Summary of recent advances
There is a growing body of data supporting a role for non-HLA antibodies in acute and chronic rejection of solid organ transplants. While many of these non-HLA antigens remain poorly defined, the principle antigenic targets are expressed on cells of the allograft including endothelium and epithelium. These non-HLA antigens are classified as either alloantigens, such as the major histocompatibility complex class I chain-related gene A (MICA) or MICB, or tissue-specific autoantigens such as vimentin, cardiac myosin (CM), collagen V (Col V), agrin and angiotensin II receptor type I (AT1). Herein we provide an overview of the non-MHC antigenic targets that have been implicated in graft rejection and discuss the interplay between alloimmunity and autoreactivity in graft rejection.
Introduction
Antibody-mediated rejection (AMR) remains a major limitation to solid organ transplantation. The development of more sensitive tests for detection of complement split products in the allograft and for the presence of circulating HLA antibodies have improved our ability to diagnose and treat AMR. Numerous studies have shown that the presence of donor specific HLA antibodies either before and/or after allograft transplantation is associated with acute and chronic AMR and decreased long-term graft survival. More recently, antibodies against non-HLA antigens have also been recognized to contribute to the pathogenesis of AMR as both hyperacute and acute rejection have occurred in the absence of detectable HLA antibodies [2,4-5]. The clinical importance of sensitization to non-HLA antigens is underscored by reports of hyperacute rejection of HLA-identical sibling grafts [1-3]. Pre-transplant sensitization to non-HLA antigens was strongly associated with long-term graft loss of HLA-identical sibling renal transplants suggesting that non-HLA immunity also plays a role in the pathogenesis of chronic rejection [6]. The targets of humoral responses against non-HLA antigens are primarily antigens expressed on endothelial cells and epithelial cells and categorized as non-HLA alloantigens or tissue-specific autoantigens.
Non-MHC Alloantigens
Anti-endothelial cell antibodies
Anti-endothelial cell antibodies (AECA) have been reported to mediate endothelial cell (EC) activation, apoptosis and cell injury. AECAs represent a heterogeneous group of antibodies comprising both IgM and IgG subclasses and are directed against a variety of antigenic determinants on ECs [7]. Pretransplant AECA are associated with increased frequency of acute renal rejection and decreased long-term graft survival [8]. To investigate how AECA are involved in acute renal allograft rejection, eluates from 25 renal allografts were tested for anti-EC antibodies. Eight of 9 patients with irreversible vascular renal allograft rejection had IgM AECAs eluted from the rejected kidney, but AECA were absent in the 13 kidneys lost to other types of rejection [9]. These AECA were able to activate EC resulting in upregulation of mRNAs encoding the adhesion molecules VACM-1 and ICAM-1. In a cohort of 57 renal transplant candidates, AECA were present in 47% of patients who were sensitized to HLA and in 16% of nonsensitzed patients [10]. Although, these antibodies were mainly of the IgG isotype and did not mediate cytotoxicity, they were able to cause apoptosis of ECs in vitro. No significant correlation was found between the presence of AECAs and graft outcome in this cohort.
In cardiac transplantation, 17/31 patients who developed posttransplant AECA experiencedAMR compared to only 9 of 49 patients without AECAs [11]. In addition, allograft survival at 2 years was significantly better in the AECA- group compared to the AECA+ group and AECA positivity was associated with cardiac allograft vasculopathy (CAV). Pretransplant cytotoxic IgM non-HLA antibodies were as associated with a diagnosis of primary graft failure and worse survival of cardiac transplant recipients [12]. Collectively, these studies suggest that AECA may cause AMR and identify a high-risk group for CAV.
A major limitation of these studies is the lack of knowledge of the antigenic specificity of the AECA. Current lymphocyte crossmatching techniques fail to detect AECA. The XM-One assay is a novel endothelial cell flow cytometry crossmatch technique that uses Tie-2 antibody coated magnetic beads to select precursor EC directly from donor blood [13]. Results of a multicenter clinical trial evaluating the association of AECAs with renal allograft rejection showed that pretransplant donor reactive AECAs were present in significantly higher proportion of patients with rejection [13]. Additional studies are needed to confirm if this crossmatch method is useful for identifying clinically relevant AECA.
MICA
MICA is encoded by genes located within the MHC region on chromosome 6 just centromeric to HLA-B. MICA is highly polymorphic with over 68 MICA alleles. MICA is considered as a plausible target of allograft response because of its polymorphic nature and the fact that endothelial cells can express MICA on their surface under stress due to ischemia reperfusion injury and rejection [14-15]. MICA antibodies associate with acute and chronic rejection of heart, renal and pancreas transplants [14,16-20]. In a large multicenter study, pretransplant MICA antibodies were found in 217 of the 1910 patients tested and was associated with renal graft rejection and lower one-year survival [20]. The long term effect of anti-MICA antibodies was investigated in a prospective multicenter study of 1319 renal transplant recipients [19]. Patients developing posttransplant MICA antibodies had a significantly lower 4-year allograft survival (86%) compared to those without antibodies (98%). A limitation of these studies is that they failed to discriminate between donor specific vs. third party anti-MICA antibodies. Anti-MICA antibodies have been found to mediate complement dependent cytotoxicity in vitro [21] suggesting that they may contribute to the pathogenesis of AMR through complement mediated injury. A recent study addressed the question of C4d deposition in kidney biopsy and donor specific antibodies (DSA) to HLA, MICA and GSTT1 [22]. They showed the majority of patients with C4d+ biopsies had DSA to HLA (47%), MICA (21%) or GSSTT1.
Two reports assessed the effect of MICA DSA on cardiac allograft outcome. In a study of 44 heart recipients, 60% of patients with acute rejection produced MICA DSA compared to 14% without rejection [18]. In the second study, pre- and posttransplant sera from 491 heart transplant recipients were studied for DSA to MICA. They found no effect of sensitization to MICA on episodes of rejection or CAV. The lack of concordance between these studies may be due to small sample size and/or differences in the timing of sample collection. Interestingly, both groups reported an absence of MICA expression on cardiac endothelial cells suggesting that MICA is not constitutively expressed in the transplanted heart. However, MICA genes contain a heat shock response element promoter and their expression can be induced in response to cellular stress. Ischemia reperfusion injury and cytokines such as IL-2, IL-4 and IL-15 that are produced during inflammation and rejection can also upregulate the expression of MICA in the graft [23-24]. Further studies are needed to determine the expression pattern of MIC in solid organ transplants during quiescence and rejection.
Since membrane bound MICA proteins can be upregulated during inflammation and rejection, and soluble MICA is increased in the circulation of transplant recipients [25] we posit that MICA alloreactive T cells responding via the indirect pathway are primed to donor derived soluble MICA antigens in the context of self MHC class II and induce anti-MICA antibody responses. Consistent with this possibility, several studies have shown that antibodies to MICA are produced after transplantation and their frequency is higher in regraft patients [26]. Furthermore, the immune response to mismatched HLA lead to the development of antibodies to MICA antigens expressed on the airway epithelial cells of lung transplants [27].
Tissue Specific Antigens
Vimentin
Vimentin is a non-polymorphic intermediate filament expressed in cytosol of endothelial, vascular smooth muscle cells, activated platelets and macrophages, renal tubular cells, mesangial cells and renal stromal cells. Vimentin is strongly expressed in the intima and media of coronary arteries where vascular smooth muscle cells and fibroblasts locate. Autoimmune responses to vimentin are associated with both acute and chronic rejection of heart and renal allografts. Cardiac transplant recipients developing CAV show significantly higher titers of anti-vimentin antibodies in the first and second year posttransplant than patients who remained disease free [28]. Production of humoral immune responses to vimentin was also accompanied by the generation of vimentin-specific autoreactive CD8 positive T cells in cardiac transplant recipients [29]. Furthermore, sera containing anti-vimentin antibodies induced leukocytes, to release of platelet activating factor which in turn cased the formation of platelet-leukocyte conjugates [30]. Studies in non-human primates confirmed the findings in human heart recipients and showed that development of cellular and humoral autoimmune responses to vimentin was a prominent feature of allograft rejection and CAV [31]. Immunization of mice with vimentin resulted in development of anti-vimentin antibodies and vimentin-specific T cells and accelerated rejection of cardiac allografts, but not isografts [32]. Furthermore, anti-vimentin antibodies were necessary to cause rejection as shown by the ability of adoptively transferred serum to restore accelerated rejection in B cell deficient mice. Thus, it appears that anti-vimentin antibodies alone are insufficient to cause graft rejection and rather act in concert with the alloimmune response. An important question that emerges from these findings is how vimentin antibodies are pathogenic to the process of rejection. One theory suggests that alloreactive immune response mediate graft injury, apoptosis of endothelial cells and subsequent exposure of neoantigens such as vimentin causing an autoimmune response. Anti-vimentin antibodies bind to vimentin positive platelets, leukocytes and endothelial cells causing complement deposition and leukocyte-platelet aggregation in the microcirculation of the graft [32]. In addition, cross-reactivity between anti-streptococcal antibodies and vimentin/cardiac myosin has been described, suggesting a possible mechanism contribute to myocyte injury [33].
Cardiac Myosin
Cardiac myosin (CM) is a heart specific antigen implicated in allograft rejection [34]. Pretransplant myosin autoantibodies correlated with acute cardiac transplant rejection [35]. The expansion of alloreactive T cells was followed by an increase of cardiac myosin reactive T cells and development of anti-myosin IgG1 autoantibodies in a mouse heart transplant model mismatched for minor histocompatibility alloantigens [36]. This supports the idea that CM released during alloimmune injury of the allograft is recognized by CD4+ T helper autoreactive cells through indirect recognition pathway and triggers the generation of autoreactive CM antibodies. Notably, mature CM is not expressed in the thymus during development which may result in incomplete negative selection [37].
Collagen V
Both cellular and humoral responses to Col V act as a major risk factor in the development ofbronchiolitis obliterans syndrome (BOS) after human lung transplantation [38]. Col V is usually interstitial and not normally exposed in healthy tissue. However, Col V is unveiled during ischemia reperfusion injury or in interstitial remodeling of lung transplants and can be detected in bronchoalveolar lavage fluid. Transfer of anti-Col V antibodies to rat lung isografts induced pathology consistent with primary graft dysfunction and mediated epithelial cell cytotoxicity [39]. Col V-specific T cells appear in human and rat lung transplant recipients before the clinical onset of BOS and adoptive transfer of Col V reactive T cells induced rejection [40]. Cellular injury to Col V was mediated by IL17A which recruits monocytes and neutrophils and acts in synergy with other local inflammatory cytokines [38,41]. Th17 cells have been implicated in a number of autoimmune or inflammatory conditions and in models of allograft rejection [42]. In the absence of Th1-mediated alloimmune responses, CD4 Th17 cells mediate an aggressive proinflammatory response leading to cardiac allograft rejection and CAV [43]. Furthermore, Th17-mediated acute lung transplant rejection could be prevented by adoptive transfer of CD4+ Col V specific T regulatory cells [40].
K-α1 tubulin
K-α1 tubulin is a glycoprotein expressed in air way epithelial cells and is constitutively associated to a guanosine triphosphate (GTP). It forms microtubules in cells and plays an important role in maintaining cellular structure, microtubule-based intracellular movement. K-α1 tubulin is not normally expressed on cell surface, however epithelial cell damage can result in the exposure of K-α1 tubulin which may promote autoimmune responses. Goers et al.[44] Showed that 12/36 lung transplant recipients developed anti-K-α1 tubulin antibodies posttransplant in the absence of HLA sensitization and was strongly associated with BOS. The binding of K-α1 tubulin antibodies to airway epithelial cells activated a PKC-driven calcium maintenance pathway and stimulated expression of transcription factors and fibrogenic growth factors culminating in cell cycle signaling and fibroproliferation. To determine if alloimmunity induces pathogenic autoimmune responses, anti-MHC antibodies were administered intrabronchially into the native lungs of mice. Lungs of mice receiving anti-MHC class I antibodies showed increased expression of IL-17 and they developed antibodies to self-antigens K-α1 tubulin, and collagen V [45]. IL-17 neutralization resulted in reduction of autoantibody and lesions induced by anti-MHC class I antibodies. These results indicate that antibodies to donor MHC can induce pathogenic autoimmune response which may play a pivotal role in chronic rejection.
Angiotensin II receptor type I
Angiotensin type 1 receptor (AT1R) is the main receptor for angiotensin II in the glomerulus and mediates arterial blood pressure and salt balance. It is also expressed in the brush border and basolateral membranes of the proximal tubules, in the vasculature, and in other components of the kidney. Anti- AT1R-antibodies were found in 16/20 recipients with renal refractory vascular rejection who had no HLA antibodies [46]. Removal of AT1R-antibodies by plasmapheresis in combination with intravenous immunoglobulin (IVIG) and pharmacologic AT1R blockade improved renal function and graft survival in 7/16 patients as compared to the remaining 9 patients with conventional treatment. In addition, passive transfer of human AT1R-antibodies into rats induced endarteritis and intravascular infiltrates within one week. The AT1R-antibodies were complement-fixing IgG1 and IgG3 isotypes, however, C4d deposition was only detected in 5 out these 16 patients, suggesting the pathogenesis of AT1R-antibodies may be complement independent. AT1R-antibodies were shown to promote inflammatory responses and contribute to allograft rejection through the phosphorylation of ERK kinase and activation of AP-1 and nuclear factor κB(NF-κB) resulting in the production of tissue factor and reactive oxygen species. Blockade of NF- κB with decoy oligodeoxynucleotides reduced tubulointerstitial infiltration in rat renal allografts.
Natural Antibodies and Ischemia Reperfusion Injury
Recent studies have implicated IgM natural antibodies as self-targets in the pathogenesis of ischemia reperfusion injury (IRI). Studies of different animal IRI models showed that reperfusion of ischemic tissues elicits an acute inflammatory response involving the complement system which is activated by autoreactive natural IgM [47]. Mice deficient in complement are protected against IRI. These studies suggest that hypoxia triggers the expression of neo-antigens and upon binding of the natural antibodies, initiates cellular recruitment and complement activation. Recent studies confirmed that human natural IgM could induce IRI injury in a murine intestinal model suggesting that innate autoimmunity may operate under pathogenic conditions in human [48] .However, whether similar mechanisms operate in humans is unknown. Candidate natural antibodies that have been shown to bind to ischemic endothelial cells include the nonmuscle myosin heavy chain type II A and C IgM antibodies.
Interplay between alloimmunity and autoreactivity in graft rejection
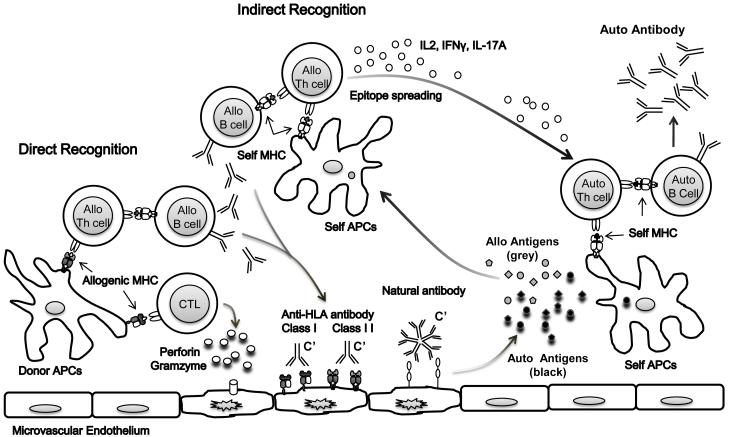
It is increasingly recognized that alloimmune responses and tissue specific autoimmune responses act in concert to promote graft rejection (Figure 1). Alloimmune responses occur through direct and indirect recognition. The direct pathway involves presentation of allogeneic MHC class I and II antigens on donor APCs to recipient T cells and is believed to be the primary mechanism of acute rejection mediated by alloreactive cytotoxic T lymphocytes and alloantibodies resulting in graft injury. Graft injury causes the release of alloantigens and self-antigens which can in turn be presented via the indirect recognition pathway to generate pathogenic allo and autoreactive cellular and humoral immune responses [45]. The indirect pathway involves processing the donor alloantigens and/or self-antigens by recipient APCs and presentation to recipient T cells and is believed to be the major pathway for chronic rejection. Once initiated, the indirect alloimmune response can spread to additional determinants within the primary target antigen called intramolecular epitope spreading, or to epitopes on other allogeneic or self antigens called intermolecular epitope spreading [49].
Figure 1.
A model for interplay between alloimmunity and autoreactivity in graft rejection. Graft damage elicited by alloreactive T and B cells primed through the direct and/or indirect allorecognition pathways results in the release of alloantigens and self-antigens. Auto and alloantigens shed from the injured graft are processed and presented through the indirect recognition pathway by host antigen presenting cells in the context of HLA class II molecules. The development of autoreactive cellular and humoral immune responses to self-antigens is secondary to alloimmune responses where repeated exposure of recipient CD4+ T cells to self-antigens and pro-inflammatory cytokines (IL-2, INF γ, IL-17a) overrides the threshold for self-tolerance.
How alloimmunity leads to loss of tolerance to self-antigens in the transplant setting is not well understood but recent studies implicate alloreactive T cells in this process [36,45,50]. Murine skin allograft studies have shown that activation of indirectly alloantigen primed T cells can result in determinant spreading and the generation of pathogenic autoreactive T cells [50]. These findings suggest that the development of humoral responses to autoantigens could result as a consequence of alloimmune-mediated graft damage where repeated exposure of recipient CD4+ T cells to self-antigens surpasses the threshold of self-tolerance and leads to autoimmunity. Although the majority of autoreactive B cells in the periphery are functionally attenuated, [51] they can pose a danger in the development of rejection if T cell tolerance is breached permitting T cell helper activation of these autoreactive B cells. Experimental studies suggest that chronic stimulation with autoantigens can break T cell self tolerance. Tsumiyama et al [52] showed that repeated stimulation of CD4+ T cells with self-antigens led to the development of autoantibody-inducing CD4+ T cells. Thus autoimmunity resulted from over-stimulating the host’s immune response by repeated immunization with antigen.
Conclusions
Autoimmunity may be a consequence of alloreactivity induced after solid organ transplant. Continued efforts to define the non-HLA alloantigens and tissue-specific autoantigens involved in transplant rejection are critical to understanding the mechanisms and pathogenesis of non-HLA antibodies and development of treatment options.
Acknowledgements
Funding Sources: This work was supported by the National Institute of Allergy and Infectious Diseases Grant RO1 AI 42819 and NIH U01AI077821 and the National Heart Lung and Blood Institute Grant RO1 HL 090995 to E. F. R.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahern AT, Artruc SB, DellaPelle P, Cosimi AB, Russell PS, Colvin RB, Fuller TC. Hyperacute rejection of HLA-AB-identical renal allografts associated with B lymphocyte and endothelial reactive antibodies. Transplantation. 1982;33:103–106. doi: 10.1097/00007890-198201000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Amico P, Honger G, Bielmann D, Lutz D, Garzoni D, Steiger J, Mihatsch MJ, Dragun D, Schaub S. Incidence and prediction of early antibody-mediated rejection due to non-human leukocyte antigen-antibodies. Transplantation. 2008;85:1557–1563. doi: 10.1097/TP.0b013e31816f612a. [DOI] [PubMed] [Google Scholar]
- 3.Grafft CA, Cornell LD, Gloor JM, Cosio FG, Gandhi MJ, Dean PG, Stegall MD, Amer H. Antibody-mediated rejection following transplantation from an HLA-identical sibling. Nephrol Dial Transplant. 25:307–310. doi: 10.1093/ndt/gfp526. [DOI] [PubMed] [Google Scholar]
- 4.Brasile L, Rodman E, Shield CF, 3rd, Clarke J, Cerilli J. The association of antivascular endothelial cell antibody with hyperacute rejection: a case report. Surgery. 1986;99:637–640. [PubMed] [Google Scholar]
- 5.Sumitran-Karuppan S, Tyden G, Reinholt F, Berg U, Moller E. Hyperacute rejections of two consecutive renal allografts and early loss of the third transplant caused by non-HLA antibodies specific for endothelial cells. Transpl Immunol. 1997;5:321–327. doi: 10.1016/s0966-3274(97)80016-0. [DOI] [PubMed] [Google Scholar]
- 6.Opelz G. Non-HLA transplantation immunity revealed by lymphocytotoxic antibodies. Lancet. 2005;365:1570–1576. doi: 10.1016/S0140-6736(05)66458-6. [DOI] [PubMed] [Google Scholar]
- 7.Bordron A, Revelen R, D’Arbonneau F, Dueymes M, Renaudineau Y, Jamin C, Youinou P. Functional heterogeneity of anti-endothelial cell antibodies. Clin Exp Immunol. 2001;124:492–501. doi: 10.1046/j.1365-2249.2001.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ismail AM, Badawi RM, El-Agroudy AE, Mansour MA. Pretransplant detection of anti-endothelial cell antibodies could predict renal allograft outcome. Exp Clin Transplant. 2009;7:104–109. [PubMed] [Google Scholar]
- 9.Lucchiari N, Panajotopoulos N, Xu C, Rodrigues H, Ianhez LE, Kalil J, Glotz D. Antibodies eluted from acutely rejected renal allografts bind to and activate human endothelial cells. Hum Immunol. 2000;61:518–527. doi: 10.1016/s0198-8859(00)00109-9. [DOI] [PubMed] [Google Scholar]
- 10.Le Bas-Bernardet S, Hourmant M, Coupel S, Bignon JD, Soulillou JP, Charreau B. Non-HLA-type endothelial cell reactive alloantibodies in pre-transplant sera of kidney recipients trigger apoptosis. Am J Transplant. 2003;3:167–177. doi: 10.1034/j.1600-6143.2003.00021.x. [DOI] [PubMed] [Google Scholar]
- 11.Fredrich R, Toyoda M, Czer LS, Galfayan K, Galera O, Trento A, Freimark D, Young S, Jordan SC. The clinical significance of antibodies to human vascular endothelial cells after cardiac transplantation. Transplantation. 1999;67:385–391. doi: 10.1097/00007890-199902150-00008. [DOI] [PubMed] [Google Scholar]
- 12.Smith JD, Hamour IM, Burke MM, Mahesh B, Stanford RE, Haj-Yahia S, Robinson DR, Kaul P, Yacoub MH, Banner NR, et al. A reevaluation of the role of IgM Non-HLA antibodies in cardiac transplantation. Transplantation. 2009;87:864–871. doi: 10.1097/TP.0b013e31819a65fa. [DOI] [PubMed] [Google Scholar]
- 13.Breimer ME, Rydberg L, Jackson AM, Lucas DP, Zachary AA, Melancon JK, Von Visger J, Pelletier R, Saidman SL, Williams WW, Jr., et al. Multicenter evaluation of a novel endothelial cell crossmatch test in kidney transplantation. Transplantation. 2009;87:549–556. doi: 10.1097/TP.0b013e3181949d4e. •• Using a novel donor specific precursor endothelial cell crossmatch, this multicenter study demonstrated that presensitization to endothelial cell antigens was associated with acute renal allograft rejection.
- 14.Hankey KG, Drachenberg CB, Papadimitriou JC, Klassen DK, Philosophe B, Bartlett ST, Groh V, Spies T, Mann DL. MIC expression in renal and pancreatic allografts. Transplantation. 2002;73:304–306. doi: 10.1097/00007890-200201270-00029. [DOI] [PubMed] [Google Scholar]
- 15.Quiroga I, Salio M, Koo DD, Cerundolo L, Shepherd D, Cerundolo V, Fuggle SV. Expression of MHC class I-related Chain B (MICB) molecules on renal transplant biopsies. Transplantation. 2006;81:1196–1203. doi: 10.1097/01.tp.0000205788.05322.42. [DOI] [PubMed] [Google Scholar]
- 16.Mizutani K, Terasaki P, Rosen A, Esquenazi V, Miller J, Shih RN, Pei R, Ozawa M, Lee J. Serial ten-year follow-up of HLA and MICA antibody production prior to kidney graft failure. Am J Transplant. 2005;5:2265–2272. doi: 10.1111/j.1600-6143.2005.01016.x. [DOI] [PubMed] [Google Scholar]
- 17.Panigrahi A, Gupta N, Siddiqui JA, Margoob A, Bhowmik D, Guleria S, Mehra NK. Post transplant development of MICA and anti-HLA antibodies is associated with acute rejection episodes and renal allograft loss. Hum Immunol. 2007;68:362–367. doi: 10.1016/j.humimm.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Suarez-Alvarez B, Lopez-Vazquez A, Gonzalez MZ, Fdez-Morera JL, Diaz-Molina B, Blanco-Gelaz MA, Pascual D, Martinez-Borra J, Muro M, Alvarez-Lopez MR, et al. The relationship of anti-MICA antibodies and MICA expression with heart allograft rejection. Am J Transplant. 2007;7:1842–1848. doi: 10.1111/j.1600-6143.2007.01838.x. [DOI] [PubMed] [Google Scholar]
- 19.Terasaki PI, Ozawa M, Castro R. Four-year follow-up of a prospective trial of HLA and MICA antibodies on kidney graft survival. Am J Transplant. 2007;7:408–415. doi: 10.1111/j.1600-6143.2006.01644.x. [DOI] [PubMed] [Google Scholar]
- 20.Zou Y, Stastny P, Susal C, Dohler B, Opelz G. Antibodies against MICA antigens and kidney-transplant rejection. N Engl J Med. 2007;357:1293–1300. doi: 10.1056/NEJMoa067160. •• A large multicenter study shows that presensitization to MICA is associated with renal allograft rejection and poorer allograft survival.
- 21.Zou Y, Mirbaha F, Lazaro A, Zhang Y, Lavingia B, Stastny P. MICA is a target for complement-dependent cytotoxicity with mouse monoclonal antibodies and human alloantibodies. Hum Immunol. 2002;63:30–39. doi: 10.1016/s0198-8859(01)00349-4. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez-Marquez A, Aguilera I, Gentil MA, Caro JL, Bernal G, Fernandez Alonso J, Acevedo MJ, Cabello V, Wichmann I, Gonzalez-Escribano MF, et al. Donor-specific antibodies against HLA, MICA, and GSTT1 in patients with allograft rejection and C4d deposition in renal biopsies. Transplantation. 2009;87:94–99. doi: 10.1097/TP.0b013e31818bd790. [DOI] [PubMed] [Google Scholar]
- 23.Jinushi M, Takehara T, Tatsumi T, Kanto T, Groh V, Spies T, Suzuki T, Miyagi T, Hayashi N. Autocrine/paracrine IL-15 that is required for type I IFN-mediated dendritic cell expression of MHC class I-related chain A and B is impaired in hepatitis C virus infection. J Immunol. 2003;171:5423–5429. doi: 10.4049/jimmunol.171.10.5423. [DOI] [PubMed] [Google Scholar]
- 24.Roberts AI, Lee L, Schwarz E, Groh V, Spies T, Ebert EC, Jabri B. NKG2D receptors induced by IL-15 costimulate CD28-negative effector CTL in the tissue microenvironment. J Immunol. 2001;167:5527–5530. doi: 10.4049/jimmunol.167.10.5527. [DOI] [PubMed] [Google Scholar]
- 25.Suarez-Alvarez B, Lopez-Vazquez A, Diaz-Molina B, Bernardo-Rodriguez MJ, Alvarez-Lopez R, Pascual D, Astudillo A, Martinez-Borra J, Lambert JL, Gonzalez S, et al. The predictive value of soluble major histocompatibility complex class I chain-related molecule A (MICA) levels on heart allograft rejection. Transplantation. 2006;82:354–361. doi: 10.1097/01.tp.0000228911.22944.23. [DOI] [PubMed] [Google Scholar]
- 26.Mizutani K, Terasaki PI, Shih RN, Pei R, Ozawa M, Lee J. Frequency of MIC antibody in rejected renal transplant patients without HLA antibody. Hum Immunol. 2006;67:223–229. doi: 10.1016/j.humimm.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Angaswamy N, Saini D, Ramachandran S, Nath DS, Phelan D, Hachem R, Trulock E, Patterson GA, Mohanakumar T. Development of antibodies to human leukocyte antigen precedes development of antibodies to major histocompatibility class I-related chain A and are significantly associated with development of chronic rejection after human lung transplantation. Hum Immunol. 2010;71:560–565. doi: 10.1016/j.humimm.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jurcevic S, Ainsworth ME, Pomerance A, Smith JD, Robinson DR, Dunn MJ, Yacoub MH, Rose ML. Antivimentin antibodies are an independent predictor of transplant-associated coronary artery disease after cardiac transplantation. Transplantation. 2001;71:886–892. doi: 10.1097/00007890-200104150-00011. [DOI] [PubMed] [Google Scholar]
- 29.Barber LD, Whitelegg A, Madrigal JA, Banner NR, Rose ML. Detection of vimentin-specific autoreactive CD8+ T cells in cardiac transplant patients. Transplantation. 2004;77:1604–1609. doi: 10.1097/01.tp.0000129068.03900.25. [DOI] [PubMed] [Google Scholar]
- 30.Leong HS, Mahesh BM, Day JR, Smith JD, McCormack AD, Ghimire G, Podor TJ, Rose ML. Vimentin autoantibodies induce platelet activation and formation of platelet-leukocyte conjugates via platelet-activating factor. J Leukoc Biol. 2008;83:263–271. doi: 10.1189/jlb.0607339. [DOI] [PubMed] [Google Scholar]
- 31.Azimzadeh AM, Pfeiffer S, Wu GS, Schroder C, Zhou H, Zorn GL, 3rd, Kehry M, Miller GG, Rose ML, Pierson RN., 3rd Humoral immunity to vimentin is associated with cardiac allograft injury in nonhuman primates. Am J Transplant. 2005;5:2349–2359. doi: 10.1111/j.1600-6143.2005.01022.x. [DOI] [PubMed] [Google Scholar]
- 32.Mahesh B, Leong HS, McCormack A, Sarathchandra P, Holder A, Rose ML. Autoantibodies to vimentin cause accelerated rejection of cardiac allografts. Am J Pathol. 2007;170:1415–1427. doi: 10.2353/ajpath.2007.060728. • These results demonstrated that autoantibodies to vimentin in concert with the alloimmune response play a pathogenic role in allograft rejection.
- 33.Guilherme L, Kalil J. Rheumatic fever and rheumatic heart disease: cellular mechanisms leading autoimmune reactivity and disease. J Clin Immunol. 2010;30:17–23. doi: 10.1007/s10875-009-9332-6. [DOI] [PubMed] [Google Scholar]
- 34.Morgun A, Shulzhenko N, Unterkircher CS, Diniz RV, Pereira AB, Silva MS, Nishida SK, Almeida DR, Carvalho AC, Franco M, et al. Pre- and post-transplant anti-myosin and anti-heat shock protein antibodies and cardiac transplant outcome. J Heart Lung Transplant. 2004;23:204–209. doi: 10.1016/S1053-2498(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 35.Warraich RS, Pomerance A, Stanley A, Banner NR, Dunn MJ, Yacoub MH. Cardiac myosin autoantibodies and acute rejection after heart transplantation in patients with dilated cardiomyopathy. Transplantation. 2000;69:1609–1617. doi: 10.1097/00007890-200004270-00015. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka M, Zwierzchoniewska M, Mokhtari GK, Terry RD, Balsam LB, Robbins RC, Fedoseyeva EV. Progression of alloresponse and tissue-specific immunity during graft coronary artery disease. Am J Transplant. 2005;5:1286–1296. doi: 10.1111/j.1600-6143.2005.00880.x. [DOI] [PubMed] [Google Scholar]
- 37.Lompre AM, Nadal-Ginard B, Mahdavi V. Expression of the cardiac ventricular alpha- and beta-myosin heavy chain genes is developmentally and hormonally regulated. J Biol Chem. 1984;259:6437–6446. [PubMed] [Google Scholar]
- 38.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, Meyer KC, Hayney MS, Braun RK, Greenspan DS, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–3506. doi: 10.1172/JCI28031. • Strong col(V)-specific responses were associated with substantially increased incidence and severity of BOS. Col(V)-specific responses were dependent on both CD4+ T cells and monocytes and required both IL-17 and the monokines TNF-alpha and IL-1beta.
- 39.Iwata T, Philipovskiy A, Fisher AJ, Presson RG, Jr., Chiyo M, Lee J, Mickler E, Smith GN, Petrache I, Brand DB, et al. Anti-type V collagen humoral immunity in lung transplant primary graft dysfunction. J Immunol. 2008;181:5738–5747. doi: 10.4049/jimmunol.181.8.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braun RK, Molitor-Dart M, Wigfield C, Xiang Z, Fain SB, Jankowska-Gan E, Seroogy CM, Burlingham WJ, Wilkes DS, Brand DD, et al. Transfer of tolerance to collagen type V suppresses T-helper-cell-17 lymphocyte-mediated acute lung transplant rejection. Transplantation. 2009;88:1341–1348. doi: 10.1097/TP.0b013e3181bcde7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sumpter TL, Wilkes DS. Role of autoimmunity in organ allograft rejection: a focus on immunity to type V collagen in the pathogenesis of lung transplant rejection. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1129–1139. doi: 10.1152/ajplung.00330.2003. [DOI] [PubMed] [Google Scholar]
- 42.Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol. 2007;148:32–46. doi: 10.1111/j.1365-2249.2007.03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan X, Paez-Cortez J, Schmitt-Knosalla I, D’Addio F, Mfarrej B, Donnarumma M, Habicht A, Clarkson MR, Iacomini J, Glimcher LH, et al. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J Exp Med. 2008;205:3133–3144. doi: 10.1084/jem.20081937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, Mohanakumar T. De novo production of K-alpha1 tubulin-specific antibodies: role in chronic lung allograft rejection. J Immunol. 2008;180:4487–4494. doi: 10.4049/jimmunol.180.7.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fukami N, Ramachandran S, Saini D, Walter M, Chapman W, Patterson GA, Mohanakumar T. Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. J Immunol. 2009;182:309–318. doi: 10.4049/jimmunol.182.1.309. •• Native murine lungs treated with anti-MHC class I antibodies showed increased expression of chemokines and chemokine receptors, growth factors and induced IL-17. Antibodies to donor MHC antigens can induce autoimmunity mediated by IL-17 which plays a role in chronic lung rejection.
- 46.Dragun D, Muller DN, Brasen JH, Fritsche L, Nieminen-Kelha M, Dechend R, Kintscher U, Rudolph B, Hoebeke J, Eckert D, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352:558–569. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 47.Zhang M, Austen WG, Jr., Chiu I, Alicot EM, Hung R, Ma M, Verna N, Xu M, Hechtman HB, Moore FD, Jr., et al. Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 2004;101:3886–3891. doi: 10.1073/pnas.0400347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang M, Alicot EM, Carroll MC. Human natural IgM can induce ischemia/reperfusion injury in a murine intestinal model. Mol Immunol. 2008;45:4036–4039. doi: 10.1016/j.molimm.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ciubotariu R, Liu Z, Colovai AI, Ho E, Itescu S, Ravalli S, Hardy MA, Cortesini R, Rose EA, Suciu-Foca N. Persistent allopeptide reactivity and epitope spreading in chronic rejection of organ allografts. J Clin Invest. 1998;101:398–405. doi: 10.1172/JCI1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valujskikh A, Fedoseyeva E, Benichou G, Heeger PS. Development of autoimmunity after skin graft rejection via an indirect alloresponse. Transplantation. 2002;73:1130–1137. doi: 10.1097/00007890-200204150-00021. •• This study shows that self-restricted, autoreactive T cells can be primed via the indirect recognition pathway. While the initial T cell priming is directed at alloantigenic determinants, chronic rejection involves autoreactive T cells.
- 51.Duty JA, Szodoray P, Zheng NY, Koelsch KA, Zhang Q, Swiatkowski M, Mathias M, Garman L, Helms C, Nakken B, et al. Functional anergy in a subpopulation of naive B cells from healthy humans that express autoreactive immunoglobulin receptors. J Exp Med. 2009;206:139–151. doi: 10.1084/jem.20080611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsumiyama K, Miyazaki Y, Shiozawa S. Self-organized criticality theory of autoimmunity. PLoS One. 2009;4:e8382. doi: 10.1371/journal.pone.0008382. • This study demonstrates that naïve CD4+ T cells can be activated through repeated antigenic exposure and acquire autoreactivity.