Abstract
Mice with genomic knockout of either melanocortin type 3 receptors (MC3R-/-), type 4 receptors (MC4R-/-) or knockout of both (double knockout, DKO) were tested for their anorectic response to the mixed MC3/4R agonist, MTII, injected into the anterior cerebral ventricle. Wild type (WT) mice showed a strong anorexia and, as expected, DKO were completely unresponsive to MTII. In contrast, both MC3R-/- and MC4R-/- showed a partial anorectic response. Induction of c-Fos immunoreactivity by MTII was examined in brain regions including paraventricular hypothalamus (PVN) and area postrema (AP). Compared with WT, MC4R-/- showed no activation in AP but showed normal activation in PVN, whereas MC3R-/- showed reduced activation in PVN but not in AP. RT-PCR analysis showed that hypothalamic mRNA for MC3R in MC4R-/- and for MC4R in MC3R-/- was unaltered from WT levels. These data suggest that both receptor subtypes are involved in the behavioral action of MTII, and that the critical receptors are in different brain regions.
Keywords: Melanocortin receptors, food intake, MTII, paraventricular nucleus, area postrema, gene expression
1. Introduction
Mice with genomic knockout of the melanocortin type 4 receptor (MC4R-/-) are hypoactive, overeat, and become obese early in life [4,6]. In contrast, knockout of the melanocortin type 3 receptor (MC3R-/-) is not associated with significant overweight, although a small increase in body fat suggests altered metabolism or energy partitioning [2,3]. Interestingly, double knockout (DKO) of both type 3 and 4 receptors yield mice that are more obese than MC4R-/- [1,2] suggesting that the two receptors may have additive roles under some conditions.
Recently, we reported that daily food intake, under conditions of different levels of working for food, was elevated in both MC4R-/- and DKO at low costs, but fell below that of wild type (WT) littermates or of MC3R-/- at higher costs [1]. We also found that the anorexia to peripherally-administered cholecystokinin was reduced in MC3R-/- relative to other genotypes, and that c-Fos induced in PVN by CCK was likewise selectively reduced. These data further the case that MC3Rs may be involved in food intake. To test this in a more direct manner, we now report studies on food intake and c-Fos following central administration of the mixed MC3R/MC4R agonist, melanotan II (MTII) [5]. We also measured hypothalamic expression of MC3R and MC4R mRNAs in single knockouts, relative to WT.
2. Material and methods
2.1. Subjects and housing
MC3R-/- and MC4R-/- mice were bred in colonies at the Cancer Genetics vivarium at the University of Florida. These originated, respectively, from colonies at Merck Pharmaceutical Co and Millennium Pharmaceuticals Co. Both are on a C56BL/129 background. DKOs were generated by crossing the MC3R-/- and MC4R-/- mice. All mice were genotyped from a tail snip taken at weaning. Mice were moved to the Psychology Department vivarium for behavioral testing. Both facilities are managed by a fully-accredited animal care program.
The Psychology vivarium was maintained on a 12:12 light cycle (on: 0700-1900) at an ambient temperature of 23±2°C and relative humidity 50-70%. For at least a week before, and throughout behavioral testing, the mice were housed singly in standard polycarbonate cages with Sani Chips (Harlan, Madison WI) bedding and a red polycarbonate Igloo® (BioServ, Frenchtown NJ) as enrichment (25). Food (5001 pellets; PMI International, Brentwood MO) and tap water were available at all times, except as noted. Animal use was in accordance with principles in the NRC Guide for Care and Use of Laboratory Animals, and was approved by the University of Florida Institutional Animal Care and Use Committee. The mice used, of both sexes, were included in the food demand studies of our previous paper [1]. Their mean (+SE) body weights (g) at the time of surgery for this experiment were WT 27.4±1.8, MC3R-/- 31.9±2.4, MC4R-/- 47.9±3.5, DKO 50.4±3.5. Additional experimentally-naïve WT, MC3R-/- and MC4R-/- were used to assess hypothalamic gene expression.
2.2. Surgery
Mice of all 4 genotypes, 4-9 mo of age, were surgically fitted with a cannula (Plastics one, 27 gauge, 2 mm length below pedestal) aimed to end just above the right lateral cerebral ventricle. The surgery was performed using aseptic and stereotaxic techniques and isoflurane in oxygen as anesthesia. The cannula was secured to the skull using a tissue bonding glue and dental acrylic. Animals were given postoperative analgesic (Ketorolac, 5 mg/kg), and allowed 1 week for recovery during which time they were handled frequently.
2.3. Feeding tests
Mice were deprived of food overnight (~18 h) and at the end of that time were held gently in a towel and injected cerebroventricularly with either sterile saline (1 μl) or MTII (1 μg/1 μl saline) over a 15 sec period. Each mouse was replaced in its home cage and a weighed pellet of chow (Purina 5001) was placed into the cage 5 min later. The pellet was removed and weighed after 30 min to determine intake. The procedure was repeated 4-5 days later, but with the alternate injection. Half the mice received saline first and half received MTII. There was no order effect, so the data were combined for analysis. Intake after saline differed between genotypes, so intakes after MTII were analyzed raw and as % of intake after vehicle.
2.4. C-Fos immunoreactivity
After completion of the acute feeding studies, the same mice now freely fed were used to study induction of c-Fos immunoreactivity. This was performed as several batches, with all genotypes represented in each batch; no differences were found as a result of batch, so data were combined, as planned. On each test day, chow pellets were removed from the home cages 1-2 h beforehand to prevent recent spontaneous meals.
Mice received central injection of MTII (5 μg/1 μl) or saline (1 μl), as described above, and were returned to their cage without food. After 60 min, mice were anesthetized (Sleepaway, 1 ml/kg; Fort Dodge), and perfused transcardially with heparinized saline followed by para-formaldehyde. The processing of coronal brain sections c-Fos immunoreactivity at the levels of the paraventricular nucleus (PVN) of the hypothalamus, area postrema (AP), and nucleus of the tractus solitarius (NTS) has been described elsewhere [1,6]. Fos positive cells in these regions were counted under a microscope by two observers; animal identifiers were obscured during this phase. The counts from the two observers showed <5% variation and were averaged. There was minimal c-Fos in saline-treated mice of any genotype, so we combined all saline-treated mice into a single basal group. These data were not included in the statistical analysis
2.5. Receptor mRNA expression
mRNA expression was assessed using RT-PCR as described elsewhere [4]. Whole hypothalamus (~20 mg) was dissected using a blade and forceps, RNA extracted, and cDNA generated. The RT-PCR results were normalized to Hprt1 housekeeping gene and the fold difference over Hprt1 calculated using the 2-ΔCt method, with threshold 10x noise level.
2.6. Statistical analysis
Data were analyzed using one-way ANOVA (SPSS) and post hoc Tukey tests were used to examine the genotype effects on the % suppression of food intake or the numbers of Fos-stained cells in each region of treated mice.
3. Results
3.1. Anorectic action MTII
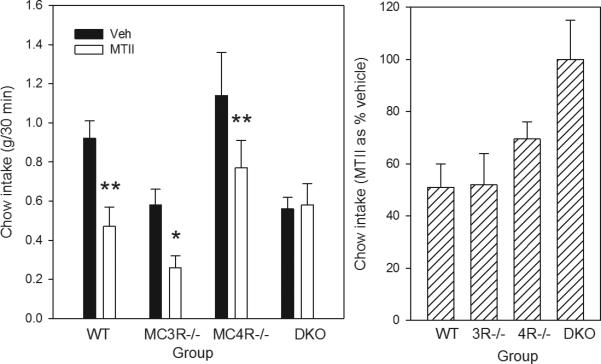
The 30 min intake of chow (Figure 1) differed as a function of genotype (F3,17=6.11, p<0.01). Mean intake after vehicle injection did not differ in any group compared with WT, but intake of MC4R-/- mice was higher than that of either MC3R-/- or DKO (Ps<0.05). Thus, MTII data were analyzed both as absolute intake (left panel) and as % decrease from saline (right panel). MTII reduced the food eaten relative to saline in WT by 47% (P<0.01, paired t-test), and in MC3R-/-and MC4R-/- by 56% and 32% respectively (Ps<0.05). Intake in DKO mice was unaffected by MTII.
Figure 1.
Left panel shows intake of chow in 30 min after 18 h food deprivation in WT, MC3R-/-, MC4R-/- and DKO mice given lateral cerebroventricular injection of either vehicle (VEH: saline) or MTII (1 nmol). *P<0.05, **P<.0.01 MTII vs Vehicle. Shown are means ± SE for group sizes of 6-8. The right panel shows the same data with the intake after MTII expressed as % of intake after vehicle.
3.2. C-Fos immunoreactivity
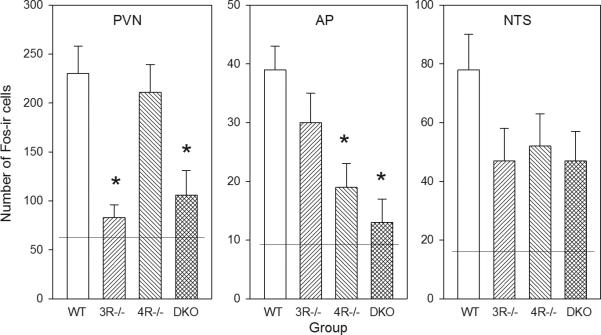
The number of Fos-ir cells in MTII-treated groups (Figure 2) differed as a function of genotype in PVN (F3,25=9.17, P<0.01) and AP (F3,24=7.54, P<0.01), but not in NTS (F3,24=1.88). In the PVN, Fos was induced strongly in WT and MC4R-/-, but much less in MC3R-/- and DKO (Ps<0.05). Fos-ir in these latter two groups did not differ from the mean in mice not receiving MTII. In contrast, in the AP, Fos was induced strongly in WT and MC3R-/- but less so in MC4R-/- and DKO (Ps<0.05). The activation in AP of MC3R-/- was significantly higher than in both DKO and the mean from mice not receiving MTII.
Figure 2.
Number of Fos-ir cells after lateral cerebroventricular injection of MTII (5 nmol). Data were averaged in PVN, AP and NTS from the section with the most cells for each mouse. Shown are means ± SE for group sizes of 5-7. *P<0.05 vs corresponding WT value. The horizontal lines are the mean numbers of cells in vehicle-injected controls.
3.3. Receptor mRNA expression
The expression of hypothalamic MC3R mRNA was 1.03±.12 in WT and 1.29±.10 in MC4R-/- mice (M±SE for Ns=5-6; data as fold-increase relative to Hprt1). The corresponding expression of MC4R mRNA was 1.01±.07 in WT and 1.02±.04 in MC3R-/-. Neither of these differences was significant by t-tests.
4. Discussion
These data suggest a dual region of action for MTII in relation to food intake. In our hands, MTII produced anorexia in both MC3R-/- and MC4R-/-, suggesting that stimulation of either receptor can produce anorexia. MTII-induced Fos-ir was absent in the PVN of MC3R-/-, but was normal in MC4R-/-, suggesting that MC3Rs alone underlie the induction of Fos in the PVN. In contrast, in the AP, only the MC4R-/- mice had reduced Fos-ir relative to WT, suggesting that MC4Rs alone underlie the induction of Fos induction in the AP. It should be noted that these Fos data do not tell us whether these are direct or indirect effects of MTII, or how far MTII may diffuse from the ventricular space.
A function for MC3Rs was first suspected from findings that, relative to +/+ littermates, MC3R -/- mice weighed the same but had up to 40% more body fat with reciprocal change in body length and lean body mass [2,3]. Ligand binding studies show that MC3Rs are abundant in forebrain regions including the hypothalamus [7]. The present results suggest an important inhibitory role for MC3Rs in the PVN in food intake. MC4R-/- mice, which express only MC3Rs, showed some inhibition of food intake to lateral cerebroventricular injection of MTII. This result was unexpected because Marsh [8] reported that food deprived MC4R-/- mice did not reduce food intake to dorsal 3rd ventricular injection of 1 μg MTII, the same dose as used in the present study. The differences in procedure include site of injection and age; it is not obvious why the lateral but not the 3rd ventricular injection might have better access to PVN. Additional studies will be needed to clarify the basis of these discrepancies. DKO mice were unresponsive to MTII, implicating both MC3 and 4Rs in the MTII anorexia.
Our Fos-ir studies further indicate functional MC3Rs in forebrain areas associated with feeding. As has been reported after 3rd ventricular injection of MTII in rats [9], Fos-ir was induced in PVN, AP, and NTS of WT mice after lateral ventricular injection. The activation in PVN was completely absent in MC3R-/- and in DKO, indicating that it is dependent on only the type 3 receptor. In contrast, Fos-ir induced by MTII was only slightly and not significantly reduced in AP or NTS of MC3R-/- compared to WT, but was completely absent in MC4R-/- and DKO. Importantly, and consistent with data from sedentary MC4R-/- mice in a previous study [4], we found no change in whole hypothalamus MC3R mRNA relative to WT. We additionally find no change in MC4R mRNA in MC3R-/- mice. These data do not rule out the possibility of changes in more discrete regions or in regions of the brain that were not sampled, but strongly suggest that absence of one receptor type does not globally influence the expression of the other.
In conclusion, we suggest that direct activation of MC3Rs in PVN is sufficient for an anorectic effect of MTII. The present data could also indicate a reduced sensitivity to MTII in either MC3R-/- or MC4R-/- compared with WT; a dose-response analysis will be needed to address this question.
Acknowledgements
This work was funded in part by NIH grants RO1DK064712 and RO1DK057080. We thank Dr. Dennis Huszar and Millennium Pharmaceuticals/Genelogic for providing the breeding stock MC4R-/- mice, Dr. Lex VanDerPloeg and Merck Research for providing the breeding stock MC3R-/- mice, and both David White at Genelogic and Merck Research for allowing us to generate and study the MC3R-/- × MC4R-/- double knockout mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Atalayer D, Robertson KL, Haskell-Luevano C, Andreasen A, Rowland NE. Food demand and meal size in mice with single or combined disruption of melanocortin type 3 and 4 receptors. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1667–74. doi: 10.1152/ajpregu.00562.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–21. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 3.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan X, Yu H, et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nature Genetics. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 4.Haskell-Luevano C, Schaub JW, Andreasen A, Haskell KR, Moore MC, Koerper LM, Rouzard F, Baker HV, Millard WJ, Walter G, Litherland SA, Xiang Z. Voluntary exercise prevents the obese and diabetic metabolic syndrome of the melanocortin-4 receptor knockout mouse. FASEB J. 2009;23:642–55. doi: 10.1096/fj.08-109686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hruby VJ, Lu D, Sharma SD, Castrucci AL, Kesterson RA, al-Obeidi FA, Hadley ME, Cone RD. Cyclic lactam alpha-melanotropin analogues of Ac-Nle4-cyclo[Asp5, D-Phe7,Lys10] alpha-melanocyte-stimulating hormone-(4-10)-NH2 with bulky aromatic amino acids at position 7 show high antagonist potency and selectivity at specific melanocortin receptors. J Med Chem. 1995;38:3454–61. doi: 10.1021/jm00018a005. [DOI] [PubMed] [Google Scholar]
- 6.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Smith FJ, Boston BA, Fang Q, Berkemeir LR, Gu W, Cone RD, Campfield LA, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–41. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 7.Lindblom J, Schioth HB, Larsson A, Wikberg JES, Bergstrom L. Autoradiographic discrimination of melanocortin receptors indicates that the MC3 subtype dominates in the medial rat brain. Brain Res. 1998;810:161–71. doi: 10.1016/s0006-8993(98)00918-4. [DOI] [PubMed] [Google Scholar]
- 8.Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KAm, Fisher SL, Burn P, Palmiter RD. Response of melanocortin-4-receptor-deficient mice to anorectic and orexigenic peptides. Nature Genetics. 1999;21:119–22. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- 9.Thiele TE, Van Dijk G, Yagaloff KA, Fisher SL, Schwartz MW, Burn P, Seeley RJ. Central infusion of melanocortin agonist MTII in rats: assessment of c-Fos expression and taste aversion. Am J Physiol Regul Integr Comp Physiol. 1998;274:R248–54. doi: 10.1152/ajpregu.1998.274.1.R248. [DOI] [PubMed] [Google Scholar]