effectively inhibit leptin-induced increased migration and invasion of HCC cells.
Figure 2. Adiponectin blocks leptin-induced migration in hepatocellular carcinoma cells.
(A) Cells were subjected to scratch-migration assay. Plates were photographed immediately following scratching (0 h). Cells were treated with 100 ng/ml leptin (L), 10 μg/ml adiponectin (Adn), leptin + adiponectin (L+Adn) or untreated media (U) and allowed to migrate. The plates were photographed at the identical location of the initial image (0h) at 24h and 48h. The histogram represents the fold change in migration. *, p<0.01, compared to untreated controls (U); #p<0.01, compared to leptin (L) treated cells. (B) Huh7 and HepG2 cells were subjected to ECIS migration assay in the presence of leptin and adiponectin treatments as above. Cells were allowed to grow to confluence on ECIS 8W1E plates and initial resistance was measured for few hours. Cells were subjected to high voltage to initiate wound at 3 hours resulting in the drop of resistance. Remaining cells were allowed to migrate in the presence or absence of treatments and resistance changes were measured for ~36 h after the creation of wound.
Figure 3. Adiponectin blocks leptin-induced invasion of hepatocellular carcinoma cells.
(A) Cells were cultured in Matrigel invasion chambers followed by treatment with 100 ng/ml leptin (L), 10 μg/ml of adiponectin alone (Adn) and in combination (L+Adn) for 24 h. The number of cells that invaded through the matrigel was counted in five different regions. The slides were blinded to remove counting bias. *p<0.005, compared with untreated (U) controls; #p<0.001, compared to leptin (L) treated cells. (B) Resistance changes in the impedance at 4 kHz as confluent layers of HUVEC cells were challenged with HepG2 and Huh7 cells suspensions. The control curve of HUVEC cells received media without HepG2 or Huh7 cells. HepG2 and Huh7 cells were treated as above and changes in resistance were monitored for 36h.
Adiponectin inhibits phosphorylation of leptin signaling molecule
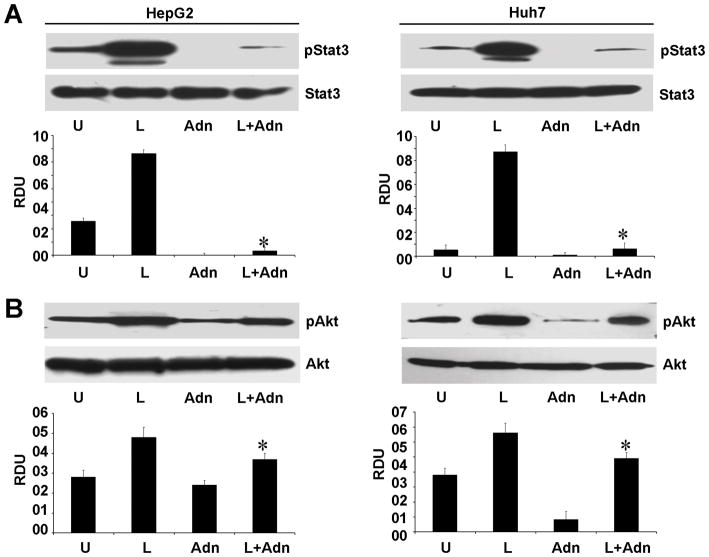
Binding of leptin to leptin receptor (Ob-Rb) phosphorylates conserved tyrosine residues (33) and these phosphorylation events are important for downstream signaling events (33). Our previous studies have shown the direct involvement of Stat3 and Akt signaling in pro-cancerous actions of leptin (8, 11). In this study, we show that adiponectin effectively inhibits the oncogenic functions of leptin such as proliferation, cell-migration and invasion. We sought to determine the underlying molecular mechanism by which adiponectin antagonizes the oncogenic actions of leptin. We found that leptin increased phosphorylation of Stat3 and Akt in comparison to untreated HCC cells whereas combined treatment with adiponectin significantly reduced leptin-induced Stat3 and Akt phosphorylation (Figure 4). We previously demonstrated that activation of Stat is upstream of the activation of Akt (11). Activation of these critical downstream effecters is interdependent such that leptin signaling can be inhibited by up-regulation of an upstream inhibitory molecules, suppressor of cytokine signaling 3 (SOCS3)(27). Overexpression of SOCS3 inhibits leptin-mediated tyrosine phosphorylation of JAK2 and subsequently Stat3 activation (27, 34, 35) which can in turn inhibit Akt activation. Thus, we examined whether adiponectin can upregulate SOCS3 expression. Indeed, adiponectin treatment increased SOCS3 expression in HCC cells (Figure 5). These results collectively show that adiponectin inhibits components of the signaling machinery used by leptin in addition to upregulating an important upstream inhibitor.
Figure 4. Evidence of adiponectin-mediated inhibition of leptin signaling.
Cells were treated with 100 ng/ml leptin (L), 10 μg/ml of adiponectin (Adn) alone and in combination (L+Adn). Untreated cells are denoted as U. Equal amount of cell lysates were subjected to immunoblot analysis using (A) anti-pStat and (B) anti-pAkt. The histogram is the mean of densitometric analysis showing relative density units (RDU) of the western blot signals for pStat3 or pAkt normalized to total Stat3 or Akt. *p<0.005 compared with leptin (L) treated cells.
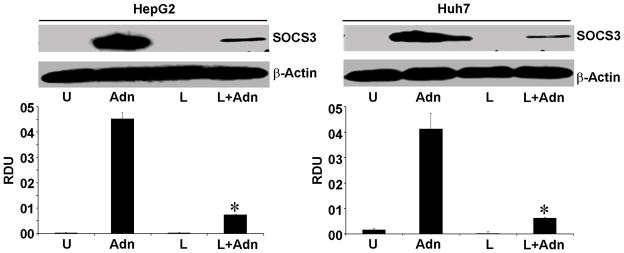
Figure 5. Adiponectin increases SOCS3 expression in HCC cells.
Cells were treated with 100 ng/ml leptin (L) and 10 μg/ml of adiponectin (Adn) alone and in combination (L+Adn). Untreated cells are denoted as U. Equal amount of cell lysates were subjected to immunoblot analysis using anti-SOCS3. The histogram is the mean of densitometric analysis showing relative density units (RDU) of the western blot signals for SOCS3 normalized to β-actin. *p<0.001 compared with leptin (L) treated cells.
Adiponectin inhibits leptin-induced HCC tumor progression in athymic nude mice
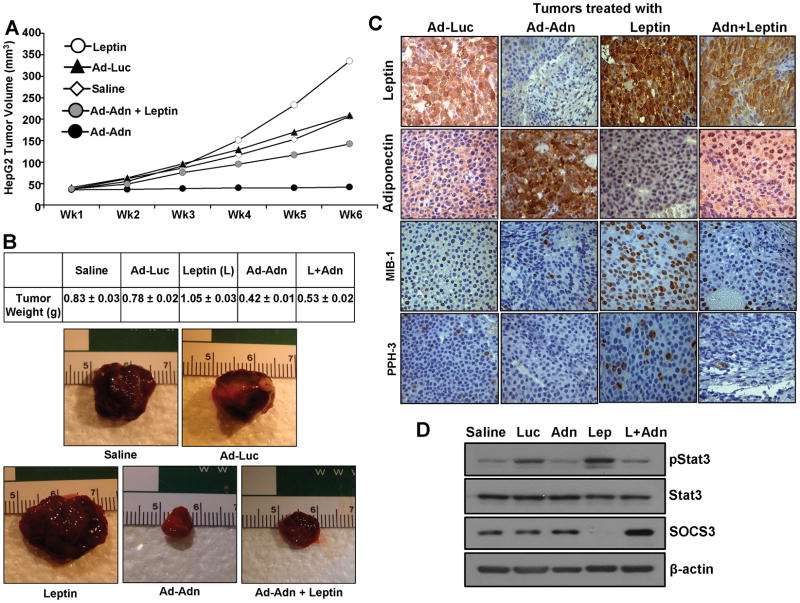
We investigated the physiological relevance of our in vitro findings by evaluating suppressing effects of adiponectin on leptin-induced development of HCC in vivo. Leptin treatment significantly increased tumor growth as compared to the saline-treated group. Adiponectin treatment (Ad-Adn) inhibited tumor growth resulting in reduced tumor size compared to saline and adenovirus-luciferase control. Importantly, adiponectin treatment efficiently inhibited leptin-induced tumor growth (Figure 6A, B). Adiponectin adenovirus-treated tumors showed elevated levels of adiponectin whereas leptin-treated tumors showed increased staining for leptin as compared to controls. The immunohistochemical assessment of tumor proliferation showed higher MIB1 and PPH3 expression in the leptin- treated group while little if any MIB1 and PPH3 expression was observed in the adiponectin-treated group (Figure 6C). We further confirmed our in vitro findings regarding important signaling molecules using tumor samples from various treatment groups. Leptin-treated tumors revealed elevated p-Stat3 levels in comparison to saline-treated controls. Adiponectin treatment, on the other hand, inhibited leptin-induced p-Stat3 levels in combined-treatment tumor-groups. Adiponectin-treated tumors and leptin-adiponectin combination-treated tumors showed elevated SOCS3 levels (Figure 6D). Analysis of signaling molecules in tumor samples provided the critical molecular link between p-Stat3 and SOCS3 in leptin-adiponectin crosstalk. Collectively, these in vivo studies demonstrated a cogent antagonistic effect of adiponectin on the potential oncogenic actions of leptin.
Figure 6. Adiponectin treatment inhibits leptin-induced HCC tumor growth in nude mice.
HepG2 cells derived tumors were developed in nude mice and treated with leptin, saline, Ad-Luc, Ad-Adn (108 pfu) and leptin+Ad-Adn. (A) Tumor growth was monitored by measuring the tumor volume for 6 weeks. (n = 8 mice per group, from three independent experiments). (B) At the end of 6 weeks, tumors were collected, measured, weighed and photographed. Ad-Adn treatment reduced tumor size as compared to Ad-Luc, * p < 0.01. Adiponectin treatment significantly reduced leptin-induced tumor size as compared to leptin alone (p < 0.01) and Ad-luc (p < 0.01). Average tumor weight and representative tumor images are shown here. (C) Tumor samples were subjected to immunohistochemical analysis using leptin, adiponectin, MIB-1 and PPH-3 antibody. Ad-Adn treated tumors showed significant increase in adiponectin expression and reduction in MIB-1 and PPH-3 expression as compared to Ad-Luc treated tumors, *p < 0.05 Ad-Adn versus Ad-Luc. Ad-Adn treated tumors showed significant reduction in leptin-induced expression of MIB-1 and PPH-3 as compared to leptin treated tumors, *p < 0.05 Ad-Adn versus Leptin. (D) Tumor lysates were subjected to immunoblot analysis using Stat3, p-Stat3 and SOCS3 antibodies.
Analysis of expression of adiponectin and leptin in human HCC and their correlation with proliferation and prognostic parameters
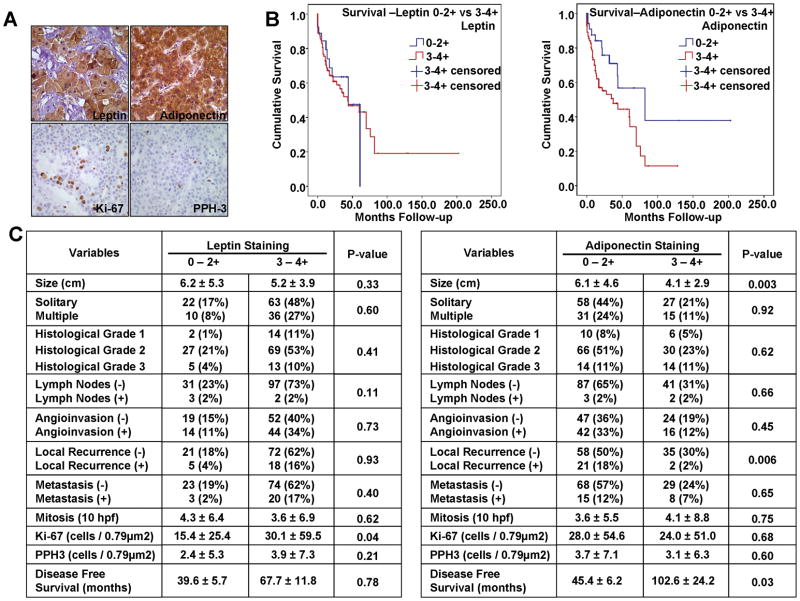
Our in vitro and in vivo data prompted us to investigate the pattern of leptin and adiponectin expression in a tissue microarray of human HCC (140 samples) to understand their importance in tumor progression. Representative photomicrographs from immunostained TMA’s are shown in Figure 7A. Adiponectin expression correlated significantly and inversely with tumor size (p=0.003) hence larger tumors showed decreased adiponectin expression as compared to smaller tumors. Analysis of clinicopathological characteristics showed an inverse correlation between adiponectin expression, tumor size and local recurrence (p=0.006). Importantly, higher adiponectinn expression directly correlated with increased disease free survival (Figure 7B and C). Immunohistochemical studies showed that 100 (74%) of HCC’s had 3–4+ leptin expression (Figure 7C); 43 (32%) had 3–4+ adiponectin expression (Figure 7C). Leptin expression correlated significantly with Ki-67 expression (p=0.04) (Figure 7C) but was not significant for PPH3. A potential limitation of TMA was the lack of adequate number of controls for NASH, HCV in addition to normal liver. Next, we analyzed the association of leptin and adiponectin expression with NASH and non-NASH groups. HCC sample cohort included 47 samples (33%) with HCV, HBV, HBV+HCV diagnosis (non-NASH group) and 21 samples (15%) with NASH related liver diseases (Cryptogenic, NASH, Steatohepatitis) while no data was available regarding underlying pathological conditions for 72 samples (52%). Based on our leptin categorization, there was an association with higher staining in the NASH group compared with the non-NASH group (p=0.03), while there was no difference in adiponectin staining between the NASH and non-NASH groups (p = 0.40) (Supplementary Figure 2). Collectively, these data demonstrate that adiponectin inhibits the progression of hepatocellular carcinoma.
Figure 7. Leptin and adiponectin expression in human HCC samples and their correlation with proliferation and prognostic parameters.
A. Representative photomicrographs of HCC samples from TMA (135 human samples) showing staining for leptin, adiponectin, Ki-67 and PPH-3 (Figure 7A). Adiponectin expression correlated significantly and inversely with tumor size (p=0.003). Analysis of clinicopathological characteristics showed that there is an inverse correlation between adiponectin expression, tumor size, local recurrence (p=0.006) and direct correlation with disease free survival (Figure 7B and C). Immunohistochemical studies showed that 100 (74%) of HCC’s had 3–4+ leptin expression (Figure 7C); 43 (32%) had 3–4+ adiponectin expression (Figure 7C). Leptin expression correlated significantly with Ki-67 expression (p=0.04) (Figure 7C) but was not significant for PPH3.
Discussion
The dynamic levels of leptin and adiponectin get modulated in obesity such that obesity is now considered to be a hyperleptinaemic and hypoadiponectinaemic state.(3), (36). In the present study, we investigated the effect of adiponectin on oncogenic actions of leptin. The following novel findings are described in this study: (a) Adiponectin treatment alone inhibits malignant properties such as proliferation, invasion and migration of hepatocellular carcinoma cells; (b) Adiponectin blocks the oncogenic effects of leptin by inhibiting leptin-induced proliferation, migration and invasion; (c) Adiponectin treatment leads to inhibition of Stat3 and Akt phosphorylation even in the presence of leptin; (d) adiponectin treatment leads to overexpression of SOCS3; (e) adiponectin treatment inhibits leptin-induced HCC tumor growth in vivo; (f) leptin expression correlates positively with HCC proliferation and NASH, while adiponectin expression correlates inversely with tumor size and local recurrence, and directly to disease-free survival in human HCC tumor samples. Taken together our results suggest an attractive molecular strategy employing adiponectin analogues for potential therapy of metastatic HCC.
Our data is important in clinical context since HCC has the highest relative-risk increase in association with obesity compared to all the cancers studied including prostate, kidney, gallbladder, colon, rectum, esophagus, stomach and pancreas (1, 37, 38). A recent clinical study examining obesity as an independent risk factor for hepatocellular carcinoma in patients with cirrhosis who underwent transplantation concluded that obesity is indeed a statistically significant independent risk factor for HCC, after multivariate analysis (38). Our earlier studies clearly show that leptin induces proliferation, migration and invasion of hepatocellular carcinoma cells. Reagents blocking leptin activity might prove useful for hepatocellular carcinoma patients with elevated leptin levels. Inhibition of leptin may be achieved with soluble leptin receptors that bind free leptin, leptin antagonists that bind leptin receptor or specific anti-leptin receptor monoclonal antibodies. Importantly, recent development of leptin muteins (39) with antagonistic properties and other proteins blocking leptin activity also offer new possibilities for research and ultimately therapy for metastatic HCC. While all these agents to counteract leptin signaling are in various stages of development, our studies demonstrate the potential antagonistic effect of adiponectin on HCC.
While the work performed here was not directed to a particular liver disease leading to HCC, we recognize the growing concern that adipocytokines play in modulating liver injury and repair. This is particularly true in metabolic syndrome-related NAFLD and its more aggressive histology NASH. There is no question that the incidence of HCC in the United States is increasing. While in part this is due to chronic hepatitis C viral infection (HCV)-related cirrhosis, we are certain that cryptogenic cirrhosis has its origins in NASH. Since NASH fibrosis appears to be exacerbated by leptin, and progression of fibrosis inhibited by adiponectin, our data are critical for future clinical and basic research inquiry concerning how obesity and its related adipocytokines promote hepatic carcinogenesis. The role of metabolic syndrome, obesity, and NASH-related liver disease, may have a significant impact not only on HCC promotion but also—as we have observed—a significant impact on HCC growth as well as other more adverse malignant properties that would increase patient related HCC mortality.
The clinical relevance of adiponectin treatment has been suggested for improving glucose/lipid homeostasis, increasing insulin sensitivity and preventing atherosclerosis in animal models (22, 40, 41). In addition to increasing adiponectin levels using adiponectin analogues, augmentation of its effectiveness, can potentially become a future beneficial treatment. Considering the high prevalence of obesity in the US, our study has the potential to significantly impact the vast majority of obese hepatocellular carcinoma patients by using adiponectin for inhibiting growth, invasion and migration of HCC cells and improving overall survival.
Supplementary Material
Acknowledgments
Financial Support: Grant Support: NIDDK NIH, K01DK77137 and R03DK089130 to NKS; NIDDK NIH, R01DK062092 and R01DK075397 to FAA; NCI NIH R01CA131294 and R21CA155686 to DS; and NIDDK, NIH, R24 to the Division of Digestive Diseases DK064399.
List of Abbreviations
- HCC
Hepatocellular Carcinoma
- ECIS
Electric Cell Substrate Impedance Sensing
- Stat3
Signal transducer and activator of transcription 3
- ERK
Extracellular signal-regulated kinases
- Akt
v-akt murine thymoma viral oncogene homolog 1
- PPH3
Phosphohistone H3
- SOCS3
Suppressors of Cytokine Signaling 3
- AdipoR1
Adiponectin Receptor 1
- Adipo R2
Adiponectin Receptor 2
- BrdU
Bromodeoxyuridine
- FBS
Fetal Bovine Serum
References
- 1.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 2.Caldwell SH, Crespo DM, Kang HS, Al-Osaimi AM. Obesity and hepatocellular carcinoma. Gastroenterology. 2004;127:S97–103. doi: 10.1053/j.gastro.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 3.Nawrocki AR, Scherer PE. Keynote review: the adipocyte as a drug discovery target. Drug Discov Today. 2005;10:1219–1230. doi: 10.1016/S1359-6446(05)03569-5. [DOI] [PubMed] [Google Scholar]
- 4.Muoio DM, Lynis Dohm G. Peripheral metabolic actions of leptin. Best Pract Res Clin Endocrinol Metab. 2002;16:653–666. doi: 10.1053/beem.2002.0223. [DOI] [PubMed] [Google Scholar]
- 5.Wolk R, Berger P, Lennon RJ, Brilakis ES, Johnson BD, Somers VK. Plasma leptin and prognosis in patients with established coronary atherosclerosis. J Am Coll Cardiol. 2004;44:1819–1824. doi: 10.1016/j.jacc.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 6.Somasundar P, McFadden DW, Hileman SM, Vona-Davis L. Leptin is a growth factor in cancer. J Surg Res. 2004;116:337–349. doi: 10.1016/j.jss.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Bjorbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997;272:32686–32695. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- 8.Saxena NK, Sharma D, Ding X, Lin S, Marra F, Merlin D, Anania FA. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007;67:2497–2507. doi: 10.1158/0008-5472.CAN-06-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saxena NK, Taliaferro-Smith L, Knight BB, Merlin D, Anania FA, O’Regan RM, Sharma D. Bidirectional crosstalk between leptin and insulin-like growth factor-I signaling promotes invasion and migration of breast cancer cells via transactivation of epidermal growth factor receptor. Cancer Res. 2008;68:9712–9722. doi: 10.1158/0008-5472.CAN-08-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saxena NK, Vertino PM, Anania FA, Sharma D. leptin-induced growth stimulation of breast cancer cells involves recruitment of histone acetyltransferases and mediator complex to CYCLIN D1 promoter via activation of Stat3. J Biol Chem. 2007;282:13316–13325. doi: 10.1074/jbc.M609798200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma D, Saxena NK, Vertino PM, Anania FA. Leptin promotes the proliferative response and invasiveness in human endometrial cancer cells by activating multiple signal-transduction pathways. Endocr Relat Cancer. 2006;13:629–640. doi: 10.1677/erc.1.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaffler A, Scholmerich J, Buechler C. Mechanisms of disease: adipokines and breast cancer - endocrine and paracrine mechanisms that connect adiposity and breast cancer. Nat Clin Pract Endocrinol Metab. 2007;3:345–354. doi: 10.1038/ncpendmet0456. [DOI] [PubMed] [Google Scholar]
- 13.Gorden P, Gavrilova O. The clinical uses of leptin. Curr Opin Pharmacol. 2003;3:655–659. doi: 10.1016/j.coph.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 15.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 16.Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem. 1996;120:803–812. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- 17.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 18.Maahs DM, Ogden LG, Kinney GL, Wadwa P, Snell-Bergeon JK, Dabelea D, Hokanson JE, et al. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation. 2005;111:747–753. doi: 10.1161/01.CIR.0000155251.03724.A5. [DOI] [PubMed] [Google Scholar]
- 19.Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 20.Spranger J, Kroke A, Mohlig M, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226–228. doi: 10.1016/S0140-6736(03)12255-6. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Lam KS, Xu JY, Lu G, Xu LY, Cooper GJ, Xu A. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem. 2005;280:18341–18347. doi: 10.1074/jbc.M501149200. [DOI] [PubMed] [Google Scholar]
- 22.Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. Br J Cancer. 2006;94:1221–1225. doi: 10.1038/sj.bjc.6603051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 24.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen MB, McAinch AJ, Macaulay SL, Castelli LA, O’Brien PE, Dixon JB, Cameron-Smith D, et al. Impaired activation of AMP-kinase and fatty acid oxidation by globular adiponectin in cultured human skeletal muscle of obese type 2 diabetics. J Clin Endocrinol Metab. 2005;90:3665–3672. doi: 10.1210/jc.2004-1980. [DOI] [PubMed] [Google Scholar]
- 26.Dos Santos E, Benaitreau D, Dieudonne MN, Leneveu MC, Serazin V, Giudicelli Y, Pecquery R. Adiponectin mediates an antiproliferative response in human MDA-MB 231 breast cancer cells. Oncol Rep. 2008;20:971–977. [PubMed] [Google Scholar]
- 27.Saxena NK, Titus MA, Ding X, Floyd J, Srinivasan S, Sitaraman SV, Anania FA. Leptin as a novel profibrogenic cytokine in hepatic stellate cells: mitogenesis and inhibition of apoptosis mediated by extracellular regulated kinase (Erk) and Akt phosphorylation. Faseb J. 2004;18:1612–1614. doi: 10.1096/fj.04-1847fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Lam JB, Lam KS, Liu J, Lam MC, Hoo RL, Wu D, et al. Adiponectin modulates the glycogen synthase kinase-3beta/beta-catenin signaling pathway and attenuates mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res. 2006;66:11462–11470. doi: 10.1158/0008-5472.CAN-06-1969. [DOI] [PubMed] [Google Scholar]
- 29.Mantzoros C, Petridou E, Dessypris N, Chavelas C, Dalamaga M, Alexe DM, Papadiamantis Y, et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab. 2004;89:1102–1107. doi: 10.1210/jc.2003-031804. [DOI] [PubMed] [Google Scholar]
- 30.Barb D, Pazaitou-Panayiotou K, Mantzoros CS. Adiponectin: a link between obesity and cancer. Expert Opin Investig Drugs. 2006;15:917–931. doi: 10.1517/13543784.15.8.917. [DOI] [PubMed] [Google Scholar]
- 31.Miyoshi Y, Funahashi T, Kihara S, Taguchi T, Tamaki Y, Matsuzawa Y, Noguchi S. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res. 2003;9:5699–5704. [PubMed] [Google Scholar]
- 32.Steeg PS, Theodorescu D. Metastasis: a therapeutic target for cancer. Nat Clin Pract Oncol. 2008;5:206–219. doi: 10.1038/ncponc1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahima RS, Osei SY. Leptin signaling. Physiol Behav. 2004;81:223–241. doi: 10.1016/j.physbeh.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 34.Bjorbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem. 1999;274:30059–30065. doi: 10.1074/jbc.274.42.30059. [DOI] [PubMed] [Google Scholar]
- 35.Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 36.Calle EE, Thun MJ. Obesity and cancer. Oncogene. 2004;23:6365–6378. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- 37.Moller H, Mellemgaard A, Lindvig K, Olsen JH. Obesity and cancer risk: a Danish record-linkage study. Eur J Cancer. 1994;30A:344–350. doi: 10.1016/0959-8049(94)90254-2. [DOI] [PubMed] [Google Scholar]
- 38.Nair S, Mason A, Eason J, Loss G, Perrillo RP. Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis? Hepatology. 2002;36:150–155. doi: 10.1053/jhep.2002.33713. [DOI] [PubMed] [Google Scholar]
- 39.Gertler A. Development of leptin antagonists and their potential use in experimental biology and medicine. Trends Endocrinol Metab. 2006;17:372–378. doi: 10.1016/j.tem.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–2468. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 41.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.