Abstract
Recent evidence suggests that the major pathways mediating cell cholesterol homeostasis respond to a common signal: active membrane cholesterol. Active cholesterol is that fraction which exceeds the complexing capacity of the polar bilayer lipids. Increments in plasma membrane cholesterol exceeding this threshold have an elevated chemical activity (escape tendency) and redistribute via diverse transport proteins to both circulating plasma lipoproteins and intracellular organelles. Active cholesterol prompts several feedback responses thereby. It is the substrate for its own esterification and for the synthesis of regulatory side-chain oxysterols. It also stimulates manifold pathways that down-regulate the biosynthesis, curtail the ingestion and increase the export of cholesterol. Thus, the abundance of cholesterol is tightly coupled to that of its polar lipid partners through active cholesterol.
How is Cell Cholesterol Sensed and Set?
Sterols such as cholesterol in animals and ergosterol in fungi are essential and tightly regulated membrane bilayer constituents (Box 1). For example, the cholesterol level in human fibroblasts remains quite constant during months of culture [1]. Several pathways that regulate cell cholesterol have been explicated in depth [2-4]. Nevertheless, how cells specify the amount of cholesterol they require and how they sense transient changes in plasma membrane cholesterol levels so as to make homeostatic adjustments has only recently come into view. It appears that plasma membrane sterols form complexes with polar bilayer lipids, mostly phospholipids, with diverse affinities. It is the cholesterol-complexing capacity of these polar lipids that defines the sterol requirement of the plasma membrane. Cholesterol molecules in excess of the threshold complexing capacity of the lipids are “active” and move to intracellular membranes, prompting resident effectors to restore plasma membrane cholesterol to its resting level.
Box 1. The Disposition of Cellular Cholesterol.
Cells increase their cholesterol levels, as needed, through biosynthesis, hydrolysis of cholesterol ester stores and receptor-mediated endocytosis of sterol-rich LDL [2]. Cellular cholesterol is not generally broken down but, rather, retained in cholesterol ester droplets, exported to plasma lipoproteins and, as appropriate, converted into steroids, oxysterols, bile acids, vitamin D3 and other functionally-important derivatives.
The cholesterol content of the cellular organelles varies widely [5]. This seems to reflect, to an unknown degree, its passive equilibration among bilayers of differing lipid composition. In particular, plasma membranes, rich in saturated sphingolipids and phosphatidylcholine, typically contain ∼0.8 mol cholesterol/mol phospholipids [1]. In contrast, the ER is deficient in those sterol-avid phospholipids [5] and may contain only ∼0.05 mol cholesterol/mol phospholipid [24, 40]. This 16-fold differential scales with the relative cholesterol affinities of the respective membrane phospholipids [6, 8]. The sterol content of mitochondria resembles that of the ER, while endocytic compartments, the trans-Golgi network and the Golgi apparatus fall between these extremes [5, 12].
Sterol molecules equilibrate across natural and synthetic bilayers on a sub-second timescale [1, 9, 14, 85]. Whereas the exofacial monolayer of the plasma membrane is rich in sterol-avid lipids and the phospholipids of the endofacial leaflet resemble those of cytoplasmic organelles [88], a recent report surprisingly assigned most of the cholesterol in the plasma membrane to its cytoplasmic leaflet [89]. This finding reminds us that, in addition to passive lipid partition, the apportionment of sterols within and among cellular membranes could be influenced by its association with proteins as well as by metabolic processes. Just as phospholipids are actively transported across plasma membrane bilayers [90], so is it conceivable that cholesterol could be pumped against its chemical gradient, even in the face of its rapid flip-flop. In addition, ingested cell cholesterol might be actively removed as it traverses the endocytic pathway to lysosomes [60]. Moreover, the delivery of sterols to the matrix-side of the inner mitochondrial membrane is mediated by an elaborate protein pathway [12, 52-54].
This review will consider evidence bearing on how active cholesterol can be recognized and gauged experimentally, how it is transported intracellularly, how it is exported and how it elicits feedback responses that mediate cholesterol homeostasis.
Active membrane cholesterol
Sterols normally intercalate into membranes with their 3-hydroxyl groups close to the heads of the polar bilayer lipids at the aqueous interface and their flat, rigid hydrocarbon rings and mobile tails aligned more-or-less parallel to the hydrocarbon chains that surround them [5]. Different phospholipids form sterol complexes of varied affinity and proportions [1, 6-9]. The strongest of these sterol interactions is with sphingolipids (glycosphingolipids and sphingomyelins), phosphatidylcholines and phosphatidylserines bearing long, saturated alkyl tails. Less favored partners include phosphatidylethanolamines and other phospholipids bearing small polar head groups and short and/or (poly) cis-unsaturated fatty acyl tails. In synthetic membrane systems, these assemblies are short-lived, have overall phospholipid:cholesterol compositions in the range of 1:1 to 2:1 and apparent dimensions of <20 nm [7, 9-11]. (These molar ratios might signify binding stoichiometries; we shall refer to them as equivalence points.) The association of sterols with phospholipids appears to be driven both by enthalpy (mostly favorable free energy changes derived from van der Waals contacts and hydrogen bonds between the sterol hydroxyl group and polar lipid head groups) and entropy (especially, favorable solvent free energy changes arising from the ability of large phospholipid head groups to shield the nonpolar aspects of the sterol molecules from contact with the aqueous phase) [1, 2, 5-7, 12]. Not surprisingly, the lipids most prone to form complexes are those that typically segregate into liquid ordered domains or rafts [6, 8, 13].
Sterol molecules exceeding the threshold complexing capacity of the polar membrane lipids remain dispersed in the bilayer, but in a novel state characterized by an increased escape tendency, chemical activity or fugacity [7, 10, 12]. We refer to these uncomplexed cholesterol molecules as active cholesterol [1]. Active cholesterol has enhanced collisional interactions with soluble reactants [1, 14] which might reflect an increase in the frequency and/or extent of its transient orthogonal projection (bobbing) from the membrane. Early evidence for this activity came from the demonstration that the rate constant for the transfer of cholesterol from mixed phospholipid monolayers to aqueous β-cyclodextrin rises markedly in proportion to the fraction of the sterol exceeding the threshold complexing capacity of the phospholipids [7, 10]. Similarly, cholesterol transfer from red blood cells to β-cyclodextrin is greatly stimulated by increasing their membrane cholesterol [15]. Furthermore, susceptibility to cholesterol oxidase grows dramatically when the cholesterol in synthetic bilayers is incremented above equivalence with phospholipids; that is, above their capacity to form complexes. Likewise, the susceptibility of plasma membranes to this oxidase rises sharply when their cholesterol exceeds its resting level, which we take to reflect its complexing capacity [1, 15, 16]. In addition, hydrolyzing a portion of plasma membrane sphingomyelin boosts cholesterol reactivity, as if freeing the sterol from its complexes [1, 12]. Scrambling the bilayer phospholipids in red blood cells so as to increase the abundance in their outer leaflets of phospholipid species with low cholesterol affinity also increases the reactivity of this sterol [17].
Along these lines, association of the perfringolysin O hemolysin with sterols in synthetic lipid vesicles rises as their cholesterol content is increased. Importantly, binding commences at a threshold determined by the phospholipid composition of the vesicles [18]. Furthermore, a wide variety of membrane-intercalating amphipaths (e.g., oxysterols, long-chain alcohols and fatty acids) increase the reactivity of synthetic membrane and plasma membrane cholesterol with cholesterol oxidase and β-cyclodextrin; apparently, they competitively displace the sterol from complexes with polar lipid partners [15, 18-21]. That these diverse agents associate with phospholipids in a manner resembling, to some extent, that of sterols is consistent with their ability to protect red blood cells against destabilization induced by sterol extraction [20, 22]. Conversely, introducing intercalating amphipaths bearing saturated alkyl chains and large polar head groups (e.g., sphingomyelin, lysophosphatides or hexadecylphosphocholine) reduces membrane sterol reactivity [15, 18-20, 23] and, as discussed below, affects diverse homeostatic processes as if associating with membrane cholesterol so as to reduce its activity.
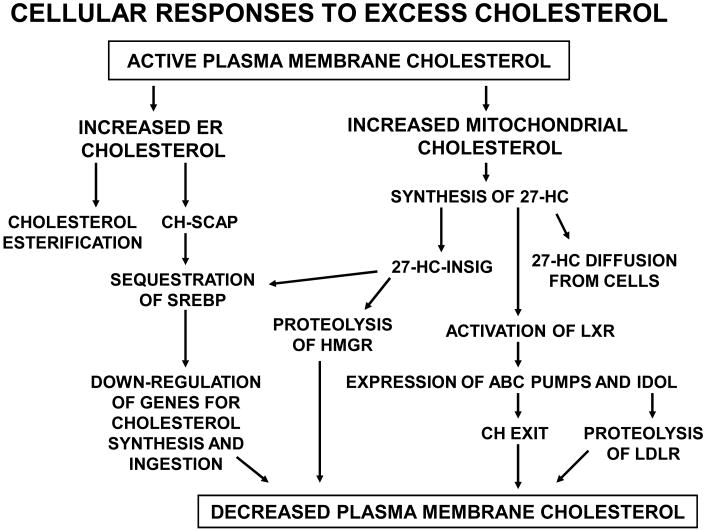
The threshold for the increased reactivity of plasma membrane cholesterol, determined experimentally using cyclodextrin and cholesterol oxidase, coincides with the cell's resting (i.e., physiologically set) cholesterol level [1]. It is a central tenet of this review that cells normally maintain cholesterol levels in their plasma membranes (and possibly their intracellular membranes as well) close to equivalence with the complexing capacity of the membrane polar lipids. When membrane cholesterol rises beyond this threshold value, the excess that forms is active and mobile. A proportional fraction equilibrates passively down its activity gradient (i.e., it redistributes without the input of metabolic energy) both to extracellular lipoproteins and to intracellular membranes. A variety of sterol-sensitive proteins in the organelles sense this increment as a signal to reduce the cellular excess through homeostatic responses until the active fraction is nulled (Figure 1) [1, 10]. Accordingly, homeostatic effectors do not act in direct proportion to total cell cholesterol but rather respond to increments above equivalence (i.e., saturation of lipid partners).
Figure 1.
Homeostatic responses to increased plasma membrane cholesterol are mediated by active cholesterol. Plasma membrane bilayer cholesterol in excess of the complexing capacity of membrane polar lipids is active. Active plasma membrane cholesterol equilibrates with intracellular membranes, increasing their cholesterol content. This triggers multiple feedback responses. High levels of ER cholesterol become esterified and also inhibit the SREBP pathway, thereby down-regulating cholesterol accretion. In parallel, increased mitochondrial cholesterol stimulates 27-HC biosynthesis which triggers multiple feedback responses. While all of the cell's diverse polar lipid species complex cholesterol with different affinities, the plasma membrane is pictured as central here because of its high level of sterol-avid lipids, its consequent high sterol content and its sharp threshold for cholesterol activation (Figure 2). The plasma membrane is also the cellular compartment most easily manipulated experimentally. Abbreviation: CH, cholesterol.
Several homeostatic systems appear to be directly or indirectly regulated by active cholesterol (Figure 2). But how can we be sure that it is active cholesterol and not some other cue that elicits these responses? Several experimental criteria can be used to address this question (Box 2). The following passages summarize the evidence for an active cholesterol feedback mechanism.
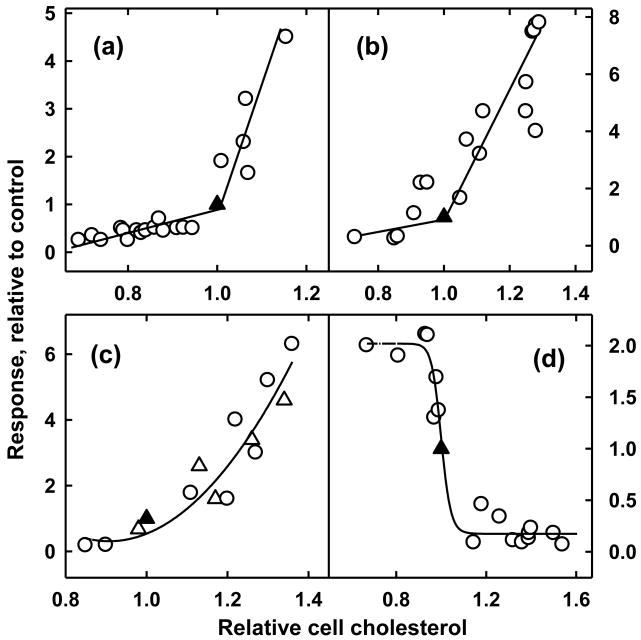
Figure 2.
Rapid homeostatic responses to alterations in plasma membrane cholesterol. Plotted are the responses of human fibroblasts over 1-4 hours to modifications of their cholesterol with hydroxypropyl-β-cyclodextrin ± cholesterol. Values are scaled to the corresponding untreated controls (▲), set at 1.0/1.0. (a) Response of the size of the ER cholesterol pool [25]. (b) Response of the rate of esterification of plasma membrane [3H]cholesterol in the ER [66]. (c) Response of mitochondrial biosynthesis of 27-HC in wild-type (○) and NPC1-deficient fibroblasts (△) [19]. (d) Inactivation of ER HMGR activity [15]. In each case, increments in plasma membrane cholesterol above resting levels elicit sharp homeostatic responses that serve to return cells to resting levels. Figures reproduced with minor modifications by permission.
Box 2. Indicators of Active Plasma Membrane Cholesterol.
The rate and extent of transfer of plasma membrane cholesterol to cyclodextrin increases sharply at a threshold cholesterol concentration near the membrane's physiological rest point.
The susceptibility of plasma membrane cholesterol to cholesterol oxidase increases sharply at a threshold cholesterol concentration near the membrane's physiological rest point.
Homeostatic responses have sharp thresholds near the physiological rest point of plasma membrane cholesterol (Figure 2).
Agents that free cholesterol from lipid complexes (e.g., sphingomyelinase) promote cholesterol transfers, cholesterol oxidase susceptibility and homeostatic responses.
A variety of intercalating amphipaths that appear to displace cholesterol from phospholipids (e.g., octanol and 25-hydroxycholesterol) promote cholesterol transfer, cholesterol oxidase susceptibility and homeostatic responses.
Intercalators that appear to complex sterols (e.g., lysophosphatides) inhibit cholesterol transfer, cholesterol oxidase susceptibility and homeostatic responses.
Endoplasmic Reticulum (ER) Responses
Cholesterol normally contributes only about 5 moles per 100 moles of phospholipid in the ER membrane of resting Chinese Hamster Ovary (CHO) cells [24] and less than 1 percent of the total cholesterol in human fibroblasts [25]. Fibroblast ER cholesterol rises sharply as plasma membrane cholesterol is increased modestly above a threshold that is situated at the cell's physiological set point (Figure 2a). As predicted from the preceding discussion, treating the cell surface with sphingomyelinase C, 25-hydroxycholesterol or 1-octanol increases the size of the ER cholesterol pool, while treatments with lysophosphatidylcholine or cholesterol oxidase reduce it [9, 15, 25, 26]. While the dose-response relationship seen between the plasma membrane and ER (Figure 2a) could reflect a more complex and uncharacterized management regime, it is parsimonious to consider that the size of the ER pool is set simply by the passive equilibration of active plasma membrane cholesterol with the ER membrane [1, 10].
In the ER, excess cell cholesterol is conjugated with fatty acids by acyl-CoA cholesterol acyl transferase (ACAT) and stored in lipid droplets. This esterification reaction is controlled by the local membrane cholesterol level [27]. The rate of cholesterol esterification increases markedly in response to modest increments of plasma membrane cholesterol above the resting level set by the cell (Figure 2b). The sterol substrate comes mostly from the plasma membrane, apparently through a brisk circulation between these compartments [25]. Esterification is also stimulated by exposing intact cells to sphingomyelinase C, a treatment that liberates, and therefore activates, a portion of the complexed plasma membrane sterol [9, 28]. Furthermore, the esterification reaction is made acutely dependent on ER cholesterol concentration by the cooperativity of ACAT [27].
Mitochondrial Oxysterol Responses
Mitochondria convert cholesterol to steroids, bile acids and oxysterols [29] and, in addition to the ER, are principal targets for intracellular homeostatic signaling by active cholesterol (Figure 1). The 27-hydroxycholesterol (27-HC) synthesized by fibroblast mitochondria is mostly derived from cell surface cholesterol, and its production is accelerated within minutes following a modest jump in plasma membrane cholesterol [19]. In one study, synthesis increased ∼30-fold when fibroblast plasma membrane cholesterol was elevated ∼60% above its resting level (Figure 2c). Further evidence that active cholesterol was the substrate for this reaction was the observation that 1-octanol stimulated, and lysophosphatidylserine inhibited, the short-term biosynthesis of 27-HC [19].
Side-chain oxysterols like 27-HC are more water-soluble (hence, more mobile) than cholesterol and, as derivatives of active cholesterol, are used by cells in several ways to feed back negatively on cholesterol accretion (Figure 1 and refs. [30,31]). Oxysterols rapidly exit cells [31, 32]; this is a particularly important pathway by which cells in the central nervous system and macrophages off-load excess sterol [33]. Upon reaching the liver, these oxysterols are converted to bile acids and excreted. In addition, side-chain oxysterols can bind to the integral ER protein, Insig, which then promotes the inactivation of 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGR) and, hence, the sequestration of sterol regulatory element binding proteins (SREBP). Side-chain oxysterols also stimulate the expression of several homeostatic proteins, such as ATP binding cassette (ABC) cholesterol pumps. Lastly, it is possible that side-chain oxysterols affect integral regulatory proteins such as Insig by perturbing bilayer organization, their specific interactions with proteins notwithstanding [34].
HMGR Responses
HMGR, a resident ER enzyme important in controlling the rate of sterol biosynthesis, is elaborately regulated by multiple feedback mechanisms. Most rapid among these homeostatic responses is its proteolytic degradation. At least in human fibroblasts, a small fraction of excess (hence, active) cholesterol must first be converted to 27-HC to trigger this proteolysis [19, 35]. The oxysterol then associates with the resident ER protein, Insig [36] which, in turn, binds to and marks HMGR for ubiquitination and subsequent proteasomal digestion [4, 37]. Consequently, the inactivation of HMGR responds sharply to small increments in the level of plasma membrane cholesterol at its rest point (Figure 2d). Supporting an active cholesterol signaling mechanism, the loss of HMGR is rapidly stimulated by amphipaths [20, 22] and countered by lysophosphatidylserine [19]. That the dose-response curve for HMGR inactivation (Figure 2d) is sharper than that for the production of 27-HC (Figure 2c) suggests an additional high-order step, possibly the multimerization of oxysterol–Insig [36, 38].
SREBP Responses
SREBP activates genes for cholesterol accretion such as those encoding low-density lipoprotein (LDL) receptors and enzymes of the sterol biosynthetic pathway [4]. This transcription factor is held inactive in the ER by its “escort”, Scap (SREBP cleavage activating protein), when Scap is complexed with cholesterol [24, 39]. Retention of SREBP in the ER is strengthened by the association of Scap-cholesterol with Insig-oxysterol complexes [36]. When cellular sterol levels fall, SREBP-Scap is transferred from the ER to the Golgi apparatus where its active domain is proteolytically liberated for transit to the nucleus [4].
The sequestration of inactive SREBP in the ER is fine-tuned by no less than four cholesterol-dependent processes. These are: i) ER cholesterol increases abruptly in proportion to the level of plasma membrane cholesterol above its baseline (Figure 2a); ii) Scap is acutely activated by ER cholesterol above its resting level [24,40]; iii) the activation of the Scap tetramer appears to be made more acute by the positive cooperativity of its sterol binding [24,40]; and iv) 27-HC production in response to active cholesterol [19] activates Insig and, hence, leads to the retention of inactive SREBP in the ER [4,36,38]. The protection of Insig from rapid proteasomal destruction might provide yet another mode of SREBP regulation in response to active cholesterol [41].
Liver X receptor (LXR) Responses
Side-chain oxysterol derivatives of cholesterol, synthesized in proportion to active cholesterol levels [19], also regulate the abundance of cellular sterols though the activation of LXRs [42]. These transcription factors promote the expression of members of the ATP-binding cassette (ABC) transporter super-family which expel cholesterol from cells. Oxysterol-activated LXRs also stimulate the production of the inducible degrader of the LDL receptor (IDOL). This protein curtails the endocytosis of LDL, thereby reducing the uptake of exogenous cholesterol [43].
Transfer of Membrane Cholesterol
Active cholesterol must be conveyed to the ER and mitochondria to elicit the regulatory responses just described (Figure 1). However, despite recent progress outlined below, the path taken by cholesterol between any two membranes in the cell is not yet clear. While cholesterol is carried in the bilayers of cytoplasmic vesicles [2,44,45], these are not generally considered to be the principal vehicles for its transport. Nor do sterol molecules appear to simply desorb from membranes and diffuse through the cytosol; such spontaneous movements would takes hours and would not be acceptor-specific [45,46]. However, sterol molecules can be rapidly transferred through the collision of donors such as the plasma membrane with acceptors [1,14]. Such a mechanism might underlie the shuttling of sterol molecules between intracellular compartments by diverse carrier proteins that function on a time scale of a few minutes without the input of metabolic energy [1,2,12,19,25,47-49]. The active (transiently-projecting) fraction of the sterol in donor membranes could be the preferred substrate for these transfer reactions.
Soluble cytoplasmic carrier proteins
An early candidate for a sterol shuttle was the sterol carrier protein-2, SCP2, however, it now appears that this protein serves to facilitate lipid transfers in peroxisomes [12, 45, 50]. Similarly, the significance of the cytoplasmic circuit of caveolin, an integral, cholesterol-bearing plasma membrane protein, has not been thoroughly clarified [45, 51], although its transit via endocytic caveolae to cholesterol-rich lipid droplets has been well characterized [51]. The steroidogenic acute regulatory protein, StAR, was also originally thought to shuttle cholesterol through the cytosol. It now seems instead that homologs of StAR, called START or STARD proteins, are in fact cytosolic cholesterol carriers. One of their functions is to deliver cholesterol to the outer mitochondrial membrane. There, the StAR protein initiates sterol transfer to the matrix side of the inner membrane through an elaborate protein complex located at intermembrane contact sites [12, 52-54].
Direct intermembrane sterol transfer
There is growing evidence that proteins imbedded in the contact sites between apposed organelle membranes can directly mediate intermembrane sterol transfers [2, 12, 45, 55-58]. Cholesterol-binding members of the family of oxysterol binding protein (OSBP)-related proteins (ORPs and, in yeast, Oshs) are leading candidates for such a role [57, 59].
Exit of cholesterol from the endocytic pathway
Cholesterol ingested as LDL, as well as that internalized in the plasma membrane-derived envelopes of endocytic vesicles, accumulates in the tubular and vesicular inclusions characteristic of late endosomes and multivesicular bodies [2, 60]. Two proteins that mobilize this sterol are NPC1 and NPC2, named for Niemann-Pick type C disease in which massive amounts of cholesterol and other membrane lipids accumulate in late endocytic compartments. NPC1 is a sterol-binding protein integral to the outer membrane of late endosomes and lysosomes [61-64]. The molecular function of NPC1 remains elusive [61]. However, a clue comes from its homolog, the Niemann-Pick C1 like protein, NPC1L1, which facilitates the transfer of extracellular (apical) cholesterol to the cytoplasm of intestinal and liver cells [65]. In contrast to NPC1, NPC2 is a water-soluble cholesterol-binding protein well suited to shuttling cholesterol from the internal vesicular membranes of late endosomes to the NPC1 in their boundary membranes [61-64].
Cholesterol still exits from NPC1-deficient, lipid-laden digestive vacuoles, driven by the large standing pool therein; however, the timescale is a few hours rather than minutes [66, 67]. A second transport pathway may operate in parallel with NPC1/NPC2, possibly utilizing a START protein [58]. Cholesterol can also be liberated from the membranous inclusions that accumulate in NPC disease by feeding cells the soluble sterol carrier, β-cyclodextrin [68, 69]. Sequestered cholesterol has also been mobilized by providing NPC1-deficient cells with exogenous acid sphingomyelinase [70]. This in vitro “enzyme therapy” might activate the cholesterol trapped in phospholipid complexes within the membranous inclusions, much like the action of sphingomyelinase on plasma membranes.
Reverse cholesterol transport
The circulation of plasma lipoproteins enables the cells of the body to share their cholesterol on a time scale of several hours [71]. A significant fraction of the cholesterol that exits cells utilizes the simplest of homeostatic mechanisms: equilibration driven by the difference in chemical activity of the active cholesterol in the plasma membrane and circulating lipoproteins. This passive efflux utilizes both uncatalyzed and facilitated pathways; an example of the latter is that mediated by the plasma membrane scavenger receptor BI, SR-BI [45, 46].
Cholesterol is also pumped from the cell to diverse acceptors by members of the large family of plasma membrane ABC proteins [72]. ABCA1 appears to transfer both cholesterol and phospholipids to lipid-poor plasma apolipoprotein A-1, thereby initiating the assembly of mature high-density lipoprotein particles [73]. ABCG1 and ABCG4 then facilitate the transfer of additional cholesterol to these nascent high-density lipoproteins [74, 75]. ABCG1 and ABCG4 can also export plasma membrane cholesterol non-specifically to a variety of other acceptors. Similarly, ABCG5–ABCG8 heterodimers transfer cholesterol from the apical brush borders of hepatocytes and enterocytes to micellar carriers in the bile and intestinal lumen. This activity rids the body of excess cholesterol as well as the deleterious plant sterol, sitosterol [76, 77].
The molecular mechanisms by which these pump proteins drive the transfer of cholesterol to extracellular acceptors is not clear. It seems likely that, rather than mediating cholesterol export directly, at least some of these pumps simply increase the accessibility of cholesterol at the cell surface for subsequent non-specific collisional transfers [78, 79], perhaps by activating it [1, 45]. This supposition is supported by the lack of acceptor specificity of some ABC-catalyzed transport reactions, at least in vitro. Such a mechanism could also explain why the action of ABCG1 qualitatively resembles unmediated efflux in many ways [75]. It is relevant in this regard that SR-BI, ABCA1 and ABCG1 make plasma membrane cholesterol more susceptible to cholesterol oxidase attack and to cyclodextrin extraction [77, 80]. Furthermore, it has been suggested that ABCG5–ABCG8 drives the partial projection of cholesterol molecules from the outer leaflet of brush border bilayers so as to favor their collisional capture by lipid micelles in the bile [81]. Finally, as predicted for an active cholesterol transport mechanism, the efficiency of plasma membrane cholesterol export by ABCA1 is stimulated by the addition of ceramides, which displace sterols from phospholipid complexes [20, 22], and is inhibited by sphingomyelin, which complexes with sterols [1, 6, 8, 9].
Other ABC transporters promote the excretion of noxious intercalators by pumping them from the cytoplasmic to the outer leaflet of the plasma membrane bilayer [72, 78, 82]. A similar mechanism has been proposed for the export of cholesterol [83, 84]. It is unlikely that ABC proteins act by facilitating the transbilayer equilibration of sterols, since sterol flip-flop is naturally quite rapid [14, 85]. However, active transfer by ABC pumps could expand the fraction of plasma membrane cholesterol in the outer leaflet. The heightened activity of this excess surface cholesterol could then drive its downhill (i.e., unenergized) movement to extracellular acceptors. Such an ATP-dependent sterol-activation mechanism might also be used by yeast in which ABC pump proteins stimulate the transfer of sterols from the plasma membrane to the ER [86].
Concluding remarks
The active cholesterol hypothesis is new, and several of its ramifications require further investigation (Box 3). Nevertheless, it appears that cells set their cholesterol at equivalence with the complexing capacity of their polar membrane lipids. Because cholesterol molecules that exceed this threshold have enhanced chemical activity or fugacity, they move to and inform homeostatic pathways based in the ER and mitochondria. Multiple feedback responses then restore lipid balance by adjusting the rates of cholesterol esterification, biosynthesis, ingestion and export (Figure 1). The biosynthesis of phospholipids similarly rises and falls in response to the level of cell cholesterol [9, 87], perhaps helping to null active cholesterol.
Box 3. Outstanding questions.
What is the sterol complexation capacity of plasma membranes and intracellular membranes?
What are the physical characteristics of active cholesterol?
To what degree is the distribution of cholesterol within the cell determined by diffusional equilibrium?
What are the cholesterol thresholds of the various cellular membranes?
Are there cholesterol thresholds for steroid or bile acid biosynthesis or for LXR-dependent gene expression?
What are the pathways and mechanisms of intracellular cholesterol transport and what are the precise functions of specific transfer proteins?
Is active cholesterol the true substrate for intermembrane transport?
Is active cholesterol the true substrate for export from the plasma membrane?
Does the export of endocytic cholesterol from late endosomal compartments require its activation?
The expression of SREBP and LXR proteins constitutes a powerful means for controlling cell cholesterol abundance [3, 4, 80, 87]. However, the regulation of transcription for homeostasis must respond to upstream information. The evidence discussed above suggests that active cholesterol can provide such a signal. The direct control of homeostatic effector activity by active cholesterol will also be more rapid and sensitive than transcriptional pathways typically allow.
Acknowledgments
We thank Will Prinz, Arun Radhakrishnan and Donald Small for their valuable comments on this manuscript. This work was supported by National Institutes of Health grant HL 28448.
Footnotes
Disclosure statement. The authors have no actual or potential conflicts of interest regarding this publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lange Y, Steck TL. Cholesterol homeostasis and the escape tendency (activity) of plasma membrane cholesterol. Prog Lipid Res. 2008;47:319–332. doi: 10.1016/j.plipres.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol. 2008;9:125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- 3.Chang TY, et al. Cholesterol Sensing, Trafficking, and Esterification. Annu Rev Cell Dev Biol. 2006;22:129–157. doi: 10.1146/annurev.cellbio.22.010305.104656. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein JL, et al. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 5.van Meer G, et al. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almeida PF. Thermodynamics of lipid interactions in complex bilayers. Biochim Biophys Acta. 2009;1788:72–85. doi: 10.1016/j.bbamem.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 7.McConnell HM, Radhakrishnan A. Condensed complexes of cholesterol and phospholipids. Biochim Biophys Acta. 2003;1610:159–173. doi: 10.1016/s0005-2736(03)00015-4. [DOI] [PubMed] [Google Scholar]
- 8.Niu SL, Litman BJ. Determination of membrane cholesterol partition coefficient using a lipid vesicle-cyclodextrin binary system: effect of phospholipid acyl chain unsaturation and headgroup composition. Biophys J. 2002;83:3408–3415. doi: 10.1016/S0006-3495(02)75340-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohvo-Rekila H, et al. Cholesterol interactions with phospholipids in membranes. Prog Lipid Res. 2002;41:66–97. doi: 10.1016/s0163-7827(01)00020-0. [DOI] [PubMed] [Google Scholar]
- 10.Radhakrishnan A, McConnell HM. Chemical activity of cholesterol in membranes. Biochemistry. 2000;39:8119–8124. doi: 10.1021/bi0005097. [DOI] [PubMed] [Google Scholar]
- 11.Sahl SJ, et al. Fast molecular tracking maps nanoscale dynamics of plasma membrane lipids. Proc Natl Acad Sci U S A. 2010;107:6829–6834. doi: 10.1073/pnas.0912894107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mesmin B, Maxfield FR. Intracellular sterol dynamics. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2009;1791:636–645. doi: 10.1016/j.bbalip.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hancock JF. Lipid rafts: contentious only from simplistic standpoints. Nat Rev Mol Cell Biol. 2006;7:456–462. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steck TL, et al. Probing red cell membrane cholesterol movement with cyclodextrin. Biophys J. 2002;83:2118–2125. doi: 10.1016/S0006-3495(02)73972-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lange Y, et al. How cholesterol homeostasis is regulated by plasma membrane cholesterol in excess of phospholipids. Proc Natl Acad Sci U S A. 2004;101:11664–11667. doi: 10.1073/pnas.0404766101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahn KW, Sampson NS. Cholesterol oxidase senses subtle changes in lipid bilayer structure. Biochemistry. 2004;43:827–836. doi: 10.1021/bi035697q. [DOI] [PubMed] [Google Scholar]
- 17.Lange Y, et al. Scrambling of phospholipids activates red cell membrane cholesterol. Biochemistry. 2007;46:2233–2238. doi: 10.1021/bi6023397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flanagan JJ, et al. Cholesterol Exposure at the Membrane Surface Is Necessary and Sufficient to Trigger Perfringolysin O Binding. Biochemistry. 2009;48:3977–3987. doi: 10.1021/bi9002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lange Y, et al. Regulation of fibroblast mitochondrial 27-hydroxycholesterol production by active plasma membrane cholesterol. J Lipid Res. 2009;50:1881–1888. doi: 10.1194/jlr.M900116-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lange Y, et al. Activation of membrane cholesterol by displacement from phospholipids. J Biol Chem. 2005;280:36126–36131. doi: 10.1074/jbc.M507149200. [DOI] [PubMed] [Google Scholar]
- 21.Ratajczak MK, et al. Cholesterol Displacement from Membrane Phospholipids by Hexadecanol. Biophys J. 2007;93:2038–2047. doi: 10.1529/biophysj.107.109553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lange Y, et al. Activation of membrane cholesterol by 63 amphipaths. Biochemistry. 2009;48:8505–8515. doi: 10.1021/bi900951r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marco C, et al. Hexadecylphosphocholine alters nonvesicular cholesterol traffic from the plasma membrane to the endoplasmic reticulum and inhibits the synthesis of sphingomyelin in HepG2 cells. The International Journal of Biochemistry & Cell Biology. 2009;41:1296–1303. doi: 10.1016/j.biocel.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Radhakrishnan A, et al. Switch-like Control of SREBP-2 Transport Triggered by Small Changes in ER Cholesterol: A Delicate Balance. Cell Metabolism. 2008;8:512–521. doi: 10.1016/j.cmet.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lange Y, et al. Regulation of endoplasmic reticulum cholesterol by plasma membrane cholesterol. J Lipid Res. 1999;40:2264–2270. [PubMed] [Google Scholar]
- 26.Lange Y, Steck TL. Quantitation of the pool of cholesterol associated with acyl-CoA:cholesterol acyltransferase in human fibroblasts. J Biol Chem. 1997;272:13103–13108. doi: 10.1074/jbc.272.20.13103. [DOI] [PubMed] [Google Scholar]
- 27.Chang TY, et al. Acyl-coenzyme A:cholesterol acyltransferases. Am J Physiol Endocrinol Metab. 2009;297:E1–9. doi: 10.1152/ajpendo.90926.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Subbaiah PV, et al. Sphingomyelinase D, a novel probe for cellular sphingomyelin: effects on cholesterol homeostasis in human skin fibroblasts. J Lipid Res. 2003;44:1574–1580. doi: 10.1194/jlr.M300103-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 30.Gill S, et al. Sterol regulators of cholesterol homeostasis and beyond: The oxysterol hypothesis revisited and revised. Progress in Lipid Research. 2008;47:391–404. doi: 10.1016/j.plipres.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Bjorkhem I. Are side-chain oxidized oxysterols regulators also in vivo? J Lipid Res. 2009;50 Suppl:S213–218. doi: 10.1194/jlr.R800025-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lange Y, et al. Movement of 25-hydroxycholesterol from the plasma membrane to the rough endoplasmic reticulum in cultured hepatoma cells. J Lipid Res. 1995;36:1092–1097. [PubMed] [Google Scholar]
- 33.Russell DW, et al. Cholesterol 24-hydroxylase: an enzyme of cholesterol turnover in the brain. Annu Rev Biochem. 2009;78:1017–1040. doi: 10.1146/annurev.biochem.78.072407.103859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gale SE, et al. Side Chain Oxygenated Cholesterol Regulates Cellular Cholesterol Homeostasis through Direct Sterol-Membrane Interactions. J Biol Chem. 2009;284:1755–1764. doi: 10.1074/jbc.M807210200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lange Y, et al. Effectors of Rapid Homeostatic Responses of Endoplasmic Reticulum Cholesterol and 3-Hydroxy-3-methylglutaryl-CoA Reductase. J Biol Chem. 2008;283:1445–1455. doi: 10.1074/jbc.M706967200. [DOI] [PubMed] [Google Scholar]
- 36.Radhakrishnan A, et al. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: Oxysterols block transport by binding to Insig. Proc Natl Acad Sci U S A. 2007;104:6511–6518. doi: 10.1073/pnas.0700899104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeBose-Boyd RA. Feedback regulation of cholesterol synthesis: sterol-accelerated ubiquitination and degradation of HMG CoA reductase. Cell Res. 2008;18:609–621. doi: 10.1038/cr.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee PC, DeBose-Boyd RA. Intramembrane glycine mediates multimerization of Insig-2, a requirement for sterol regulation in Chinese hamster ovary cells. J Lipid Res. 2010;51:192–201. doi: 10.1194/jlr.M900336-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radhakrishnan A, et al. Direct binding of cholesterol to the purified membrane region of SCAP: mechanism for a sterol-sensing domain. Mol Cell. 2004;15:259–268. doi: 10.1016/j.molcel.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 40.Sokolov A, Radhakrishnan A. Accessibility of cholesterol in endoplasmic reticulum (ER) membranes and activation of SREBP-2 switch abruptly at a common cholesterol threshold. J Biol Chem. 2010 doi: 10.1074/jbc.M110.148254. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gong Y, et al. Sterol-regulated ubiquitination and degradation of Insig-1 creates a convergent mechanism for feedback control of cholesterol synthesis and uptake. Cell Metab. 2006;3:15–24. doi: 10.1016/j.cmet.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 42.Edwards PA, et al. LXRs; oxysterol-activated nuclear receptors that regulate genes controlling lipid homeostasis. Vascul Pharmacol. 2002;38:249–256. doi: 10.1016/s1537-1891(02)00175-1. [DOI] [PubMed] [Google Scholar]
- 43.Zelcer N, et al. LXR Regulates Cholesterol Uptake Through Idol-Dependent Ubiquitination of the LDL Receptor. Science. 2009;325:100–104. doi: 10.1126/science.1168974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schnabl M, et al. Multiple lipid transport pathways to the plasma membrane in yeast. Biochim Biophys Acta. 2005;1687:130–140. doi: 10.1016/j.bbalip.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 45.Prinz WA. Non-vesicular sterol transport in cells. Prog Lipid Res. 2007;46:297–314. doi: 10.1016/j.plipres.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yancey PG, et al. Importance of Different Pathways of Cellular Cholesterol Efflux. Arterioscler Thromb Vasc Biol. 2003;23:712–719. doi: 10.1161/01.ATV.0000057572.97137.DD. [DOI] [PubMed] [Google Scholar]
- 47.Hao M, et al. Vesicular and non-vesicular sterol transport in living cells. The endocytic recycling compartment is a major sterol storage organelle. J Biol Chem. 2002;277:609–617. doi: 10.1074/jbc.M108861200. [DOI] [PubMed] [Google Scholar]
- 48.Wustner D, et al. Direct observation of rapid internalization and intracellular transport of sterol by macrophage foam cells. Traffic. 2005;6:396–412. doi: 10.1111/j.1600-0854.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- 49.Baumann NA, et al. Transport of newly synthesized sterol to the sterol-enriched plasma membrane occurs via nonvesicular equilibration. Biochemistry. 2005;44:5816–5826. doi: 10.1021/bi048296z. [DOI] [PubMed] [Google Scholar]
- 50.Seedorf U, et al. Sterol carrier protein-2. Biochim Biophys Acta. 2000;1486:45–54. doi: 10.1016/s1388-1981(00)00047-0. [DOI] [PubMed] [Google Scholar]
- 51.Le Lay S, et al. Filling up adipocytes with lipids. Lessons from caveolin-1 deficiency. Biochim Biophys Acta. 2009;1791:514–518. doi: 10.1016/j.bbalip.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 52.Rone MB, et al. Cholesterol transport in steroid biosynthesis: role of protein-protein interactions and implications in disease states. Biochim Biophys Acta. 2009;1791:646–658. doi: 10.1016/j.bbalip.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller WL. Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2007;1771:663–676. doi: 10.1016/j.bbalip.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 54.Lavigne P, et al. Mammalian StAR-Related Lipid Transfer (START) Domains with Specificity for Cholesterol: Structural Conservation and Mechanism of Reversible Binding. Subcell Biochem. 2010;51:425–437. doi: 10.1007/978-90-481-8622-8_15. [DOI] [PubMed] [Google Scholar]
- 55.Holthuis JCM, Levine TP. Lipid traffic: floppy drives and a superhighway. Nat Rev Mol Cell Biol. 2005;6:209–220. doi: 10.1038/nrm1591. [DOI] [PubMed] [Google Scholar]
- 56.Levine T, Loewen C. Inter-organelle membrane contact sites: through a glass, darkly. Curr Opin Cell Biol. 2006;18:371–378. doi: 10.1016/j.ceb.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 57.Schulz TA, et al. Lipid-regulated sterol transfer between closely apposed membranes by oxysterol-binding protein homologues. J Cell Biol. 2009;187:889–903. doi: 10.1083/jcb.200905007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Charman M, et al. MLN64 mediates egress of cholesterol from endosomes to mitochondria in the absence of functional Niemann-Pick Type C1 protein. J Lipid Res. 2010;51:1023–1034. doi: 10.1194/jlr.M002345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schulz TA, Prinz WA. Sterol transport in yeast and the oxysterol binding protein homologue (OSH) family. Biochim Biophys Acta. 2007;1771:769–780. doi: 10.1016/j.bbalip.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mobius W, et al. Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic. 2003;4:222–231. doi: 10.1034/j.1600-0854.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 61.Lloyd-Evans E, Platt FM. Lipids on trial: the search for the offending metabolite in Niemann-Pick type C disease. Traffic. 2010;11:419–428. doi: 10.1111/j.1600-0854.2010.01032.x. [DOI] [PubMed] [Google Scholar]
- 62.Karten B, et al. Mechanisms and consequences of impaired lipid trafficking in Niemann-Pick type C1-deficient mammalian cells. Biochim Biophys Acta. 2009;1791:659–670. doi: 10.1016/j.bbalip.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 63.Storch J, Xu Z. Niemann-Pick C2 (NPC2) and intracellular cholesterol trafficking. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2009;1791:671–678. doi: 10.1016/j.bbalip.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kwon HJ, et al. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 2009;137:1213–1224. doi: 10.1016/j.cell.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Betters JL, Yu L. NPC1L1 and cholesterol transport. FEBS Lett. 2010;584:2740–2747. doi: 10.1016/j.febslet.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lange Y, et al. Cholesterol movement in Niemann-Pick type C cells and in cells treated with amphiphiles. J Biol Chem. 2000;275:17468–17475. doi: 10.1074/jbc.M000875200. [DOI] [PubMed] [Google Scholar]
- 67.Cruz JC, et al. Role of Niemann-Pick type C1 protein in intracellular trafficking of low density lipoprotein-derived cholesterol. J Biol Chem. 2000;275:4013–4021. doi: 10.1074/jbc.275.6.4013. [DOI] [PubMed] [Google Scholar]
- 68.Liu B, et al. Cyclodextrin overcomes the transport defect in nearly every organ of NPC1 mice leading to excretion of sequestered cholesterol as bile acid. J Lipid Res. 2010;51:933–944. doi: 10.1194/jlr.M000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rosenbaum AI, et al. Endocytosis of beta-cyclodextrins is responsible for cholesterol reduction in Niemann-Pick type C mutant cells. Proceedings of the National Academy of Sciences. 2010;107:5477–5482. doi: 10.1073/pnas.0914309107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Devlin C, et al. Improvement in lipid and protein trafficking in Niemann-Pick C1 cells by correction of a secondary enzyme defect. Traffic. 2010;11:601–615. doi: 10.1111/j.1600-0854.2010.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dietschy JM, Turley SD. Control of cholesterol turnover in the mouse. J Biol Chem. 2002;277:3801–3804. doi: 10.1074/jbc.R100057200. [DOI] [PubMed] [Google Scholar]
- 72.Aye ILMH, et al. Transport of lipids by ABC proteins: Interactions and implications for cellular toxicity, viability and function. Chemico-Biological Interactions. 2009;180:327–339. doi: 10.1016/j.cbi.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 73.Vedhachalam C, et al. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high density lipoprotein particles. J Biol Chem. 2007;282:25123–25130. doi: 10.1074/jbc.M704590200. [DOI] [PubMed] [Google Scholar]
- 74.Tarr PT, et al. Emerging new paradigms for ABCG transporters. Biochim Biophys Acta. 2009;1791:584–593. doi: 10.1016/j.bbalip.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sankaranarayanan S, et al. Effects of acceptor composition and mechanism of ABCG1-mediated cellular free cholesterol efflux. J Lipid Res. 2009;50:275–284. doi: 10.1194/jlr.M800362-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van der Veen JN, et al. Activation of the liver X receptor stimulates trans-intestinal excretion of plasma cholesterol. J Biol Chem. 2009;284:19211–19219. doi: 10.1074/jbc.M109.014860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oram JF, Vaughan AM. ATP-Binding cassette cholesterol transporters and cardiovascular disease. Circ Res. 2006;99:1031–1043. doi: 10.1161/01.RES.0000250171.54048.5c. [DOI] [PubMed] [Google Scholar]
- 78.van Meer G, et al. ABC lipid transporters: Extruders, flippases, or flopless activators? FEBS Letters. 2006;580:1171–1177. doi: 10.1016/j.febslet.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 79.Tall AR, et al. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 2008;7:365–375. doi: 10.1016/j.cmet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 80.Rothblat GH, et al. Cell cholesterol efflux: integration of old and new observations provides new insights. J Lipid Res. 1999;40:781–796. [PubMed] [Google Scholar]
- 81.Small DM. Role of ABC transporters in secretion of cholesterol from liver into bile. Proc Natl Acad Sci U S A. 2003;100:4–6. doi: 10.1073/pnas.0237205100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Katzir H, et al. Role of the plasma membrane leaflets in drug uptake and multidrug resistance. Febs Journal. 2010;277:1234–1244. doi: 10.1111/j.1742-4658.2009.07555.x. [DOI] [PubMed] [Google Scholar]
- 83.Garrigues A, et al. The multidrug transporter, P-glycoprotein, actively mediates cholesterol redistribution in the cell membrane. PNAS. 2002;99:10347–10352. doi: 10.1073/pnas.162366399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kosters A, et al. The mechanism of ABCG5/ABCG8 in biliary cholesterol secretion in mice. J Lipid Res. 2006;47:1959–1966. doi: 10.1194/jlr.M500511-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bruckner RJ, et al. Flip-Flop-Induced Relaxation of Bending Energy: Implications for Membrane Remodeling. Biophysical Journal. 2009;97:3113–3122. doi: 10.1016/j.bpj.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Y, Prinz WA. ATP-binding cassette (ABC) transporters mediate nonvesicular, raft-modulated sterol movement from the plasma membrane to the endoplasmic reticulum. J Biol Chem. 2004;279:45226–45234. doi: 10.1074/jbc.M407600200. [DOI] [PubMed] [Google Scholar]
- 87.Nohturfft A, Zhang SC. Coordination of Lipid Metabolism in Membrane Biogenesis. Annual Review of Cell and Developmental Biology. 2009;25:539–566. doi: 10.1146/annurev.cellbio.24.110707.175344. [DOI] [PubMed] [Google Scholar]
- 88.Quinn PJ. Plasma membrane phospholipid asymmetry. Subcell Biochem. 2002;36:39–60. doi: 10.1007/0-306-47931-1_3. [DOI] [PubMed] [Google Scholar]
- 89.Mondal M, et al. Sterols Are Mainly in the Cytoplasmic Leaflet of the Plasma Membrane and the Endocytic Recycling Compartment in CHO Cells. Mol Biol Cell. 2009;20:581–588. doi: 10.1091/mbc.E08-07-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Devaux PF, et al. Proteins involved in lipid translocation in eukaryotic cells. Chem Phys Lipids. 2006;141:119–132. doi: 10.1016/j.chemphyslip.2006.02.007. [DOI] [PubMed] [Google Scholar]