Abstract
Because leptin reduces food intake and body weight, the coexistence of elevated leptin levels with obesity is widely interpreted as evidence of “leptin resistance.” Indeed, obesity promotes multiple cellular processes that attenuate leptin signaling (referred to here as “cellular leptin resistance”), and which amplify the extent of weight gain induced by genetic and environmental factors. As commonly employed, however, the term “leptin resistance” embraces a range of phenomena that are distinct with respect both to underlying mechanisms and pathophysiological implications. Moreover, the induction of cellular leptin resistance by obesity complicates efforts to distinguish the mechanisms that predispose to weight gain from those that result from it. We suggest a framework for approaching these issues and important avenues for future investigation.
Keywords: Leptin, obesity, genetic models, inflammation, SOCS3, behaviour, diet
Leptin Action and the concept of “gleptin resistance”
Leptin, a polypeptide hormone that is produced by adipocytes in proportion to their triglyceride content, links changes in body energy (fat) stores to adaptive responses in the central control of energy balance (1–4). By binding to and activating the long form of its receptor (LEPR-B) in the brain, leptin decreases food intake while increasing energy expenditure. Evolutionary considerations, together with a large body of experimental data, indicate that a major physiologic role of leptin is to respond to and defend against reductions of body fat (and thus leptin) that might impair survival and reproductive fitness. With the notable exception that body fat mass is markedly increased, the phenotypes of humans and rodents that lack leptin or LEPR-B mirror the physiological response to starvation (hunger, decreased metabolic rate, infertility, immune dysfunction, insulin resistance, etc.). Thus, leptin action is required for energy stores to be sensed in the central nervous system (CNS), and as such is essential for normal energy homeostasis, reproduction, and the like.
Leptin replacement effectively reverses the altered physiology associated with low-leptin states, including in genetic leptin deficiency (e.g., Lepob/ob mice and rare humans with loss of function mutations in the leptin gene)(5–7), lipodystrophic syndromes (the lack of adipose tissue results in a corresponding diminution of circulating leptin)(8–9), and otherwise normal weight-reduced humans whose circulating leptin is decreased due to diminished fat mass (10–12). Moreover, exogenous leptin acutely decreases feeding and body weight in normal animals, and is a powerful determinant of energy expenditure in fasted animals (5, 13–14). These observations establish leptin deficiency as a key regulator of metabolic and neuroendocrine responses to states characterized by negative energy balance and weight loss.
Although leptin administration reduces food intake in normal animals, food intake ultimately returns toward normal during prolonged leptin administration, once body fat stores have been substantially depleted (5). Moreover, treatment with leptin alone (even at very high doses) is ineffective as a means to decrease food intake and body weight in obese animals and humans, although congenital leptin-deficiency states represent an exception to this rule (15). Indeed, the subset of overweight and obese human subjects who demonstrate the strongest catabolic response to leptin are those at the lower end of the obese BMI range, and those with relatively low leptin levels for any given BMI/level of adiposity (16–17). Together with the aforementioned finding of elevated circulating leptin levels in obese subjects (commensurate with their adipose mass) (18–19), these observations have inspired the notion of “leptin resistance” in common forms of obesity (20)- analogous to the insulin resistance that contributes to type 2 diabetes, and which often coexists with “leptin resistance” in obese individuals. Indeed, similar cellular mechanisms may attenuate the action of both hormones, as detailed next.
LEPR-B signaling
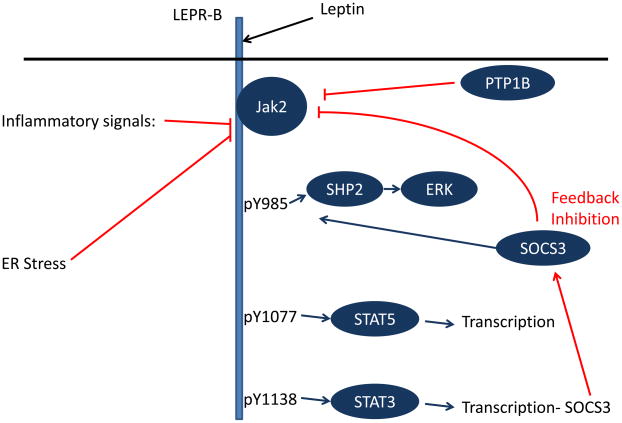
LEPR-B is a type 1 cytokine receptor that, upon leptin binding to its extracellular domain, undergoes a conformational change to activate its associated Jak2 tyrosine kinase (21). Activated Jak2 promotes the tyrosine phosphorylation of a number of intracellular residues on LEPR-B (as well as on Jak2 itself), and each tyrosine phosphorylation site recruits a specific set of downstream molecules to promote specific intracellular signals (Figure 1). LEPR-B contains three distinct tyrosine phosphorylation sites: Tyr985, Tyr1077, and Tyr1138 (22). Tyr1138 recruits signal transducer and activator of transcription-3 (STAT3), a latent transcription factor, which subsequently becomes tyrosine phosphorylated (pSTAT3) by Jak2, enabling its nuclear translocation and promoting its transcriptional effects. The detection of pSTAT3 is used as an important bioassay of LEPR-B signaling in vivo (23). Similarly, Tyr1077 recruits and mediates the phosphorylation and activation of a related transcription factor, STAT5 (22, 24). Tyr985 recruits the tyrosine phosphatase PTPN11 (a.k.a., SHP2; which controls ERK activation), and also binds the suppressor of cytokine signaling-3 (SOCS3; an inhibitor of LepRb/Jak2 signaling) (25–26).
Figure 1.
Schematic diagram of LepRb signaling and mechanisms of cellular leptin resistance. Leptin binding promotes the activation of LEPR-B-associated Jak2, which phosphorylates three tyrosine residues on the intracellular tail of LEPR-B. Each of these phosphorylated residues recruits a unique set of downstream signaling molecules. Phosphorylated Tyr985 (pY985) recruits SHP2 (which participates in ERK activation) and SOCS3 (an inhibitor of LEPR-B signaling). pY1077 recruits the transcription factor STAT5, while pY1138 recruits STAT3. A variety of processes contribute to the attenuation of LEPR-B signaling (red lines), including the feedback inhibition that occurs by STAT3-promoted SOCS3 accumulation. PTP1B, ER stress, and inflammatory signals may also participate in the inhibition of LEPR-B signaling in obesity.
Processes that attenuate LEPR-B signaling
LEPR-B Tyr1138-STAT3 signaling promotes the transcription and accumulation of SOCS3, which binds to Tyr985. SOCS3 binding to Tyr985 attenuates LEPR-B signaling, completing a negative feedback loop (25–26); indeed, disruption of LEPR-B Tyr985 or CNS SOCS3 in mice decreases food intake and adiposity (27–29). Furthermore, disruption of the afferent limb of this feedback pathway (i.e., Tyr1138-STAT3) also increases the amplitude and duration of Jak2 activation in cultured cells, and some leptin effects (e.g., on the immune system) are enhanced in Tyr1138 mutant animals (30).
Similarly, the protein tyrosine phosphatase, PTP1B, mediates the dephosphorylation of Jak2, limiting the extent of leptin action in cultured cells and in vivo (31–32). As is true for SOCS3, inactivation of PTP1B in the brain of mice increases leptin signaling and decreases adiposity, implying a physiological role for both proteins to limit signaling via LEPR-B (33–34).
Other pathways limit cellular leptin action as well. In peripheral tissues (such as adipose, liver, and muscle), obesity promotes both endoplasmic reticulum (ER) stress and a state of chronic low-level inflammation that contributes to insulin resistance; both of these processes may also participate in the attenuation of CNS LEPR-B signaling in obesity (35–37) (Figure 1). For example, obesity is associated with hypothalamic ER stress, which impairs LEPR-B signaling in cultured cells; conversely, attenuation of ER stress improves leptin signaling and leptin action in vivo. Increased activity of inflammatory signaling pathways in the hypothalamus of obese animals can impair leptin signaling both in vivo and in cultured cell models, whereas genetic or pharmacological blockade of inflammatory signals in the brain of obese rodents promotes leptin action and protects against DIO (35–37). The story is more complicated here, however, as some forms of systemic inflammation (acute infection, cancer cachexia, etc.) promote anorexia and weight loss via mechanisms involving hypothalamic systems that are also targets for leptin action (38).
Thus, SOCS3, PTP1B, ER stress, and inflammation represent some of the molecular and cellular mediators that directly attenuate LEPR-B signaling in states of obesity, and as such represent mediators of cellular leptin resistance. While they clearly contribute to diminished leptin action in obesity, the degree to which these responses themselves enable weight gain and/or the maintenance of increased adiposity in obese individuals remains incompletely understood.
Assessing leptin resistance in genetic models
Recent years have witnessed a dramatic increase in the number of genetic mouse models of obesity, and measures of leptin sensitivity (Box 1) have become a routine component of efforts to investigate mechanisms underlying such obesity. To our knowledge, measures of leptin sensitivity are diminished across obese animal models- including both diet-induced obesity (DIO) and models of monogenic or polygenic obesity in both rats and mice (the exception being Lepob/ob mice). Consequently, labeling these model organisms “leptin resistant” is synonymous with calling them “obese,” and adds little to our understanding of underlying mechanisms.
BOX 1. Measuring “gleptin resistance” in vivo.
Assessment of LEPR-B signaling and leptin action in vivo essentially relies on two assays: Detection of leptin-stimulated pSTAT3 in the hypothalamus (by immunoblotting or immunohistochemical methods), and responses of food intake and body weight/fat content to leptin administration (23). The assessment of pSTAT3 levels has emerged as the gold standard experimental readout for cellular LEPR-B action in vivo. The strength of this approach is that hypothalamic pSTAT3 is engaged rapidly and directly by LEPR-B, and most detectable hypothalamic pSTAT3 is typically attributable to leptin action (21, 68). Although hypothalamic pSTAT3 represents a sensitive and specific readout of LEPR-B signaling, STAT3 is not the sole mediator of cellular leptin action, however (21). Leptin action in the hypothalamus also mediates signaling by STAT5, ERK, PI3 kinase, mTOR, AMPK, and potentially other pathways that are partially or completely independent of STAT3 (69–73). Leptin also controls the membrane potential and firing of its target neurons, and such rapid effects do not involve nuclear STAT3 signaling (74). Unfortunately, many of these other pathways are more difficult to detect than pSTAT3, can be influenced by factors other than leptin (e.g., PI3 kinase is strongly activated by insulin, and mTOR is controlled by amino acid availability), and may be mediated trans-synaptically, which confounds their use as readouts of cellular leptin signaling (71, 75). Consequently, the field relies primarily on pSTAT3 as the readout for cellular leptin signaling despite ongoing uncertainty as to how this readout reflects the responsiveness of these other leptin-regulated pathways that may (or may not) be affected in obesity. Indeed, since leptin administration produces both acute effects (generally mediated by fast-acting cellular kinase cascades, such as ERK, PI3 kinase, and mTOR) and longer-term, transcriptional signals (e.g., via STAT3 and STAT5), acute and chronic leptin signals may be affected differently by mediators of cellular leptin resistance (76). Unfortunately, acute leptin action is poorly studied in chronic obese states. Thus, while pSTAT3 remains a crucial readout for LEPR-B signaling in vivo, additional assays are required to enable the examination of LEPR-B signaling and cellular leptin resistance more completely.
Furthermore, because obesity promotes various pathways of cellular leptin resistance (enumerated already in this review), it is unsurprising that leptin action should be compromised in obese animals. As importantly, the question of whether the altered parameters of leptin action in a particular animal model of obesity reflect the underlying initiating mechanism of obesity, a consequence of obesity, or some combination thereof remains largely unanswered. Hence, indices of leptin action in obese animals are of limited value unless they are obtained before weight gain occurs or in animals whose adiposity is otherwise matched to controls.
To facilitate heuristic analysis of these models, we consider several classes of genetic obesity (which occur in humans as well as in animal models): 1) Alterations in LEPR-B or LEPR-B signaling, 2) disruption of neural pathways known to participate in leptin action, 3) alterations in peripheral tissues that promote adiposity independently of changes of food intake, and 4) changes that are potentially, but not definitively, related to leptin action.
1. Alterations in LEPR-B or LEPR-B signaling
Animals (or very rare humans) with primary hypomorphic LEPR-B mutations are perhaps the most straightforward to classify, since such individuals have cellular leptin resistance in its purest form, and there can be no question that the failure of cellular leptin action is causal to obesity pathogenesis in these animals. One could similarly link alterations that compromise LEPR-B trafficking or downstream LEPR-B signaling (e.g., interference with LEPR-B → STAT3 signaling, activation of inflammatory signals, etc.) (36, 39–41) to obesity arising as a primary consequence of cellular leptin resistance. In each of these cases, diminished LEPR-B signaling (e.g., pSTAT3) and leptin action is observed under all conditions.
2. Disruption of neural pathways participating in leptin action
For disruption of neural pathways involved in leptin action, the hypothalamic melanocortin system affords an informative example. In this system, proopiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus (ARC) project to downstream targets (such as the paraventricular hypothalamic nucleus, PVH) where they release POMC-derived peptides, including α-MSH, that activate CNS melanocortin receptors to reduce food intake and increase energy expenditure (42–43). Many ARC POMC neurons express LEPR-B, and leptin increases the activity of the melanocortin system. Disruption of melanocortin action by physical lesions of the ARC or PVH, by pharmacological means, or by various genetic alterations at the level of the melanocortin peptide or its receptors, causes obesity and proportionate hyperleptinemia. In the case of such disruptions (for instance, in the case of animals null for the melanocortin 4 receptor (44)), although obese animals display cellular leptin resistance and severe attenuation of leptin action on feeding, pre-obese animals will have normal cellular LEPR-B signaling and only modestly diminished impact of leptin on feeding and body weight. Terming this form of obesity “leptin resistance” obscures a great deal of mechanistic detail regarding the primarily affected (e.g., melanocortin) pathway, which lies downstream of cellular LEPR-B signaling/action.
3. Alterations in peripheral tissues that operate independently of food intake
Under certain conditions, genetic alterations that affect energy metabolism in peripheral tissues can promote increased adiposity. For instance, disruption of mitochondrial uncoupling protein-1 (Ucp1), which mediates a mitochondrial proton leak to convert fat energy to heat in brown adipose tissue, diminishes energy expenditure and promotes increased adiposity in animals housed at thermoneutrality (although these effects are difficult to detect under other conditions) (45–46). While the potential “leptin resistance” of such animals is rarely examined (why would you, given that the lesion clearly lies outside of the leptin pathways?), one would predict that these animals should exhibit a blunting of cellular leptin action and leptin effects on feeding when studied in the obese state, but that both should be normal in the pre-obese, lean state.
4. Alterations in CNS pathways with no clear link to leptin action
Alterations in CNS pathways without a clear primary relationship to leptin action include impairment of brain-derived neurotrophic factor (BDNF) or its receptor, TrkB (47–48); disruption of pathways involved in Bardet-Biedel Syndrome (49), Prader-Willi Syndrome (50), and other obesity-provoking genetic alterations. While the cellular and anorectic response to leptin under the obese condition in these models can be expected to (and does) reveal cellular leptin resistance and decreased leptin action on energy balance (49), such studies do not address whether this impairment is secondary to obesity, or occurs independently of obesity and may thereby contribute to obesity pathogenesis. Testing for a primary defect in cellular LEPR-B action (manifest as decreased pSTAT3) that operates independently of obesity can shed light on which comes first: leptin resistance versus obesity. The observation of partial reduction of food intake in response to leptin in the presence of normal LEPR-B signaling requires cautious interpretation, however, as this may reflect disruption of either leptin-regulated downstream neural pathways or other neural systems that modulate feeding.
In summary, while mechanisms of cellular leptin resistance are likely to have important implications for energy balance, it is important to distinguish cellular leptin resistance that is caused by obesity from the often distinct primary processes that promote obesity in genetic (as well as other, e.g., diet-induced) models.The value of obese genetic models lies in the identification of underlying molecular mechanisms that control energy balance and, when defective, can cause or predispose to obesity. To effectively determine the potential primary effect of a genetic lesion on leptin action, per se, the assays must be performed in non-obese animals.
The elephant in the room: cellular leptin resistance in context-dependent obesity, including DIO
While genetic alterations in humans and animals have taught us a great deal about mechanisms of severe obesity and the systems that govern energy balance, it appears that the changed environment, not altered genetics, underlies the burgeoning epidemic of obesity in developed and developing countries. During the last 50 years, two major changes have shifted the energy balance equation: the decreased requirement for physical energy expenditure and the increased availability and abundance of palatable calorically-dense foods. A common research model of obesity investigators, diet-induced obesity (DIO), mirrors the ubiquity of highly palatable calorie-dense foods in modern societies. In this paradigm, animals remain lean when maintained on standard chow, but increase their caloric intake and rapidly gain adipose mass when provided a calorically dense diet (generally high in both fat and sugar content). While genetic predispositions to DIO clearly exist (some rodent strains gain little weight on high-calorie compared to normal chow, while others rapidly progress to obesity) (51), it is the availability of a highly palatable diet that drives overeating and subsequent obesity in these models.
It is debated to what extent cellular mechanisms of leptin resistance (i.e., impaired LEPR-B and downstream signaling) cause and/or facilitate the obese phenotype in DIO models. Clearly, we cannot resort to examining adiposity-matched animals on each diet (as one could for genetic models), since the major factor driving the body weight difference between DIO and chow-fed lean animals is the hedonically-driven excess consumption and consequent adiposity, rather than an innate difference of leptin signaling. How then to distinguish the causes from the consequences of obesity?
Perturbing the known mechanisms of cellular leptin resistance has lent some insight here, since interfering with neuronal LEPR-B Tyr985/SOCS3, PTP1B, ER stress, and some inflammatory pathways protects against obesity and augments the response to exogenous leptin in animals fed highly palatable diets (27–29, 35–36). The development of cellular leptin resistance with increasing obesity in DIO limits the ability of leptin to control adiposity, thereby magnifying the extent of weight gain or, to be more specific, participating in the determination of the new level of body fat stores following the change in diet.
To what extent might cellular leptin resistance function as a primary factor in weight gain in DIO? In fact, several lines of evidence argue against such a causative role. First, while the pSTAT3 response to exogenous leptin may be diminished in DIO animals, baseline ARC pSTAT3 levels in the absence of exogenous leptin administration (i.e., LEPR-B signaling due to endogenous circulating leptin) are actually increased in DIO animals compared to chow-fed controls (23). Thus, the reduction in LEPR-B signaling observed with exogenous leptin administration reflects a response to pharmacologic manipulation; while the mediators of cellular leptin resistance may restrain LEPR-B signaling in obesity (i.e., baseline pSTAT3 in DIO animals should perhaps be higher yet in the absence of cellular leptin resistance), these mechanisms of cellular leptin resistance do not reduce LEPR-B activity to levels below those in chow-fed animals and thus cannot account for DIO on their own. Second, DIO animals tend to reduce their food intake and body weight when switched to a less palatable diet (52). Hence, the presence of cellular leptin resistance in DIO animals is insufficient to maintain the full obesity phenotype in the absence of the primary precipitant (palatable high-calorie chow).
We propose that increased food intake and associated adiposity promotes cellular leptin resistance in DIO, and that this cellular leptin resistance prevents LEPR-B signaling from reaching the level that it would otherwise attain in response to the increased ambient leptin, thereby further facilitating the weight gain associated with the consumption of high-calorie diet. This model has the advantage of incorporating the potential relevance of cellular leptin resistance in the pathogenesis of common forms of obesity while acknowledging that it cannot explain the entire pathogenesis of DIO. It also recognizes the potential for mechanisms of cellular leptin resistance as therapeutic targets, since mitigating these processes should enhance LEPR-B signaling, thereby reducing the degree of obesity.
The initiation of DIO
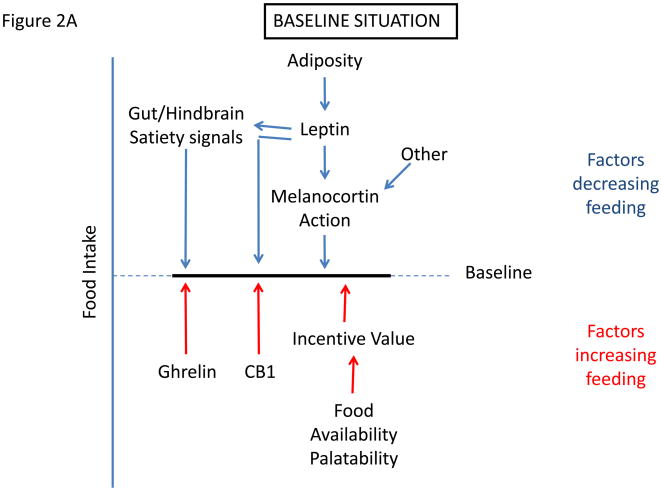
What are the key mechanisms that drive the development and maintenance of obesity in DIO? Many factors can affect food intake, only some of which are subject to biological regulation, and the amount of food that is ultimately consumed represents the integrated impact of these factors (Figure 2a). Tending to increase food intake is the hedonic attractiveness and availability of food, learned preferences, and molecular mediators of hunger, such as ghrelin and cannabinoid signaling (as well as relative reductions in ambient leptin levels with weight loss) (2–3, 53–55). Opposing these factors are leptin action, along with other mediators that promote satiety, including insulin and numerous gut-derived signals. Altering the strength of, or sensitivity to, any of these factors will alter the amount of food consumed and hence affect adiposity. Just as decreasing the strength of the leptin signal will increase feeding by diminishing anorectic drive, so will increases in the palatability and/or availability of food, even when the cellular efficacy of leptin signaling remains unchanged.
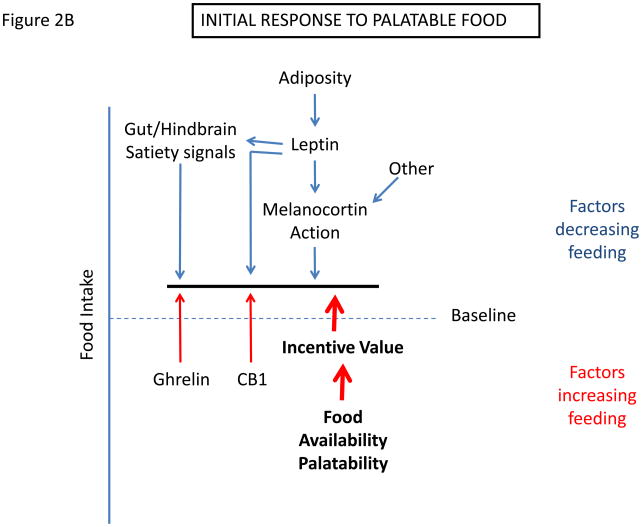
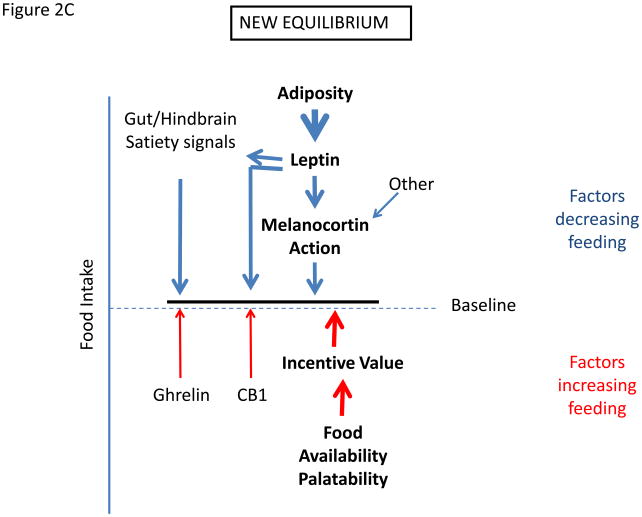
Figure 2.
Determination of settling point for food intake. (a) Schematic of mechanisms that contribute to food intake, with those factors that exert pressure to decrease feeding listed on top, and those that tend to increase feeding listed on bottom. (c) Initial response to increased palatability/availability of food. This increases feeding by increasing the drive to eat. (c) New equilibrium for food intake in the continued presence of increased food palatability/availability. Increased feeding promotes increased adiposity, which increases leptin action to promote earlier satiation and additional effects to decrease feeding toward the initial baseline. With obesity, cellular mediators of leptin resistance are promoted in the hypothalamus, limiting leptin action and increasing the amount of leptin/adiposity required to suppress feeding.
Hence, in DIO (and presumably in much of human obesity), the presence of palatable food favors increased food intake; as this increase in feeding causes fat stores to increase, the resultant rise in signals of energy repletion (including leptin) will eventually favor the return of food intake toward levels matched to energy expenditure, creating a new steady-state (albeit, at the price of higher adiposity) (Figure 2b and 2c). Indeed, when rodents are switched to a high-calorie diet, their energy intake initially increases dramatically, followed by a gradual return toward baseline values (normalized to metabolic mass). This return toward baseline feeding reflects the establishment of a new equilibrium at which the heightened incentive to feed mediated by the palatable food is balanced by the effects of increased anorectic signals such as leptin, gut satiety signals, etc. Thus, increased adiposity is a predictable response to the enhanced palatability of available food, even in the absence of molecular and cellular pathways that might directly interfere with leptin signaling and action.
An important corollary in this context is that if the diet-promoted increase in adiposity also induces cellular leptin resistance, the amount of circulating leptin needed to achieve this new equilibrium is proportionately increased, and this increase must occur through the further expansion of fat mass. Hence, diet-induced cellular leptin resistance leads to the defense of an even higher level of body fat stores than would otherwise occur. Thus, although cellular leptin resistance is not the primary cause of weight gain in this scenario, it influences both the amount of weight (fat) gained and possibly the subsequent defense of that elevated weight. Removal of the palatable food diminishes the strength of the orexigenic drive, allowing the now elevated (secondary to increased adiposity) leptin action to drive feeding down towards a new equilibrium value. The extent of weight loss will therefore depend on the associated diminution of cellular leptin resistance and other processes that are induced during the adaptation to obesity, with the consequence that normalization of body weight upon the removal of palatable, calorically-dense food may be incomplete.
With this background, we return to the issue of whether DIO can aptly be described as a state of leptin resistance. We suggest that this description is appropriate when referring to mechanisms that limit cellular leptin action per se, but that it is essential to distinguish this phenomenon from the initiating insult (palatable food, the genetic lesion predisposing certain animals to obesity, etc.), which may not, in and of itself, impair LEPR-B signaling and cellular leptin action.
Obesity and the notion of “gselective” leptin resistance
It has been proposed that the maintenance of reproductive function, energy expenditure, sympathetic outflow, and other leptin-regulated processes in the setting of DIO indicates impaired leptin action in obesity restricted to the control of feeding. Note that while the effect of genetic lesions that impair specific leptin-regulated pathways may produce selective leptin resistance, these represent a different case than DIO (39, 56). Several lines of evidence argue against a meaningful selectivity in leptin resistance in DIO. First, a variety of data suggest that leptin acts on both energy expenditure and feeding via overlapping sites and mechanisms (43, 57), and the nature of a process that might interfere with feeding but not energy expenditure is thus unclear. Indeed, while the ARC represents a major site of cellular leptin resistance in DIO (23), ARC leptin action modulates energy expenditure, glucose homeostasis, and other aspects of leptin action in addition to participating in food intake. Also, endogenous leptin clearly plays a role to limit appetite in DIO, as food intake quickly re-stabilizes after the initial increase of feeding on high palatability, energy-dense chow. Indeed, increasing the palatability of food promotes increased food intake despite the integrity of cellular leptin action, although food intake returns toward normal as adiposity increases (with the attendant increase of leptin levels and action) (Figure 2). The decrease of feeding and body weight upon the reinstatement of a normal chow diet suggests that the initial increase of food intake and subsequent adiposity represents a predictable response to hedonic characteristics of the novel diet, rather than the response to a diminution of leptin action. Thus, the transient increase and subsequent return of energy intake toward baseline during DIO support a model in which elevated leptin levels in obesity contribute to the control of hunger as well as energy expenditure in this setting. Furthermore, the response of obese humans to weight loss (which causes increased hunger as well as cold intolerance, decreased thyroid and sympathetic tone, etc.) is fundamentally intact (12), suggesting that the “extra” leptin in obese individuals exerts biologically relevant effects on parameters additional to those involved in the control of feeding. If one postulates that the effect of weight loss from the obese state to increase hunger reflects ongoing leptin resistance, the same must also be true for the diminished thyroid tone, cold intolerance, and so on, that also accompany weight loss. Thus, a variety of data argue against a meaningful selectivity (i.e. to the control of feeding only) in the attenuation of leptin action in DIO and common human obesity.
Potential mechanisms contributing to the maintenance of obesity with dietary intervention
If much of the obesity in developed societies represents a response to plentiful, available and palatable food, why does the withdrawal of such foods, as occurs with dieting, generally fail to achieve sustained weight loss in obese individuals? This is a difficult issue to investigate in humans, since palatable foods are readily available and virtually omni-present, even to most dieters -- a tasty, calorically-dense treat is only as far away as the nearest refrigerator, vending machine, convenience store, or dessert menu. This ubiquity of palatable, energy-dense foods likely contributes to the failure or relapse of many dieters, especially since weight loss itself potently increases the drive to eat.
To control for the vagaries of food availability, obese individuals have been hospitalized for study. When subjected to a 10% or greater weight loss (to within the high-normal weight range), such patients exhibit decreased thyroid and sympathetic tone, cold intolerance, and increased hunger (12, 58). Since these effects are reversed by the administration of low dose exogenous (“replacement”) leptin, many of these changes are attributable to the associated decreased circulating leptin concentrations that occur with weight loss. Thus, decreasing leptin concentrations from obese values provokes a physiologic response that tends to defend the obese levels of adiposity.
This adaptive response to weight loss (or rather, to the associated decrease of circulating leptin) could theoretically reflect a different baseline settling point (“threshold” for leptin action) in individuals who are predisposed to obesity or, alternatively could indicate that obesity and/or hyperleptinemia induce longer-term changes in neural systems that modulate energy balance, which, in turn, resets the system to a new and elevated defended level of adiposity. These possibilities are not mutually exclusive.
In at least some rodent models, the restoration of normal chow to DIO animals reduces food intake and adiposity (52), but not necessarily to the levels observed in animals that were never exposed to the obesogenic diet. Once obesity becomes established, therefore, an upward re-regulation of the defended level of body fat stores may occur. Indeed, there exist a number of otherwise confusing observations that can be accounted for by proposing that long-term reprogramming occurs in chronic obesity/hyperleptinemia: One example is the finding that chronic leptin overexpression in rodents, which initially promotes leanness, results in increased adiposity in the long-term (59–61). How the homeostatic system might become reset to a new and elevated level of adiposity and/or ambient leptin remains a key open question. While many components of cellular leptin resistance would be expected to diminish with decreasing adiposity (e.g., the activation of Tyr985/SOCS3-dependent feedback inhibition), other contributory processes may be relatively fixed and, hence, more difficult to reverse.
Important issues for obesity research
One implication of the foregoing discussion is that the potential causes of common obesity are myriad. Indeed, recent genome-wide association studies (GWAS) have identified common polymorphisms at numerous loci, each with very modest contributions to adiposity (62). The common polymorphisms/loci identified to date collectively account for little more than 10% of the heritable predisposition to obesity, consistent with the notion that the genetic variability in obesity susceptibility likely represents the sum of multiple small changes, each of which impacts different molecular determinants of feeding (or potentially energy expenditure). These GWAS focused on alleles with >5% frequency in the studied populations; hence, it remains to be seen whether lower frequency alleles of the same and/or other genes can account for the remainder of the genetic susceptibility.
Genetic differences affecting neural pathways controlling the perceived reward value of food can be expected to modify the magnitude of the response to a change in the type or availability of palatable foods. Such gene variants might be among many factors that influence this response, including, for example, developmentally-encoded differences in the function of the ARC melanocortin pathway; sensitivity to meal-related satiety signals; variation within pathways involved in cellular mechanisms of leptin action or resistance; learned preferences (and the factors that underlie them); and so forth. Genetic differences in the leptin receptor and its signaling pathway could also play a role in affecting obesity risk, by promoting cellular leptin resistance, although minimal genetic data currently exist to support this mechanism (with the exception of a potential role for SH2B1, a LEPR-B -associated signaling molecule that promotes leptin action and for which common polymorphisms are associated with obesity in GWAS)(63–64).
Along similar lines, there are many potential ways to lower food intake so as to achieve therapeutic benefit. A sustained increase in the strength of anorexic signals will favor the maintenance of a reduced level of body fat stores, as would interference with orexigenic signals and mediators of cellular leptin resistance; intervention at multiple independent points is likely to produce synergistic effects. Hence, many systems deserve a more detailed integrated analysis as we seek to better understand mechanisms governing food intake and to modify them therapeutically, including the neural pathways that modulate food palatability and reward. It will also be important to clarify how physiological signals, including leptin, ghrelin, etc., interact with these brain systems to modulate the hedonic drive to eat. Similarly, nutritional status and hormonal cues such as leptin and ghrelin are now recognized to impact behavior and emotion beyond feeding, including anxiety- and depression-related behaviors (65–66). It will therefore be useful to improve our understanding of these pathways and gain mechanistic insight into how mood contributes to overeating and/or eating disorders (and vice-versa).
Beyond changes in diet and lifestyle, it is clear that other environmental influences can also modulate the predisposition to obesity. Perinatal nutrition and other exposures contribute to the lifelong risk for obesity and metabolic disease (67). The mechanisms by which the early environment programs the later metabolic outcome remains unclear, although imprinting of key genes or altering the architecture of the neural circuits that control feeding and energy expenditure (or both) represent reasonable possibilities. The mechanisms and consequences of these developmental perturbations represent important avenues for future research.
Lastly, much remains to be learned about mechanisms underlying cellular leptin resistance and the relative importance of various mediators of cellular leptin resistance. Major issues in this area include mechanisms by which overfeeding and obesity promote ER stress and inflammation in key neuronal subsets, and how mechanisms of anorexigenic (e.g., sepsis) and orexigenic (i.e., that associated with cellular leptin resistance) hypothalamic inflammation differ in terms of their amplitude, timing, molecular pathways, and cell-specificity. Related questions pertain to roles for specific nutrients (e.g., fatty acids) in cellular leptin resistance in the hypothalamus, and much remains to be learned regarding the importance of such processes in obesity-associated attenuation of leptin action. It will also be crucial to determine whether and how chronic obesity and/or hyperleptinemia promotes a durable program to reset the neural expectation for higher levels of adiposity (leptin, etc.), which could occur at the level of cellular action, neural circuitry/plasticity, genomic imprinting, or the like. In this context, it is important to consider that not all such obesity-induced mechanisms that promote feeding and/or interfere with anorectic processes will necessarily alter LEPR-B signaling. If and when such processes are identified, it will be important to label them in precise, mechanistic terms, rather than lumping them together with “leptin resistance.”
Acknowledgments
Supported by NIH DK057768, DK056731, DK078056 (M.G.M.), DK083042 and DK052989 (M.W.S.), DK52431 (R.L.), grants from the American Diabetes Association (M.G.M., R.L.), American Heart Association, the Marilyn H. Vincent Foundation (M.G.M.), and the Russell Berrie Foundation (R.L.). We thank other participants in the PRISM 2008 meeting and members of the Myers lab for helpful discussions.
Footnotes
Conflicts: RJS receives research support, consults, and is on the speakers’ bureau for Amylin Pharmaceuticals, and is on the speakers’ bureau and scientific advisory board for Novo Nordisk.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Myers MG, Jr, Munzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab. 2009;9:117–123. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 3.Rosenbaum M, Leibel RL. The role of leptin in human physiology. N Engl J Med. 1999;341:913–915. doi: 10.1056/NEJM199909163411211. [DOI] [PubMed] [Google Scholar]
- 4.Ahima RS, Saper CB, Flier JS, Elmquist JK. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol. 2000;21:263–307. doi: 10.1006/frne.2000.0197. [DOI] [PubMed] [Google Scholar]
- 5.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 6.Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nature Genetics. 1996;12:318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- 7.Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O'Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 8.Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, Depaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 9.Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy [In Process Citation] Nature. 1999;401:73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 10.Chan JL, Heist K, Depaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest. 2003;111:1409–1421. doi: 10.1172/JCI17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351:987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 12.Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab. 2002;87:2391–2394. doi: 10.1210/jcem.87.5.8628. [DOI] [PubMed] [Google Scholar]
- 13.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 14.Kaiyala KJ, Morton GJ, Leroux BG, Ogimoto K, Wisse B, Schwartz MW. Identification of Body Fat Mass as a Major Determinant of Metabolic Rate in Mice. Diabetes. 2010 doi: 10.2337/db09-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bluher S, Mantzoros CS. Leptin in humans: lessons from translational research. Am J Clin Nutr. 2009;89:991S–997S. doi: 10.3945/ajcn.2008.26788E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravussin E, Smith SR, Mitchell JA, Shringarpure R, Shan K, Maier H, Koda JE, Weyer C. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity (Silver Spring) 2009;17:1736–1743. doi: 10.1038/oby.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oral EA, Chan JL. Rationale for leptin-replacement therapy for severe lipodystrophy. Endocr Pract. 2010;16:324–333. doi: 10.4158/EP09155.RA. [DOI] [PubMed] [Google Scholar]
- 18.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 19.Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, Leibel RL. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab. 1996;81:3424–3427. doi: 10.1210/jcem.81.9.8784109. [DOI] [PubMed] [Google Scholar]
- 20.Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1:1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 21.Robertson SA, Leinninger GM, Myers MG., Jr Molecular and neural mediators of leptin action. Physiol Behav. 2008 doi: 10.1016/j.physbeh.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong Y, Ishida-Takahashi R, Villanueva EC, Fingar DC, Munzberg H, Myers MG., Jr The long form of the leptin receptor regulates STAT5 and ribosomal protein S6 via alternate mechanisms. J Biol Chem. 2007;282:31019–31027. doi: 10.1074/jbc.M702838200. [DOI] [PubMed] [Google Scholar]
- 23.Munzberg H, Flier JS, Bjorbaek C. Region-Specific Leptin Resistance within the Hypothalamus of Diet-Induced-Obese Mice. Endocrinology. 2004 doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 24.Hekerman P, Zeidler J, Bamberg-Lemper S, Knobelspies H, Lavens D, Tavernier J, Joost HG, Becker W. Pleiotropy of leptin receptor signalling is defined by distinct roles of the intracellular tyrosines. FEBS J. 2005;272:109–119. doi: 10.1111/j.1742-4658.2004.04391.x. [DOI] [PubMed] [Google Scholar]
- 25.Banks AS, Davis SM, Bates SH, Myers MG., Jr Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 26.Bjorbaek C, Lavery HJ, Bates SH, Olson RK, Davis SM, Flier JS, Myers MG., Jr SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J Biol Chem. 2000;275:40649–40657. doi: 10.1074/jbc.M007577200. [DOI] [PubMed] [Google Scholar]
- 27.Bjornholm M, Munzberg H, Leshan RL, Villanueva EC, Bates SH, Louis GW, Jones JC, Ishida-Takahashi R, Bjorbaek C, Myers MG., Jr Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest. 2007;117:1354–1360. doi: 10.1172/JCI30688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjorbaek C, Flier JS. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med. 2004 doi: 10.1038/nm1072. [DOI] [PubMed] [Google Scholar]
- 29.Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med. 2004 doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- 30.Dunn SL, Bjornholm M, Bates SH, Chen Z, Seifert m, Myers MG., Jr Feedback inhibition of leptin receptor/Jak2 signaling via Tyr1138 of the leptin receptor and suppressor of cytokine signaling 3. Mol Endocrinol. 2005;19:925–938. doi: 10.1210/me.2004-0353. [DOI] [PubMed] [Google Scholar]
- 31.Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin DCA, imms-Hagen J, Chan C, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1b gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 32.Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, Moghal N, Lubkin M, Kim YB, Sharpe AH, Stricker-Krongrad A, Shulman GI, Neel BG, Kahn BB. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol. 2000;20:5479–5489. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med. 2006;12:917–924. doi: 10.1038/nm1435. [DOI] [PubMed] [Google Scholar]
- 34.Banno R, Zimmer D, De Jonghe BC, Atienza M, Rak K, Yang W, Bence KK. PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. J Clin Invest. 2010;120:720–734. doi: 10.1172/JCI39620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG, Jr, Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogimoto K, Harris MK, Jr, Wisse BE. MyD88 is a key mediator of anorexia, but not weight loss, induced by lipopolysaccharide and interleukin-1 beta. Endocrinology. 2006;147:4445–4453. doi: 10.1210/en.2006-0465. [DOI] [PubMed] [Google Scholar]
- 38.Grossberg AJ, Scarlett JM, Marks DL. Hypothalamic mechanisms in cachexia. Physiol Behav. 2010 doi: 10.1016/j.physbeh.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bates SH, Stearns WH, Schubert M, Tso AWK, Wang Y, Banks AS, Dundon TA, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signaling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 40.Cui Y, Huang L, Elefteriou F, Yang G, Shelton JM, Giles JE, Oz OK, Pourbahrami T, Lu CY, Richardson JA, Karsenty G, Li C. Essential role of STAT3 in body weight and glucose homeostasis. Mol Cell Biol. 2004;24:258–269. doi: 10.1128/MCB.24.1.258-269.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, Fu XY. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci USA. 2004;101:4661–4666. doi: 10.1073/pnas.0303992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 43.Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol. 2005;493:63–71. doi: 10.1002/cne.20786. [DOI] [PubMed] [Google Scholar]
- 44.Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, Burn P, Palmiter RD. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet. 1999;21:119–122. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- 45.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 46.Kozak LP, Anunciado-Koza R. UCP1: its involvement and utility in obesity. Int J Obes (Lond) 2008;32(Suppl 7):S32–38. doi: 10.1038/ijo.2008.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Unger TJ, Calderon GA, Bradley LC, Sena-Esteves M, Rios M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J Neurosci. 2007;27:14265–14274. doi: 10.1523/JNEUROSCI.3308-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seo S, Guo DF, Bugge K, Morgan DA, Rahmouni K, Sheffield VC. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum Mol Genet. 2009;18:1323–1331. doi: 10.1093/hmg/ddp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldstone AP. Prader-Willi syndrome: advances in genetics, pathophysiology and treatment. Trends Endocrinol Metab. 2004;15:12–20. doi: 10.1016/j.tem.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 51.Levin BE, Dunn-Meynell AA, McMinn JE, Alperovich M, Cunningham-Bussel A, Chua SC., Jr A new obesity-prone, glucose-intolerant rat strain (F.DIO) Am J Physiol Regul Integr Comp Physiol. 2003;285:R1184–1191. doi: 10.1152/ajpregu.00267.2003. [DOI] [PubMed] [Google Scholar]
- 52.Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 53.Cota D, Tschop MH, Horvath TL, Levine AS. Cannabinoids, opioids and eating behavior: The molecular face of hedonism? Brain Res Brain Res Rev. 2006;51:85–107. doi: 10.1016/j.brainresrev.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 54.Figlewicz DP, Benoit SC. Insulin, leptin, and food reward: update 2008. Am J Physiol Regul Integr Comp Physiol. 2009;296:R9–R19. doi: 10.1152/ajpregu.90725.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grill HJ. Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity (Silver Spring) 2006;14(Suppl 5):216S–221S. doi: 10.1038/oby.2006.312. [DOI] [PubMed] [Google Scholar]
- 56.Rahmouni K, Morgan DA, Morgan GM, Liu X, Sigmund CD, Mark AL, Haynes WG. Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. J Clin Invest. 2004;114:652–658. doi: 10.1172/JCI21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bates SH, Dundon TA, Seifert M, Carlson M, Maratos-Flier E, Myers MG., Jr LRb-STAT3 signaling is required for the neuroendocrine regulation of energy expenditure by leptin. Diabetes. 2004;53:3067–3073. doi: 10.2337/diabetes.53.12.3067. [DOI] [PubMed] [Google Scholar]
- 58.Rosenbaum M, Nicolson M, Hirsch J, Murphy E, Chu F, Leibel RL. Effects of weight change on plasma leptin concentrations and energy expenditure. J Clin Endocrinol Metab. 1997;82:3647–3654. doi: 10.1210/jcem.82.11.4390. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y, Scarpace PJ. The role of leptin in leptin resistance and obesity. Physiol Behav. 2006;88:249–256. doi: 10.1016/j.physbeh.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 60.Ogus S, Ke Y, Qiu J, Wang B, Chehab FF. Hyperleptinemia precipitates diet-induced obesity in transgenic mice overexpressing leptin. Endocrinology. 2003;144:2865–2869. doi: 10.1210/en.2002-0178. [DOI] [PubMed] [Google Scholar]
- 61.Knight ZA, Hannan KS, Greenberg ML, Friedman JM. Hyperleptinemia is required for the development of leptin resistance. PLoS One. 2010;5:e11376. doi: 10.1371/journal.pone.0011376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Monda KL, North KE, Hunt SC, Rao DC, Province MA, Kraja AT. The genetics of obesity and the metabolic syndrome. Endocr Metab Immune Disord Drug Targets. 2010;10:86–108. doi: 10.2174/187153010791213100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Renstrom F, Payne F, Nordstrom A, Brito EC, Rolandsson O, Hallmans G, Barroso I, Nordstrom P, Franks PW. Replication and extension of genome-wide association study results for obesity in 4923 adults from northern Sweden. Hum Mol Genet. 2009;18:1489–1496. doi: 10.1093/hmg/ddp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Z, Zhou Y, Carter-Su C, Myers MG, Jr, Rui L. SH2B1 enhances leptin signaling by both Janus kinase 2 Tyr813 phosphorylation-dependent and -independent mechanisms. Mol Endocrinol. 2007;21:2270–2281. doi: 10.1210/me.2007-0111. [DOI] [PubMed] [Google Scholar]
- 65.Liu J, Garza JC, Bronner J, Kim CS, Zhang W, Lu XY. Acute administration of leptin produces anxiolytic-like effects: a comparison with fluoxetine. Psychopharmacology (Berl) 2010;207:535–545. doi: 10.1007/s00213-009-1684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu XY, Kim CS, Frazer A, Zhang W. Leptin: a potential novel antidepressant. Proc Natl Acad Sci USA. 2006;103:1593–1598. doi: 10.1073/pnas.0508901103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simerly RB. Hypothalamic substrates of metabolic imprinting. Physiol Behav. 2008;94:79–89. doi: 10.1016/j.physbeh.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Faouzi M, Leshan R, Bjornholm M, Hennessey T, Jones J, Munzberg H. Differential Accessibility of Circulating Leptin to Individual Hypothalamic Sites. Endocrinology. 2007 doi: 10.1210/en.2007-0655. [DOI] [PubMed] [Google Scholar]
- 69.Robertson SA, Koleva RI, Argetsinger LS, Carter-Su C, Marto JA, Feener EP, Myers MG., Jr Regulation of Jak2 function by phosphorylation of Tyr317 and Tyr637 during cytokine signaling. Mol Cell Biol. 2009 doi: 10.1128/MCB.00278-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Villanueva EC, Munzberg H, Cota D, Leshan RL, Kopp K, Ishida-Takahashi R, Jones JC, Fingar DC, Seeley RJ, Myers MG., Jr Complex regulation of mammalian target of rapamycin complex 1 in the basomedial hypothalamus by leptin and nutritional status. Endocrinology. 2009;150:4541–4551. doi: 10.1210/en.2009-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 72.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signallingKey enzyme in leptin-induced anorexia. Nature. 2001;413:794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- 73.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 74.Munzberg H, Jobst EE, Bates SH, Jones J, Villanueva E, Leshan R, Bjornholm M, Elmquist J, Sleeman M, Cowley MA, Myers MG., Jr Appropriate inhibition of orexigenic hypothalamic arcuate nucleus neurons independently of leptin receptor/STAT3 signaling. J Neurosci. 2007;27:69–74. doi: 10.1523/JNEUROSCI.3168-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG, Jr, Seeley RJ, Schwartz MW. Insulin Activation of Phosphatidylinositol 3-Kinase in the Hypothalamic Arcuate Nucleus: A Key Mediator of Insulin-Induced Anorexia. Diabetes. 2003;52:227–231. doi: 10.2337/diabetes.52.2.227. [DOI] [PubMed] [Google Scholar]
- 76.Cota D, Matter EK, Woods SC, Seeley RJ. The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. J Neurosci. 2008;28:7202–7208. doi: 10.1523/JNEUROSCI.1389-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]