Abstract
The hypothalamic paraventricular nucleus (PVN) coordinates autonomic and neuroendocrine systems to maintain homeostasis and to respond to stress. Neuroanatomic and neurophysiologic experiments have provided insight into the mechanisms by which the PVN acts. The PVN projects directly to the spinal cord and brainstem and, specifically, to sites that control cardiorespiratory function: the intermediolateral cell columns and phrenic motor nuclei in the spinal cord and rostral ventrolateral medulla (RVLM) and the rostral nuclei in the ventral respiratory column (rVRC) in the brainstem. Activation of the PVN increases ventilation (both tidal volume and frequency) and blood pressure (both heart rate and sympathetic nerve activity). Excitatory and inhibitory neurotransmitters including glutamate and GABA converge in the PVN to influence its neuronal activity. However, a tonic GABAergic input to the PVN directly modulates excitatory outflow from the PVN. Further, even within the PVN, microinjection of GABAA receptor blockers increases glutamate release suggesting an indirect mechanism by which GABA control contributes to PVN functions. PVN activity alters blood pressure and ventilation during various stresses, such as maternal separation, chronic intermittent hypoxia (CIH), dehydration and hemorrhage. Among the several PVN neurotransmitters and neurohormones, vasopressin and oxytocin modulate ventilation and blood pressure. Here, we review our data indicating that increases in vasopressin and vasopressin type 1A (V1A) receptor signaling in the RVLM and rVRC are mechanisms increasing blood pressure and ventilation after exposure to CIH. That blockade of V1A receptors in the medulla normalizes baseline blood pressure as well as blunts PVN-evoked blood pressure and ventilatory responses in CIH-conditioned animals indicate the role of vasopressin in cardiorespiratory control. In summary, morphological and functional studies have found that the PVN integrates sensory input and projects to the sympathetic and respiratory control systems with descending projections to the medulla and spinal cord.
Keywords: Rostral ventrolateral medulla, Intermediolateral cell column of the spinal cord, Ventilatory control, Blood pressure, Sympathetic nerve activity
1. Introduction
The paraventricular nucleus (PVN) of the hypothalamus is a major integrative site for autonomic function in maintaining homeostasis. Neuroanatomic and electrophysiologic data indicate that the PVN is reciprocally connected to areas of the central nervous system (CNS) involved in cardiorespiratory function (Kc et al., 2002, 2010; Luiten et al., 1985; Mack et al., 2002, 2007; Swanson and Kuypers, 1980; Swanson and Sawchenko, 1983; Yeh et al., 1997). The PVN contributes to the maintenance of homeostasis via bidirectional interactions between inhibitory and excitatory connectivity. Imbalance in this counterbalanced system may be pathologic; increasing sympathetic nerve activity (SNA) and ventilation. In this review, we discuss the interaction between excitatory and inhibitory neurotransmission within the PVN as well as the neuronal pathways between PVN and the CNS sites that are involved in regulating sympathetic nerve discharge, vasomotor tone, and the control of breathing. A better understanding of the PVN neuronal connections, neurotransmitters and receptors will provide valuable information regarding the role of the PVN in the exaggerated sympathetic outflow associated with congestive heart failure, hypertension as well as genetic disorders including Prader-Willi syndrome.
2. PVN Connectivity
2.1 Cytoarchitecture of the PVN
The PVN, a hypothalamic structure, is located bilaterally bordering the third ventricle and is composed of magno- and parvo- cellular divisions. The magnocellular division is subdivided into the anterior, medial and posterior regions, all of which are composed predominantly of either oxytocinergic or vasopressinergic cells. The parvocellular division is subdivided into the periventricular, anterior, medial, dorsal and lateral regions. The parvocellular nuclei express more than 30 putative neurotransmitters/neurohormones including: oxytocin, vasopressin, somatostatin, corticotrophin releasing factor, thyrotrophin releasing hormone, and dopamine (Sawchenko and Swanson, 1982b; Swanson and Kuypers, 1980; Swanson and Sawchenko, 1983).
2.2 Efferent Projections from the PVN
The PVN integrates higher-order sensory and central inputs related to autonomic function. The magnocellular neurons project to the posterior pituitary and secrete vasopressin and oxytocin into the blood stream. Parvocellular neurons project to sites within the CNS, including regions that modulate the autonomic system. Specifically, PVN neurons in the dorsal, ventral and lateral parvocellular regions project to the intermediolateral (IML) cell column of the thoraco-lumbar spinal cord and pressor region of the rostral ventrolateral medulla (RVLM). Approximately 30% of spinally-projecting PVN neurons have collateral fibers to the RVLM. Therefore, PVN neurons can directly influence SNA via a PVN-IML pathway, indirectly via a PVN-RVLM pathway and both directly and indirectly via collaterals to the RVLM and IML (Figure 1) (Badoer 2001; Card et al., 2006; Coote et al., 1998; Cruz et al., 2008; Moon et al., 2002; Pyner and Coote, 1999; Ross et al., 1984; Yang and Coote, 1998). In addition to the vasomotor regions, descending projections from the PVN innervate dorsal motor nucleus of the vagus, nTS, nucleus ambiguus, central gray matter, Edinger-Westphal nucleus, pedunculopontine tegmental nucleus, nucleus of the locus coeruleus and parabrachial nucleus (Zheng et al., 1995). Thus, separate groups of cytoarchitecturally distinct neurons in the PVN project to the posterior pituitary, median eminence, and to autonomic control nuclei in the brainstem and spinal cord, and modulate cardio-sympatho, visceral, respiratory, and behavioral responses (Sawchenko 1982; Sawchenko and Swanson 1982a; Swanson and Mogenson 1981).
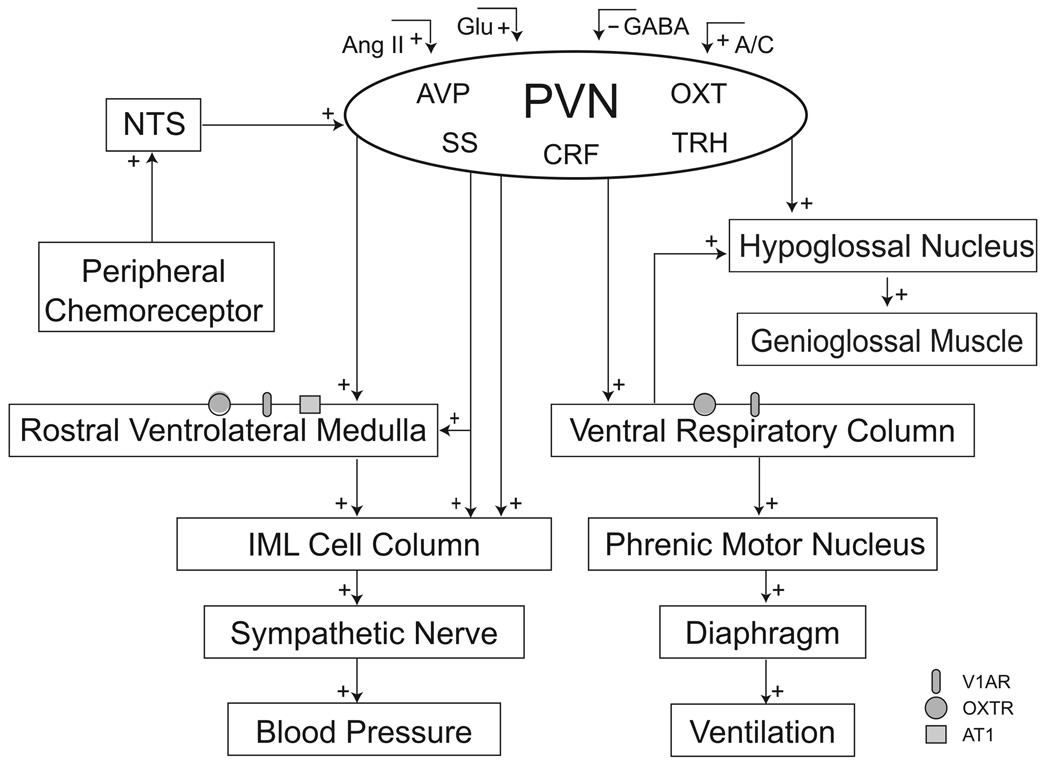
Figure 1.
A schematic diagram showing afferent and efferent neural pathways to and from the PVN. Glutamatergic (Glu), GABAergic, angiotensinergic (Ang II) as well as the noradrenergic (A)/adrenergic (C) afferent innervations modulates the activity of the PVN. PVN neurons can directly influence sympathetic nerve activity via a PVN-IML pathway, indirectly via a PVN-RVLM pathway and both directly and indirectly via collaterals to the RVLM and IML. In addition, PVN neurons project to the rostral ventral respiratory column and hypoglossal nucleus regulating the respiratory drive and genioglossal muscle activity respectively. AVP, vasopressin; OXT, oxytocin; CRF, corticotrophin releasing factor; SS, somatostatin; TRH, thyrotrophin releasing hormone; V1AR, vasopressin type 1A receptor; OXTR, oxytocin receptor; AT1, angiotensin type 1 receptor .
2.3 Afferent Input to the PVN
Subdivisions of PVN nuclei are innervated differentially by adrenergic (C) and noradrenergic (A) fibers (McNeill and Sladek 1980; Swanson et al., 1981). The A1 and C1, the A2 and C2, as well as the A6 cell groups (the locus coeruleus) provide almost all of the noradrenergic and adrenergic innervation to the PVN (Berk and Finkelstein 1981; Palkovits et al., 1980a, b; Sakumoto et al., 1978; Swanson and Hartman 1980; Takagi et al., 1980; Tribollet and Dreifuss 1981). In the magnocellular division of the PVN, noradrenergic fibers terminate only on vasopressin-containing cell bodies, whereas serotonergic and adrenocorticotropic-stained fibers terminate preferentially on oxytocinergic cell bodies. These data indicate differential synaptic control of specific cell types even within the magnocellular subdivision of the PVN.
Angiotensin II acts as a neurotransmitter involved in the regulation of sympathetic activity of the cardiovascular system. Anatomical studies have identified angiotensin type 1 (AT1) receptors in the PVN (Lenkei et al., 1995; Pfister et al., 1997) and electrophysiological studies have demonstrated that angiotensin II influences PVN neurons (Cato and Toney, 2005). Thus, angiotensin II can act in the PVN to regulate SNA and cardiovascular function (Zhu et al., 2004). In addition, circulating levels of angiotensin II, which increase during hemorrhage or dehydration, act centrally to release vasopressin (Malvin et al., 1977; Simonnet et al., 1979; Yamamoto et al., 1978). However, systemic angiotensin II does not appear to have direct access to the PVN as it neither crosses the blood-brain barrier nor enters the third ventricle by way of the choroid plexus (Schelling et al., 1977). Thus, angiotensin II from an unidentified source or transport process modulates activity of PVN neurons via its AT1 receptor.
3. PVN Activity; a Balance of Glutamatergic and GABAergic Systems
Integration between GABAergic and glutamatergic systems determines the sympatho-respiratory-excitatory drive from the PVN. These regions controlling autonomic function express GABAA receptors (Cullinan and Wolfe, 2000; Genest et al., 2007) as well as NMDA and non-NMDA receptors (Herman et al., 2000). Neurons that project from the PVN are quiescent under basal conditions, indicating that GABAergic tone dominates the inhibitory-excitatory balance in the PVN (Bains and Ferguson, 1995; Lovick and Coote, 1988). Furthermore, blocking GABAA receptors with bicuculline, results in activity. However, this activity is blocked by kynurenic acid, a nonselective ionotropic excitatory amino acid (EAA) receptor antagonist (Chen et al., 2003). Thus, disinhibition of PVN neurons evokes activity that requires the activation of EAA receptors. These data indicate that the PVN has: 1) a functionally high level of inhibitory GABAergic tone, 2) tonic excitation from extrinsic synaptic input that is unmasked by GABAA receptor antagonists and 3) neurons with a resting membrane potential below threshold. Indeed, the PVN has a dense concentration of excitatory glutamatergic nerve terminals (Boudaba et al., 1997; Herman et al., 2000; Van den Pol, 1991). Finally, bicuculline infusion into the PVN increases glutamate release, suggesting that GABA inhibits both PVN inter- and projecting-neurons (Li et al., 2006; Figure 2). Given the wealth of inhibitory GABAergic and excitatory glutamatergic inputs to the PVN (Chen et al., 2003; Decavel and Van den Pol, 1990, 1992; Li et al., 2006; Patel et al., 2000; Zhang et al., 1998, 2002), the interactions between these two opposing influences provide an important mechanism to regulate neuronal excitability and determine outflow from the PVN.
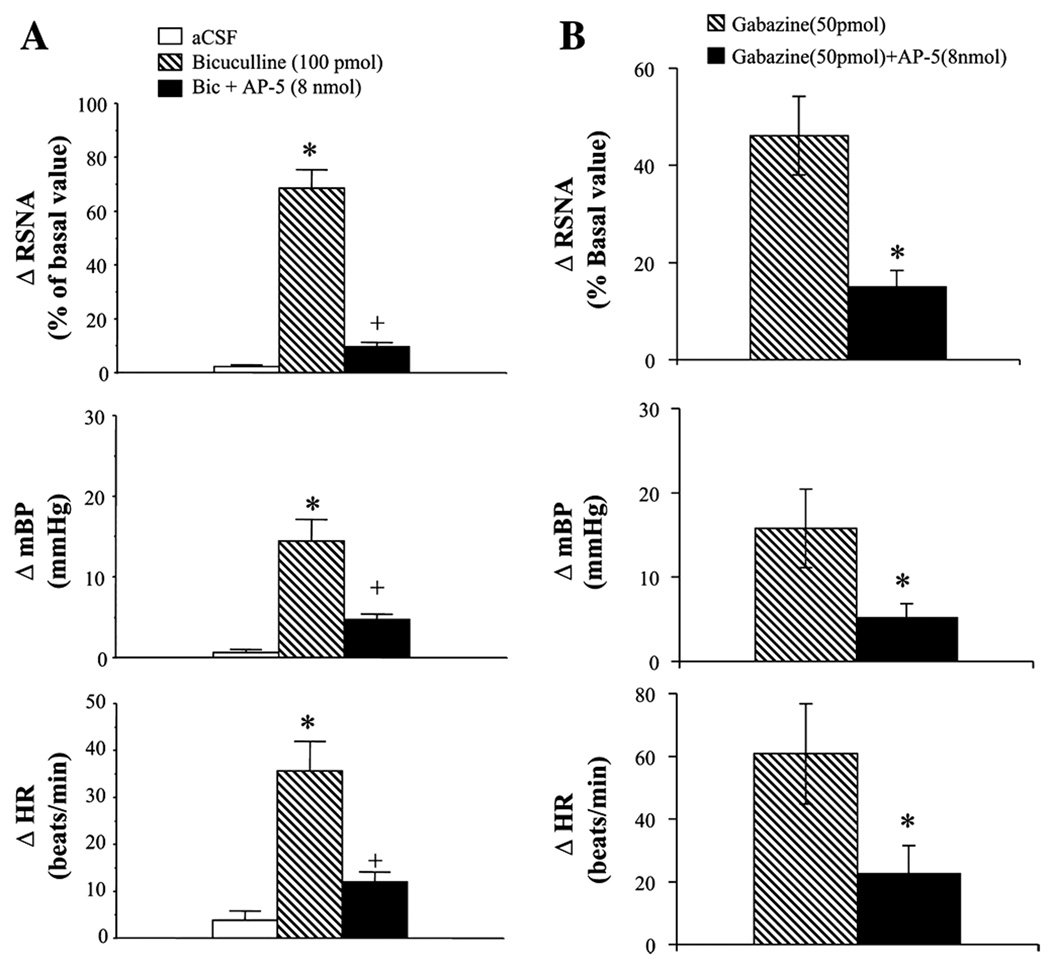
Figure 2.
A: Renal sympathetic nerve activity (RSNA), blood pressure (BP), and heart rate (HR) responses to microinjection of bicuculline (100 pmol, n = 5), bicuculline + AP-5 (8 nmol, n = 6), and aCSF (n = 5) into the PVN. B: RSNA, BP, and HR responses to microinjection of gabazine (50 pmol, n = 5) and gabazine + AP-5 (8 nmol, n = 5) into the PVN. Values are means ± SE. *, p < 0.05 . Note, microinjection of bicuculline significantly increased RSNA, BP, and HR responses, however, with prior blockade of NMDA receptors with AP-5, RSNA, BP, and HR responses to bicuculline were significantly attenuated. Similar responses were also seen with GABAA receptor blocker, gabazine. [Adapted from Li et al., 2006]
The balance between GABA and glutamate release appears to be modulated by NO. Inhibition of NOS activity, reducing NO production, decreases the release of GABA. Microinjection of L-NAME, a NOS inhibitor, in the PVN increases renal sympathetic nerve activity, blood pressure and heart rate significantly (Li et al., 2001). This is consistent with tonic EAA release evoking excitatory responses with decreased GABAergic activity. Thus, blockade of EAA receptors reduces the bicuculline-evoked responses due to interruption of tonic excitation that is revealed on GABAergic inhibition. Similarly, microinjection of glutamate increases the release of NO (Li et al., 2001) which inhibits NMDA-mediated increases in sympathetic nerve activity. Thus, a negative feedback, perhaps through NO facilitating GABA release within the PVN, may contribute to the maintenance of the overall balance and tone of sympathetic outflow.
4. PVN Modulation of Sympatho-Respiratory Function
The PVN modulates blood flow by multiple direct and indirect pathways to sympathetic preganglionic neurons (summarized in Section 2.2); but the mechanism by which it modulates ventilation are not elucidated fully. Studies conducted nearly fifty years ago showed that decreased hypothalamic function by either electrolytic lesions or thiopental injections depresses ventilation in anesthetized rats (Redgate, 1963). Kastella and colleagues (1974) recorded respiratory-modulated neuronal activity in the anterior hypothalamus, in an area that appears to receive higher-order baroreceptor and chemoreceptor inputs. Even though the ventilatory reflexes evoked by baroreceptor and chemoreceptor inputs do not depend on an intact hypothalamus, these investigators suggested the hypothalamus shows respiratory-modulated activity and speculated that respiratory and cardiovascular integration may occur in this portion of the hypothalamus in a similar manner to that described for the lower brainstem. In support of this interpretation, Yeh and coworkers (1997) showed direct connections between the PVN and phrenic motoneurons, and indirect connections between the PVN and brainstem bulbospinal neurons. In addition, the activity of PVN changes during phasic respiratory events. Together, these data suggest a link between the PVN and respiratory function (Kristensen et al., 1997).
Activation of PVN alters cardiorespiratory function in parallel. Electrical stimulation of the PVN in anesthetized rabbits increases respiratory rate and blood pressure (Duan et al., 1997). Yeh and coworkers (1997) microinjected L-glutamate bilaterally in the PVN in anesthetized rats and found that ‘fictive’ ventilation increased; respiratory frequency increased (Te decreased by 26%) and peak diaphragmatic electromyographic activity (a surrogate of tidal volume) doubled. Concomitantly, blood pressure and heart rate increased in these rabbits. In conscious rats, disinhibition of the PVN with bicuculline doubled respiratory frequency and increased tidal volume (69%) and increased mean arterial pressure (maximum Δ= 19±6 mmHg)) and heart rate (150%) (Schlenker et al., 2001). These studies agree regarding the effect of PVN activation on sympatho-respiratory function. Their differences in the magnitude of the responses could be due to differences between anesthetized and conscious rats, because anesthesia appears to influence PVN-mediated responses (Kannan et al., 1989). On the other hand, these differences could also be related to activation of a different population of PVN neurons, and the stimulant used to activate the PVN neurons; for example, glutamate may activate all neurons in the PVN whereas bicuculline only activates those neurons tonically inhibited by GABA. Nevertheless both of these studies demonstrated that PVN-induced modulation on both respiration and blood pressure.
Our studies have identified two phenotypes of PVN neurons projecting to sympatho-respiratory sites in the brainstem. In particular, vasopressin and oxytocin-containing PVN neurons project to the pre-Bötzinger complex and phrenic motoneurons (Kc et al., 2002, Mack et al., 2002). Most recently we reported a direct connection from the vasopressin-containing PVN neurons to the RVLM (Kc et al., 2010). Most of the labelled neurons projecting to both cardiorespiratory sites were identified in the dorsal, ventral and medial parvocellular region of the PVN, regions of the PVN that are involved in autonomic control. To complement these projections, vasopressin type 1A (V1A) and oxytocin receptors are expressed by the rostral ventral respiratory column (rVRC) neurons including those in the pre-Bötzinger complex and by RVLM neurons. The co-localization of axons with the neurotransmitter and receptors in these regions support that the hypothesis that vasopressin and oxytocin may be released and act as neurotransmitters in respiratory and vasomotor control nuclei (Andreatta-Van Leyen et al., 1990; Gomez et al., 1993; Kc et al., 2002, 2010; Mack et al., 2002). Microinjections of these ligands increased respiration, blood pressure and heart rate (Kc et al., 2002; Mack et al., 2007)(Figures 3 and 4). Similarly, microinjection of V1A receptor antagonist into the RVLM attenuated PVN-evoked increases in RVLM neuronal discharge and blood pressure, heart rate and ventilation (Kc et al., 2010). Taken together, these data suggest that vasopressin and oxytocin-containing PVN neurons can affect respiratory and cardiovascular functions.
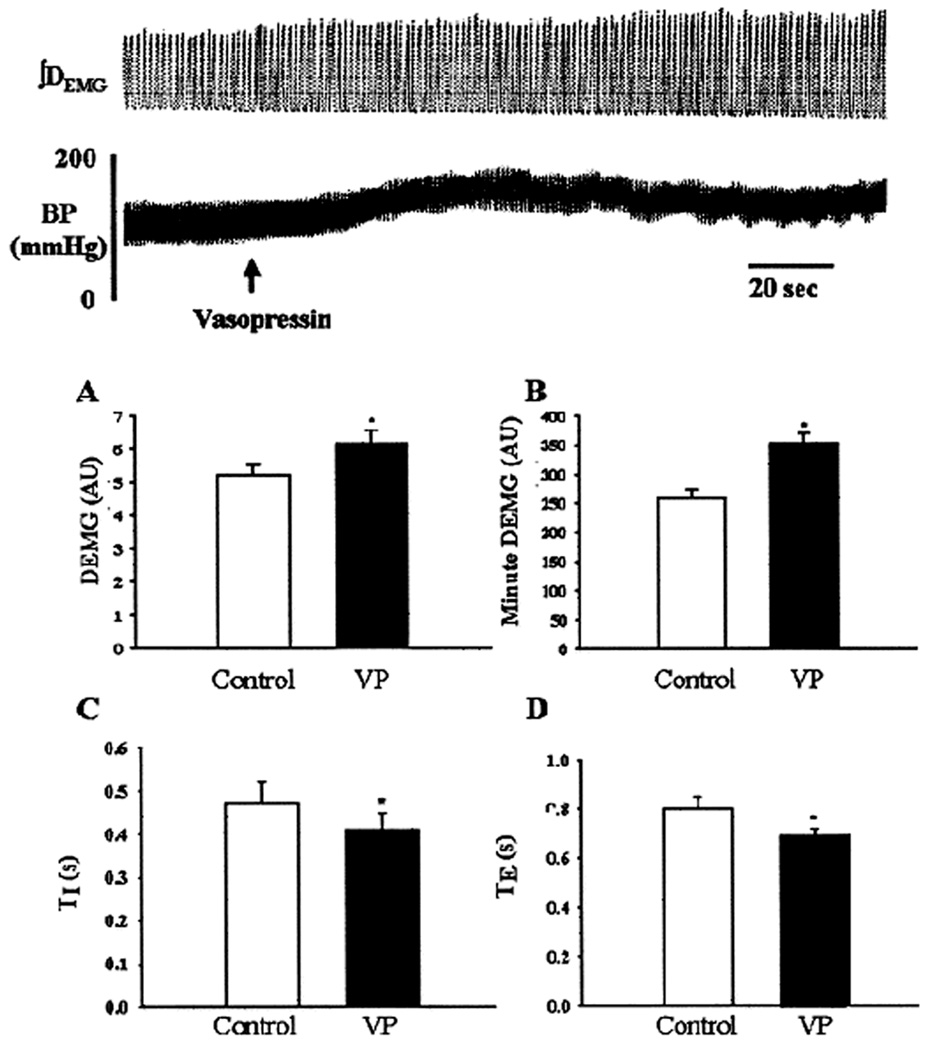
Figure 3.
Upper panel: A representative recording of integrated diaphram electromyographic activity (∫DEMG) and arterial BP showing response to unilateral microinjection of vasopressin (VP, 0.2 nmol per site, 200 nl) into the pre-Bötzinger complex. Arrow indicates time of injection. Lower panel: Mean ± SD of changes in peak DEMG (A), minute DEMG (B), inspiratory duration (TI; C), and expiratory duration (TE; D) duration before (open bars) and after (hatched bars) microinjection (n = 7). AU, Arbitrary units. *, Statistical difference, p <0.05). Note, microinjection of vasopressin into the pre-Bötzinger complex significantly increased DEMG activity, respiratory frequency discharge, blood pressure and heart rate. [Adapted from Kc et al., 2002]
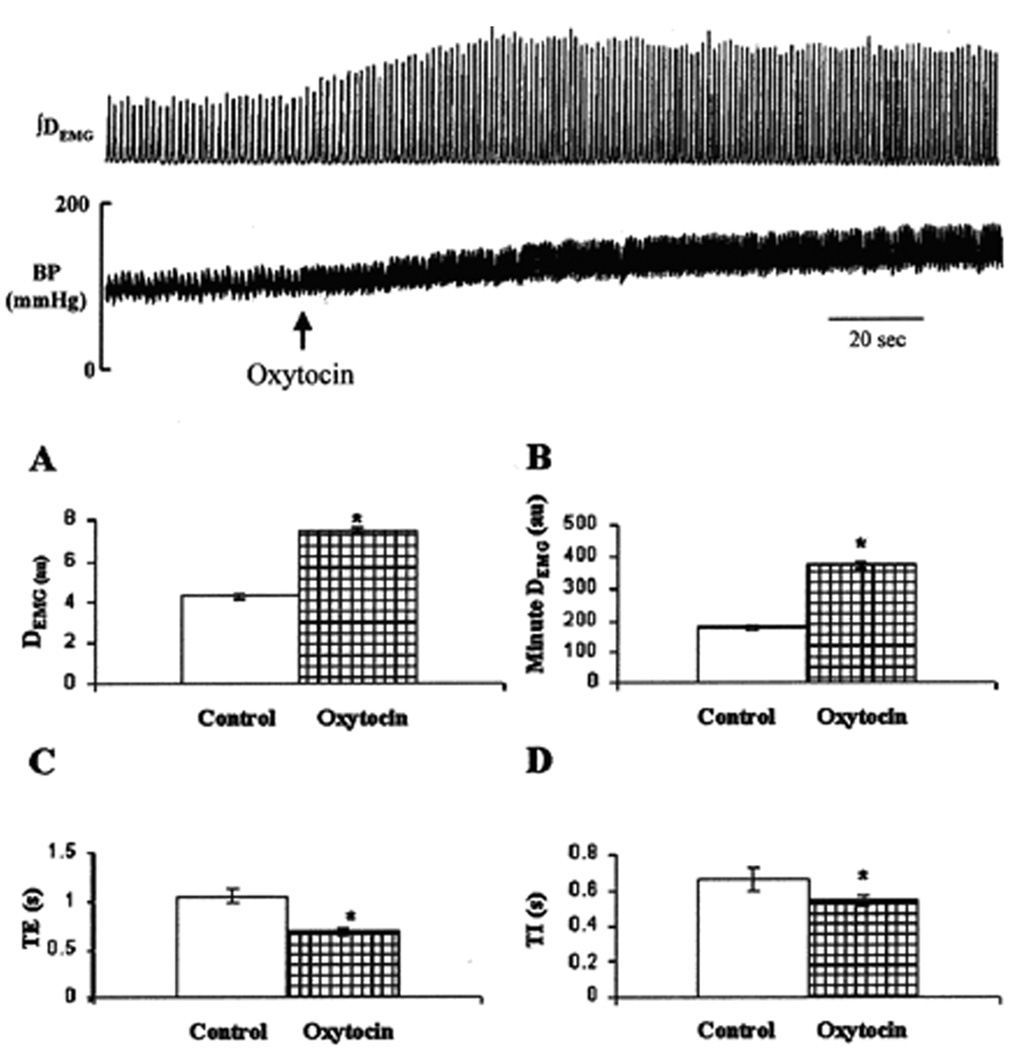
Figure 4.
Upper panel: A representative recording of integrated diaphragm electromyographic activity (∫DEMG) and arterial blood pressure (BP) showing response to unilateral microinjection of oxytocin (0.2 nmol/site, 200 nl) into the pre-Bötzinger complex. Arrow indicates time of injection. Lower panel: means ± SD of changes in peak DEMG (A), minute DEMG (B), expiratory duration (TE; C), and inspiratory duration (TI; D) duration before (open bars) and after (hatched bars) microinjection (n = 10 animals). au, Arbitrary units. *, Statistical difference, p < 0.05. Note, activation of pre-Bötzinger complex oxytocin significantly increased DEMG activity, respiratory frequency discharge, blood pressure and heart rate. [Adapted from Mack et al., 2002]
As indicated earlier, PVN activation increases sympatho-respiratory activity, the mechanisms that are involved in initiating and limiting the evoked responses may depend on nitric oxide (NO). Nitric oxide, an endothelial cell derived relaxing factor, within the PVN regulates the activity of PVN neurons. Perfusion of PVN with NO-containing CSF or microinjection of sodium nitroprusside, a NO donor, into the PVN elicits a significant reduction of arterial blood pressure, renal sympathetic nerve activity, and heart rate (Horn et al., 1994; Zhang et al., 1997) (Figure 5). Nitric oxide synthase (NOS), an enzyme that synthesizes NO, is densely localized in the PVN (Bredt et al., 1990; Sanchez et al., 1994; Vincent and Kimura, 1992). In the PVN, NO evokes the release of GABA, which could account for the inhibitory effect on sympatho-respiratory activity (Ohkuma 1995; Ohkuma et al., 1996; Zhang and Patel, 1998). To determine the role of NO in control of SNA, Zhang et al., (1998) microinjected two inhibitors of NOS, L-NMMA or L-NAME into the PVN and reported increases in renal sympathetic nerve activity, blood pressure and heart rate (Figure 6). Furthermore as a control experiment, these responses were not observed in the presence of an inactive isomer, D-NMMA (Zhang et al., 1997). To determine if GABAA receptors acted in the mechanism by which NO inhibits SNA, bicuculline was microinjected into the PVN prior to injection of L-NAME or sodium nitroprusside. The sympatho-excitatory response to L-NAME as well as the sympathoinhibitory response to sodium nitroprusside was attenuated by bicuculline (Zhang and Patel, 1998). Thus, NO may be acting by increasing GABA release and binding at GABAA receptors.
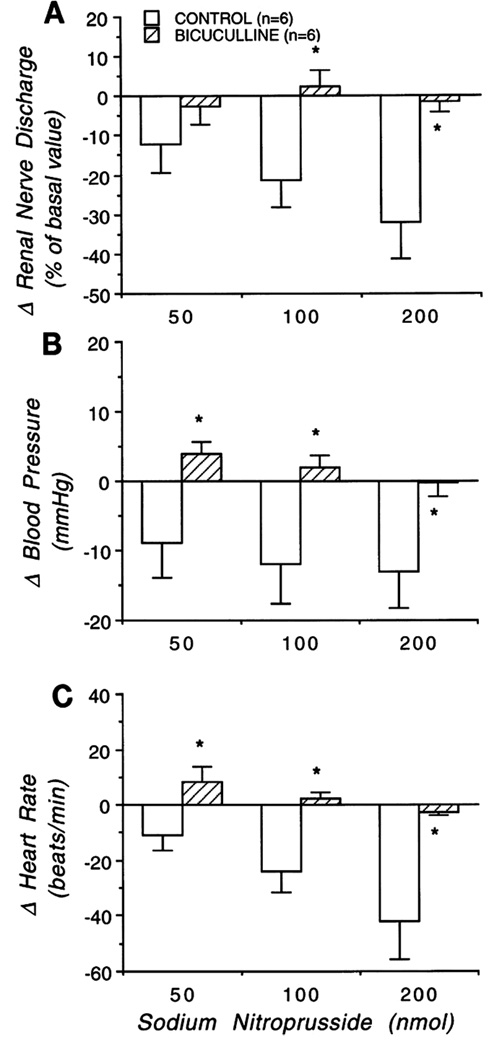
Figure 5.
Change of efferent renal sympathetic nerve discharge (RSND; A), blood pressure (B), and heart rate (C) to microinjection of sodium nitroprusside into the PVN both in absence (open bars) and presence (hatched bars) of blockade of endogenous GABA system with bicuculline in the PVN. Graphs represent mean value for each group ± SE. * p < 0.05 compared with control. Note, microinjection of sodium nitroprusside into the PVN significantly decreased RSND, arterial blood pressure, and heart rate at the highest dose. These responses were eliminated by blockade of the GABA system. [Adapted from Zhang and Patel, 1998]
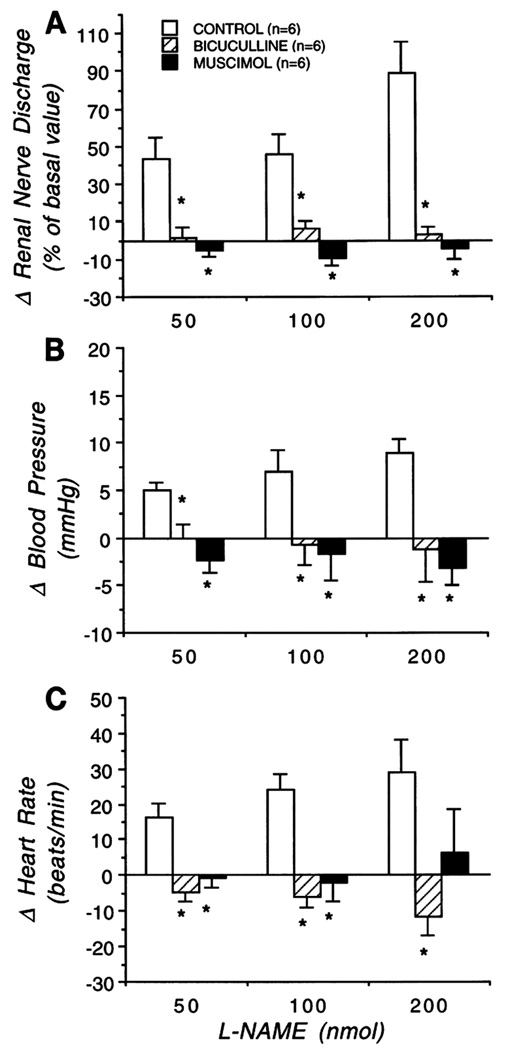
Figure 6.
Change of efferent renal sympathetic nerve discharge (RSND, A), arterial blood pressure (B), and heart rate (C) to microinjection of L-NAME into the PVN in absence (open bars) of muscimol and bicuculline (control), and presence of bicuculline (hatched bars) or muscimol (solid bars) into the PVN. Graphs represent mean value for each group ± SE. *, p< 0.05 compared with control. Note, microinjection of L-NAME (50, 100, and 200 nmol) into the PVN significantly increased RSND, arterial blood pressure, and heart rate at the highest dose. These sympathoexcitatory effects of L-NAME were masked by prior blockade of the GABA system with bicuculline and activation of the GABA system with muscimol. [Adapted from Zhang and Patel, 1998]
An interaction between NO and GABA is dynamic and may precipitate sympathoexcitation during stressful situations, such as exposure to chronic intermittent hypoxic (CIH) or maternal separation. Neuronal NOS mRNA and protein levels decrease in the PVN following CIH exposure for 35 days (Huang et al., 2007). Further, chronic stress alters the efficacy of GABAA receptors in rat hypothalamic-pituitary-adrenocortical axis (Cullinan and Wolfe, 2000). A decrease in GABA concentration as well as GABAA receptors are seen in spontaneously hypertensive rats, indicating imbalance in inhibitory input (Cullinan and Wolfe, 2000; Genest et al., 2007; Huang et al., 2007; Kunkler and Hwang, 1995; Li and Pan, 2006; Zhang et al., 1998, 2002). Decreases in NO production resulting in decreases in inhibitory GABA transmission may explain the increased activity of PVN neurons during CIH conditioning or maternal separation. Therefore, NO regulates the sympathetic outflow from the PVN via activation of the GABA system. However, the sympathoinhibitory effect of NO may not be limited by evoking a GABA release, but also by inhibiting release of excitatory neurotransmitters such as glutamate and angiotensin II (Bains and Ferguson, 1994; Martin and Haywood, 1992). Thus, elevated sympathetic nerve activity associated chronic stress states may result from decreased NO disrupting the balance of inhibitory/excitatory activity, i.e., decreased GABA release and increased excitatory glutamate release.
5. PVN Vasopressin-V1A signalling in the VRC and RVLM
The vasopressinergic fibers and its receptor, V1A, are expressed in the RVLM and rVRC. Activation of V1A receptor signalling increases blood pressure and respiration (Kc et al., 2002; Yang and Coote, 2003). Further, V1A receptor in RVLM/rVRC is upregulated in rats that have increased SNA after exposure to CIH. In CIH-conditioned rats, activation of PVN significantly increases blood pressure and respiratory output greater than in naïve animals (Figures 7 and 8) (Kc et al., 2010). Moreover, application of V1A receptor antagonist in the RVLM/rVRC attenuates blood pressure and respiratory responses to disinhibition of the PVN indicating a possible vasopressin-V1A signalling mechanism manifested in inducing enhanced cardiovascular output to CIH exposure. Blockade of those V1A receptors in the RVLM normalized the baseline blood pressure only in CIH-conditioned rats (Kc et al., 2010). This is consistent with the results of Yang et al., (2001), in which the ongoing activity of RVLM-spinal cord neurons was unaffected by V1A receptor antagonist microinjected into the RVLM. Thus, these physiological studies complement the neuroanatomical studies indicating that under normal conditions, vasopressin may not provide an effective tonic drive to the RVLM region, at least in anesthetized animal whereas after conditioning with CIH the upregulation of receptor results in effective tonic drive to the RVLM.
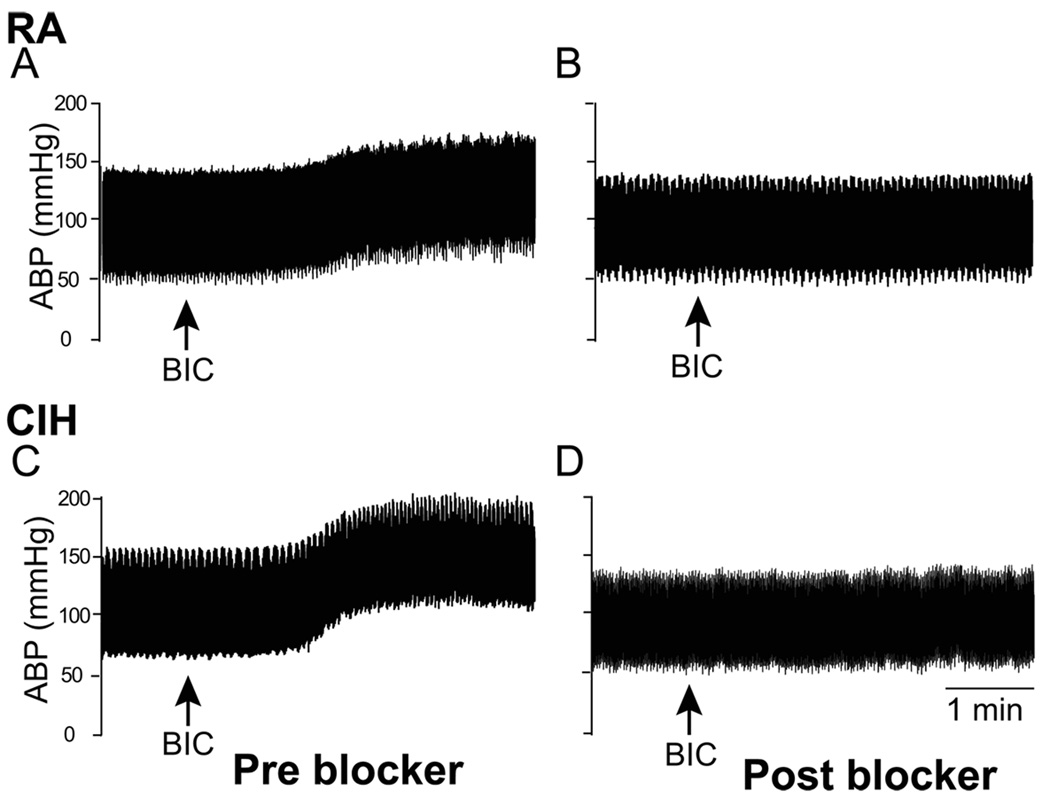
Figure 7.
Representative chart recordings from a room air (RA, A and B)- and chronic intermittent hypoxia-conditioned (CIH, C and D) rat showing arterial blood pressure (ABP) during baseline and following disinhibition of PVN with bicuculline (BIC), before and after blockade of vasopressin V1A receptors into the RVLM/rVRC with 0.2 nmol in RA- and 0.4 nmol in CIH-conditioned rat. Arrows indicate BIC injection. Note, it required a significantly higher dose of blocker to blunt the ABP increase to PVN disinhibition in CIH-conditioned rats. Also microinjection of blocker into the RVLM normalized the ABP in CIH-conditioned rat (D). [Adapted from Kc et al., 2010]
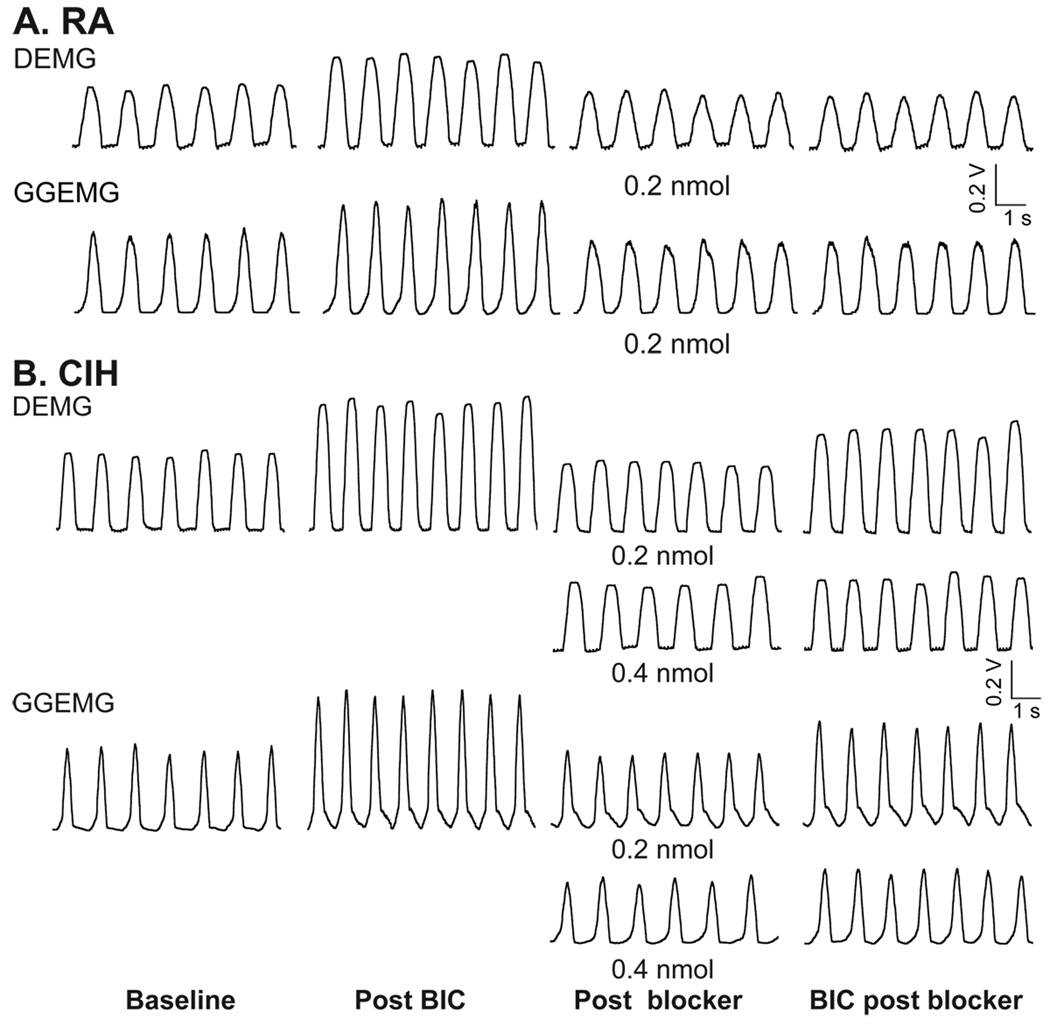
Figure 8.
Representative chart recording from a room air (RA, A)- and chronic intermittent hypoxia (CIH, B) conditioned rat showing integrated diaphragmatic (DEMG) and genioglossal muscle (GGEMG) during baseline and following disinhibition of PVN with bicuculline (BIC) and after blockade of vasopressin V1A receptors into the RVLM (0.2 nmol in RA rat and 0.2 nmol and 0.4 nmol in CIH-conditioned rat) to PVN disinhibition. Note, it required a significantly higher dose of blocker to blunt DEMG and GGEMG activity to PVN disinhibition in CIH-conditioned rats. [Adapted from Kc et al., 2010]
In addition to vasopressin, other neurotransmitters such as glutamate, angiotensin and oxytocin may also act in the PVN’s modulation of RVLM activity (Amano et al., 1994; Chen and Toney, 2003; Mack et al., 2002, 2007; McMullan et al., 2007; Stocker et al., 2006; Tagawa and Dampney, 1999; Yang et al., 2001, 2002). Systemic angiotensin II regulates blood pressure centrally by augmenting SNA (McMullan et al., 2007) and by stimulating the release of vasopressin from the PVN (Shi et al., 2006; Schiavone et al., 1988; Sladek and Joynt 1979). Pretreatment with d(CH2)5VDAVP, a specific vasopressin antagonist, partially reduces the blood pressure response to intracerebrovascular injection of angiotensin II, suggesting vasopressin contributes to the central angiotensin II pressure responses (Unger et al., 1981). Angiotensin II receptor blocker when microinjected into the RVLM region partially decreases the blood pressure (Tagawa and Dampney, 1999) whereas vasopressin antagonist blunts the blood pressure responses (Kc et al., 2010) evoked by PVN disinhibition. One possibility of this observed difference between these two studies could be due to unilateral verses bilateral microinjection of the blocker in the RVLM. This unilateral injection may have caused angiotensin II to evoke release of vasopressin as indicated earlier causing only partial decrease in the blood pressure response. It appears that the excitability of RVLM-spinal vasomotor neurons depends on a number of chemically discrete synaptic inputs. Future studies with microdialysates and chemical detection using HPLC would provide valuable information about an equipotencial or differential role of vasopressin, angiotensin II or other neurotransmitters involved mediating these responses.
6. PVN Modulation of Sympathoexcitation via Peripheral Chemoreflex
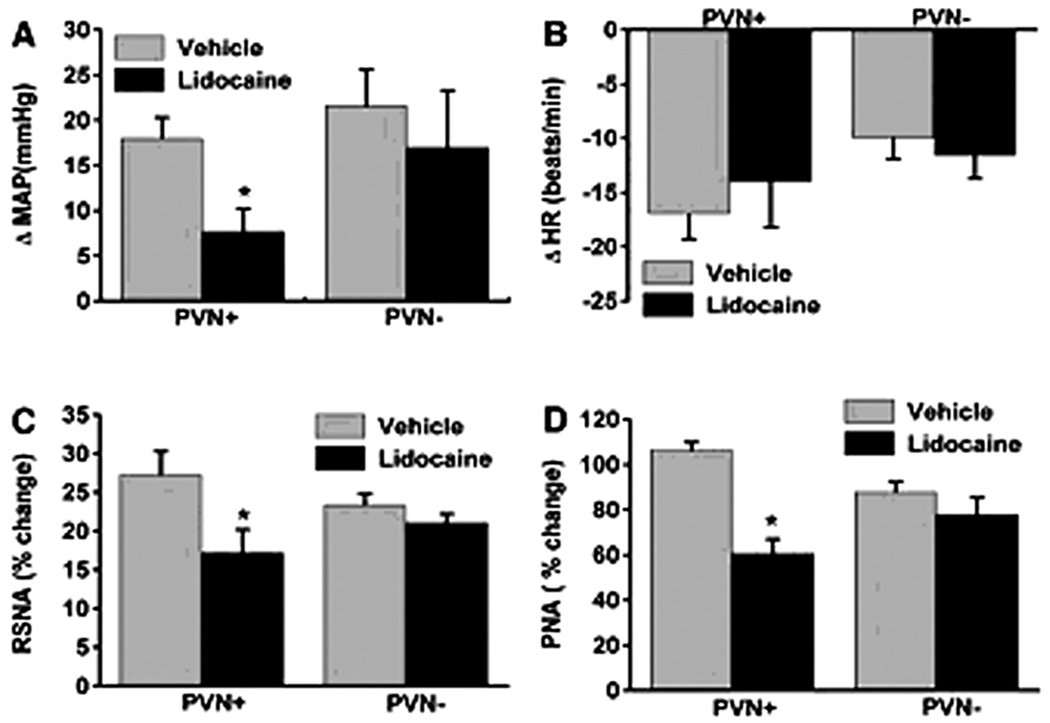
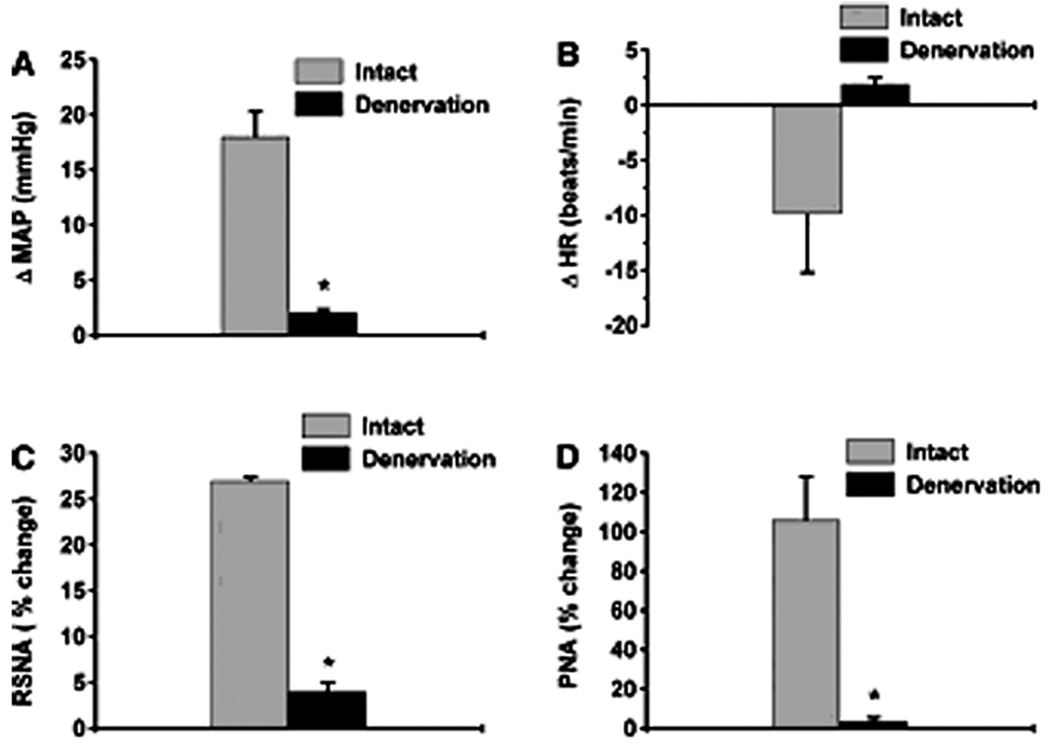
The PVN integrates chemosensory afferent signals (Schlenker, 2001). Hypoxia stimulates carotid bodies, which via the carotid sinus nerves activate the commissural nuclei of the solitary tract (nTS) (Swanson and Sawchenko, 1983), and evokes an increase in sympatho-respiratory motor activity via respiratory sites (Koshiya and Guyenet, 1996). In addition, chemoreceptor-activated nTS neurons also activate PVN neurons via a noradrenergic pathway (Chen et al., 2004). In unanesthetized rats, bilateral lesions of the PVN prevent potassium cyanide (KCN) from evoking the chemoreflex in spite of an intact brainstem network (Olivan, 2001). Similarly in anesthetized rats, the pressor, sympathoexcitatory and respiratory responses to KCN are dependent, in part, on the PVN (Reddy et al. 2005). Specifically, 2% lidocaine in the PVN attenuates increases in RSNA, MAP, and PNA in response to systemic KCN (Reddy et al., 2005) (Figure 9). Furthermore, the role of the PVN in mediating chemoreflex may be specific for hypoxic but not for hypercapnic stimulation. This was tested by comparing the cardiopulmonary responses to stimulation of central chemoreceptors with 10% CO2 in carotid denervated animals (Figure 10). The PVN neurotransmission blockade with lidocaine has no effect on hypercapnia-induced central chemoreflex responses indicating that the PVN is not needed for modulating cardiopulmonary and RSNA responses to hypercapnia. In summary, the role of the PVN may be selective for processing sympathoexcitatory and ventilatory responses evoked by the peripheral but not central chemoreflex.
Figure 9.
Changes in mean arterial pressure (ΔMAP, mmHg; A), heart rate (ΔHR, beats/min; B), renal sympathetic nerve activity (RSNA, %change, C), and phrenic nerve activity (PNA, %change, D) in response to chemoreflex activation before (vehicle) and after microinjection of lidocaine into the PVN (PVN +) (n = 8). Also illustrated, reflex responses in animals where microinjections were targeted outside the PVN (PVN−) (n = 5). Data are means ± SE. *Comparison of reflex response to KCN before and after lidocaine injection within the PVN (P < 0.05). Note, bilateral microinjection of lidocaine into the PVN significantly decreased MAP, RSNA and PNA with no change in HR to evoked stimulation of peripheral stimulation with potassium cyanide. [Adapted from Reddy et al., 2005]
Figure 10.
Effect of bilateral carotid sinus denervation on reflex responses to potassium cyanide (KCN, n = 5). ΔMAP (mmHg, A), ΔHR (beats/min, B), RSNA (%change, C), and PNA (%change, D) in response to chemoreflex activation before and after sinus denervation (n = 5). *Comparison of reflex responses to KCN between intact and denervated carotid sinus. Data are means ± SE. (P < 0.05). Note, carotid sinus nerve denervation significantly decreased MAP, RSNA and PNA with no change in HR to evoked stimulation of peripheral stimulation with potassium cyanide. [Adapted from Reddy et al., 2005]
7. Stress-induced Plasticity of the PVN Neurons
PVN neurons exhibit marked biochemical plasticity in response to stress (Boudaba et al., 1996; Cullinan et al., 2008; Patel 2000; Zhang et al., 2002). Stress alters the plasticity of receptor expression; it decreases GABA levels as well as mRNA for GABAA receptors in the PVN, leading to increased excitability of the PVN neurons (Cullinan and Wolfe, 2000; Genest et al., 2007; Huang et al., 2007; Li and Pan, 2006; Zhang et al., 1998). Indeed, a decrease in GABAergic mechanisms in the PVN is related to the pathogenesis of a variety of clinical manifestations related to increased sympathetic nerve activity (Haywood et al., 2001; Zhang et al., 1998, 2002). Similarly, increases in GABAA receptor binding site also increase PVN activity to exogenously administered bicuculline (Cullinan and Wolfe, 2000; Genest et al., 2007). Conceivably, bicuculline microinjection in the PVN induces release of glutamate which could increase sympathetic nerve activity (Li et al., 2006), supporting the notion of a GABAA-mediated tonic inhibition of excitatory input. Based on these studies, it can be concluded that a decrease in GABA levels and/or increase in GABAA receptor binding sites would cause an imbalance in the PVN homeostasis. Although GABA activity/levels was not measured, increases in blood pressure and diaphragmatic and genioglossal muscle activity evoked by bicuculline microinjection in the PVN in CIH-conditioned rats could be mediated by a similar mechanism (Kc et al., 2010). However, changes in the other excitatory neurotransmitters such as glutamate (Herman et al., 2000), norepinephrine (Han et al., 2002) or neuropeptide such as angiotensin II (Chen and Toney, 2003; McMullan et al., 2007) or corticotrophin-releasing factor (Moga and Saper, 1994; Swanson and Sawchenko, 1983) could also be altered and needs further investigation.
Acknowledgements
This work is supported by the National Heart, Lung and Blood Institute Grants 4R00HL087620-03 (to P. Kc) and HL-090554 (to T. E. Dick).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amano M, Asari T, Kubo T. Excitatory amino acid receptors in the rostral ventrolateral medulla mediate hypertension induced by carotid body chemoreceptor stimulation. Naunyn Schmiedebergs Arch. Pharmacol. 1994;349:549–554. doi: 10.1007/BF01258457. [DOI] [PubMed] [Google Scholar]
- Andreatta-van Leyen S, Averill DB, Ferrario CM. Cardiovascular actions of vasopressin at the ventrolateral medulla. Hypertension. 1990;15:I102–I106. doi: 10.1161/01.hyp.15.2_suppl.i102. [DOI] [PubMed] [Google Scholar]
- Badoer E. Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin. Exp. Pharmacol. Physiol. 2001;28:95–99. doi: 10.1046/j.1440-1681.2001.03413.x. [DOI] [PubMed] [Google Scholar]
- Bains JS, Ferguson AV. Paraventricular nucleus neurons projecting to the spinal cord receive excitatory input from the subfornical organ. Am. J Physiol. 1995;268:R625–R633. doi: 10.1152/ajpregu.1995.268.3.R625. [DOI] [PubMed] [Google Scholar]
- Berk ML, Finkelstein JA. Afferent projections to the preoptic area and hypothalamic regions in the rat brain. Neuroscience. 1981;6:1601–1624. doi: 10.1016/0306-4522(81)90227-x. [DOI] [PubMed] [Google Scholar]
- Boudaba C, Szabo K, Tasker JG. Physiological mapping of local inhibitory inputs to the hypothalamic paraventricular nucleus. J. Neurosci. 1996;16:7151–7160. doi: 10.1523/JNEUROSCI.16-22-07151.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudaba C, Schrader LA, Tasker JG. Physiological evidence for local excitatory synaptic circuits in the rat hypothalamus. J Neurophysiol. 1997;77:3396–3400. doi: 10.1152/jn.1997.77.6.3396. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Card JP, Sved JC, Craig B, Raizada M, Vazquez J, Sved AF. Efferent projections of rat rostroventrolateral medulla C1 catecholamine neurons: Implications for the central control of cardiovascular regulation. J Comp Neurol. 2006;499:840–859. doi: 10.1002/cne.21140. [DOI] [PubMed] [Google Scholar]
- Cato MJ, Toney GM. Angiotensin II excites paraventricular nucleus neurons that innervate the rostral ventrolateral medulla: an in vitro patch-clamp study in brain slices. J Neurophysiol. 2005;93:403–413. doi: 10.1152/jn.01055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QH, Toney GM. Responses to GABA-A receptor blockade in the hypothalamic PVN are attenuated by local AT1 receptor antagonism. Am. J Physiol Regul. Integr. Comp Physiol. 2003;285:R1231–R1239. doi: 10.1152/ajpregu.00028.2003. [DOI] [PubMed] [Google Scholar]
- Chen XQ, Du JZ, Wang YS. Regulation of hypoxia-induced release of corticotropin-releasing factor in the rat hypothalamus by norepinephrine. Regul. Pept. 2004;119:221–228. doi: 10.1016/j.regpep.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Coote JH, Yang Z, Pyner S, Deering J. Control of sympathetic outflows by the hypothalamic paraventricular nucleus. Clin. Exp. Pharmacol. Physiol. 1998;25:461–463. doi: 10.1111/j.1440-1681.1998.tb02235.x. [DOI] [PubMed] [Google Scholar]
- Cruz JC, Bonagamba LG, Machado BH, Biancardi VC, Stern JE. Intermittent activation of peripheral chemoreceptors in awake rats induces Fos expression in rostral ventrolateral medulla-projecting neurons in the paraventricular nucleus of the hypothalamus. Neuroscience. 2008;157:463–472. doi: 10.1016/j.neuroscience.2008.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan WE, Wolfe TJ. Chronic stress regulates levels of mRNA transcripts encoding beta subunits of the GABA(A) receptor in the rat stress axis. Brain Res. 2000;887:118–124. doi: 10.1016/s0006-8993(00)03000-6. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Ziegler DR, Herman JP. Functional role of local GABAergic influences on the HPA axis. Brain Struct. Funct. 2008;213:63–72. doi: 10.1007/s00429-008-0192-2. [DOI] [PubMed] [Google Scholar]
- Decavel C, Van den Pol AN. GABA: a dominant neurotransmitter in the hypothalamus. J. Comp Neurol. 1990;302:1019–1037. doi: 10.1002/cne.903020423. [DOI] [PubMed] [Google Scholar]
- Decavel C, Van den Pol AN. Converging GABA-and glutamate-immunoreactiveaxons make synaptic contact with identified hypothalamic neurosecretary neurons. J. Comp Neurol. 1992;316:104–116. doi: 10.1002/cne.903160109. [DOI] [PubMed] [Google Scholar]
- Duan YF, Winters R, McCabe PM, Green EJ, Huang Y, Schneiderman N. Cardiorespiratory components of defense reaction elicited from paraventricular nucleus. Physiol Behav. 1997;61:325–330. doi: 10.1016/s0031-9384(96)00410-6. [DOI] [PubMed] [Google Scholar]
- Genest SE, Balon N, Laforest S, Drolet G, Kinkead R. Neonatal maternal separation and enhancement of the hypoxic ventilatory response in rat: the role of GABAergic modulation within the paraventricular nucleus of the hypothalamus. J Physiol. 2007;583:299–314. doi: 10.1113/jphysiol.2007.135160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez RE, Cannata MA, Milner TA, Anwar M, Reis DJ, Ruggiero DA. Vasopressinergic mechanisms in the nucleus reticularis lateralis in blood pressure control. Brain Res. 1993;604:90–105. doi: 10.1016/0006-8993(93)90356-r. [DOI] [PubMed] [Google Scholar]
- Han SK, Chong W, Li LH, Lee IS, Murase K, Ryu PD. Noradrenaline excites and inhibits GABAergic transmission in parvocellular neurons of rat hypothalamic paraventricular nucleus. J Neurophysiol. 2002;87:2287–2296. doi: 10.1152/jn.2002.87.5.2287. [DOI] [PubMed] [Google Scholar]
- Haywood JR, Mifflin SW, Craig T, Calderon A, Hensler JG, Hinojosa-Laborde C. gamma-Aminobutyric acid (GABA)--A function and binding in the paraventricular nucleus of the hypothalamus in chronic renal-wrap hypertension. Hypertension. 2001;37:614–618. doi: 10.1161/01.hyp.37.2.614. [DOI] [PubMed] [Google Scholar]
- Herman JP, Eyigor O, Ziegler DR, Jennes L. Expression of ionotropic glutamate receptor subunit mRNAs in the hypothalamic paraventricular nucleus of the rat. J Comp Neurol. 2000;422:352–362. [PubMed] [Google Scholar]
- Herman JP, Tasker JG, Ziegler DR, Cullinan WE. Local circuit regulation of paraventricular nucleus stress integration: glutamate-GABA connections. Pharmacol. Biochem. Behav. 2002;71:457–468. doi: 10.1016/s0091-3057(01)00681-5. [DOI] [PubMed] [Google Scholar]
- Horn T, Smith PM, McLaughlin BE, Bauce L, Marks GS, Pittman QJ, Ferguson AV. Nitric oxide actions in paraventricular nucleus: cardiovascular and neurochemical implications. Am. J Physiol. 1994;266:R306–R313. doi: 10.1152/ajpregu.1994.266.1.R306. [DOI] [PubMed] [Google Scholar]
- Huang J, Tamisier R, Ji E, Tong J, Weiss WJ. Chronic intermittent hypoxia modulates nNOS mRNA and protein expression in the rat hypothalamus. Respir. Physiol Neurobiol. 2007;158:30–38. doi: 10.1016/j.resp.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Kannan H, Hayashida Y, Yamashita H. Increase in sympathetic outflow by paraventricular nucleus stimulation in awake rats. Am. J Physiol. 1989;256:R1325–R1330. doi: 10.1152/ajpregu.1989.256.6.R1325. [DOI] [PubMed] [Google Scholar]
- Kastella KG, Spurgeon HA, Weiss GK. Respiratory-related neurons in anterior hypothalamus of the cat. Am. J Physiol. 1974;227:710–713. doi: 10.1152/ajplegacy.1974.227.3.710. [DOI] [PubMed] [Google Scholar]
- Kc P, Haxhiu MA, Tolentino-Silva FP, Wu M, Trouth CO, Mack SO. Paraventricular vasopressin-containing neurons project to brain stem and spinal cord respiratory-related sites. Respir. Physiol Neurobiol. 2002;133:75–88. doi: 10.1016/s1569-9048(02)00131-3. [DOI] [PubMed] [Google Scholar]
- Kc P, Balan KV, Tjoe SS, Martin RJ, LaManna JC, Haxhiu MA, Dick TE. Increased vasopressin transmission from the paraventricular nucleus to the rostral medulla augments cardiorespiratory outflow in chronic intermittent hypoxia-conditioned rats. J Physiol. 2010;588:725–740. doi: 10.1113/jphysiol.2009.184580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiya N, Guyenet PG. NTS neurons with carotid chemoreceptor inputs arborize in the rostral ventrolateral medulla. Am. J Physiol. 1996;270:R1273–R1278. doi: 10.1152/ajpregu.1996.270.6.R1273. [DOI] [PubMed] [Google Scholar]
- Kristensen MP, Poe GR, Rector DM, Harper RM. Activity changes of the cat paraventricular hypothalamus during phasic respiratory events. Neuroscience. 1997;80:811–819. doi: 10.1016/s0306-4522(97)00085-7. [DOI] [PubMed] [Google Scholar]
- Kunkler PE, Hwang BH. Lower GABAA receptor binding in the amygdala and hypothalamus of spontaneously hypertensive rats. Brain Res. Bull. 1995;36:57–61. doi: 10.1016/0361-9230(94)00164-v. [DOI] [PubMed] [Google Scholar]
- Lenkei Z, Corvol P, Llorens-Cortes C. The angiotensin receptor subtype AT1A predominates in rat forebrain areas involved in blood pressure, body fluid homeostasis and neuroendocrine control. Brain Res. Mol. Brain Res. 1995;30:53–60. doi: 10.1016/0169-328x(94)00272-g. [DOI] [PubMed] [Google Scholar]
- Li DP, Pan HL. Plasticity of GABAergic control of hypothalamic presympathetic neurons in hypertension. Am. J Physiol Heart Circ. Physiol. 2006;290:H1110–H1119. doi: 10.1152/ajpheart.00788.2005. [DOI] [PubMed] [Google Scholar]
- Li YF, Mayhan WG, Patel KP. NMDA-mediated increase in renal sympathetic nerve discharge within the PVN: role of nitric oxide. Am. J Physiol Heart Circ. Physiol. 2001;281:H2328–H2336. doi: 10.1152/ajpheart.2001.281.6.H2328. [DOI] [PubMed] [Google Scholar]
- Li YF, Jackson KL, Stern JE, Rabeler B, Patel KP. Interaction between glutamate and GABA systems in the integration of sympathetic outflow by the paraventricular nucleus of the hypothalamus. Am. J. Physiol Heart Circ. Physiol. 2006;291:H2847–H2856. doi: 10.1152/ajpheart.00625.2005. [DOI] [PubMed] [Google Scholar]
- Lovick TA, Coote JH. Electrophysiological properties of paraventriculo-spinal neurones in the rat. Brain Res. 1988;454:123–130. doi: 10.1016/0006-8993(88)90810-4. [DOI] [PubMed] [Google Scholar]
- Luiten PG, ter Horst GJ, Karst H, Steffens AB. The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Res. 1985;329:374–378. doi: 10.1016/0006-8993(85)90554-2. [DOI] [PubMed] [Google Scholar]
- Mack SO, Kc P, Wu M, Coleman BR, Tolentino-Silva FP, Haxhiu MA. Paraventricular oxytocin neurons are involved in neural modulation of breathing. J. Appl. Physiol. 2002;92:826–834. doi: 10.1152/japplphysiol.00839.2001. [DOI] [PubMed] [Google Scholar]
- Mack SO, Wu M, Kc P, Haxhiu MA. Stimulation of the hypothalamic paraventricular nucleus modulates cardiorespiratory responses via oxytocinergic innervation of neurons in pre-Botzinger complex. J. Appl. Physiol. 2007;102:189–199. doi: 10.1152/japplphysiol.00522.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvin RL, Mouw D, Vander AJ, Gregg C. Hypothalamic stimulation of ADH release by angiotensin II. In: Buckley JP, Ferrario CM, editors. Central Actions of Angiotensin and Related Hormones. New York: Pergamon; 1977. pp. 257–266. [Google Scholar]
- Martin DS, Haywood JR. Sympathetic nervous system activation by glutamate injections into the paraventricular nucleus. Brain Res. 1992;577:261–267. doi: 10.1016/0006-8993(92)90282-e. [DOI] [PubMed] [Google Scholar]
- McMullan S, Goodchild AK, Pilowsky PM. Circulating angiotensin II attenuates the sympathetic baroreflex by reducing the barosensitivity of medullary cardiovascular neurones in the rat. J. Physiol. 2007;582:711–722. doi: 10.1113/jphysiol.2007.128983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill TH, Sladek JR., Jr Simultaneous monoamine histofluorescence and neuropeptide immunocytochemistry: II. Correlative distribution of catecholamine varicosities and magnocellular neurosecretory neurons in the rat supraoptic and paraventricular nuclei. J Comp Neurol. 1980;193:1023–1033. doi: 10.1002/cne.901930414. [DOI] [PubMed] [Google Scholar]
- Moga MM, Saper CB. Neuropeptide-immunoreactive neurons projecting to the paraventricular hypothalamic nucleus in the rat. J Comp Neurol. 1994;346:137–150. doi: 10.1002/cne.903460110. [DOI] [PubMed] [Google Scholar]
- Moon EA, Goodchild AK, Pilowsky PM. Lateralisation of projections from the rostral ventrolateral medulla to sympathetic preganglionic neurons in the rat. Brain Res. 2002;929:181–190. doi: 10.1016/s0006-8993(01)03388-1. [DOI] [PubMed] [Google Scholar]
- Ohkuma S, Narihara H, Katsura M, Hasegawa T, Kuriyama K. Nitric oxide-induced [3H] GABA release from cerebral cortical neurons is mediated by peroxynitrite. J Neurochem. 1995;65:1109–1114. doi: 10.1046/j.1471-4159.1995.65031109.x. [DOI] [PubMed] [Google Scholar]
- Ohkuma S, Katsura M, Guo JL, Narihara H, Hasegawa T, Kuriyama K. Role of peroxynitrite in [3H] gamma-aminobutyric acid release evoked by nitric oxide and its mechanism. Eur. J Pharmacol. 1996;301:179–188. doi: 10.1016/0014-2999(96)00013-1. [DOI] [PubMed] [Google Scholar]
- Olivan MV, Bonagamba LG, Machado BH. Involvement of the paraventricular nucleus of the hypothalamus in the pressor response to chemoreflex activation in awake rats. Brain Res. 2001;895:167–172. doi: 10.1016/s0006-8993(01)02067-4. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Mezey E, Zaborszky L, Feminger A, Versteeg DH, Wijnen HJ, de Jong W, Fekete MI, Herman JP, Kanyicska B. Adrenergic innervation of the rat hypothalamus. Neurosci. Lett. 1980a;18:237–243. doi: 10.1016/0304-3940(80)90291-8. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Zaborszky L, Feminger A, Mezey E, Fekete MI, Herman JP, Kanyicska B, Szabo D. Noradrenergic innervation of the rat hypothalamus:experimental biochemical and electron microscopic studies. Brain Res. 1980b;191:161–171. doi: 10.1016/0006-8993(80)90320-0. [DOI] [PubMed] [Google Scholar]
- Patel KP. Role of paraventricular nucleus in mediating sympathetic outflow in heart failure. Heart Fail. Rev. 2000;5:73–86. doi: 10.1023/A:1009850224802. [DOI] [PubMed] [Google Scholar]
- Pfister J, Spengler C, Grouzmann E, Raizada MK, Felix D, Imboden H. Intracellular staining of angiotensin receptors in the PVN and SON of the rat. Brain Res. 1997;754:307–310. doi: 10.1016/s0006-8993(97)00180-7. [DOI] [PubMed] [Google Scholar]
- Pyner S, Coote JH. Identification of an efferent projection from the paraventricular nucleus of the hypothalamus terminating close to spinally projecting rostral ventrolateral medullary neurons. Neuroscience. 1999;88:949–957. doi: 10.1016/s0306-4522(98)00255-3. [DOI] [PubMed] [Google Scholar]
- Reddy MK, Patel KP, Schultz HD. Differential role of the paraventricular nucleus of the hypothalamus in modulating the sympathoexcitatory component of peripheral and central chemoreflexes. Am. J. Physiol Regul. Integr. Comp Physiol. 2005;289:R789–R797. doi: 10.1152/ajpregu.00222.2005. [DOI] [PubMed] [Google Scholar]
- Redgate ES. Hypothalamic influence on respiration. Ann. N. Y. Acad. Sci. 1963;109:606–618. doi: 10.1111/j.1749-6632.1963.tb13491.x. [DOI] [PubMed] [Google Scholar]
- Ross CA, Ruggiero DA, Joh TH, Park DH, Reis DJ. Rostral ventrolateral medulla: selective projections to the thoracic autonomic cell column from the region containing C1 adrenaline neurons. J. Comp Neurol. 1984;228:168–185. doi: 10.1002/cne.902280204. [DOI] [PubMed] [Google Scholar]
- Sakumoto T, Tohyama M, Satoh K, Kimoto Y, Kinugasa T, Tanizawa O, Kurachi K, Shimizu N. Afferent fiber connections from lower brain stem to hypothalamus studied by the horseradish peroxidase method with special reference to noradrenaline innervation. Exp. Brain Res. 1978;31:81–94. doi: 10.1007/BF00235806. [DOI] [PubMed] [Google Scholar]
- Sanchez F, Alonso JR, Arevalo R, Blanco E, Aijon J, Vazquez R. Coexistence of NADPH-diaphorase with vasopressin and oxytocin in the hypothalamic magnocellular neurosecretory nuclei of the rat. Cell Tissue Res. 1994;276:31–34. doi: 10.1007/BF00354781. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE. Anatomic relationships between the paraventricular nucleus of the hypothalamus and visceral regulatory mechanisms: Implications for the control of feeding behavior. In: Hoebel BG, Novin D, editors. Neural Basis of Feeding and Reward. Brunswick, ME: Haer Inst; 1982. pp. 259–2574. [Google Scholar]
- Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res. 1982a;257:275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 1982b;205:260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- Schelling P, Ganten D, Heckl R, Haydirk K, Hutchinson JS, Sponer G, Ganten U. On the origin of angiotensin-like peptides in cerebrospinal fluid. In: Buckley JP, Ferrario CM, editors. Central Actions of Angiotensin and Related Hormones. NewYork: Pergamon; 1977. pp. 519–526. [Google Scholar]
- Schiavone MT, Santos RA, Brosnihan KB, Khosla MC, Ferrario CM. Release of vasopressin from the rat hypothalamo-neurohypophysial system by angiotensin-(1–7) heptapeptide. Proc. Natl. Acad. Sci. U. S. A. 1988;85:4095–4098. doi: 10.1073/pnas.85.11.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenker E, Barnes L, Hansen S, Martin D. Cardiorespiratory and metabolic responses to injection of bicuculline into the hypothalamic paraventricular nucleus (PVN) of conscious rats. Brain Res. 2001;895:33–40. doi: 10.1016/s0006-8993(01)02011-x. [DOI] [PubMed] [Google Scholar]
- Shi L, Mao C, Wu J, Morrissey P, Lee J, Xu Z. Effects of i.c.v. losartan on the angiotensin II-mediated vasopressin release and hypothalamic fos expression in near-term ovine fetuses. Peptides. 2006;27:2230–2238. doi: 10.1016/j.peptides.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Simonnet G, Rodriguez F, Fumoux F, Czernichow P, Vincent JD. Vasopressin release and drinking induced by intracranial injection of angiotensin II in monkey. Am. J Physiol. 1979;237:R20–R25. doi: 10.1152/ajpregu.1979.237.1.R20. [DOI] [PubMed] [Google Scholar]
- Sladek CD, Joynt RJ. Angiotensin stimulation of vasopressin release from the rat hypothalamo-neurohypophyseal system in organ culture. Endocrinology. 1979;104:148–153. doi: 10.1210/endo-104-1-148. [DOI] [PubMed] [Google Scholar]
- Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol. 2006;494:673–685. doi: 10.1002/cne.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Hartman BK. Biochemical specificity in central pathways related to peripheral and intracerebral homeostatic functions. Neurosci. Lett. 1980;16:55–60. doi: 10.1016/0304-3940(80)90100-7. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Mogenson GJ. Neural mechanisms for the functional coupling of autonomic, endocrine and somatomotor responses in adaptive behavior. Brain Res. 1981;228:1–34. doi: 10.1016/0165-0173(81)90010-2. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Berod A, Hartman BK, Helle KB, Vanorden DE. An immunohistochemical study of the organization of catecholaminergic cells and terminal fields in the paraventricular and supraoptic nuclei of the hypothalamus. J Comp Neurol. 1981;196:271–285. doi: 10.1002/cne.901960207. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu. Rev. Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Tagawa T, Dampney RA. AT(1) receptors mediate excitatory inputs to rostral ventrolateral medulla pressor neurons from hypothalamus. Hypertension. 1999;34:1301–1307. doi: 10.1161/01.hyp.34.6.1301. [DOI] [PubMed] [Google Scholar]
- Takagi H, Shiosaka S, Tohyama M, Senba E, Sakanaka M. Ascending components of the medial forebrain bundle from the lower brain stem in the rat, with special reference to raphe and catecholamine cell groups. A study by the HRP method. Brain Res. 1980;193:315–337. doi: 10.1016/0006-8993(80)90168-7. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Dreifuss JJ. Localization of neurones projecting to the hypothalamic paraventricular nucleus area of the rat: a horseradish peroxidase study. Neuroscience. 1981;6:1315–1328. doi: 10.1016/0306-4522(81)90190-1. [DOI] [PubMed] [Google Scholar]
- Unger T, Rascher W, Schuster C, Pavlovitch R, Schomig A, Dietz R, Ganten D. Central blood pressure effects of substance P and angiotensin II: role of the sympathetic nervous system and vasopressin. Eur. J Pharmacol. 1981;71:33–42. doi: 10.1016/0014-2999(81)90384-8. [DOI] [PubMed] [Google Scholar]
- Van den Pol AN. Glutamate and aspartate immunoreactivity in hypothalamic presynaptic axons. J Neurosci. 1991;11:2087–2101. doi: 10.1523/JNEUROSCI.11-07-02087.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SR, Kimura H. Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience. 1992;46:755–784. doi: 10.1016/0306-4522(92)90184-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Share L, Shade RE. Effect of ventriculo-cisternal perfusion with angiotensin II and indomethacin on the plasma vasopressin concentration. Neuroendocrinology. 1978;25:166–173. doi: 10.1159/000122738. [DOI] [PubMed] [Google Scholar]
- Yang Z, Coote JH. Influence of the hypothalamic paraventricular nucleus on cardiovascular neurones in the rostral ventrolateral medulla of the rat. J Physiol. 1998;513(Pt 2):521–530. doi: 10.1111/j.1469-7793.1998.521bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Bertram D, Coote JH. The role of glutamate and vasopressin in the excitation of RVL neurones by paraventricular neurones. Brain Res. 2001;908:99–103. doi: 10.1016/s0006-8993(01)02593-8. [DOI] [PubMed] [Google Scholar]
- Yang Z, Wheatley M, Coote JH. Neuropeptides, amines and amino acids as mediators of the sympathetic effects of paraventricular nucleus activation in the rat. Exp. Physiol. 2002;87:663–674. doi: 10.1113/eph8702439. [DOI] [PubMed] [Google Scholar]
- Yang Z, Coote JH. The influence of vasopressin on tonic activity of cardiovascular neurones in the ventrolateral medulla of the hypertensive rat. Auton. Neurosci. 2003;104:83–87. doi: 10.1016/S1566-0702(02)00288-6. [DOI] [PubMed] [Google Scholar]
- Yeh ER, Erokwu B, LaManna JC, Haxhiu MA. The paraventricular nucleus of the hypothalamus influences respiratory timing and activity in the rat. Neurosci. Lett. 1997;232:63–66. doi: 10.1016/s0304-3940(97)00579-x. [DOI] [PubMed] [Google Scholar]
- Yoshimatsu H, Niijima A, Oomura Y, Yamabe K, Katafuchi T. Effects of hypothalamic lesion on pancreatic autonomic nerve activity in the rat. Brain Res. 1984;303:147–152. doi: 10.1016/0006-8993(84)90222-1. [DOI] [PubMed] [Google Scholar]
- Zhang K, Mayhan WG, Patel KP. Nitric oxide within the paraventricular nucleus mediates changes in renal sympathetic nerve activity. Am. J Physiol. 1997;273:R864–R872. doi: 10.1152/ajpregu.1997.273.3.R864. [DOI] [PubMed] [Google Scholar]
- Zhang K, Patel KP. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: role of GABA. Am. J Physiol. 1998;275:R728–R734. doi: 10.1152/ajpregu.1998.275.3.R728. [DOI] [PubMed] [Google Scholar]
- Zhang K, Zucker IH, Patel KP. Altered number of diaphorase (NOS) positive neurons in the hypothalamus of rats with heart failure. Brain Res. 1998;786:219–225. doi: 10.1016/s0006-8993(97)01449-2. [DOI] [PubMed] [Google Scholar]
- Zhang K, Li YF, Patel KP. Reduced endogenous GABA-mediated inhibition in the PVN on renal nerve discharge in rats with heart failure. Am. J Physiol Regul. Integr. Comp Physiol. 2002;282:R1006–R1015. doi: 10.1152/ajpregu.00241.2001. [DOI] [PubMed] [Google Scholar]
- Zheng JQ, Seki M, Hayakawa T, Ito H, Zyo K. Descending projections from the paraventricular hypothalamic nucleus to the spinal cord: anterograde tracing study in the rat. Okajimas Folia Anat. Jpn. 1995;72:119–135. doi: 10.2535/ofaj1936.72.2-3_119. [DOI] [PubMed] [Google Scholar]
- Zhu GQ, Gao L, Patel KP, Zucker IH, Wang W. ANG II in the paraventricular nucleus potentiates the cardiac sympathetic afferent reflex in rats with heart failure. J Appl. Physiol. 2004;97:1746–1754. doi: 10.1152/japplphysiol.00573.2004. [DOI] [PubMed] [Google Scholar]