Abstract
Vasoactive intestinal peptide (VIP) is a basic 28 amino acid peptide that binds to a member of the class II family of G protein-coupled receptors (GPCRs). It is widely expressed throughout the body and plays an important role in numerous biological functions. VIP acts via three different GPCRs: VPAC1, VPAC2, and PAC1, which have been identified in various tissues, including brain, lung, kidney, gastrointestinal tract, tongue, and also on immunocompetent cells such as macrophages and lymphocytes. There is mounting evidence that VIP expression and signaling is altered in numerous neurological disorders, and it is becoming apparent that VIP and its receptors could be therapeutic loci for the treatment of several pathological conditions of the central nervous system. In this review, we describe the pathology of several major neurological disorders and discuss the potential pharmacotherapeutic role of VIP and its receptors for the treatment of disorders such as Alzheimer’s disease, Parkinson’s disease, and Autism Spectrum Disorders.
Keywords: Alzheimer’s disease, Autism Spectrum Disorders, neurological disorders, Parkinson’s disease, pharmacotherapeutics, pituitary adenylate cyclase-activating peptide, vasoactive intestinal peptide
Introduction
In many neurological and neurodegenerative disorders of the central nervous system (CNS), widespread neuronal cell death initiates irreparable and life-altering damage. In most cases, the current treatment strategies for these diseases have very low efficacy. However, recent research and clinical data demonstrate a large therapeutic potential for Class II G protein-coupled receptors (GPCR) and their ligands. This class of transmembrane sensory proteins offers promise as drug targets for disorders that involve both the nervous and endocrine systems, such as improper energy metabolism, cardiac dysfunction, neurodegenerative disorders, inflammation, and immune dysfunction (for a review, see [1]). One example of a GPCR ligand that is currently the subject of widespread pharmacological investigation is vasoactive intestinal peptide (VIP). This peptide is widely expressed throughout the body and is a key player in a vast range of biological functions. Due to its widespread and potent actions on the CNS, VIP and its receptors could be promising therapeutic targets for the treatment of various neurological disorders. In this review, we summarize the physiology of VIP and its receptors, and discuss their potential pharmacotherapeutic role(s) in key neurological disorders, including Alzheimer’s disease (AD), Parkinson’s disease (PD), and Autism Spectrum Disorders (ASD).
Overview of tissue expression and actions of VIP
VIP is a basic 28-amino acid peptide that was first extracted from pig small intestine and is expressed in numerous metabolically active organs such as the intestines, pancreas, urogenital tract, thyroid, adrenal glands, and the hypothalamus [2–4]. VIP is highly conserved across many different animal phyla, suggesting an important function for this peptide throughout evolution. The primary amino acid structure of VIP is identical in almost all mammals studied to date [5]. VIP is synthesized as part of a larger pro-peptide that also contains peptide histidine isoleucine (PHI) in most mammals [6], or its equivalent peptide histidine methionine (PHM) in humans [7]. The conserved nature of VIP in a number of different mammalian species not only highlights its importance throughout evolution, but may also offer a strategic advantage for the potential use of VIP-targeted therapies in disorders of the CNS.
VIP plays a role in numerous biological activities, including vasodilation and smooth muscle relaxation, stimulation of pepsinogen secretion by the chief cells of the gut, and secretion of water and electrolytes into the intestines [8]. Together, these actions help to facilitate overall gut motility and digestion. VIP has also been shown to play an important role in modulating the immune system [9]. It has been detected in several key organs of the immune system, including the thymus, spleen, and lymph nodes [10]. VIP is released from nerve terminals and immune cells within lymphoid organs and acts as a potent anti-inflammatory factor, by regulating the production of both anti- and pro-inflammatory mediators [11]. Additionally, VIP has also recently been shown to be present within the taste buds of the tongue, and VIP null mice have been shown to be hypersensitive to sweet tastants [12, 13].
Although VIP was initially classified as a gut hormone, evidence from many studies over the past 30 years has demonstrated that it also acts as a neurotransmitter in both the central (CNS) and peripheral (PNS) nervous system. VIP is widely expressed throughout numerous brain regions, with the highest expression levels found in the cerebral cortex, hippocampus, amygdala, and hypothalamus [8]. It has been shown to enhance glycogen metabolism in the cerebral cortex, regulate embryonic growth, and promote neuronal survival [14, 15]. Many studies have demonstrated that the neuroprotective properties of VIP are facilitated by promoting the expression and secretion of astroglia-derived factors in the presence of toxins [16]. Likewise, VIP is also known to play a crucial role in driving mammalian circadian rhythm, which is partly regulated by the hypothalamus-pituitary-adrenal axis. Mice lacking VIP do not exhibit the daily rise in circulating corticosterone in response to light and have deficiencies in circadian behaviors, such as poor motor rhythmicity in constant darkness and improper responses to phase-shifting light pulses [17, 18]. Thus, it is apparent that VIP functions as both an important endocrine and neuronal factor in many locations throughout the body.
VIP and its receptors: VPAC1 and VPAC2
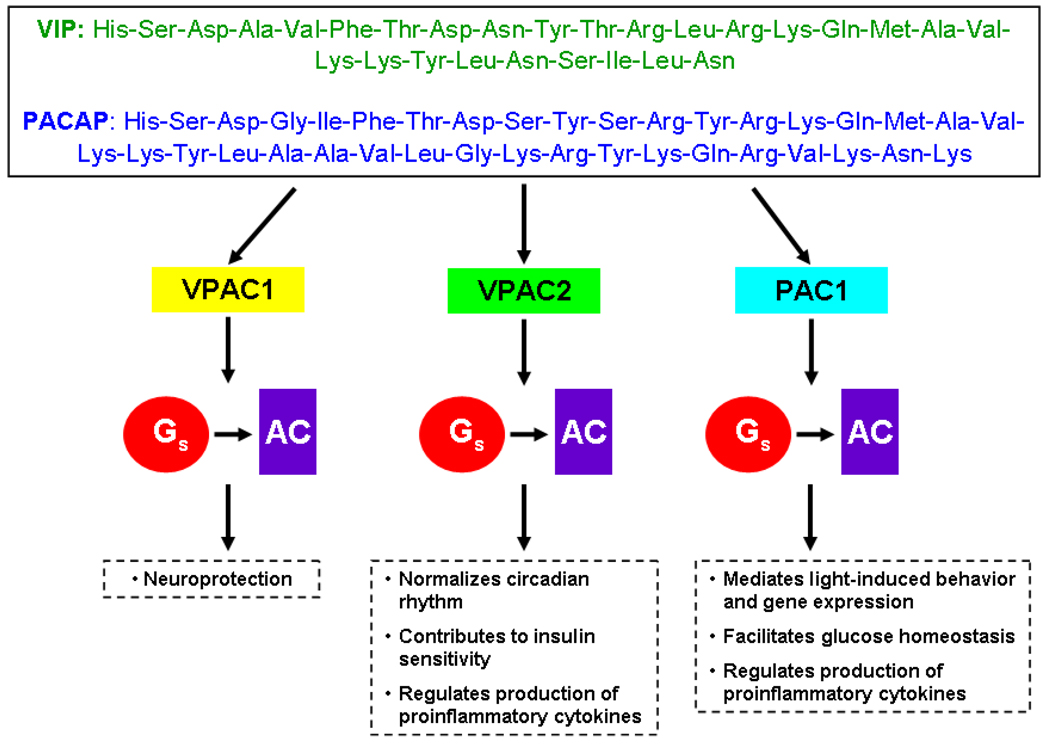
VIP exerts its biological effects through specific membrane receptors belonging to the super-family of G protein-coupled receptors (GPCRs). More specifically, VIP activates class II GPCRs or the ‘secretin receptor family’, which predominantly consists of peptide hormone and neuropeptide receptors. These receptors are comprised of seven α-helical transmembrane domains and oligopeptide intra- and extracellular loops. Other structurally related peptides include secretin, glucagon and glucagon-like peptide-1 and -2 (GLPs), growth hormone releasing hormone (GHRH), corticotropin-releasing hormone (CRH), parathyroid hormone (PTH), gastric inhibiting polypeptide (GIP), and calcitonin family ligands (for a review on GPCRs and ligands, see [1, 19]). However, the most homologous peptide to VIP is pituitary adenylate cyclase-activating peptide (PACAP), which shares 68% identity at the amino acid level. The peptides activate two common receptors: VPAC1 and VPAC2 [20, 21]. These receptors stimulate adenylate cyclase (AC), which increases intracellular cyclic adenosine monophosphate (cAMP) concentrations. In addition, activation of VPAC receptors stimulates the production of intracellular calcium [22] and also modulates the activity of phospholipase D (PLD) [23]. While VIP binds both receptor subtypes with high affinity, VPAC receptors 1 and 2 can be distinguished based on helodermin binding affinity. Helodermin is the bioactive peptide extracted from the venom of the Gila monster (Heloderma suspectum) which exhibits VIP-like physiological effects (e.g., stimulates AC and cAMP). VPAC2 is considered to be helodermin-preferring compared to VPAC1 [20]. In addition to helodermin binding efficacy, VPAC receptors can also be differentiated based upon activation by distinctive agonists and antagonists (see Table 1 for an overview of some VIP receptor agonists and antagonists). In addition to binding to VPAC1 and VPAC2, VIP can also bind with low affinity to the pituitary adenylate cyclase activating peptide (PACAP) receptor, PAC1. PACAP is a VIP-like peptide that exhibits extensive similarities to VIP and shares VIP receptors and functions [24]. PAC1 binds PACAP with high affinity and also VIP with low affinity [20]. These receptors also recognize other structurally related peptides and bind them with lower affinities than that of VIP (for overview, see [25]). Figure 1 provides an overview of VIP and PACAP-activated receptors.
Table 1. Summary of some agonists and antagonists of VIP-activated receptors.
Some agonistic and antagonistic ligands with the greatest binding affinities to VPAC1, VPAC2, and PAC1 receptors are listed. For a complete overview, see [26].
| VPAC1 | VPAC2 | PAC1 | |
|---|---|---|---|
| Agonists | VIP | [125I]BAY 55- 9837 |
PACAP-38 |
| PACAP-27 | N-stearyl- [Nle17]VIP |
||
| Ro25 1553 | Maxadilan | ||
| [K15, R16, L17]VIP(3- 7)/GRF(8-27) |
Helodermin | ||
| Antagonists | N-stearyl- [Nle17] neurotensin(6- 11)/VIP(7-28) |
N-stearyl- Nle17] neurotensin(6- 11)/VIP(7-28 |
N-stearyl-[Nle17] neurotensin(6-11)/VIP(7-28) |
| Max.d.4 | |||
| PG 97-269 | PG 99-465 | PACAP(6-38) | |
| PG 97-269 | PG 97-269 |
Figure 1. Overview of VIP, PACAP, and their receptors.
The amino acid sequences of vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating peptide (PACAP) are given, demonstrating the high degree of homology shared by the peptides. Both VIP and PACAP bind VPAC1, VPAC2, and PAC1 receptors, initiating a signaling pathway which is transduced by the s subunit of the G protein complex (Gs), and Gs then stimulates adenylate cyclase (AC). Some of the putative functions of each receptor are also summarized.
Although receptors activated by VIP are widely distributed throughout the entire body, VPAC1 and VPAC2 receptors exhibit slightly different expression patterns. VPAC1 receptors are found in many tissues and organs, such as the liver, kidney, prostate, breast, spleen, lung, gastrointestinal tract, and lymph nodes [27]. Within the CNS, VPAC1 is expressed in the pyriform cortex, putamen, supraoptic nucleus, choroid plexus, dentate gyrus, and the pineal gland [28–30]. PAC2 receptors are predominantly present in smooth muscle layers of organs and blood vessels as well as the cerebral cortex, periventricular nucleus, suprachiasmatic nucleus (SCN), thalamus, hypothalamus, and amygdala of the CNS [29–32]. Nevertheless, anatomical mapping of these receptors demonstrates one or both receptors in regions of the body in which VIP effects are described, such as the gastrointestinal tract and CNS [28].
While the expression pattern of VPAC receptors has been extensively studied, much less detail is known about the biological functions of these receptors. Many studies to date have used mutant mouse models in order to validate putative physiological workings of VIP-activated receptors. Studies of VPAC2 knockout mice have demonstrated that this receptor is involved in generating normal circadian rhythms of electrical activity, clock gene expression, and behavior [33–36]. PAC1 also appears to play a similar functional role by facilitating light-induced behavioral rhythm and gene expression, which has been investigated using PAC1 receptor and PACAP ligand deficient mice [37–39]. Mice lacking the VPAC2 receptor were also shown to have greater insulin sensitivity, as they were able to maintain a normal glucose response in the presence of lower insulin levels in comparison to wild-type mice [40]. In contrast, PAC1 null mice exhibited deficient insulin levels in response to glucose, reduced glucose tolerance, and an impaired glucagon response to insulin-induced hypoglycaemia [41, 42]. VIP and PACAP are also thought to play a role in regulating immunity and inflammation. VIP helps to modulate the production of cytokines by T helper 1 (Th1) and T helper 2 (Th2) cells. Studies using VPAC2 receptor knockout mice and transgenic mice overexpressing the VPAC2 receptor have revealed that the receptor regulates the balance between Th1 and Th2 by stimulating production of more Th2-type cytokines, which mediate hypersensitivity reactions (e.g. allergy) [43–45]. Likewise, upon activating the PAC1 receptor, endotoxin-induced septic shock appears to be prevented, which is thought to occur by reducing the production of proinflammatory interleukin-6 (IL-6) [46]. Because VIP and PACAP are widely expressed throughout the body and obviously play an important role in a myriad of peripheral and CNS functions, VPAC and PAC receptors offer prime targets for the development of novel pharmacotherapeutics.
VIP and its role in the treatment of neurological disorders of the CNS
Within the realm of drug discovery and pharmacotherapeutics, recent research and clinical findings have focused on the exploitation of class II G protein-coupled receptors (GPCRs) in the development of new therapeutic agents. Ligands that activate these receptors, such as VIP, have been shown to be beneficial drug targets for the treatment of numerous neurological disorders. For the remainder of this review, we will discuss the role of VIP in the pathology and treatment of some major neurological disorders, including Alzheimer’s disease, Parkinson’s disease, and Autism Spectrum Disorders.
Alzheimer’s disease and VIP
Alzheimer’s disease (AD) is a neurodegenerative disorder that is typically diagnosed later in life and affects more than 35 million people worldwide [47]. The disease is characterized by extracellular deposits of fibrillar β-amyloid (Aβ) that compact into senile plaques in the brain. These plaques activate microglia and astrocytes that release proinflammatory cytokines and chemokines. This initiates the inflammatory process, resulting in neurodegeneration over time. The neuronal loss associated with AD results in gross atrophy of affected regions within the temporal and parietal lobes. Functionally, the disease primarily affects the hippocampus and neocortex, causing progressive loss in cognitive ability and memory function, ultimately leading to dementia [48, 49]. Therefore, therapeutic agents that target these inflammatory processes present great potential for slowing AD pathophysiology.
VIP has been implicated in the treatment of AD on account of its neuroprotective properties. In a study by Delgado and colleagues [49], VIP was shown to effectively limit harmful Aβ-induced microglia activation and the subsequent release of neurotoxins, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and nitric oxide (NO). Thus, VIP prevented the severe neuronal cell death that leads to AD pathology in the brain. It has been postulated that the protective effect of VIP occurs upon activation of the VPAC1 receptor by initiating the cAMP/protein kinase A (PKA) signaling pathway. In turn, this stimulates neuroprotective glial proteins, such as activity-dependent neurotrophic factor (ADNF). ADNF influences neuronal survival by recognizing neurotoxic substances, including β-amyloid [50]. VPAC1-mediated cAMP/PKA signaling also impairs the nuclear translocation and DNA binding of NFkB by inhibiting IKK-mediated IkB phosphorylation and blocks both p38 MAPK and p42/p44 ERK by inhibiting upstream regulatory kinases [49]. Because VIP exhibits both anti-inflammatory and neuroprotective properties, this pluripotent peptide offers promise as a therapeutic approach for the treatment of AD.
Once released in the body, however, VIP is quickly degraded by enzymes, leaving the peptide with a very short half-life. In recent years, VIP analogs have been created to address these obstacles to therapeutic use. For example, fatty acid acylation of the native peptide has proven to be successful in addressing issues such as poor metabolic stability and reduced biological activity [1]. The VIP analog stearyl-Nle17-VIP (SNV) is 100-fold more potent than the native peptide and has been shown to provide significant neuroprotection by raising cellular resistance against oxidative stress [51]. With further careful research, derivatives of VIP could potentially offer greater therapeutic benefit than the native peptide for the treatment of devastating neurodegenerative disorders such as AD.
Parkinson’s disease and VIP
Like AD, Parkinson’s disease (PD) is a common neurodegenerative disease of the CNS and primarily impairs an individual’s motor skills and speech. Parkinsonian movement is characterized by tremor at rest, stiffness, postural instability, and akinesia or bradykinesia, which are thought to be caused by dopamine deficiency. This is supported by an observed decrease in neuronal density due to widespread cell death in the substantia nigra of individuals diagnosed with PD [52]. More specifically, the abnormal firing patterns of (GABA)ergic neurons are altered by the degeneration of the nigrostriatal dopaminergic neurons. When these neurons are damaged, activation of upper motor neurons is abnormally changed, leading to parkinsonian symptoms [53]. Current treatments for PD are mainly aimed at temporarily alleviating some symptoms of the disease, as chronic drug use has not proven to be very efficacious in slowing the progression of this disease.
As VIP has been shown to protect against neuronal cell death and act as a modulator of the inflammatory immune response, it has been implicated as a viable treatment option for Parkinson’s disease. In a 2003 study, Delgado and Ganea demonstrated that VIP could protect dopaminergic cells from bacterial endotoxin LPS-induced inflammation in mouse embryonic neurons. LPS treatment typically results in profound neuronal cell loss and is thought to be mediated by increased microglial activation. Therefore, VIP most likely prevents neurodegeneration by deactivating microglia and the production of proinflammatory mediators via binding of VPAC1 [54]. This has important therapeutic implications, as a loss of dopamine-producing neurons in the substantia nigra is a primary cause of PD. In a study using a rat model of Parkinson’s disease, systemically administered VIP was successful at reversing motor deficits but did not stop the decline in striatal dopamine levels. In addition, the peptide was found to preserve neurons by possibly inducing the secretion of neuroprotective agents from brain mast cells, such as nerve growth factor (NGF) [53, 55]. In a similar study using a mouse model of PD, VIP treatment significantly decreased dopaminergic neuronal loss in the substantia nigra. Once again, it was found that the therapeutic abilities of the peptide were imparted through VPAC1-mediated microglia deactivation and the subsequent production of cytotoxic mediators, such as iNOS, interleukin 1β, and tumor necrosis factor α [56].
Autism Spectrum Disorder and VIP
In contrast to AD and PD, Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder that is typically diagnosed within the first three years of life based on three areas of behavior: social deficits, impaired language and communication, and stereotyped and repetitive behaviors. Over the past 20 years, the frequency of diagnosis of ASD in children in the United States and Europe has greatly increased, with prevalence rates estimated between ten and twenty per 10,000 children [57]. To date, there is no definitive cause of ASD, but this disorder has been linked to both genetic and environmental factors. Studies of affected families and twins have indicated that ASD is highly genetic, as the relative risk of having a second child with ASD, particularly if both children are male, is 20–50 times higher than the population base rate [58]. In terms of environmental causes, incidents of ASD have been linked to thalidomide or valproic acid exposure, prenatal alcohol use, infections, and vaccinations [59]. However, it is likely that susceptibly to ASD is due to many interacting genes with the resulting phenotype modified by complex environmental factors [60].
While there is no known universal origin of the disorder, neuroanatomical and physiological studies of ASD patients have revealed several striking differences from normal subjects. For example, levels of neuropeptides, such as VIP, calcitonin gene-regulating peptide, brain-derived neurotrophic factor (BDNF), and neurotrophin 4/5 were higher than normal in the blood of newborns who were later diagnosed with ASD or mental retardation [61]. Likewise, an additional link between VIP and ASD is exemplified in a subset of ASD individuals who suffer from inflammatory bowel disorders, as VIP is an important gut peptide and immunomodulatory factor [59]. There is also evidence to suggest a connection between polymorphisms in the upstream region of the VPAC2 receptor gene with the gastrointestinal and stereotypical behaviors observed in autistic patients. In a 2001 study, Asano and colleagues investigated alterations in the VPAC2 gene in a small sample of ASD individuals and discovered 10 new polymorphisms. The most frequent deviation was a single-nucleotide polymorphism, and no change in amino acids was observed. Preliminary data showed that three polymorphisms in the upstream region of the gene in particular may have a role in autism. However, they were unable to demonstrate a significant difference in the frequencies of VPAC2 polymorphisms between ASD patients (n = 14) and unrelated control patients (n = 52) [62]. In mice, a deficit in social behavior has been observed following a blockage of VIP during neural tube closure. This is analogous to the period in human development during which the improper neural development resulting in ASD is thought to occur [59]. Therefore, the strong associations between VIP and ASD suggest that this pluripotent peptide could have pharmacotherapeutic potential. Although VIP and VIP-based analogs are not currently used to treat ASD, this peptide could potentially be a viable target for future drug design and development.
Conclusions
Although we have made great strides in uncovering the pathological nature of several disorders of the CNS in recent years, many neurological diseases that affect so many people worldwide have little or no effective treatment options. However, findings from current research have indicated a promising pharmacotherapeutic role for VIP and its receptors for treating several neurological disorders, such as AD, PD, and ASD. In AD and PD, microglia activation stands out as one of the histopathological hallmarks, causing widespread cell death. VIP has shown to be a major neuroprotective factor against this inflammatory response by inhibiting microglia-derived proinflammatory factors. Therefore, this peptide presents good potential as a therapeutic agent for inflammation-induced neurodegenerative disorders of the CNS. Likewise, alterations in VIP have also been associated with other neurological disorders, such as ASD. Although there is currently no cure or effective treatment for ASD, this peptide is worthy of future pharmacotherapeutic exploration and design, as several studies have indicated a strong link between this peptide and ASD. VIP and its receptors are widely distributed throughout the body, demonstrating that this peptide is important for a myriad of biological functions. A greater understanding of VIP, VIP receptor signaling, and their role in neurological pathologies is needed for the development of novel and efficacious therapeutics for the treatment of complex CNS disorders.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute on Aging.
Abbreviations
- AD
Alzheimer’s disease
- ASD
Autism Spectrum Disorders
- cAMP
cyclic adenosine monophosphate
- CNS
Central nervous system
- GPCR
G protein-coupled receptor
- PACAP
Pituitary adenylate cyclase-activating peptide
- PD
Parkinson’s disease
- VIP
Vasoactive intestinal peptide
Footnotes
The authors have no conflicts of scientific interest with respect to the manuscript.
References
- 1.Chapter MC, White CM, DeRidder A, Chadwick W, Martin B, Maudsley S. Chemical modification of Class II G protein-coupled receptor ligands: Frontiers in the development of peptide analogs as neuroendocrine pharmacological therapies. Pharmacol. Ther. 2010;125(1):39–54. doi: 10.1016/j.pharmthera.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Said SI, Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science. 1970;169(951):1217–1218. doi: 10.1126/science.169.3951.1217. [DOI] [PubMed] [Google Scholar]
- 3.Fahrenkrug J. Transmitter role of vasoactive intestinal peptide. Pharmacol. Toxicol. 1993;72(6):354–363. doi: 10.1111/j.1600-0773.1993.tb01344.x. [DOI] [PubMed] [Google Scholar]
- 4.Gozes I, Brenneman DE. VIP: molecular biology and neurobiological function. Mol. Neurobiol. 1989;3(4):201–236. doi: 10.1007/BF02740606. [DOI] [PubMed] [Google Scholar]
- 5.Du BH, Eng J, Hulmes JD, Chang M, Pan YC, Yalow RS. Guinea pig has a unique mammalian VIP. Biochem. Biophys. Res. Commun. 1985;128(3):1093–1098. doi: 10.1016/0006-291x(85)91052-6. [DOI] [PubMed] [Google Scholar]
- 6.Nishizawa M, Hayakawa Y, Yanaihara N, Okamoto H. Nucleotide sequence divergence and functional constraint in VIP mRNA evolution between human and rat. FEBS Lett. 1985;183(1):55–59. doi: 10.1016/0014-5793(85)80953-4. [DOI] [PubMed] [Google Scholar]
- 7.Itoh N, Obata K, Yanaihara N, Okamoto H. Human preprovasoactive intestinal peptide contains a novel PHI-27-like peptide, PHM-27. Nature. 1983;304(5926):547–549. doi: 10.1038/304547a0. [DOI] [PubMed] [Google Scholar]
- 8.Dickson L, Finlayson K. VPAC and PAC receptors: from ligands to function. Pharmacol. Ther. 2009;121(3):294–316. doi: 10.1016/j.pharmthera.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Rey E, Varela N, Chorny A, Delgado M. Therapeutic approaches of vasoactive intestinal peptide as a pleiotropic immunomodulator. Curr. Pharm. Des. 2007;13(11):1113–1139. doi: 10.2174/138161207780618966. [DOI] [PubMed] [Google Scholar]
- 10.Gomariz RP, Martinez C, Abad C, Leceta J, Delgado M. Immunology of VIP: a review and therapeutical perspectives. Curr. Pharm. Des. 2001;7(2):89–111. doi: 10.2174/1381612013398374. [DOI] [PubMed] [Google Scholar]
- 11.Delgado M, Jonakait GM, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit chemokine production in activated microglia. Glia. 2002;39(2):148–161. doi: 10.1002/glia.10098. [DOI] [PubMed] [Google Scholar]
- 12.Martin B, Shin YK, White CM, Ji S, Kim W, Carlson OD, Napora JK, Chadwick W, Chapter M, Waschek JA, Mattson MP, Maudsley S, Egan JM. Vasoactive intestinal peptide null mice demonstrate enhanced sweet taste preference, dysglycemia and reduced taste bud leptin receptor expression. Diabetes. 2010 doi: 10.2337/db09-0807. DOI: 10.2337/db09-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen T, Kaya N, Zhao FL, Lu SG, Cao Y, Herness S. Co-expression patterns of the neuropeptides vasoactive intestinal peptide and cholecystokinin with the transduction molecules alpha-gustducin and T1R2 in rat taste receptor cells. Neuroscience. 2005;130(1):229–238. doi: 10.1016/j.neuroscience.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Sorg O, Magistretti PJ. Vasoactive intestinal peptide and noradrenaline exert long-term control on glycogen levels in astrocytes: blockade by protein synthesis inhibition. J. Neurosci. 1992;12(12):4923–4931. doi: 10.1523/JNEUROSCI.12-12-04923.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenneman DE, Hill JM, Gressens P, Gozes I. Neurotrophic action of VIP: from CNS ontogeny to therapeutic strategy. In: Said SI, editor. Proinflammatory and Anti-inflammatory Peptides. New York: Marcel Dekker; 1997. pp. 383–408. [Google Scholar]
- 16.Dejda A, Sokołowska P, Nowak JZ. Neuroprotective potential of three neuropeptides PACAP, VIP and PHI. Pharmacol. Rep. 2005;57(3):307–320. [PubMed] [Google Scholar]
- 17.Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Liu X, Waschek JA. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285(5):R939–R949. doi: 10.1152/ajpregu.00200.2003. [DOI] [PubMed] [Google Scholar]
- 18.Loh DH, Abad C, Colwell CS, Waschek JA. Vasoactive intestinal peptide is critical for circadian regulation of glucocorticoids. Neuroendocrinology. 2008;88(4):246–255. doi: 10.1159/000140676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin B, Lopez de Maturana R, Brenneman R, Walent T, Mattson MP, Maudsley S. Class II G protein-coupled receptors and their ligands in neuronal function and protection. Neuromolecular Med. 2005;7(1–2):3–36. doi: 10.1385/nmm:7:1-2:003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP, Wank SA, Waschek JA. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol. Rev. 1998;50(2):265–270. [PMC free article] [PubMed] [Google Scholar]
- 21.Laburthe M, Couvineau A, Tan V. Class II G protein-coupled receptors for VIP and PACAP: structure, models of activation and pharmacology. Peptides. 2007;28(9):1631–1639. doi: 10.1016/j.peptides.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Dickson L, Aramori I, McCulloch J, Sharkey J, Finlayson K. A systematic comparison of intracellular cyclic AMP and calcium signaling highlights complexities in human VPAC/PAC receptor pharmacology. Neuropharmacology. 2006;51(6):1086–1098. doi: 10.1016/j.neuropharm.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 23.McCulloch DA, Lutz EM, Johnson MS, MacKenzie CJ, Mitchell R. Differential activation of phospholipase D by VPAC and PAC1 receptors. Ann. N. Y. Acad. Sci. 2000;921:175–185. doi: 10.1111/j.1749-6632.2000.tb06964.x. [DOI] [PubMed] [Google Scholar]
- 24.Arimura A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn. J. Physiol. 1998;48(5):301–331. doi: 10.2170/jjphysiol.48.301. [DOI] [PubMed] [Google Scholar]
- 25.Laburthe M, Couvineau A, Marie JC. VPAC receptors for VIP and PACAP. Receptors Channels. 2002;8(3–4):137–153. [PubMed] [Google Scholar]
- 26.Harmar AJ, Hills RA, Rosser EM, Jones M, Buneman OP, Dunbar DR, Greenhill SD, Hale VA, Sharman JL, Bonner TI, Catterall WA, Davenport AP, Delagrange P, Dollery CT, Foord SM, Gutman GA, Laudet V, Neubig RR, Ohlstein EH, Olsen RW, Peters J, Pin JP, Ruffolo RR, Searls DB, Wright MW, Spedding M. IUPHAR-DB: the IUPHAR database of G protein-coupled receptors and ion channels. Nucl. Acids Res. 2009;37:D680–D685. doi: 10.1093/nar/gkn728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reubi JC. In vitro evaluation of VIP/PACAP receptors in healthy and diseased human tissues. Clinical implications. Ann. N. Y. Acad. Sci. 2000;921:1–25. doi: 10.1111/j.1749-6632.2000.tb06946.x. [DOI] [PubMed] [Google Scholar]
- 28.Usdin TB, Bonner TI, Mezey E. Two receptors for vasoactive intestinal polypeptide with similar specificity and complementary distributions. Endocrinology. 1994;135(6):2662–2680. doi: 10.1210/endo.135.6.7988457. [DOI] [PubMed] [Google Scholar]
- 29.Vertongen P, Schiffman SN, Gourlet P, Robberecht P. Autoradiographic visualization of the receptor subclasses for vasoactive intestinal polypeptide (VIP) in rat brain. Ann. N. Y. Acad. Sci. 1998;865:412–415. doi: 10.1111/j.1749-6632.1998.tb11206.x. [DOI] [PubMed] [Google Scholar]
- 30.Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol. Rev. 2000;52(2):269–324. [PubMed] [Google Scholar]
- 31.Sheward WJ, Lutz EM, Harmar AJ. The distribution of vasoactive intestinal peptide 2 receptor messenger RNA in the rat brain and pituitary gland as assessed by in situ hybridization. Neuroscience. 1995;67(2):409–418. doi: 10.1016/0306-4522(95)00048-n. [DOI] [PubMed] [Google Scholar]
- 32.Harmar AJ, Sheward WJ, Morrison CF, Waser B, Gugger M, Reubi JC. Distribution of the VPAC2 receptor in peripheral tissues of the mouse. Endocrinology. 2004;145(3):1203–1210. doi: 10.1210/en.2003-1058. [DOI] [PubMed] [Google Scholar]
- 33.Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, Morrison CF, Dorin JR, Piggins HD, Reubi JC, Kelly JS, Maywood ES, Hastings MH. The VPAC2 receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell. 2002;109(4):497–508. doi: 10.1016/s0092-8674(02)00736-5. [DOI] [PubMed] [Google Scholar]
- 34.Cutler DJ, Haraura M, Reed HE, Shen S, Sheward WJ, Morrison CF, Marston HM, Harmar AJ, Piggins HD. The mouse VPAC2 receptor confers suprachiasmatic nuclei cellular rhythmicity and responsiveness to vasoactive intestinal polypeptide in vitro. Eur. J. Neurosci. 2003;17(2):197–204. doi: 10.1046/j.1460-9568.2003.02425.x. [DOI] [PubMed] [Google Scholar]
- 35.Hughes AT, Fahey B, Cutler DJ, Coogan AN, Piggins HD. Aberrant gating of photic input to the suprachiasmatic circadian pacemaker of mice lacking the VPAC2 receptor. J. Neurosci. 2004;24(14):3522–3526. doi: 10.1523/JNEUROSCI.5345-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat. Neurosci. 2005;8(4):476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hannibal J, Jamen F, Nielsen HS, Journot L, Brabet P, Fahrenkrug J. Dissociation between light-induced phase shift of the circadian rhythm and clock gene expression in mice lacking the pituitary adenylate cyclase activating polypeptide type 1 receptor. J. Neurosci. 2001;21(13):4883–4890. doi: 10.1523/JNEUROSCI.21-13-04883.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Waschek JA. Selective deficits in the circadian light response in mice lacking PACAP. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287(5):R1194–R1201. doi: 10.1152/ajpregu.00268.2004. [DOI] [PubMed] [Google Scholar]
- 39.Kawaguchi C, Tanaka K, Isojima Y, Shintani N, Hashimoto H, Baba A, Nagai K. Changes in light-induced phase shift of circadian rhythm in mice lacking PACAP. Biochem. Biophys. Res. Commun. 2003;310(1):169–175. doi: 10.1016/j.bbrc.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Asnicar MA, Koster A, Heiman ML, Tinsley F, Smith DP, Galbreath E, Fox N, Ma YL, Blum WF, Hsiung HM. Vasoactive intestinal polypeptide/pituitary adenylate cyclase-activating peptide receptor 2 deficiency in mice results in growth retardation and increased basal metabolic rate. Endocrinology. 2002;143(10):3994–4006. doi: 10.1210/en.2002-220354. [DOI] [PubMed] [Google Scholar]
- 41.Jamen F, Persson K, Bertrand G, Rodriguez-Henche N, Puech R, Bockaert J, Ahren B, Brabet P. PAC1 receptor-deficient mice display impaired insulinotropic response to glucose and reduced glucose tolerance. J. Clin. Invest. 2000;105(9):1307–1315. doi: 10.1172/JCI9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Persson K, Ahren B. The neuropeptide PACAP contributes to the glucagon response to insulin-induced hypoglycaemia in mice. Acta. Physiol. Scand. 2002;175(1):25–28. doi: 10.1046/j.1365-201X.2002.00977.x. [DOI] [PubMed] [Google Scholar]
- 43.Goetzl EJ, Voice JK, Shen S, Dorsam G, Kong Y, West KM, Morrison CF, Harmar AJ. Enhanced delayed-type hypersensitivity and diminished immediate-type hypersensitivity in mice lacking the inducible VPAC2 receptor for vasoactive intestinal peptide. Proc. Natl. Acad. Sci. USA. 2001;98(24):13854–13859. doi: 10.1073/pnas.241503798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voice JK, Dorsam G, Chan RC, Grinninger C, Kong Y, Goetzl EJ. Immunoeffector and immunoregulatory activities of vasoactive intestinal peptide. Regul. Pept. 2002;109(1–3):199–208. doi: 10.1016/s0167-0115(02)00182-9. [DOI] [PubMed] [Google Scholar]
- 45.Voice JK, Grinninger C, Kong Y, Bangale Y, Paul S, Goetzl EJ. Roles of vasoactive intestinal peptide (VIP) in the expression of different immune phenotypes by wild-type mice and T cell-targeted type II VIP receptor transgenic mice. J. Immunol. 2003;170(1):308–314. doi: 10.4049/jimmunol.170.1.308. [DOI] [PubMed] [Google Scholar]
- 46.Martinez C, Abad C, Delgado M, Arranz A, Juarranz MG, Rodriguez-Henche N, Brabet P, Leceta J, Gomariz RP. Anti-inflammatory role in septic shock of pituitary adenylate cyclase-activating polypeptide receptor. Proc. Natl. Acad. Sci. USA. 2002;99(2):1053–1058. doi: 10.1073/pnas.012367999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Querfurth HW, LaFerla FM. Alzheimer’s disease. N. Engl. J. Med. 2010;362(4):329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 48.Wenk GL. Neuropathologic changes in Alzheimer's disease. J. Clin. Psychiatry. 2003;64 Suppl 9:7–10. [PubMed] [Google Scholar]
- 49.Delgado M, Nieves V, Gonzalez-Rey E. Vasoactive intestinal peptide protects against β-amyloid-induced neurodegeneration by inhibiting microglia activation at multiple levels. Glia. 2008;56(10):1091–1103. doi: 10.1002/glia.20681. [DOI] [PubMed] [Google Scholar]
- 50.Gozes I. Neuroprotective peptide drug delivery and development: potential new therapeutics. Trends Neurosci. 2001;24(12):700–705. doi: 10.1016/s0166-2236(00)01931-7. [DOI] [PubMed] [Google Scholar]
- 51.Offen D, Sherki Y, Melamed E, Fridkin M, Brenneman DE, Gozes I. Vasoactive intestinal peptide (VIP) prevents neurotoxicity in neuronal cultures: relevance to neuroprotection in Parkinson's disease. Brain Res. 2000;854(1–2):257–262. doi: 10.1016/s0006-8993(99)02375-6. [DOI] [PubMed] [Google Scholar]
- 52.Jankovic J. Parkinson’s disease: clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry. 2008;79(4):368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 53.Korkmaz OT, Tunçel N, Tunçel M, Oncü EM, Sahintürk V, Celik M. Vasoactive intestinal peptide (VIP) treatment of Parkinsonian rats increases thalamic gamma-aminobutyric acid (GABA) levels and alters the release of nerve growth factor (NGF) by mast cells. J. Mol. Neurosci. 2009 doi: 10.1007/s12031-009-9307-3. DOI: 10.1007/s12031-009-9307-3. [DOI] [PubMed] [Google Scholar]
- 54.Delgado M, Ganea D. Vasoactive intestinal peptide prevents activated microglia-induced neurodegeneration under inflammatory conditions: potential therapeutic role in brain trauma. FASEB J. 2003a;17(13):1922–1924. doi: 10.1096/fj.02-1029fje. [DOI] [PubMed] [Google Scholar]
- 55.Tunçel N, Sener E, Cerit C, Karasu U, Gürer F, Sahintürk V, Bayçu C, Ak D, Filiz Z. Brain mast cells and therapeutic potential of vasoactive intestinal peptide in a Parkinson's disease model in rats: brain microdialysis, behavior, and microscopy. Peptides. 2005;26(5):827–836. doi: 10.1016/j.peptides.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 56.Delgado M, Ganea D. Neuroprotective effect of vasoactive intestinal peptide (VIP) in a mouse model of Parkinson’s disease by blocking microglial activation. FASEB J. 2003b;17(8):944–946. doi: 10.1096/fj.02-0799fje. [DOI] [PubMed] [Google Scholar]
- 57.Newschaffer CJ, Croen LA, Daniels J, Giarelli E, Grether JK, Levy SE, Mandell DS, Miller LA, Pinto-Martin J, Reaven J, Reynolds AM, Rice CE, Schendel D, Windham GC. The epidemiology of autism spectrum disorders. Ann. Rev. Public Health. 2007;28:235–258. doi: 10.1146/annurev.publhealth.28.021406.144007. [DOI] [PubMed] [Google Scholar]
- 58.O’Roak BJ, State MW. Autism genetics: strategies, challenges, and opportunities. Autism Res. 2008;1(1):4–17. doi: 10.1002/aur.3. [DOI] [PubMed] [Google Scholar]
- 59.Hill JM. Vasoactive intestinal peptide in neurodevelopmental disorders: therapeutic potential. Curr. Pharm. Des. 2007;13(11):1079–1089. doi: 10.2174/138161207780618975. [DOI] [PubMed] [Google Scholar]
- 60.Rodier PM, Hyman SL. Early environmental factors in autism. Ment. Retard. Devel. Dis. Res. Rev. 1998;4(2):121–128. [Google Scholar]
- 61.Nelson KB, Grether JK, Croenn LA, Dambrosia JM, Dickens BF, Jelliffe LL, Hansen RL, Phillips TM. Neuropeptides and neurotrophins in neonatal blood of children with autism or mental retardation. Ann. Neurol. 2001;49(5):597–606. [PubMed] [Google Scholar]
- 62.Asano E, Kuivaniemi H, Huq AHMM, Tromp G, Behen M, Rothermel R, Herron J, Chugani DC. A study of novel polymorphisms in the upstream region of vasoactive intestinal peptide receptor type 2 gene in autism. J. Child. Neurol. 2001;16(5):357–363. doi: 10.1177/088307380101600509. [DOI] [PubMed] [Google Scholar]